Abstract
Aim
Non‐steroidal anti‐inflammatory drugs (NSAIDs) are commonly used to control postoperative pain; however, their postoperative use has been associated with anastomotic leakage after gastrointestinal surgery. This systematic review and meta‐analysis aimed to determine the correlation between the use of NSAIDs and anastomotic leakage.
Methods
We conducted a comprehensive electronic literature search up to August 2018 to identify studies comparing anastomotic leakage in patients with and without postoperative NSAID use following gastrointestinal surgery. We then carried out a meta‐analysis using random‐effects models to calculate odds ratios (OR) with 95% confidence intervals (CI).
Results
Twenty‐four studies were included in this meta‐analysis, including a total of 31 877 patients. Meta‐analysis showed a significant association between NSAID use and anastomotic leakage (OR 1.73; 95% CI = 1.31‐2.29, P < .0001). Subgroup analyses showed that non‐selective NSAIDs, but not selective cyclooxygenase‐2 inhibitors, were significantly associated with anastomotic leakage. However there was no significant subgroup difference between selective and non‐selective NSAIDs.
Conclusion
Results of this meta‐analysis indicate that postoperative NSAID use is associated with anastomotic leakage following gastrointestinal surgeries. Caution is warranted when using NSAIDs for postoperative analgesic control in patients with gastrointestinal anastomoses.
Keywords: anastomotic leakage, cyclooxygenase inhibitor, gastrointestinal surgery, meta‐analysis, non‐steroidal anti‐inflammatory drugs
The results of this meta‐analysis indicate that postoperative NSAID use is associated with anastomotic leakage following gastrointestinal surgeries. Although the difference was not significant, selective NSAIDs may be safer than non‐selective NSAIDs.

1. INTRODUCTION
Anastomotic leakage has long been a concern among gastrointestinal surgeons. Its occurrence not only causes postoperative morbidity and mortality, but also lengthens hospital stay and increases hospital costs.1, 2 Importantly, anastomotic leakage worsens oncological outcomes in patients with resectable and curable malignancies, leading to poorer disease‐free survival, overall survival, and functional outcome.3, 4
Multiple factors contribute to anastomotic leakage, and its incidence varies depending on the location of the anastomosis. Esophageal anastomoses have the highest incidence of leakage, and gastric anastomoses the lowest incidence, whereas the incidence of colorectal anastomotic leakage differs among publications and anastomosis sites, ranging from 1% to 20%.5
The early recovery after surgery protocol has been proposed to reduce postoperative stress. The protocol aims to promote postoperative recovery, reduce hospital stay and, most importantly, reduce postoperative complications, especially cardiovascular and pulmonary complications.6 Non‐steroidal anti‐inflammatory drugs (NSAIDs) play a major part in this protocol as a means of postoperative pain control. However, application of the early recovery after surgery protocol has been associated with an increased incidence of anastomotic leakage,7 and it has been suggested that NSAIDs may be a causative factor in impaired anastomotic healing.
Many potential mechanisms have been proposed to explain how postoperative NSAID use may cause anastomotic leakage. NSAIDs decreased protective prostaglandins, and inhibited mucosal cyclooxygenase (COX)‐1, intestinal epithelial cell migration, and mucosal restitution in animal models8 which, in turn, reduced anastomotic tensile strength and collagen deposition causing delayed anastomotic healing.9, 10, 11
Previous reviews have examined the correlation between postoperative NSAID use and anastomotic leakage, but most have considered colorectal anastomoses only.7, 12 However, we suggest that the mechanisms shown in animal models may be applicable to all gastrointestinal anastomoses. Furthermore, it is also possible that selective COX‐2 inhibitors may be safer than non‐selective NSAIDs in terms of preventing anastomotic leakage based on the above‐mentioned mechanism.
The primary objective of this systematic review and meta‐analysis was to determine the effect of postoperative NSAID use on gastrointestinal anastomotic leakage, regardless of the site of anastomosis. The secondary objective was to compare the anastomotic leakage risk between non‐selective NSAIDs and selective COX‐2 inhibitors.
2. METHODS
2.1. Search strategy
We conducted a literature search of the Medline, PubMed, Cochrane Library, http://clinicaltrial.gov, and Web of Science databases up to August 2018. The search was limited to English language and human studies. The search terms used were “Anastomosis or anastomotic leakage” AND “NSAIDs” [MesH term]. Additional articles were retrieved by manually searching the reference lists of the included studies and other reviews.
2.2. Selection criteria
Studies were included if they met the following criteria: (i) study with anastomosis of the gastrointestinal tract; (ii) study compared postoperative NSAID use with non‐use; and (iii) investigations reported anastomotic leakage. Case reports or reports with incomplete data were excluded.
2.3. Data extraction
The studies were independently and critically assessed by two authors using a standard protocol and discrepancies were resolved by consensus. Extracted data included study design, number of institutes, definition of anastomotic leakage, operative diagnosis, location of anastomosis, urgency of surgery, type of NSAIDs, sample size, and numbers of anastomotic leakage per group.
2.4. Quality assessment
Qualities of the included studies were assessed using the Jadad score13 and the Newcastle‐Ottawa scale (NOS)14 for randomized controlled trials (RCT) and observational studies, respectively. Studies were considered to be high quality if they had a Jadad score ≥3 or NOS ≥7.
2.5. Data synthesis and meta‐analysis
Meta‐analysis was done by computing the OR from the original data using the Cochrane‐Mantel‐Haenszel method, with 95% CI. P ≤ .05 was considered significant in all analyses. Data analysis was carried out using Review Manager (RevMan) v5.3 software (Cochrane Collaboration) and a random‐effect model was used for graphical presentation. Statistical heterogeneity was quantified using I2 statistics and Cochrane Q tests. I2 values >50% indicated heterogeneity.15 In the presence of heterogeneity, we conducted subgroup and meta‐regression analyses to determine if the inter‐study variation could be explained by certain co‐variates, including type of study, NSAID class, NSAID administration, urgency of surgery, location of anastomosis, and operative diagnosis. Sensitivity analyses were done to assess the impact of individual potential confounding variables. Publication bias was assessed visually by funnel plot, and asymmetry was assessed formally by rank correlation test (Begg’s test).16 Publication bias was analyzed using WINPEPI software.17
3. RESULTS
3.1. Study selection
The initial systematic search identified 430 studies and an additional search for reviews identified a further five studies. After adjusting for duplicates and critical assessment, a total of six RCT18, 19, 20, 21, 22, 23 and 18 observational studies24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41 were included in the meta‐analysis. The PRISMA flow diagram of the detailed literature search and selection process is shown in Figure 1. Of 27 full‐text article reviews, three were excluded from the quantitative analysis because we could not extract the original data from two, and the other study compared multimodal interventions in which NSAIDs were also distributed to the control group.
