Abstract
Background:
Some studies have investigated the effects of iron on breast carcinogenesis and reported different findings about the association between Fe and breast cancer risk. This study was conducted to estimate this effect using meta-analysis method.
Methods:
A total of 20 articles published between 1984 and 2017 worldwide were selected through searching PubMed, Scopus, Embase, Web of Science, and Cochrane Library. Keywords such Breast Cancer, Neoplasm, Trace elements, Iron, Breast tissue concentration, Plasma concentration, Scalp hair concentration, toenail concentration and their combination were used in the search.
Results:
The total number of participants was 4,110 individuals comprising 1,624 patients with breast cancer and 2,486 healthy subjects. Fe concentration was measured in the various subgroups in both case and control groups. There were significant correlations between Fe concentration and breast cancer in breast tissue subgroup (SMD: 0.67 [95% CI: 0.17 to 1.17; P=0.009]). Whereas, there was no meaningful difference in Fe status between women with and without breast cancer related to scalp hair and plasma subgroups; (SMD: -3.74 [95% CI: -7.58 to 0.10; P=0.056] and (SMD:-1.14[95% CI: -2.30 to 0.03; P=0.055], respectively.
Conclusion:
The present meta-analysis indicated a positive and straight association between iron concentrations and risk of breast cancer but because of high heterogeneity we recommend more accurate future studies.
Key Words: Breast Cancer, Neoplasm, trace elements, Iron, Meta-analysis
Breast cancer has the most cancer incidence in women and also the second most common global cancer (1, 2). It represents the fifth cause of cancer deaths and the first cause of cancer death in women around the world (3, 4). Although, mortality rate of breast cancer has decreased in developed regions but it still represents the first cause of death due to cancer in less developed regions and the second cause in developed regions (5, 6). So, understanding the etiology and factors involved in breast carcinogenesis can contribute to treatment options (7). Some studies have shown that not only inherited genetic factors increase the breast cancer risk; but also, lifestyle and environment are important risk factors for breast cancer (8, 9). Numerous studies have established involvement of metallic compounds like trace elements in the development of breast cancer (10-12). Trace elements elicit several biological functions; participate in the synthesis of hormones and vitamins, regulate gene expression, modulate cell membrane permeability and take part in electron transport (13); also are involved in hormonal and enzymatic function because they compete with other metals for potential interaction sites (14).
These elements can be essential and benefit in small concentrations or toxic and carcinogenic, when taken in excess (15). Researchers demonstrated that trace elements are able to influence breast carcinogenesis in multiple ways; they act as estrogen disruptors and active estrogen signaling which a network of important biological mechanisms in the carcinogenesis process like, proliferation and migration of breast cancer cells trigger (10, 16).
One of them is Fe- in carcinogenesis of breast cancer. Iron works as a structural and functional cofactor for various proteins and enzymes, a prosthetic group in many enzymes and an important constituent of succinate dehydrogenase as well as a part of the hem of hemoglobin, myoglobin, and the cytochromes (15, 17). It is a key element in different biochemical cell processes and the integrity of various cell apparatus, and also involved in many physiological functions like oxygen transportation, oxidative phosphorylation and xenobiotic metabolism (17). However, high Fe intake can have carcinogenic effects and leads to development of cancer due to the fact that Fe is crucial for regulating of cell growth (18). Furthermore, iron may act as a catalyst, exert toxicity by generating highly toxic molecules and involve in the repress of host defense cells (19). Due to the high incidence of breast carcinomas and its mortality and morbidity rate in the world, prevention planning and treatment this disease seemed necessary. The management of risk factors is an appropriate option for prevention of non-communicable diseases, thus determining the risk factors involved in breast carcinogenesis can contribute to treatment options.
There are many studies with different findings concerning the influence of Fe in the incidence of breast cancer; and lack of systematic review and meta-analysis in this area to provide an overall and valid result, conducting a meta-analysis is important; because a meta-analysis study leads to a large sample size and overall resolution. The aim of this study was to investigate the influence of Fe on breast cancer by reviewing the available studies.
