Abstract
Aim
Impact of several immune‐inflammatory markers on long‐term outcome has been reported in various malignancies. The aim of the present study was to evaluate through a meta‐analysis the oncological outcome of immune‐inflammatory markers, such as neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), and C‐reactive protein to albumin ratio (CAR) in esophageal cancer.
Methods
A systematic electronic search for relevant studies was carried out in PubMed, Cochrane library, Embase, and Google scholar. Meta‐analysis was done using hazard ratio (HR) and 95% confidence interval (CI) as effect measures. A systematic review and meta‐analysis were undertaken according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses protocol. P‐values <.01 were considered statistically significant.
Results
A total of 10 retrospective articles (n = 4551) were included in this study. Synthesized results showed that higher NLR and CAR were significantly associated with poor overall survival (HR 1.47, 95% CI = 1.32‐1.63, P < .00001) and HR 1.88, 95% CI = 1.28‐2.77, P < .001, respectively). On the contrary, PLR was not a prognostic factor in our analysis (HR 1.25, 95% CI = 1.01‐1.54, P < .01). Elevated NLR, PLR, and CAR were strongly associated with a higher T stage (HR 2.28, 95% CI = 1.67‐3.11; HR 1.57, 95% CI = 1.29‐1.90; HR 1.76, 95% CI = 1.16‐2.67, respectively). Begg’s funnel plots identified significant publication bias in NLR, but not in PLR and CAR.
Conclusion
NLR and CAR represent useful guides for the management of esophageal cancer, although publication bias should be considered. Further prospective studies are needed to confirm the results of the present study.
Keywords: C‐reactive protein to albumin ratio, esophageal cancer, neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, prognostic factor
Forest plot for the association between C‐reactive protein to albumin ratio (CAR) and overall survival of patients treated by surgery for esophageal cancer. Only three studies (n = 1033 patients) evaluated prognostic value of CAR. Cut‐off value of the included studies ranged from 0.085 to 0.5 (median, 0.22). Higher CAR was strongly associated with poorer survival versus lower CAR (HR 1.88, 95% CI = 1.28‐2.77, P = .001).
![]()
1. INTRODUCTION
Esophageal cancer is a highly aggressive disease with poor prognosis. According to the latest global cancer statistics, each year, an estimated 455 800 new esophageal cancer cases and 400 200 deaths occur globally. In males, it is the seventh most prevalent and sixth most highly mortal cancer, whereas in females it is the ninth most common cause of mortality.1
Numerous prognostic factors, including TNM stage, have been reported.2 However, recently, inflammatory and nutritional markers such as neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), and C‐reactive protein to albumin ratio (CAR) have been recognized as useful prognostic markers for esophageal cancer patients worldwide.3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19 Of note, the majority of these investigations were retrospective cohort studies. Only a few carried out a systematic review and meta‐analysis. As a consequence, the consistency and magnitude of the prognostic impact of these markers currently remain unclear. Additionally, a systematic review and meta‐analysis including CAR in esophageal cancer have not been carried out to date.
As a consequence, we carried out a systematic review and meta‐analysis to assess the prognostic values of NLR, PLR, and CAR for esophageal cancer.
2. MATERIALS AND METHODS
2.1. Search strategy
In the present study, the search strategy was based on the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) 2009 guidelines.20 Literature databases such as PubMed, Cochrane library, Embase, and Google scholar were searched from 2003 to 2018. The following medical subject headings were searched: “esophageal cancer (or carcinoma)” and “neutrophil to lymphocyte ratio (or NLR),” “esophageal cancer (or carcinoma)” and “platelet to lymphocyte ratio (or PLR),” and “esophageal cancer (or carcinoma)” and “C‐reactive protein to albumin ratio (or CAR).” Furthermore, references in the cited articles were overlooked. A total of 341 manuscripts were identified, and 331 manuscripts were excluded according to our exclusion criteria. (Figure 1).
Figure 1.