Figure 1.
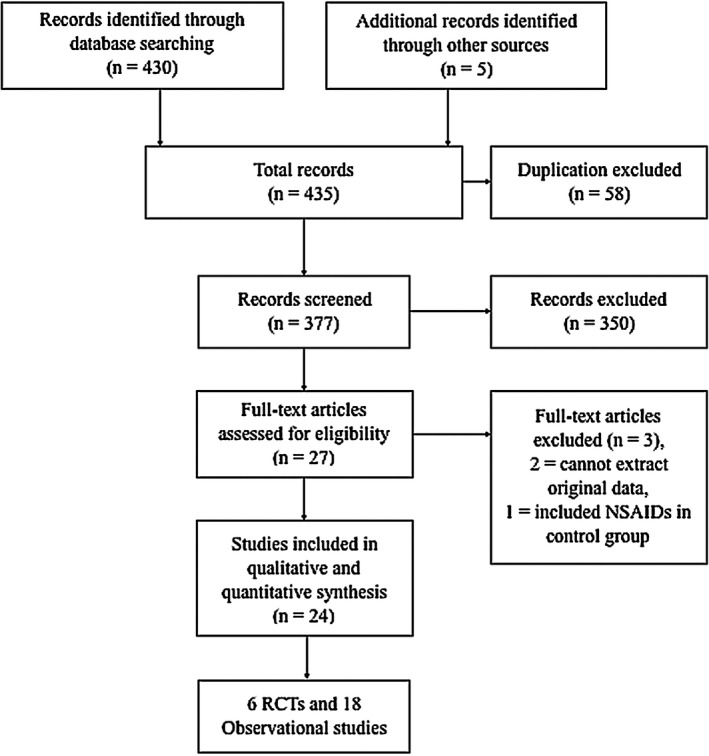
PRISMA flow shows study selection process. NSAIDs, non‐steroidal anti‐inflammatory drugs; RCT, randomized controlled trial
3.2. Characteristics of included studies
Six RCT and 18 observational studies were included in this meta‐analysis. Sample sizes varied from 40 to 220 for the RCT and from 75 to 13 082 for the observational studies. Most studies included the anastomotic location as colorectal anastomoses (four RCT,18, 19, 21, 22 13 observational studies24, 25, 26, 27, 28, 29, 31, 32, 34, 35, 36, 38, 40), a diagnosis of malignancy (three RCT,19, 20, 21 six observational studies24, 28, 36, 38, 40, 41), and surgery carried out as an elective procedure (all RCT, 12 observational studies24, 25, 26, 28, 29, 31, 32, 35, 36, 38, 40, 41). Most studies reported the classes of NSAIDs used, except for five observational studies, from some of which we were able to extract the original data. Data on non‐selective NSAIDs were extracted from 15 studies18, 19, 21, 22, 23, 24, 26, 27, 28, 31, 36, 37, 38, 40, 41 and on selective COX‐2 inhibitors from eight studies.20, 23, 25, 27, 29, 35, 38, 40 Quality assessment showed that all the RCT and all but two of the observational studies were high quality,24, 35 with the two observational studies considered low quality. Characteristics of the included studies are outlined in Table 1.
Table 1.
Characteristics of included studies to determine the correlation between the use of NSAIDs and anastomotic leakage
| Author, year | Study design | Country, Institute | Recruitment period | Definition of AL | Diagnosis | Location of anastomosis | Urgency of surgery | N | NSAIDs administration | Quality assessmenta |
|---|---|---|---|---|---|---|---|---|---|---|
| Chen,18 2005 | RCT, Double‐blind | Taiwan, single | 2003 | NR | Mixed | Colorectal | Elective | 74 | PCA: ketolorac 1.2 g/mL + morphine 1 mg/mL 2 mL bolus and 10 min lockout until pain score <3 | 5 |
| Schlachta,19 2007 | RCT, Double‐blind | Canada, single | 2002‐2005 | NR | Mixed (Cancer 50%) | Colorectal | Elective | 44 | Ketolorac 30 mg IV every 6 h for 2 d after operation | 3 |
| Sim,20 2007 | RCT, Double‐blind | Singapore, single | 2002‐2004 | NR | Mixed (Cancer 94.9%) | Mixed (Colorectal 94.9%) | Elective | 79 | Valdecoxib 40 mg orally once pre‐operation and once daily for 5 d after operation | 2 |
| Xu,21 2008 | RCT, Double‐blind | China, single | 2006‐2007 | NR | Cancer | Colorectal | Elective | 40 | Flurbiprofen 1 mg/kg IV 30 min before and 6 h after skin incision | 5 |
| Chen,22 2009 | RCT, Double‐blind | Taiwan, single | 2006‐2007 | NR | Mixed | Colorectal | Elective | 102 | PCA: ketolorac 1.2 g/mL + morphine 1 mg/mL 2 mL bolus and 10 min lockout until pain score <3 | 4 |
| Wattchow,23 2009 | RCT, Double‐blind | Australia, 2 institutes | 2003‐2006 | NR | Mixed | Mixed (Colorectal 99%, Small intestine 1%) | Elective | 220 | Celecoxib 100 mg or Diclofenac 50 mg orally twice daily for 7 d or until discharge | 4 |
| Rosenberg,24 2007 | Retrospective cohort | Denmark, Single | 2004‐2006 | NR | Cancer | Colorectal | Elective | 310 | Diclofenac 75 mg twice daily, Not reported duration | 5 |
| Klein,26 2009 | Retrospective case‐control | Denmark, Single | 2004‐2007 | Leak requiring reoperation | Mixed (Cancer 96%) | Colorectal | Elective | 75 | Diclofenac 150 mg/d, Not reported duration | 7 |
| Holte,25 2009 | Retrospective cohort | Denmark, Single | 1997‐2006 | Radiologic finding or intra‐operative finding or clinical finding | NR | Colon | Elective | 502 | Ibuprofen 600 mg every 8 h or Celecoxib 200 mg every 12 h at POD 2‐8 | 7 |
| Gorissen,27 2012 | Retrospective cohort | Netherlands, 2 institutes | 2008‐2010 | Radiologic finding or intra‐operative finding or clinical finding | Mixed (Cancer 72%) | Colorectal | Mixed (Elective 86.4%) | 795 | NSAIDs use within POD 5 | 8 |
| Klein,28 2012 | Retrospective cohort | Denmark, 6 institutes | 2006‐2009 | Leak requring reoperation | Cancer | Colorectal | Elective | 2752 | NSAIDs use at least 2 d within POD 7 | 9 |
| Zittel,29 2013 | Retrospective cohort | Sweden, single | 2008‐2009 | NR | Mixed (Cancer 57.6%) | Colorectal | Elective | 205 | Etoricoxib 120 mg once daily, Not reported duration | 8 |