Methods
Study selection: We performed a literature search with citations in the Scopus, PubMed, Web of Science, Embase, and Cochrane Library databases for case-control and cross sectional published between 1984 and 2017. The search strategy was done via keywords: Breast Cancer, Trace elements, Iron, Breast tissue concentration, Plasma concentration, Scalp hair concentration, toenail concentration and their combination. The search scope was developed using the wildcard symbol ‘*’ and an advanced search was performed with the combination of words or phrases using Boolean operators (‘AND’, ‘OR’, ‘NOT’). The titles from searching were scanned to be appropriated for inclusion in the study. Furthermore, the lists of reference from all related reviews and main articles were manually searched for more references.
Inclusion and exclusion criteria: All studies that analyzed Fe levels in breast Cancer patients were reviewed. Each screened study for inclusion in final analysis must present the data on the relation between Fe levels and breast cancer risk by measuring this element concentration in any of three types of sample specimens: serum, breast tissue and hair. Study was limited to conduct articles in humans. We excluded studies if they were duplicate publications or that were meta-analyses or systematic considerations; if they perform on non-human creatures (i.e. animal studies); if they presented insufficient data; and if published in languages other than English. We searched gray literature as far as possible in search engineers, but access to all of the gray literature sours was not possible.
Data extraction: The extracted data for all studies (case- control and cross sectional) include the first author, year of publication, sample size, sample age, location, iron concentration, mean difference, type of sample specimens, and iron screening method (figure 1-study flowchart). Two of the authors independently extracted information from each article and compared findings. In which cases, the results were discordant, papers were reviewed jointly and discrepancies between researchers were resolved via group discussions.
Evaluation of the quality of selected studies: In this study, Newcastle–Ottawa Scale checklist was used to assess the quality of studies (table 1). The NOS (Newcastle–Ottawa Scale) ranges from zero to nine stars. Selected papers were ranked in three groups according to NOS quality assessment: 1- low quality (up to 3 stars), 2- medium quality (4-6 stars) and 3- high quality (more than seven stars).
Table 1.
Quality assessment of included articles according to the Newcastle–Ottawa Scale checklist
| Selection | Comparability | Exposure | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Author (References) | 1 | 2 | 3 | 4 | A | B | 1 | 2 | 3 |
| Rizk.Sh.L (23) | * | * | * | * | |||||
| N.Drake II.E (24) | * | * | * | * | * | ||||
| Singh.V (25) | * | * | * | * | |||||
| Geraki.K (20) | * | * | * | ||||||
| Wen Kuo.HW (21) | * | * | * | * | * | * | * | ||
| Ionescu.J.G (39) | * | * | * | ||||||
| Cui.Y (27) | * | * | * | * | * | * | |||
| Ebrahim.A.M (28) | * | * | * | * | * | ||||
| Magalhaes.T (29) | * | * | * | * | * | ||||
| Arinola, O. G (30) | * | * | * | * | * | * | |||
| Joo.N.S (22) | * | * | * | * | * | * | * | ||
| Pasha. Q (31) | * | * | * | * | * | ||||
| Feng.J (32) | * | * | * | * | * | * | * | ||
| Silva.M.P (33) | * | * | * | * | * | ||||
| Rehman.S (34) | * | * | * | ||||||
| Pavithra. V(35) | * | * | * | * | |||||
| Karki.K (36) | * | * | * | * | * | * | |||
| Romanjuk.A (37) | * | * | * | ||||||
| Jablonska.E (40) | * | * | * | * | * | ||||
| Quintana Pacheco. DA (38) | * | * | * | * | * | * | * | ||
The criteria considered to measure bias in the authors' intended studies included: reference to the time and place of the study, describing exit and entry criteria of the participants, how to measure the variables, what statistical methods used and preparing reports on standard deviation or confidence intervals of the estimates.
Statistical analysis: Studies were combined based on the sample size, mean and standard deviation. The formula of two integrated variance was used to calculate the difference between the average variance of the normal distribution. To assess heterogeneity between studies, the chi-square test and I2 index was used. In this study, the analysis was performed using a random-effects model, considering the significant heterogeneity between the studies. Integrated estimations and the related confidence interval of 95% were evaluated using forest plots as visuals. Sensitivity analyses were also performed. For evaluating publication bias, Funnel plots and Egger test were used. P<0.05 were considered as valid for heterogeneity tests. STATA (version 12) was used for statistical analyses.