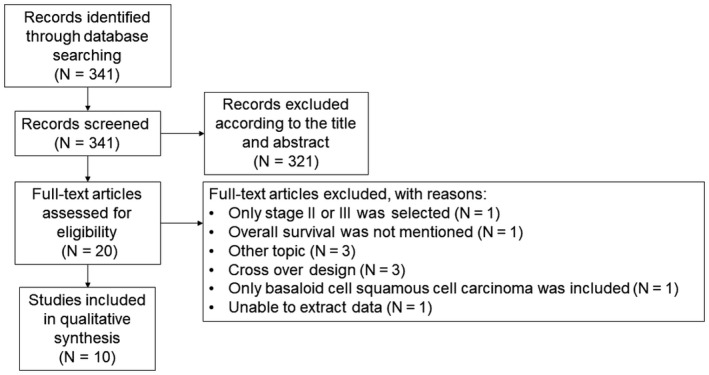
Flow diagram of the search strategy for the included studies
2.2. Inclusion and exclusion criteria
Inclusion criteria for selecting the articles for our analysis were as follows: (i) diagnosis of esophageal cancer was made based on pathological examination; (ii) correlation of pretreatment NLR, PLR, and CAR with overall survival (OS) was reported; (iii) publications were in English language. Exclusion criteria were as follows: only stage II or III was selected (n = 1); survival outcomes were not mentioned (n = 1); other topic (n = 3); cross‐over design (n = 3); only basaloid cell squamous cell carcinoma was selected (n = 1); and unable to extract data (n = 1).
2.3. Data extraction and quality evaluation
Two authors (Y.I. and H.T.) independently evaluated and extracted all candidate studies. Quality of the included studies was assessed through the Newcastle‐Ottawa Quality Assessment Scale (NOS). The latter consists of three parts as follows: selection, compatibility, and outcome assessments.21 Maximum score was 9 points and a NOS score >5 indicated acceptable quality studies.
2.4. Statistical analysis
Hazard ratio (HR) and 95% confidence interval (CI) for OS were directly summarized from each published study. We measured heterogeneity between the included studies using Cochran’s Q test with P‐value and I 2 statistic.22 P‐value <.1 for Cochran’s Q test and I 2 > 50% for the I 2 test suggested significant heterogeneity among the included studies. Furthermore, we used the random‐effects model (DerSimonian‐Laird method) for cases with significant heterogeneity (Cochran’s Q test <0.1 or I 2 >50%).21 Otherwise, we adopted the fixed‐effects model (Mantel‐Haenszel method).23 Finally, we used Begg’s funnel plots to visually assess the publication bias.24 All analyses were carried out by Review Manager (RevMan) 5.3.5 (Cochrane Collaboration, Software Update) and JMP 12.0 (SAS Institute Inc). P‐values <.01 were considered statistically significant.
2.5. Risk of bias
Appropriateness of the included studies was assessed by two authors (Y.I. and H.T.) by means of the Quality in Prognostic Studies (QUIPS) tool.25 All studies were scored as low, moderate, or high risk. Each included the following six domains: study participation, study attrition, prognostic factor measurement, outcome measurement, study confounding, and statistical analysis and reporting.
3. RESULTS
Flow diagram of the search strategy for the included studies is shown in Figure 1. A total of 341 articles were identified in the databases. Subsequently, in line with the inclusion and exclusion criteria, 10 retrospective cohort studies (n = 4551 patients with esophageal cancer) were included in the present meta‐analysis (Table 1).
Table 1.
Detailed data of the included studies reporting the relationship of NLR, PLR, or CAR and prognosis after an esophageal cancer resection
| Authors | Year | Study period | Histology | NLR cut‐off | PLR cut‐off | CAR cut‐off | Outcome | Measures | Number | Age | Gender | Stage | Included patients were all performed curative resection | Adjuvant therapy | Median follow up month (range) | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ishibashi et al17 | 2018 | 2009‐2014 | All types | 3 | 135 | 0.085 | OS and CSS | NLR, PLR, CAR | 143 |
<65 = 29 ≥65 = 114 |
Female = 22 Male= 121 |
I = 33 II = 33 III = 60 IV = 17 |
Yes |