| Subendran,32 2014 | Retrospective case‐control | Canada, single | 2001‐2012 | Radiologic finding or intra‐operative finding | Mixed (IBD 65.6%, cancer 34.4%) | Colorectal | Elective | 262 | NSAIDs use within POD 5 | 8 |
| Saleh,31 2014 | Retrospective cohort | Canada, single | 2004‐2011 | Document at reoperation or Radiological finding | Mixed (Cancer 65.5%) | Colorectal | Elective | 731 | NSAIDs use within POD 5 | 8 |
| STARSurg UK,30 2014 | Prospective cohort | UK, multi‐institutes | 2013 | Radiologic finding or intra‐operative finding or clinical finding | Mixed (Cancer 62.1%) | Mixed (Colorectal 75.9%) | Mixed (Elective 72.1%) | 1503 | NSAIDs use within POD 2 | 8 |
| Paulsir,34 2015 | Retrospective cohort | USA, multi‐institutes | 2012‐2014 | Leaks requiring antibiotic or intervention or reoperation | NR | Colorectal | Mixed (Elective 78.6%) | 4360 | NSAIDs use within POD 1 | 9 |
| Hakkarainen,33 2015 | Retrospective cohort | USA, 47 institutes | 2006‐2010 | Leak requiring percutaneous drainage or reoperaion | NR | Bariatic, Colorectal | Mixed (Elective 87.6%) | 13082 | NSAIDs use within POD 1 | 9 |
| Raju,35 2015 | Retrospective cohort | Australia, 2 institutes | 2008‐2014 | Leak requiring percutaneous drainage or reoperaion | Mixed (Cancer 70.6%) | Colorectal | Elective | 267 | Celecoxib 100 mg twice daily start at 2 h before operation to POD 7 | 6 |
| Bakker,36 2016 | Retrospective cohort | Netherlands, single | 2006‐2013 | Leak requiring percutaneous drainage or reoperaion | Cancer | Colorectal | Elective | 856 | NSAIDs use at least 2 d until discharge | 8 |
| Rutegard,38 2016 | Retrospective cohort | Sweden, multi‐institutes | 2007‐2012 | Leak requiring percutaneous drainage or reoperaion | Cancer | Rectum | Elective | 2605 | NSAIDs use within POD 10 | 8 |
| Rushfeldt,37 2016 | Retrospective cohort with propensity score analysis | Norway, Single | 2007‐2009 | NR | Mixed (Cancer 52.8%) | Mixed (Colorectal 73.4%) | Mixed (Elective 88%) | 428 | NSAIDs use within POD 5 | 8 |
| Haddad,39 2017 | Retrospective cohort | USA, multi‐institutes | 2013‐2015 | NR | Trauma | Mixed (Small intestine 93.4%, Colorectal 6.6%) | Emergency | 533 | NSAIDs use 7 d prior to operation up to POD 14 | 7 |
| Fjederholt,41 2018 | Retrospective cohort | Denmark, 2 institutes | 2003‐2012 | Radiologic finding or endoscopic finding | Cancer | Esophagojejunostomy | Elective | 556 | NSAIDs use within POD 7 | 9 |
| Hultberg,40 2017 | Retrospective cohort | Sweden, 15 institutes | 2007‐2013 | Radiologic finding or intra‐operative finding or clinical finding or Endoscopic finding | Cancer | Rectal | Elective | 1495 | NSAIDs use at least 2 d within POD 7 | 9 |
Abbreviations: AL, anastomotic leakage; IBD, inflammatory bowel disease; NR, not reported; NSAIDs, non‐steroidal anti‐inflammatory drugs; PCA, patient controlled analgesia; POD, postoperative day; RCT, randomised controlled trial.
Quality assessment for RCT and observational studies using Jadad score and Newcastle‐Ottawa scale (NOS) for randomised controlled trials (RCTs) and observational studies, respectively.
3.3. Association of NSAIDs with anastomotic leakage
Overall anastomotic leakage rate in this study was 6.0% (1922/31 877). Patients who received NSAIDs postoperatively had a higher leakage rate (7.5%; 777/10 318) than those without NSAIDs (5.3%; 1145/21 558). Meta‐analysis showed a significantly higher rate of anastomotic leakage after postoperative NSAID use (pooled OR 1.73, 95% CI 1.31‐2.29, P < .001), but with evidence of heterogeneity across the included studies (I2 = 80%, Cochrane Q test P < .00001) (Figure 2). The funnel plot appeared relatively symmetrical, suggesting no publication bias, as confirmed by Begg’s test (P = .444) (Figure 3). There was some discrepancy in the results between the study types: RCT showed a non‐significant difference in anastomotic leakage between the NSAID and placebo groups (pooled OR 1.91, 95%CI 0.69‐5.35, P = .67) without heterogeneity (I2 = 0%, Cochrane Q test P = .67), whereas observational studies found a significantly higher leakage rate after postoperative NSAID use (OR 1.72, 95%CI 1.28‐2.31, P < .001) with evidence of heterogeneity (I2 = 84%, Cochrane Q test P < .001) (Figure 2).
Figure 2.