Results
In initial search process, 71 studies were identified. Of these studies, 9 duplicates were excluded. We excluded other 42 articles (review article, lack of enough information and comparison with other elements). After detailed review of selected articles, 20 published studies between 1984 and 2018 including four cross-sectional (20-22) and 17 case control (23-25), (26-30), (31-38) were selected for the final analysis (figure 1). Considering all the included studies, the total number of participants was 4,110 individuals containing 1,624 patients with breast cancer and 2,486 healthy subjects. Selected studies were conducted in different countries. Of the 20 studies, nine were conducted in Asia (25), (21, 22, 30-32), (34-36), six in Europe (20, 29, 39), (37, 38, 40), four in America (23, 24, 27, 34), and one were performed in Africa (18). Among the reviewed studies, in eight of them iron status was measured in serum and plasma (21, 25) (30, 32, 35, 36, 38, 39); in two studies scalp hair iron status was used (22, 31) and in the remaining ten breast tissues was the sample specimen used (18, 20, 23, 24, 27, 29, 33, 34, 37, 40). The quality of studies were assessed using the NOS; accordingly, three studies had a score of 7 , four studies had a score of 6), six studies had a score of 5 , three studies had a score of 4 and the remaining four studies had a score of 3. The baseline characteristics of these studies are summarized in table 2.
Table 2.
Baseline characteristics of studies included in this meta-analysis
| First author, (Reference) | Country (year of publication) | Case | Control | Matrix | Iron concentration (Mean±SD) | Unit | Type of measurement | ||
|---|---|---|---|---|---|---|---|---|---|
| Case | Control | ||||||||
| Rizk.Sh.L (23) | Chicago (1984) | 25 | 25 | Breast tissue | 238.5±113 | 218.4±149.3 | µg/g | EDXRF | |
| N.Drake II.E(24) | Texas (1989) | 26 | 26 | Breast tissue | 239±113 | 218±149 | µg/g | INAA | |
| Singh.V (25) | India (1998) | 10 | 10 | Serum | 2250±350 | 1710±7 | µg/g | AAS | |
| Geraki.K (20) | UK (2002) | 20 | 20 | Breast tissue | 16.40±14.11 | 6.06±6.57 | ppm | TXRF | |
| Wen Kuo.HW (21) | China (2002) | 43 | 26 | Serum Benign |
1055.81±104.05 | 1040.38±99.94 | µg/l | ICP-AES | |
| Wen Kuo.HW (21) | China (2002) | 43 | 25 | Serum Malignant |
114.71±107.12 | 1040.38±99.94 | µg/l | ICP-AES | |
| Ionescu.J.G (39) | Prague, Czech Republic, Germany (2006) | 20 | 8 | Plasma | 53.17±10.20 | 10.94±8.10 | µg/kg | AAS- ICP-MS | |
| Cui.Y (27) | USA (2007) | 251 | 249 | Breast tissue | 2.38±2.10 | 2.12±2 | ng/cm2 | TXRF | |