No = 71 Yes = 72 |
22.8 mo (0.6‐87.2 mo) |
7 |
| Hirahara et al3 | 2018 | 2006‐2014 | SCC | 1.6 | 147 | NA | OS | NLR, PLR | 147 |
<70 = 56 ≥70 = 91 |
Female = 15 Male= 132 |
I = 59 II = 33 III = 55 |
Yes | None | NR | 6 |
| Wang et al5 | 2017 | 2012‐2013 | SCC | 2 | 159 | NA | OS and DFS | NLR, PLR | 280 | 64.1 ± 7.4 |
Female = 47 Male = 233 |
0/I /II = 179 III /IV = 101 |
Yes |
No = 166 Yes = 114 |
NR | 6 |
| Gao et al6 | 2017 | 2005‐2015 | SCC | 2.86 | NA | NA | OS | NLR | 1281 |
NLR <2.86 = 58.1 ± 9.1 NLR ≥ 2.86 = 60.4 ± 31.17 |
Female = 276 Male = 1005 |
0 = 27 I = 125 II = 586 III = 520 IV = 23 |
No | NR | NR | 6 |
| Miyazaki et a.7 | 2016 | 2004‐2014 | All types | 3.49 | NA | NA | OS | NLR | 192 | 65.8 (42‐86) |
Female = 19 Male = 173 |
I = 58 II = 50 III = 60 IV = 24 |
Yes | None | 26.5 mo (1‐108 mo) | 7 |
| Geng et al11 | 2016 | 2002‐2012 | SCC | 1.7 | 120 | NA | OS | NLR, PLR | 916 |
<60 = 455 ≥60 = 461 |
Female = 220 Male = 696 |
0‐I = 168 II = 395 III = 353 |
Yes | None | 39 mo (3‐146 mo) | 6 |
| Wei et al13 | 2015 | 2006‐2010 | SCC | 1.835 | 163.8 | 0.095 | OS | NLR, PLR, CAR | 423 |
<54 = 146 ≥54 = 277 |
Female = 82 Male = 341 |
I = 54 II = 168 III = 142 IV = 59 |
No | NR | 35.7 mo (0.6‐95.6 mo) | 7 |
| Xu et al14 | 2015 | 2000‐2010 | SCC | 2.4 | 147 | 0.5 | OS | CAR, NLR, PLR | 468 |
<58 = 227 ≥58 = 241 |
Female = 52 Male = 416 |
I = 24 II = 181 III = 142 |
Yes |
No=272 Yes=196 |
49.9 mo (10.9‐88 mo) | 6 |
| Han et al16 | 2015 | 2007‐2008 | SCC | 2.6 | 244 | NA | OS and DFS | NLR, PLR | 218 |
<60 = 109 ≥60 = 109 |
Female = 41 Male = 177 |
I+II =133 III = 85 |
Yes |
No=136 Yes=82 |
38.6 mo (3‐71 months) | 6 |
| Feng et al15 | 2014 | 2005‐2008 | SCC | 3.5 | 150 | NA | OS | NLR and PLR | 483 |
<60 = 273 ≥60 = 210 |
Female = 72 Male = 411 |
NR | Yes | NR | NR | 6 |
Abbreviations: CAR, C‐reactive protein to albumin ratio; CSS, cancer‐specific survival; DFS, disease‐free survival; NA, not applicable; NLR, neutrophil to lymphocyte ratio; NOS, Newcastle‐Ottawa Quality Assessment Scale; OS, overall survival; PLR, platelet to lymphocyte ratio; SCC, squamous cell carcinoma.
3.1. Neutrophil to lymphocyte ratio
As shown in Figure 2, a total of nine studies (n = 4042 patients) reported the prognostic value of NLR. The cut‐off value of the included studies ranged from 1.7 to 3.5 (median, 2.57). Patients treated for esophageal cancer with higher pretreatment NLR had a significant association with poorer prognosis in (HR 1.47, 95% CI = 1.32‐1.63, P < .00001). As heterogeneity was not significant, the analysis was estimated using a fixed‐effects model (P = .1, I 2 = 40%; Figure 2). We observed that a higher NLR was significantly associated with male gender (OR 1.6, 95% CI = 1.13‐2.27, P = .008) and T3 or T4 of tumor depth (OR 2.28, 95% CI = 1.67‐3.11, P < .00001; Table 2). In contrast, age, tumor location, tumor differentiation, and lymph node metastasis were not associated with higher NLR. OS subgroup analysis was carried out using histology, curative resection, cut‐off value, sample size, and HR from multivariate analysis (Table S1). All subgroups with the exception of small sample size, strengthened the prognostic value of NLR for OS.
Figure 2.
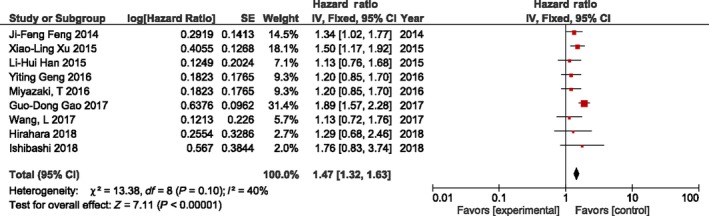
Forest plot for the association between neutrophil to lymphocyte ratio (NLR) and overall survival of patients treated by surgery for esophageal cancer
Table 2.