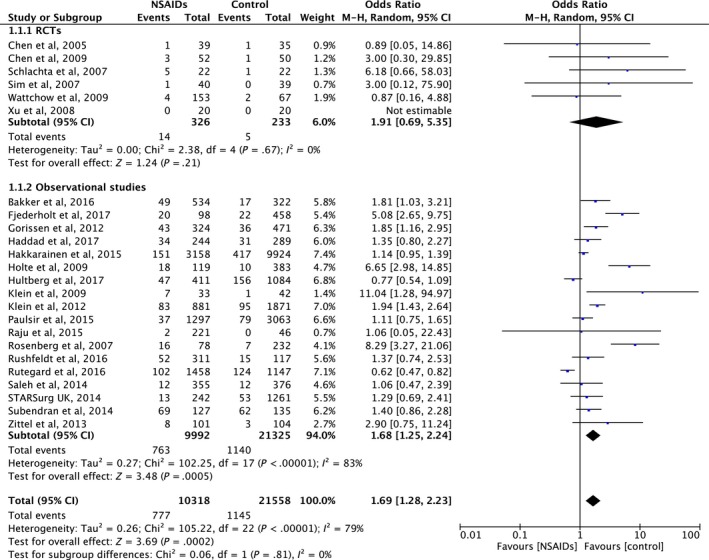
Forrest plot of meta‐analysis between randomized controlled trials (RCT) and observational studies. NSAIDs, non‐steroidal anti‐inflammatory drugs
Figure 3.
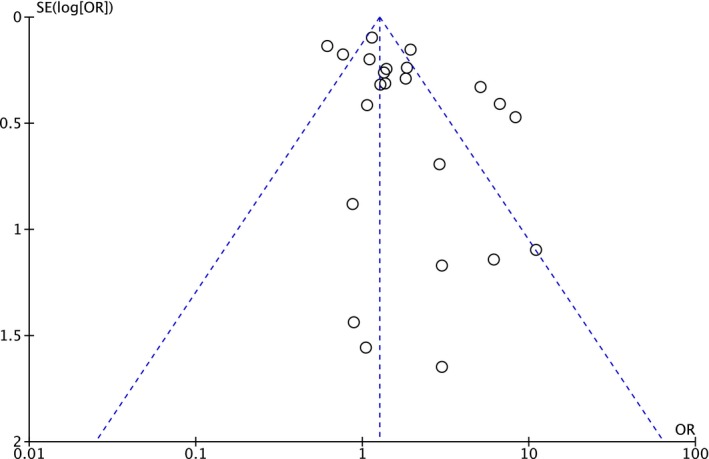
Funnel plot with pseudo 95% CI (random‐effect model). OR, odds ratio; SE, study effect
3.4. Protocol‐based versus non‐systematic NSAIDs use
To investigate the effect of NSAID dose on anastomotic leakage, we categorized NSAID use in the included studies into protocol‐based and non‐systematic use. In the protocol‐based group, NSAIDs were given according to the institutional protocol (11 studies; n = 1918), whereas in the non‐systematic group, NSAIDs were given at any given time during the postoperative period (13 studies; n = 30 140). Details of NSAID use are shown in Table 1. The protocol‐based group had a significantly higher anastomotic leakage rate compared with non‐users (pooled OR 4.67, 95% CI 2.84‐7.67, P < .001) without evidence of heterogeneity (I2 = 5%, Cochrane Q test P = .40), whereas the non‐systematic group also had a significantly increased risk for anastomotic leakage compared with non‐users (pooled OR 1.38, 95% CI 1.06‐0.181, P = .02), but with evidence of heterogeneity (I2 = 82%, Cochrane Q test P < .001). However, there was a statistically significant subgroup difference between the protocol‐based group and the non‐systematic group (P < .001) (Figure 4).
Figure 4.
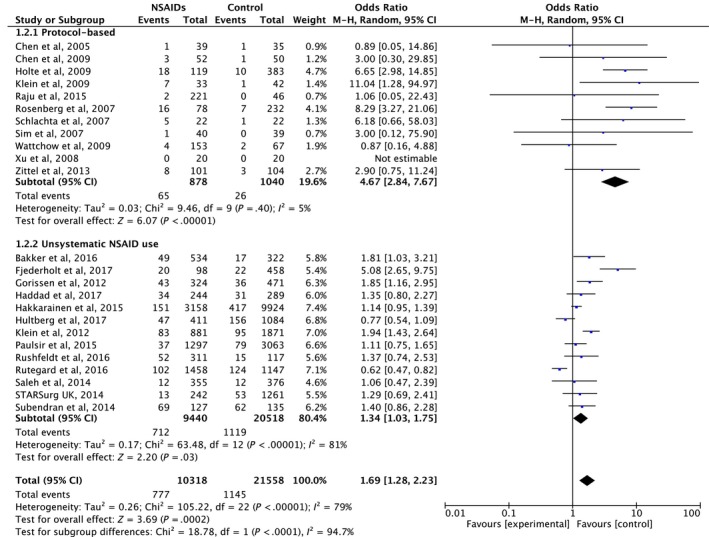
Forrest plot of meta‐analysis between protocol‐based non‐steroidal anti‐inflammatory drugs (NSAIDs) use and non‐systematic NSAIDs use
3.5. Non‐selective NSAIDs versus selective COX‐2 inhibitors
Among all the included studies, we extracted information on non‐selective NSAID use from 15 (n = 4110) and on selective COX‐2 inhibitor use from eight (n = 1063) studies. Subgroup analysis showed that patients who received postoperative non‐selective NSAIDs had a significantly higher rate of anastomotic leakage than patients who did not receive NSAIDs (pooled OR 1.80, 95% CI 1.12‐2.91, P = .02) with evidence of heterogeneity (I2 = 85%, Cochrane Q test P < .00001). In contrast, the anastomotic leakage rate in patients taking selective COX‐2 inhibitors was not significantly higher than in those not taking NSAIDs (pooled OR = 1.67, 95% CI 0.90‐3.13, P = .11), with evidence of heterogeneity (I2 = 67%, Cochrane Q test P = .004). However, comparison between users of non‐selective and selective NSAIDs showed no significant subgroup difference (P = .85) (Figure 5).
Figure 5.