| Ebrahim.A.M(18) | Sudan (2007) | 40 | 40 | Breast tissue | 66.10±12.79 | 57.92±7.23 | ppm | INAA | |
| Magalhaes.T (29) | Portugal, Germany (2008) | 15 | 15 | Breast tissue | 43.20±9.8 | 34±7.90 | µg/g | TXRF | |
| Arinola, O. G (30) | Nigeria(2008) | 29 | 30 | Plasma | 59.62±2.53 | 59.05±2.50 | µg/l | AAS | |
| Joo,N (22) | South Korea (2009) | 40 | 144 | Hair | 6.45±0.43 | 9.15±0.28 | µg/g | - | |
| Pasha. Q (31) | Pakistan (2010) | 33 | 35 | Scalp Hair Malignant |
114±24.50 | 129±28.10 | µg/g | AAS | |
| Pasha. Q (31) | Pakistan (2010) | 36 | 35 | Scalp Hair Benign |
80.40±13 | 129±28.10 | µg/g | AAS | |
| Feng.J.F (32) | China (2012) | 32 | 20 | Serum | 1146.30±156.2 | 1062.3±59.2 | µg/l | M6 AAS | |
| Silva.M (33) | Brazil (2012) | 34 | 38 | Breast tissue Malignant |
33±8.30 | 15.60±6.50 | mg/kg | TXRF | |
| Silva.M (33) | Brazil (2012) | 9 | 38 | Breast tissue Benign |
15.1±6.30 | 15.60±6.50 | mg/kg | ||
| Rehman.S (34) | Pakistan (2014) | 15 | - | Breast tissue Benign |
49.1±11.4 | - | mg/l | AAS | |
| Rehman.S (34) | Pakistan (2014) | 20 | - | Breast tissue Malignant |
225±121 | - | mg/l | AAS | |
| Pavithra.V(35) | India (2015) | 54 | 54 | Serum | 85.47±47.45 | 67.49±28.24 | - | Ferrozo | |
| Karki. K (36) | India (2015) | 70 | 70 | Serum Benign |
57.26 ±3.69 | 69.33±3.62 | µg/l | ferrozine | |
| Karki. K (36) | India (2015) | 70 | 70 | Serum Malignant |
46.73±1.32 | 69.33±3.62 | µg/l | ferrozine | |
| Romanjuk.A (37) | Ukraine (2016) | 20 | - | Breast tissue | 2.0 ±0.26 | - | g/mol | EDXRF | |
| Jablonska.E (40) | Poland (2017) | 42 | 42 | Breast tissue | 66.50 | 41.20 | µg/g | AAS | |
| Quintana Pacheco. DA (38) | Germany (2018) | 627 | 1466 | Plasma | 17.80±6.60 | 17.4±6.60 | µmol/l | The Roche Cobas 6000 analytical system | |
Abbreviations: Atomic absorption spectrophotometry (AAS); inductively coupled plasma–atomic emission spectrometry (ICP-AES); X-ray fluorescence spectrometry (TXRF); Instrumental neutron activation analysis (INAA); Energy dispersive X-ray fluorescence (EDXRF).
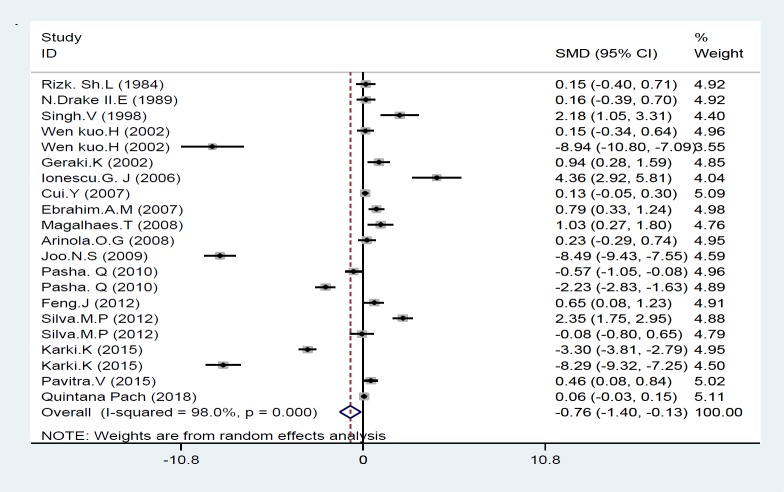
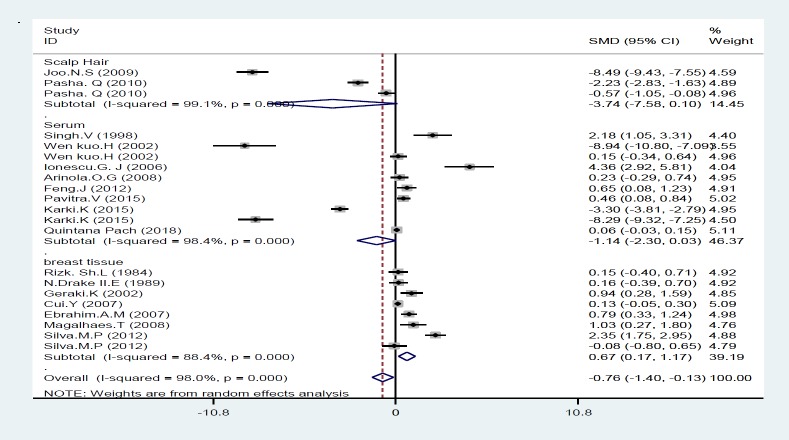
In this study, the levels of iron in various studies in both the case and control groups were identified and standard mean difference (SMD) was measured in analysis. Among the included studies, two of them were excluded from analysis because they did not report the iron concentration in control group (34, 37). Also, one study presented data as mean and did not present standard deviation, so it was excluded from final analysis (40); overall, seventeen of 20 studies presented their findings as means±SD and were included in final analysis (table 2). As seen in figure 2, the present meta-analysis using a random effects model showed a significant association between Fe statuses and risk of breast cancer; the standard mean difference (SMD) was -0.76 [95% CI: -1.40 to -0.13; P<0.001] and there was a significant heterogeneity [I2=98.0%; P=0.000] (figure 2). We conducted a subgroup analysis according to the type of sample specimens; accordingly Fe concentrations were measured in three groups 1: serum and plasma, 2: breast tissue and 3: scalp hair. Our meta-analysis found a meaningful difference in Fe concentrations between individuals with and those without breast cancer only in breast tissue subgroup which SMD was 0.67 [95% CI: 0.17 to 1.17; P=0.009], whereas there was no meaningful difference in Fe statuses between women with and without breast cancer related to scalp hair and plasma subgroups; their mean difference was -3.74 [95% CI: -7.58 to 0.10; P=0.056] and -1.14[95% CI: -2.30 to 0.03; P=0.055], respectively (figure 3).
Figure 2.
Forest plot of the association of iron with breast cancer risk. Square represents effect estimate of individual studies with their 95 % confidence intervals. And the diamond shows the overall estimate of SMD in this study. In this chart, studies are stored in order of the year of publication and author’s names, based on a random effects model
Figure 3.
Forest plot of the association of iron with breast cancer risk based on sample type (serum and plasma, breast tissues, scalp hair)
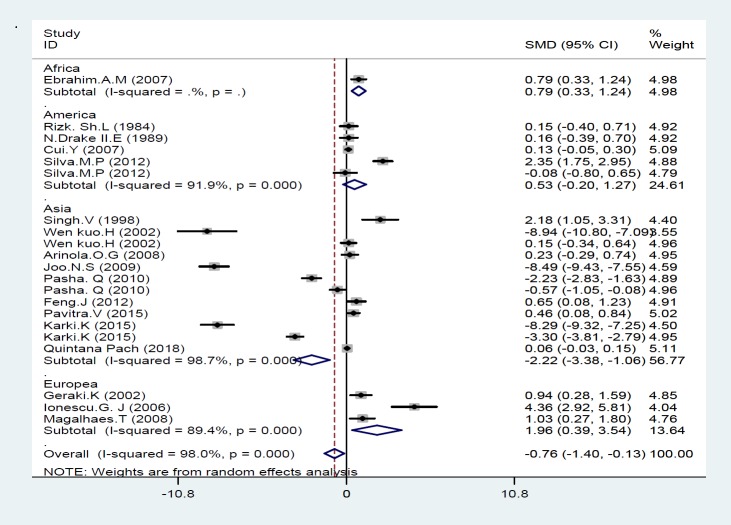
Figure 4 shows the result of meta-analysis for each continent. As seen, there was significant statistical difference in Fe concentration between cancer patients and healthy controls in Asia, Europe and Africa; their SMD was -2.22 [95% CI: -3.38 to -1.06; P=0.000], 1.96 [95% CI: 0.39 to 3.53; P=0.014] and 0.79 [95% CI: 0.33 to 1.24; P=0.001], while no significant difference was observed in Fe concentration between cancer patients and healthy women in America; the mean difference was 0.53 [95% CI: -0.2 to 1.27; P=0.154]. We performed a subgroup analysis based on the quality score; the results of meta-analysis showed a meaningful difference in Fe status between breast cancer and control group only in high quality studies (≥7 stars) which the mean difference was -4.19 [95% CI: -7.27 to -1.10; P=0.00], suggested that Fe status was associated with an increased risk of breast cancer: Only in studies with high quality score.