Link between clinicopathological features and elevated NLR
| Clinical features | No. of studies | No. of patients | Pooled results | Analytical effects model | ||
|---|---|---|---|---|---|---|
| OR | 95%CI | P‐value | ||||
| Male (vs Female) | 7 | 3294 | 1.60 | 1.13‐2.27 | .008 | Random |
| Age (y) ≥60 vs <60 | 3 | 1617 | 0.92 | 0.75‐1.13 | .40 | Fixed |
| Tumor depth | ||||||
| T3, T4 (vs T1, T2) | 6 | 2097 | 2.28 | 1.67‐3.11 | <.00001 | Random |
| Lymph node metastasis | ||||||
| N0, N1 (vs N2, N3) | 4 | 1398 | 1.35 | 1.01‐1.81 | .04 | Fixed |
| Differentiation | ||||||
| Poor (vs well, moderate) | 5 | 2951 | 1.24 | 1.01‐1.53 | .04 | Fixed |
| Location | ||||||
| Upper (vs middle, lower) | 7 | 3294 | 0.96 | 0.75‐1.24 | .77 | Random |
Abbreviations: Fixed, fixed‐effects model; NLR, neutrophil to lymphocyte ratio; Random, random‐effects model.
3.2. Platelet to lymphocyte ratio
Platelet to lymphocyte ratio was reported in seven studies (n = 2655 patients), and the cut‐off value of the included studies ranged from 135 to 244 (median, 157.4). Results of the meta‐analysis show an absence of association between PLR and OS (Figure 3). Due to significant heterogeneity, the analysis was carried out with a random‐effects model (P = .03, I 2 = 58%). We observed that a higher PLR was strongly associated with deeper tumor depth (OR 1.57, 95% CI = 1.29‐1.90, P < .00001). In contrast, PLR was not associated with gender, age, lymph node metastasis, tumor differentiation, and main tumor location (Table 3). OS subgroup analysis was done using histology, cut‐off value, sample size, and HR from multivariate analysis (Table S2). PLR could not indicate a prognostic value for OS in any of the subgroups.
Figure 3.
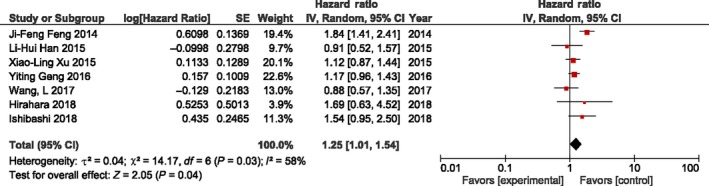
Forest plot for the association between platelet to lymphocyte ratio (PLR) and overall survival of patients treated by surgery for esophageal cancer
Table 3.
Link between clinicopathological features and elevated PLR
| Clinical features | No. of studies | No. of patients | Pooled results | Analytical effects model | ||
|---|---|---|---|---|---|---|
| OR | 95%CI | P‐value | ||||
| Male (vs Female) | 5 | 1675 | 0.79 | 0.41‐1.51 | .47 | Random |
| Age (y) ≥60 vs <60 | 3 | 1617 | 0.94 | 0.77‐1.15 | .56 | Fixed |
| Tumor depth | ||||||
| T3, T4 (vs T1, T2) | 5 | 1907 | 1.57 | 1.29‐1.90 | <.00001 | Fixed |
| Lymph node metastasis | ||||||
| N0, N1 (vs N2, N3) | 3 | 1206 | 1.37 | 1.03‐1.83 | .03 | Fixed |
| Differentiation | ||||||
| Poor (vs well, moderate) | 4 | 1760 | 1.22 | 0.99‐1.52 | .07 | Fixed |
| Location | ||||||
| Upper (vs middle, lower) | 5 | 1907 | 1.08 | 0.76‐1.55 | .66 | Fixed |
Abbreviations: Fixed, fixed‐effects model, PLR, platelet to lymphocyte ratio; Random, random‐effects model.
3.3. C‐reactive protein to albumin ratio
Only three studies (n = 1033 patients) evaluated the prognostic value of CAR. The cut‐off value of the included studies ranged from 0.085 to 0.5 (median, 0.22). Higher CAR was strongly associated with poorer survival versus lower CAR groups (HR 1.88, 95% CI = 1.28‐2.77, P = .001). (Figure 4) A random‐effects model for significant heterogeneity was used to carry out the analysis (P = .03, I 2 = 71%). Our results show that CAR had significant association with gender (OR 1.76, 95% CI = 1.16‐2.67, P = .008), tumor depth (OR 2.44, 95% CI = 1.25‐4.77, P = .009), and tumor differentiation (OR 1.7, 95% CI = 1.24‐2.32, P = .0009; Table 4). Due to an insufficient number of studies for CAR in esophageal cancer, subgroup analysis could not be carried out.
Figure 4.