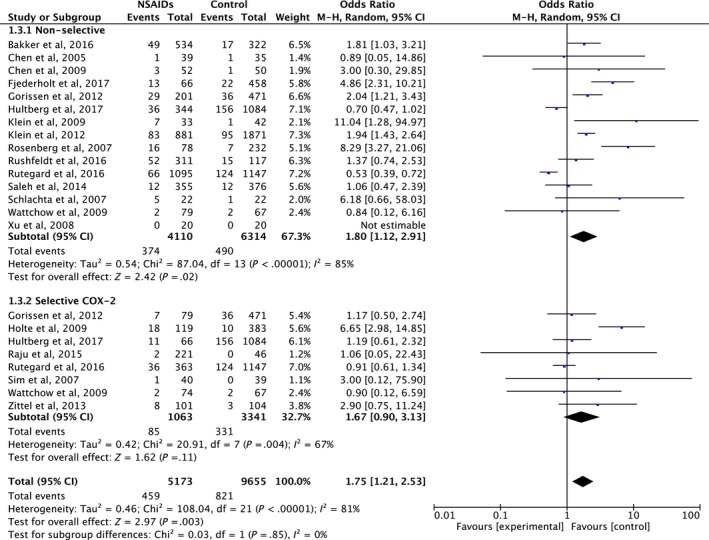
Forrest plot of meta‐analysis between non‐selective non‐steroidal anti‐inflammatory drugs (NSAIDs) and selective COX‐2 NSAIDs
3.6. Colorectal anastomoses versus other gastrointestinal anastomoses
We carried out subgroup analyses between studies restricted to colorectal anastomoses (17 studies; n = 15 475) and studies with anastomoses not limited to colorectal (seven studies; n = 16 538). Studies with colorectal anastomoses had significantly increased anastomotic leakage rates when perioperative NSAIDs were used (pooled OR 1.80, 95% CI 1.22‐2.66, P = .003), with evidence of heterogeneity (I2 = 83%, Cochrane Q test P < .00001). Studies of anastomoses of all sites also showed significantly higher rates of anastomotic leakage (pooled OR 1.61, 95% CI 1.25‐2.66, P = .02), with evidence of heterogeneity (I2 = 72%, Cochrane Q test P = .002). There were no subgroup differences between the two groups of studies (P = .85) (Figure S1).
3.7. Meta‐regression and sensitivity analyses
Meta‐regression analysis stratified by location of anastomoses showed pooled OR for anastomotic leakage of 1.80 (95% CI 1.22‐2.66, I2 = 83%) for colorectal anastomoses and 1.70 (95% CI 1.09‐2.66, I2 = 72%) for studies that were not limited to colorectal anastomoses. Meta‐regression analysis showed no significant difference between various anastomotic sites (P = .85). Furthermore, separate stratified and meta‐regression analyses showed no significant differences in the OR of anastomotic leakage rates after postoperative NSAID use in relation to the type of study, NSAID class, urgency of surgery, or operative diagnosis (Table 2).
Table 2.
Stratified analysis and meta‐regression of included studies
| Studies | N | OR (95% CI) | I 2 | Heterogeneity | |||
|---|---|---|---|---|---|---|---|
| χ2 | I 2 | P value | |||||
| 1. Type of studies | |||||||
| RCTS | 6 | 559 | 1.91 (0.69‐5.35) | 0 | 0.06 | 0 | .81 |
| Cohort studies | 18 | 31 317 | 1.68 (1.25‐2.24) | 83 | |||
| 2. NSAIDs class | |||||||
| Non selective | 15 | 10 424 | 1.80 (1.12‐2.91) | 85 | 0.03 | 0 | .85 |
| Selective COX‐2 | 8 | 4404 | 1.67 (0.90‐3.13) | 67 | |||
| 3. Urgency of surgery | |||||||
| Elective | 18 | 11 175 | 2.08 (1.31‐3.29) | 84 | 4.55 | 72 | .03 |
| Not limit to elective surgery | 6 | 20 701 | 1.23 (1.06‐1.42) | 0 | |||
| 4. Location of anastamoses | |||||||
| Colorectal | 17 | 15 475 | 1.80 (1.22‐2.66) | 83 | 0.20 | 0 | .66 |
| Not limit to colorectal | 7 | 16 401 | 1.58 (1.04‐2.42) | 72 | |||
| 5. Diagnosis | |||||||
| Cancer | 7 | 8614 | 1.88 (0.96‐3.69) | 93 | 0.31 | 0 | .58 |
| Not limit to cancer | 17 | 23 262 | 1.54 (1.21‐1.96) | 44 | |||
| 6. NSAIDs administration | |||||||
| Protocol based | 11 | 1918 | 4.67 (2.84‐7.67) | 5 | 18.78 | 94.7 | <.0001 |
| Unsystematic | 13 | 29 958 | 1.34 (1.03‐1.75) | 81 | |||
Abbreviations: CI, confidence interval; NSAIDs, non‐steroidal anti‐inflammatory drugs; OR, odds ratio; RCT, randomized controlled trial
Sensitivity analyses were carried out to assess the impact of low‐quality studies (Table 1). Exclusion of the two low‐quality studies did not affect the significance of the results (pooled OR 1.61, 95% CI 1.22‐2.11, P < .001).
4. DISCUSSION
Numerous mechanisms have shown how NSAIDs can damage human intestines, although some remain controversial. Non‐selective NSAIDs have been associated with enterocyte mitochondrial dysfunction leading to increased epithelial permeability, invasion of luminal bacteria, neutrophil infiltration, and free radical production.42, 43, 44 Inhibition of COX by NSAIDs also decreases protective prostaglandins.45 Non‐selective NSAIDs and their acidic compounds can cause topical mucosal injury.9 However, most COX in the intestinal mucosal layer are COX‐1, and selective COX‐2 inhibitors may thus be more tolerable in the normal gastrointestinal tract.
Selective COX‐2 inhibitors and non‐selective NSAIDs confound the anastomotic healing process. Submucosal collagen fibers provide a core structure that determines tensile strength, and both selective COX‐2 inhibitors and non‐selective NSAIDs adversely affected this structure in an animal model which, in turn, led to decreased tensile strength of the anastomoses and reduced bursting pressure.46, 47, 48 NSAIDs also inhibited epithelial cell migration and mucosal restitution by depolarization and decreased surface expression of potassium channels.8 However, unlike in normal tissue, enterocytes express high levels of COX‐2 during inflammation, which catalyzes prostaglandin E2, resulting in increased vascular endothelial growth factor expression and angiogenesis.49
The above results and hypotheses shed doubt on the safety of postoperative NSAID use for analgesic control. Numerous previous meta‐analyses have shown significantly higher anastomotic leakage rates in patients given NSAIDs.7, 12, 50 The current systematic review and meta‐analysis confirmed the association between postoperative NSAID use and higher anastomotic leakage (pooled OR 1.73, 95% CI 1.31‐2.29, P < .001). However, our analysis of RCT did not show a significant effect of postoperative NSAIDs on anastomotic leakage rate compared with placebo. This meta‐analysis included only six RCT. Furthermore, the primary outcome of all RCT were not anastomotic leakage; therefore, we extracted corresponding data from each RCT. Finally, the sample size from RCT was very small compared to observational studies (n = 559 vs 31 499), which makes it relatively reasonable to integrate both study designs in order to make a conclusion from current evidence. From the result of no significant subgroup difference between studies, RCT and all designs, we believe that the controversial result may be explainable by the small sample sizes of the RCT, thus limiting their statistical power, rather than by the absence of a relationship between NSAIDs use and anastomotic leakage.