Figure 4.
Forest Plot of the association of iron with breast cancer risk based on continent
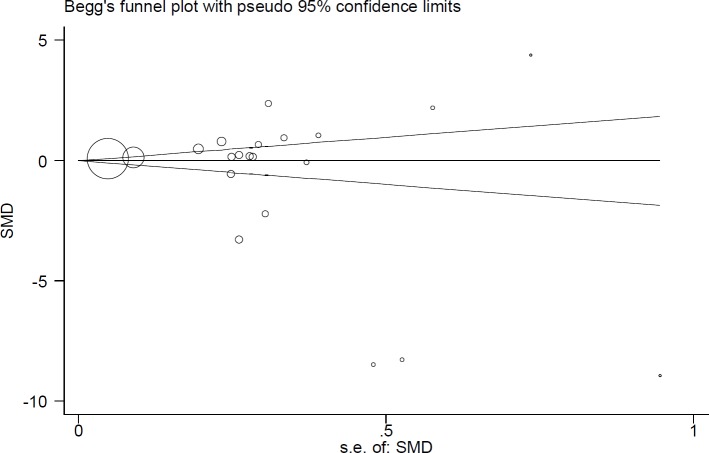
Figure 5 presents the Beggs funnel plot of tests related to Fe statuses in cancer patients. Interpretation of this plot showed no signs of publication bias in these studies (p=0.24); this means that studies with negative and positive results have been published (figure 5).
Figure 5.
Begg’s funnel plot for publication bias
Discussion
This study systematically reviewed the effects of iron in breast cancer and quantitatively analyze the association between iron levels and the risk of breast cancer. The current review including 20 studies (figure 2), showed that high levels of iron have significant relationship with increased risk of breast cancer (P=0.053). In this regard, serum, breast tissues and scalp hair status of iron were examined. We observed a meaningful association between iron levels in breast tissues and an increased risk of breast cancer (p≤0.05). Whereas, analysis of Fe concentrations in serum and scalp hair did not reveal any significant relationship between iron statuses and the risk of breast cancer (p>0.05). So far, some studies have evaluated the relationship between iron concentration and the risk of breast cancer and have reported contradictory results. Our findings were consistent with previous studies as demonstrated iron status can increase the incidence of breast cancer (18, 20-23, 29, 31-33, 35, 36, 39); higher levels of iron were detected in breast tumor tissues relative to the normal tissues in some studies (41, 42), also higher concentrations of serum iron was observed in women with breast tumor compared to healthy subjects (43, 44). Whereas others did not report a positive link between Fe concentration and breast cancer (24, 25, 27, 30, 34, 37).
The present study via meta-analysis method proved a direct relationship between high iron levels and greater risk of breast cancer. Similarly, a systematic review summarized the evidence regarding the relationship between Fe and breast cancer risk and concluded that this trace element can apply a stimulatory function in breast cancer (10). Accordingly, it was established that higher iron concentrations can enhance risk of breast cancer in women (41-44). However, iron abundantly exists in all human organisms and performs many functions. Overload iron may have toxic effects; for example, it contributes in generating free radicals, a reaction that is regulated by antioxidant mechanisms whenever iron concentration is in a normal state (15, 17-19).