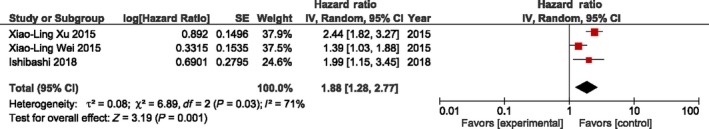
Forest plot for the association between C‐reactive protein to albumin ratio (CAR) and overall survival of patients treated by surgery for esophageal cancer
Table 4.
Link between clinicopathological features and elevated CAR
| Clinical features | No. of studies | No. of patients | Pooled results | Analytical effects model | ||
|---|---|---|---|---|---|---|
| OR | 95%CI | P‐value | ||||
| Male (vs Female) | 3 | 1033 | 1.76 | 1.16‐2.67 | .008 | Fixed |
| Tumor depth | ||||||
| T3, T4 (vs T1, T2) | 3 | 1033 | 2.44 | 1.25‐4.77 | .009 | Random |
| Lymph node metastasis | ||||||
| N0, N1 (vs N2, N3) | 3 | 1033 | 1.96 | 1.05‐3.67 | .03 | Random |
| Differentiation | ||||||
| Poor (vs well, moderate) | 3 | 1033 | 1.7 | 1.24‐2.32 | .0009 | Fixed |
Abbreviations: CAR, C‐reactive protein to albumin ratio; Fixed, fixed‐effects model; Random, random‐effects model.
3.4. Publication bias
Begg’s funnel plots were used to visually assess the publication bias in the present study. (Figure S1) A significant publication bias was found in NLR for OS, as the funnel plots of NLR were asymmetrical. No obvious publication bias was found in PLR and CAR for OS, although there were a relatively small number of included studies.
3.5. Risk of bias
Risk of bias summary and graph using the QUIPS tool are described (Figure S2A,B). A lower risk of bias was present in study participation, study attrition, prognostic factor measurement, outcome measurement, and statistical analysis and reporting. However, in the study‐confounding section, 40% of the high‐risk studies were included.6, 13, 16, 17
4. DISCUSSION
Predicting prognosis using preoperative factors should be pivotal in determining perioperative treatment strategy. TNM tumor staging has been recognized to have the most predictive power for prognosis; however, it is well known that preoperative staging is not always consistent with postoperative staging.26
In recent years, the influence of systemic inflammatory responses on the short‐ and long‐term outcomes of various malignancies has been widely recognized.27 Immune‐inflammatory measures (eg, NLR, PLR, and CAR) are easily obtained from peripheral blood tests and have been widely recognized as significant prognostic markers in solid tumors such as gastric,28, 29, 30, 31 colorectal,32, 33, 34 liver,35 and lung36, 37 cancers.
In esophageal cancer, there are currently a few systematic reviews and meta‐analyses of immune‐inflammatory measures as prognostic factors.38 In the present study, we investigated and summarized the prognostic powers of NLR, PLR, and CAR for esophageal cancer using meta‐analysis. Results of the meta‐analysis showed a strong association between poor prognosis and high pretreatment NLR and CAR. However, PLR was not a significant prognostic marker for OS, which was not consistent with the result of a meta‐analysis by Yodying et al38 We speculated the reasons for these conflicting results as follows. Unlike NLR and CAR, many studies showed less impact of PLR on the prognosis than the other immune‐inflammatory markers in various malignancies, including esophageal cancer.39, 40, 41, 42, 43, 44 We previously reported that NLR and CAR were significant prognostic measures in esophageal cancer. On the contrary, similar to the current meta‐analysis, PLR did not play the same role in esophageal cancer.17 Interestingly, we previously reported that patients who did not undergo antiplatelet or anticoagulant therapy and who had a higher PLR value had a significantly poorer OS versus those with a lower PLR. However, such differences were not observed in patients who received antiplatelet and/or anticoagulant therapies. Of the studies included in the present meta‐analysis, none has described the use of antiplatelet or anticoagulant therapy. Antiplatelet or anticoagulant therapy may affect the function of the platelet and coagulation systems. Further studies investigating in more detail antiplatelet or anticoagulant therapy may help clarify the actual prognostic value of PLR for survival.