Subgroup analysis showed that patients taking NSAIDs according to hospital protocol had significantly higher rates of anastomotic leakage than those not taking NSAIDs (pooled OR 4.67, 95% CI 2.84‐7.67, P < .001), without evidence of heterogeneity (I2 = 5%, Cochrane Q test P = .40). Patients in the protocol‐based group were supposedly given NSAIDs in a regular way, with higher cumulative doses compared with the non‐systematic group. This suggests that the association between NSAID use and anastomotic leakage may be dose‐related, although further studies are needed to confirm this theory.
Subgroup analysis also showed that patients taking non‐selective NSAIDs had a significantly higher rate of anastomotic leakage than patients not taking NSAIDs (pooled OR 1.80, 95% CI 1.12‐2.91, P = .02). In contrast, selective COX‐2 inhibitors tended to increase the risk of anastomotic leakage, but the effect was not significant (pooled OR = 1.67, 95% CI 0.90‐3.13, P = .11). However, there was no significant subgroup difference between patients taking non‐selective and COX‐2‐selective NSAIDs. These results support the hypotheses that both classes of NSAIDs had adverse effects on anastomotic healing, leading to increased anastomotic leakage; however, non‐selective NSAIDs might cause greater damage then selective COX‐2 inhibitors by causing intestinal mucosal injury, at least in part.
In animal models, adverse effects of NSAIDs were found in both small intestine and colon resulting in increased anastomotic leakage rate.8, 9, 11, 42, 44 In human studies, consistent results were also reported regardless of anastomotic site; however, the majority were colorectal anastomoses. In our study, studies with colorectal anastomoses had significantly increased anastomotic leakage rates when perioperative NSAIDs were used (pooled OR 1.80, 95% CI 1.22‐2.66, P = .003). Consistently, studies of anastomoses of all sites also showed significantly higher rates of anastomotic leakage (pooled OR 1.61, 95% CI 1.25‐2.66, P = .02). There were no subgroup differences between the two groups of studies (P = .85). In fact, Fjederholt et al41 reported a strong association between NSAIDs use and the risk of anastomotic leakage (ketorolac; OR 6.05, 95% CI 2.71‐13.5) (other NSAIDs; OR 5.24, 95% CI 1.85‐14.8) after surgery for gastroesophageal junction only. Two other studies33, 39 of which majority of anastomosis site is not colorectal, were also included in our meta‐analysis. These results support our hypothesis that NSAIDs were associated with increased anastomotic leakage in all gastrointestinal anastomoses.
The present study had several limitations. First, our conclusions were mainly based on observational studies; however, subgroup analysis showed no significant subgroup difference between RCT and observational studies, suggesting that this potential bias was not significant. Second, there was statistical heterogeneity, and the included observational studies were clinically heterogenous in terms of patient characteristics, indications for surgery, and location of anastomoses. Although stratified and meta‐regression analyses showed no significant differences, heterogeneity decreased the validity of the results. Third, most of the included studies (17/24) only considered colorectal anastomoses, and the implication of the results for all gastrointestinal anastomoses might not be completely accurate.
In conclusion, postoperative NSAID use appears to be associated with an increased incidence of anastomotic leakage following gastrointestinal surgery. Selective COX‐2 inhibitors might be safer than non‐selective NSAIDs, although the results were inconclusive. Caution is warranted when using NSAIDs for postoperative analgesic control in patients with gastrointestinal anastomoses.
DISCLOSURE
Conflicts of Interest: Authors declare no conflicts of interest for this article.
Supporting information
ACKNOWLEDGEMENT
We thank Susan Furness, PhD, from Edanz Group (http://www.edanzediting.com/ac) for editing a draft of this manuscript.
Jamjittrong S, Matsuda A, Matsumoto S, et al. Postoperative non‐steroidal anti‐inflammatory drugs and anastomotic leakage after gastrointestinal anastomoses: Systematic review and meta‐analysis. Ann Gastroenterol Surg. 2020;4:64–75. 10.1002/ags3.12300
Jamjittrong and Matsuda contributed equally to this work.