Interestingly, this meta‐analysis showed that NLR, PLR, and CAR were significantly associated with T stages. Tumor invasion is a neoplastic process, closely related to inflammatory cells. The latter orchestrate the tumor microenvironment, namely cancer‐related inflammation. It has been reported that cancer‐related inflammation suppresses effective antitumor immunity by increasing regulatory T cells and activating cytokines in various malignancies.27 Additionally, inflammatory mediators or immunocompetent cells are involved in migration and invasion. As a consequence, local cancer‐related inflammation and/or mediators spill out of the systemic circulation potentially linking immune‐inflammatory measures and tumor progression.45
Various limitations can be identified in the present systematic review and meta‐analysis. First, in esophageal cancer, a smaller number of studies on immune‐inflammatory measures for prognosis have been reported compared to other gastroenterological malignancies. Second, all studies were retrospective investigations, and clinicopathologically detailed covariates were not adequately adjusted. A high risk of bias regarding study confounding affected nearly half of the included studies. As a consequence, higher quality studies focusing on these confounding factors or prospectively carried out studies are needed. Third, the optimal cut‐off values for each immune‐inflammatory measure are still under debate. Seven studies used time‐dependent receiver operating characteristics curve, two studies used online cut‐off finding software, and one study used median value to determine the cut‐off value. According to the reports, there were also differences in cut‐off values. In order to apply these markers in the clinical setting, in future, it will be necessary to determine the ideal cut‐off values.
In conclusion, NLR and CAR, but not PLR, are useful prognostic markers for esophageal cancer. Further prospective studies are required in order to confirm the results of this systematic review and meta‐analysis.
DISCLOSURE
Conflicts of Interest: Authors have no conflicts of interest to disclose and received no financial support for this study. All authors certify that they have no commercial associations that might pose a conflict of interest with respect to the submitted article.
The protocol of the present study was registered in PROSPERO and conforms to provisions of the Declaration of Helsinki.
Supporting information
ACKNOWLEDGEMENTS
The authors thank Shinsuke Nomura, Keita Kouzu, Yujiro Itazaki, Satoshi Tsuchiya, Mayu Tashiro, Takao Sugihara, Nozomi Ito, Hiroyuki Horiguchi, and Shuichi Hiraki for their critical review of this manuscript.
Ishibashi Y, Tsujimoto H, Yaguchi Y, Kishi Y, Ueno H. Prognostic significance of systemic inflammatory markers in esophageal cancer: Systematic review and meta‐analysis. Ann Gastroenterol Surg. 2020;4:56–63. 10.1002/ags3.12294
REFERENCES
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. [DOI] [PubMed] [Google Scholar]
- 2. Sobin LH, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours. Hoboken, NJ: John Wiley & Sons; 2011. [Google Scholar]
- 3. Hirahara N, Tajima Y, Fujii Y, Yamamoto T, Hyakudomi R, Hirayama T, et al. A novel prognostic scoring system using inflammatory response biomarkers for esophageal squamous cell carcinoma. World J Surg. 2018;42(1):172–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Feng JF, Chen S, Yang X. Systemic immune‐inflammation index (SII) is a useful prognostic indicator for patients with squamous cell carcinoma of the esophagus. Medicine (Baltimore). 2017;96(4):e5886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang L, Wang C, Wang J, Huang X, Cheng Y. A novel systemic immune‐inflammation index predicts survival and quality of life of patients after curative resection for esophageal squamous cell carcinoma. J Cancer Res Clin Oncol. 2017;143(10):2077–86. [DOI] [PubMed] [Google Scholar]
- 6. Gao GD, Sun B, Wang XB, Wang SM. Neutrophil to lymphocyte ratio as prognostic indicator for patients with esophageal squamous cell cancer. Int J Biol Markers. 2017;32(4):e409–14. [DOI] [PubMed] [Google Scholar]
- 7. Miyazaki T, Sakai M, Sohda M, Tanaka N, Yokobori T, Motegi Y, et al. Prognostic significance of inflammatory and nutritional parameters in patients with esophageal cancer. Anticancer Res. 2016;36(12):6557–62. [DOI] [PubMed] [Google Scholar]
- 8. Kosumi K, Baba Y, Ishimoto T, Harada K, Nakamura K, Ohuchi M, et al. Neutrophil/lymphocyte ratio predicts the prognosis in esophageal squamous cell carcinoma patients. Surg Today. 2016;46(4):405–13. [DOI] [PubMed] [Google Scholar]
- 9. Ikeguchi M, Kouno Y, Kihara K, Suzuki K, Endo K, Nakamura S, et al. Evaluation of prognostic markers for patients with curatively resected thoracic esophageal squamous cell carcinomas. Mol Clin Oncol. 2016;5(6):767–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jung J, Park SY, Park SJ, Park J. Prognostic value of the neutrophil‐to‐lymphocyte ratio for overall and disease‐free survival in patients with surgically treated esophageal squamous cell carcinoma. Tumour Biol. 2016;37(6):7149–54. [DOI] [PubMed] [Google Scholar]
- 11. Geng Y, Shao Y, Zhu D, Zheng X, Zhou Q, Zhou W, et al. Systemic immune‐inflammation index predicts prognosis of patients with esophageal squamous cell carcinoma: a propensity score‐matched analysis. Sci Rep. 2016;6:39482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Duan H, Zhang X, Wang FX, Cai MY, Ma GW, Yang H, et al. Prognostic role of neutrophil‐lymphocyte ratio in operable esophageal squamous cell carcinoma. World J Gastroenterol. 2015;21(18):5591–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wei XL, Wang FH, Zhang DS, Qiu M‐Z, Ren C, Jin Y, et al. A novel inflammation‐based prognostic score in esophageal squamous cell carcinoma: the C‐reactive protein/albumin ratio. BMC Cancer. 2015;15:350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xu XL, Yu HQ, Hu W, Song Q, Mao WM. A novel inflammation‐based prognostic score, the C‐reactive protein/albumin ratio predicts the prognosis of patients with operable esophageal squamous cell carcinoma. PLoS One. 2015;10(9):e0138657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Feng JF, Huang Y, Chen QX. Preoperative platelet lymphocyte ratio (PLR) is superior to neutrophil lymphocyte ratio (NLR) as a predictive factor in patients with esophageal squamous cell carcinoma. World J Surg Oncol. 2014;12:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Han LH, Jia YB, Song QX, Wang JB, Wang NN, Cheng YF. Prognostic significance of preoperative lymphocyte‐monocyte ratio in patients with resectable esophageal squamous cell carcinoma. Asian Pac J Cancer Prev. 2015;16(6):2245–50. [DOI] [PubMed] [Google Scholar]
- 17. Ishibashi Y, Tsujimoto H, Hiraki S, Kumano I, Yaguchi Y, Horiguchi H, et al. Prognostic value of preoperative systemic immunoinflammatory measures in patients with esophageal cancer. Ann Surg Oncol. 2018;25(11):3288–99. [DOI] [PubMed] [Google Scholar]
- 18. Sharaiha RZ, Halazun KJ, Mirza F, Port JL, Lee PC, Neugut AI, et al. Elevated preoperative neutrophil: lymphocyte ratio as a predictor of postoperative disease recurrence in esophageal cancer. Ann Surg Oncol. 2011;18(12):3362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen H, He J. Preoperative neutrophil‐to‐lymphocyte ratio as a prognostic predictor after radical resection of esophageal squamous cell carcinoma. Chin J Oncol. 2014;36(4):294–7. [PubMed] [Google Scholar]
- 20. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–34. [DOI] [PubMed] [Google Scholar]
- 21. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. [DOI] [PubMed] [Google Scholar]
- 22. Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127(9):820–6. [DOI] [PubMed] [Google Scholar]
- 23. Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719–48. [PubMed] [Google Scholar]
- 24. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994; 50:1088–101. [PubMed] [Google Scholar]
- 25. Hayden JA, van der Windt DA, Cartwright JL, Cote P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158(4):280–6. [DOI] [PubMed] [Google Scholar]
- 26. Tsujimoto H, Sugasawa H, Ono S, Ichikura T, Yamamoto J, Hase K. Has the accuracy of preoperative diagnosis improved in cases of early‐stage gastric cancer? World J Surg. 2010;34(8):1840–6. [DOI] [PubMed] [Google Scholar]
- 27. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer‐related inflammation. Nature. 2008;454(7203): 436–44. [DOI] [PubMed] [Google Scholar]
- 28. Miyamoto R, Inagawa S, Sano N, Tadano S, Adachi S, Yamamoto M. The neutrophil‐to‐lymphocyte ratio (NLR) predicts short‐term and long‐term outcomes in gastric cancer patients. Euro J Surg Oncol. 2018;44(5):607–12. [DOI] [PubMed] [Google Scholar]