REFERENCES
- 1. Bakker I, Grossmann I, Henneman D, Havenga K, Wiggers T. Risk factors for anastomotic leakage and leak‐related mortality after colonic cancer surgery in a nationwide audit. Br J Surg. 2014;101(4):424–32. [DOI] [PubMed] [Google Scholar]
- 2. Hammond J, Lim S, Wan Y, Gao X, Patkar A. The burden of gastrointestinal anastomotic leaks: an evaluation of clinical and economic outcomes. J Gastrointest Surg. 2014;18(6):1176–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kube R, Mroczkowski P, Granowski D, Benedix F, Sahm M, Schmidt U, et al. Anastomotic leakage after colon cancer surgery: a predictor of significant morbidity and hospital mortality, and diminished tumour‐free survival. Eur J Surg Oncol. 2010;36(2):120–4. [DOI] [PubMed] [Google Scholar]
- 4. Ramphal W, Boeding JRE, Gobardhan PD, Rutten HJT, de Winter LJMB, Crolla RMPH, et al. Oncologic outcome and recurrence rate following anastomotic leakage after curative resection for colorectal cancer. Surg Oncol. 2018;27(4):730–6. [DOI] [PubMed] [Google Scholar]
- 5. Turrentine FE, Denlinger CE, Simpson VB, Garwood RA, Guerlain S, Agrawal A, et al. Morbidity, mortality, cost, and survival estimates of gastrointestinal anastomotic leaks. J Am Coll Surg. 2015;220(2):195–206. [DOI] [PubMed] [Google Scholar]
- 6. Gustafsson UO, Scott MJ, Hubner M, Nygren J, Demartines N, Francis N, et al. Guidelines for Perioperative care in elective colorectal surgery: Enhanced Recovery After Surgery (ERAS((R))) society recommendations: 2018. World J Surg. 2019;43(3):659–95. [DOI] [PubMed] [Google Scholar]
- 7. Modasi A, Pace D, Godwin M, Smith C, Curtis B. NSAID administration post colorectal surgery increases anastomotic leak rate: systematic review/meta‐analysis. Surg Endosc. 2019;33(3):879–85. [DOI] [PubMed] [Google Scholar]
- 8. Freeman LC, Narvaez DF, McCoy A, von Stein FB, Young S, Silver K, et al. Depolarization and decreased surface expression of K+ channels contribute to NSAID‐inhibition of intestinal restitution. Biochem Pharmacol. 2007;74(1):74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tibble J, Sigthorsson G, Foster R, Bjarnason I. Comparison of the intestinal toxicity of celecoxib, a selective COX‐2 inhibitor, and indomethacin in the experimental rat. Scand J Gastroenterol. 2000;35(8):802–7. [DOI] [PubMed] [Google Scholar]
- 10. Busti AJ, Hooper JS, Amaya CJ, Kazi S. Effects of perioperative antiinflammatory and immunomodulating therapy on surgical wound healing. Pharmacotherapy. 2005;25(11):1566–91. [DOI] [PubMed] [Google Scholar]
- 11. İnan A, Koca C, Şen M. Effects of diclofenac sodium on bursting pressures of anastomoses and hydroxyproline contents of perianastomotic tissues in a laboratory study. Int J Surg. 2006;4(4):222–7. [DOI] [PubMed] [Google Scholar]
- 12. Huang Y, Tang SR, Young CJ. Nonsteroidal anti‐inflammatory drugs and anastomotic dehiscence after colorectal surgery: a meta‐analysis. ANZ J Surg. 2018;88(10):959–65. [DOI] [PubMed] [Google Scholar]
- 13. Jadad AR, Moore RAndrew, Carroll D, Jenkinson C, Reynolds DM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. [DOI] [PubMed] [Google Scholar]
- 14. Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. Ottawa, ON: Ottawa Hospital Research Institute;2009. [Google Scholar]
- 15. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327(7414):557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101. [PubMed] [Google Scholar]
- 17. Abramson JH. WINPEPI updated: computer programs for epidemiologists, and their teaching potential. Epidemiol Perspect Innov. 2011;8(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen J‐Y, Wu G‐J, Mok MS, Chou Y‐H, Sun W‐Z, Chen P‐L, et al. Effect of adding ketorolac to intravenous morphine patient‐controlled analgesia on bowel function in colorectal surgery patients–a prospective, randomized, double‐blind study. Acta Anaesthesiol Scand. 2005;49(4):546–51. [DOI] [PubMed] [Google Scholar]
- 19. Schlachta C, Burpee S, Fernandez C, Chan B, Mamazza J, Poulin E. Optimizing recovery after laparoscopic colon surgery (ORAL‐CS). Surg Endosc. 2007;21(12):2212–9. [DOI] [PubMed] [Google Scholar]
- 20. Sim R, Cheong D, Wong K, Lee B, Liew Q. Prospective randomized, double‐blind, placebo‐controlled study of pre‐and postoperative administration of a COX‐2‐specific inhibitor as opioid‐sparing analgesia in major colorectal surgery. Colorectal Dis. 2007;9(1):52–60. [DOI] [PubMed] [Google Scholar]
- 21. Xu Y, Tan Z, Chen J, Lou F, Chen W. Intravenous flurbiprofen axetil accelerates restoration of bowel function after colorectal surgery. Can J Anesth. 2008;55(7):414–22. [DOI] [PubMed] [Google Scholar]
- 22. Chen J‐Y, Ko T‐L, Wen Y‐R, Wu S‐C, Chou Y‐H, Yien H‐W, et al. Opioid‐sparing effects of ketorolac and its correlation with the recovery of postoperative bowel function in colorectal surgery patients: a prospective randomized double‐blinded study. Clin J Pain. 2009;25(6):485–9. [DOI] [PubMed] [Google Scholar]
- 23. Wattchow D, De Fontgalland D, Bampton P, Leach P, McLaughlin K, Costa M. Clinical trial: the impact of cyclooxygenase inhibitors on gastrointestinal recovery after major surgery–a randomized double blind controlled trial of celecoxib or diclofenac vs. placebo. Aliment Pharmacol Ther. 2009;30(10):987–98. [DOI] [PubMed] [Google Scholar]
- 24. Rosenberg J, Harvald T. Severe complications with diclofenac after colonic resection. Dis Colon Rectum. 2007;50(5):685. [DOI] [PubMed] [Google Scholar]
- 25. Holte K, Andersen J, Jakobsen DH, Kehlet H. Cyclo‐oxygenase 2 inhibitors and the risk of anastomotic leakage after fast‐track colonic surgery. Br J Surg. 2009;96(6):650–4. [DOI] [PubMed] [Google Scholar]
- 26. Klein M, Andersen LPH, Harvald T, Rosenberg J, Gögenur I. Increased risk of anastomotic leakage with diclofenac treatment after laparoscopic colorectal surgery. Dig Surg. 2009;26(1):27–30. [DOI] [PubMed] [Google Scholar]
- 27. Gorissen KJ, Benning D, Berghmans T, Snoeijs MG, Sosef MN, Hulsewe KWE, et al. Risk of anastomotic leakage with non‐steroidal anti‐inflammatory drugs in colorectal surgery. Br J Surg. 2012;99(5):721–7. [DOI] [PubMed] [Google Scholar]
- 28. Klein M, Gögenur I, Rosenberg J. Postoperative use of non‐steroidal anti‐inflammatory drugs in patients with anastomotic leakage requiring reoperation after colorectal resection: cohort study based on prospective data. BMJ. 2012;345:e6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zittel TT, Razavi D, Papp A, Lundberg K. Increased risk for complications after colorectal surgery with selective cyclo‐oxygenase 2 inhibitor etoricoxib. Dis Colon Rectum. 2013;56(6):761–7. [DOI] [PubMed] [Google Scholar]
- 30. STARSurg Collaborative ; Chapman S, Glasbey J, Kelly M, Khatri C, Nepogodiev D, et al. Impact of postoperative non‐steroidal anti‐inflammatory drugs on adverse events after gastrointestinal surgery. Br J Surg. 2014;101(11):1413–23. [DOI] [PubMed] [Google Scholar]
- 31. Saleh F, Jackson TD, Ambrosini L, Gnanasegaram JJ, Kwong J, Quereshy F, et al. Perioperative nonselective non‐steroidal anti‐inflammatory drugs are not associated with anastomotic leakage after colorectal surgery. J Gastrointest Surg. 2014;18(8):1398–404. [DOI] [PubMed] [Google Scholar]
- 32. Subendran J, Siddiqui N, Victor JC, McLeod RS, Govindarajan A. NSAID use and anastomotic leaks following elective colorectal surgery: a matched case‐control study. J Gastrointest Surg. 2014;18(8):1391–7. [DOI] [PubMed] [Google Scholar]
- 33. Hakkarainen TW, Steele SR, Bastaworous A, Dellinger EP, Farrokhi E, Farjah F, et al. Nonsteroidal anti‐inflammatory drugs and the risk for anastomotic failure: a report from Washington State’s Surgical Care and Outcomes Assessment Program (SCOAP). JAMA Surg. 2015;150(3):223–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Paulasir S, Kaoutzanis C, Welch KB, Vandewarker JF, Krapohl G, Lampman RM, et al. Nonsteroidal anti‐inflammatory drugs: do they increase the risk of anastomotic leaks following colorectal operations? Dis Colon Rectum. 2015;58(9):870–7. [DOI] [PubMed] [Google Scholar]
- 35. Raju DP, Hakendorf P, Costa M, Wattchow DA. Efficacy and safety of low‐dose celecoxib in reducing post‐operative paralytic ileus after major abdominal surgery. ANZ J Surg. 2015;85(12):946–50. [DOI] [PubMed] [Google Scholar]
- 36. Bakker N, Deelder JD, Richir MilanC, Cakir H, Doodeman HJ, Schreurs WH, et al. Risk of anastomotic leakage with nonsteroidal anti‐inflammatory drugs within an enhanced recovery program. J Gastrointest Surg. 2016;20(4):776–82. [DOI] [PubMed] [Google Scholar]
- 37. Rushfeldt CF, Agledahl UC, Sveinbjørnsson B, Søreide K, Wilsgaard T. Effect of perioperative dexamethasone and different NSAIDS on anastomotic leak risk: a propensity score analysis. World J Surg. 2016;40(11):2782–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rutegård M, Westermark S, Hultberg DK, Haapamäki M, Matthiessen P, Rutegård J. Non‐steroidal anti‐inflammatory drug use and risk of anastomotic leakage after anterior resection: a protocol‐based study. Dig Surg. 2016;33(2):129–35. [DOI] [PubMed] [Google Scholar]
- 39. Haddad NN, Bruns BR, Enniss TM, Turay D, Sakran JV, Fathalizadeh A, et al. Perioperative use of nonsteroidal anti‐inflammatory drugs and the risk of anastomotic failure in emergency general surgery. J Trauma Acute Care Surg. 2017;83(4):657–61. [DOI] [PubMed] [Google Scholar]
- 40. Hultberg DK, Angenete E, Lydrup M‐L, Rutegård J, Matthiessen P, Rutegård M. Nonsteroidal anti‐inflammatory drugs and the risk of anastomotic leakage after anterior resection for rectal cancer. Eur J Surg Oncol. 2017;43(10):1908–14. [DOI] [PubMed] [Google Scholar]
- 41. Fjederholt KT, Okholm C, Svendsen LB, Achiam MP, Kirkegård J, Mortensen FV. Ketorolac and other NSAIDs increase the risk of anastomotic leakage after surgery for GEJ cancers: a cohort study of 557 patients. J Gastrointest Surg. 2018;22(4):587–94. [DOI] [PubMed] [Google Scholar]
- 42. Somasundaram S, Sigthorsson G, Simpson R, Watts J, Jacob M, Tavares I, et al. Uncoupling of intestinal mitochondrial oxidative phosphorylation and inhibition of cyclooxygenase are required for the development of NSAID‐enteropathy in the rat. Aliment Pharmacol Ther. 2000;14(5):639–50. [DOI] [PubMed] [Google Scholar]
- 43. Rainsford K. Discovery, mechanisms of action and safety of ibuprofen. Int J Clin Pract Suppl. 2003;135:3–8. [PubMed] [Google Scholar]
- 44. Basivireddy J, Jacob M, Ramamoorthy P, Balasubramanian KA. Alterations in the intestinal glycocalyx and bacterial flora in response to oral indomethacin. Int J Biochem Cell Biol. 2005;37(11):2321–32. [DOI] [PubMed] [Google Scholar]
- 45. Klein M, Krarup P‐M, Kongsbak MB, Ågren MS, Gögenur I, Jorgensen LN, et al. Effect of postoperative diclofenac on anastomotic healing, skin wounds and subcutaneous collagen accumulation: a randomized, blinded, placebo‐controlled, experimental study. Eur Surg Res. 2012;48(2):73–8. [DOI] [PubMed] [Google Scholar]
- 46. Mastboom W, Hendriks T, van Elteren P, De Boer H. The influence of NSAIDs on experimental intestinal anastomoses. Dis Colon Rectum. 1991;34(3):236–43. [DOI] [PubMed] [Google Scholar]
- 47. de Sousa JB, Soares EG, Aprilli F. Effects of diclofenac sodium on intestinal anastomotic healing. Dis Colon Rectum. 1991;34(7):613–7. [DOI] [PubMed] [Google Scholar]
- 48. Cahill R, Sheehan K, Scanlon R, Murray F, Kay E, Redmond H. Effects of a selective cyclo‐oxygenase 2 inhibitor on colonic anastomotic and skin wound integrity. Br J Surg. 2004;91(12):1613–8. [DOI] [PubMed] [Google Scholar]
- 49. Ji C, Xiong Y, Pan X, Guo X, Li Z, Qian S, et al. Effect of non‐steroidal anti‐inflammatory drugs on the increasing the incidence of colonic anastomosis in rats. Int J Clin Exp Pathol. 2015;8(6):6126. [PMC free article] [PubMed] [Google Scholar]
- 50. Smith SA, Roberts DJ, Lipson ME, Buie WD, MacLean AR. Postoperative nonsteroidal anti‐inflammatory drug use and intestinal anastomotic dehiscence: a systematic review and meta‐analysis. Dis Colon Rectum. 2016;59(11):1087–97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