- 29. Magdy M, Hussein T, Ezzat A, Gaballah A. Pre‐treatment peripheral neutrophil–lymphocyte ratio as a prognostic factor in gastric cancer. J Gastrointest Cancer. 2018. [DOI] [PubMed] [Google Scholar]
- 30. Graziosi L, Marino E, De Angelis V, Rebonato A, Cavazzoni E, Donini A. Prognostic value of preoperative neutrophils to lymphocytes ratio in patients resected for gastric cancer. Am J Surg. 2015;209(2):333–7. [DOI] [PubMed] [Google Scholar]
- 31. Kim EY, Lee JW, Yoo HM, Park CH, Song KY. The platelet‐to‐lymphocyte ratio versus neutrophil‐to‐lymphocyte ratio: which is better as a prognostic factor in gastric cancer? Ann Surg Oncol. 2015;22(13):4363–70. [DOI] [PubMed] [Google Scholar]
- 32. Diakos C, Wilson K, Asher R, Gebski V, Yip S, van Hazel G, et al. Is baseline neutrophil to lymphocyte ratio (NLR) an independent prognostic biomarker for progression free survival (PFS) and overall survival (OS) in metastatic colorectal cancer (mCRC)? Analysis of the AGITG MAX study. Ann Oncol. 2016;27(suppl 6):589. [Google Scholar]
- 33. You J, Zhu G‐Q, Xie L, Liu WY, Shi L, Wang OC, et al. Preoperative platelet to lymphocyte ratio is a valuable prognostic biomarker in patients with colorectal cancer. Oncotarget. 2016;7(18):25516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ishizuka M, Nagata H, Takagi K, Iwasaki Y, Shibuya N, Kubota K. Clinical significance of the C‐reactive protein to albumin ratio for survival after surgery for colorectal cancer. Ann Surg Oncol. 2016;23(3):900–7. [DOI] [PubMed] [Google Scholar]
- 35. Min GT, Li YM, Yao N, Wang J, Wang HP, Chen W. The pretreatment neutrophil‐lymphocyte ratio may predict prognosis of patients with liver cancer: a systematic review and meta‐analysis. Clin Transplant. 2018;32(1):e13151. [DOI] [PubMed] [Google Scholar]
- 36. Yamauchi Y, Safi S, Muley T, Warth A, Herth FJF, Dienemann H, et al. C‐reactive protein‐albumin ratio is an independent prognostic predictor of tumor recurrence in stage IIIA‐N2 lung adenocarcinoma patients. Lung Cancer. 2017;114:62–7. [DOI] [PubMed] [Google Scholar]
- 37. Ozyurek BA, Ozdemirel TS, Ozden SB, Erdogan Y, Kaplan B, Kaplan T. Prognostic value of the neutrophil to lymphocyte ratio (NLR) in lung cancer cases. Asian Pac J Cancer Prev. 2017;18(5):1417–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yodying H, Matsuda A, Miyashita M, Matsumoto S, Sakurazawa N, Yamada M, et al. Prognostic significance of neutrophil‐to‐lymphocyte ratio and platelet‐to‐lymphocyte ratio in oncologic outcomes of esophageal cancer: a systematic review and meta‐analysis. Ann Surg Oncol. 2016;23(2):646–54. [DOI] [PubMed] [Google Scholar]
- 39. Carruthers R, Tho L, Brown J, Kakumanu S, McCartney E, McDonald A. Systemic inflammatory response is a predictor of outcome in patients undergoing preoperative chemoradiation for locally advanced rectal cancer. Colorectal Dis. 2012;14(10):e701‐e707. [DOI] [PubMed] [Google Scholar]
- 40. Son H‐J, Park JW, Chang HJ, Kim DY, Kim BC, Kim SY, et al. Preoperative plasma hyperfibrinogenemia is predictive of poor prognosis in patients with nonmetastatic colon cancer. Ann Surg Oncol. 2013;20(9):2908–13. [DOI] [PubMed] [Google Scholar]
- 41. Mohamed Z, Pinato D, Mauri F, Chen K, Chang PM, Sharma R. Inflammation as a validated prognostic determinant in carcinoma of unknown primary site. Br J Cancer. 2014;110(1):208–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sanchez‐Lara K, Turcott JG, Juarez E, Guevara P, Núñez‐Valencia C, Oñate‐Ocaña LF, et al. Association of nutrition parameters including bioelectrical impedance and systemic inflammatory response with quality of life and prognosis in patients with advanced non‐small‐cell lung cancer: a prospective study. Nutr Cancer. 2012;64(4):526–34. [DOI] [PubMed] [Google Scholar]
- 43. Smith RA, Ghaneh P, Sutton R, Raraty M, Campbell F, Neoptolemos JP. Prognosis of resected ampullary adenocarcinoma by preoperative serum CA19‐9 levels and platelet‐lymphocyte ratio. J Gastrointest Surg. 2008;12(8):1422–8. [DOI] [PubMed] [Google Scholar]
- 44. Wang DS, Luo HY, Qiu MZ, Wang ZQ, Zhang DS, Wang FH, et al. Comparison of the prognostic values of various inflammation based factors in patients with pancreatic cancer. Med Oncol. 2012;29(5):3092–100. [DOI] [PubMed] [Google Scholar]
- 45. Tsujimoto H, Ono S, Ichikura T, Matsumoto Y, Yamamoto J, Hase K. Roles of inflammatory cytokines in the progression of gastric cancer: friends or foes? Gastric Cancer. 2010;13(4):212–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