Iron is necessary for physiological cellular functions because it is an inseparable part of many proteins and enzymes (45). It fundamentally contributes in the arrangement of cellular growth and differentiation (46). Physiological keeping of fairly stable iron levels is a vital condition so, both insufficiency and excess intake of iron have negative effects and can lead to disease expansion (15). High iron load is related to many chronic conditions, like imperfect control of the immune system (44) and cancer (45, 46). Iron can be carcinogenic. Several studies evaluated the relationship between elevated iron levels and risk of cancer; some of these studies reported that iron overload was linked to greater risk of overall cancer and cancer mortality (43, 47), while others did not observe this association (48). Iron overload is able to contribute in the generation of reactive oxygen species, involve in the repress of cellular immune functions, and raise tumor growth (49). Oxidative and reduction reactions involving in Fe storage and transport exhibits that it involved in the generation of free radical according to the equations that is known as Fenton and Haber-Weiss reactions (41): Fe2+ + H2O2 Fe3+ + OH + OH –
These reactions produce hydroxyl radicals that lead to lipid peroxidation, DNA damage, inactivate enzymes, and depolymerize polysaccharides and apoptosis (49-51). In breast cancer patients, free iron may be released from the storage form during the metabolism of estradiol, this reaction induces oxidative stress that leads to the generation of mutagenic radicals, and then the produced free radicals cause DNA damage and mutations in the breast (12, 48). It is accepted that the expression of several iron-regulatory proteins such as ferritin, hepcidin and ferroportin are deregulated in breast cancer subjects (41). Thus, high concentration of iron is considered as a major risk factor in the progression of breast cancer.
Iron as a nutritious substance participates in the feeding of microbial and neoplastic cells and thereby leading to disease progress (52). Iron also plays an important role in simulating angiogenesis (53). Bio activation of phenolic/quinonic compounds near the tumor location can abundantly produce the radicals of superoxide and semiquinone which have detrimental effects on the metal-rich cancer cells (54, 55). This causes inflammation and increased growth of cancer cells (56). Some studies have reported that excessive agglomeration of iron in humans is related to a high risk of cancer (57-59). However, others have reported conflicting results and not found any significant relationship between accumulation of iron and breast cancer risk (60, 61).
We conducted subgroup analysis based on the type of sample specimens to address the observed heterogeneity, and we could not find a meaningful association between iron levels in serum and scalp hair with breast cancer, whereas our analysis revealed a significant relationship between high levels of iron and greater risk of breast cancer in breast tissue and also a relatively mild association in benign breast tissue. One possible reason for this observation is that excessive accumulation of free iron in benign breast tissue due to their catalytic effects can generate mutagenic radicals, and also can repress host defense cells and, thus enhances the risk of breast cancer (28).
One of the other possible reasons is that both benign and cancerous cells may request enhanced concentration of iron for maintaining their proliferation due to iron is essential for ribonucleotide reductase which is a main enzyme in DNA synthesis (62). Therefore, it is likely that benign breast tissue with high iron accumulation be prone to breast cancer.
We also conducted subgroup analysis based on geographic region; analysis of the association Fe statuses with breast cancer based on continent showed a significantly difference in Fe concentration between breast cancer and healthy women in Asia, Europe and Africa (p<0.05); while, no meaningful difference was found in Fe concentration between women with and without breast cancer in America (P>0.05). In Asia, low levels of Fe and in Europe and Africa high levels of Fe had positive association with the risk of breast cancer. Geographical and residential socioeconomic inequalities may potentially reflect the observed differences. Also, demographic characteristics and genetic background of different populations can also explain the observed heterogeneity. In addition, our analysis based on the quality score showed a meaningful correlation between Fe statuses and breast cancer risk only in high quality studies that this may also affect the results of heterogeneity.
It has been hypothesized that dietary iron intake was positively associated with an increased breast cancer risk and several studies were performed in this regard; some of them found null results (63-65). Whereas several large prospective cohort studies reported a positive association between iron intake and the risk of breast cancer (66-68). These findings propose that higher iron intake for example what is found at red meat may enhance the breast cancer risk. Along with confirming this, the current study showed a high concentration of iron in human is positively associated with an enhanced risk of breast cancer. Thus, high iron accumulation in the body according to their effect in the progression of breast cancer is important among women, especially those who are prone to developing cancer. Since, deregulation of iron metabolism related biomolecules is one of the most important factors for breast cancer, cancer can be prevented through regulating iron metabolism related proteins and prevention of iron accumulation in the tissue. This information can be used by researchers to manufacture drugs that can control the pathways related to iron accumulation and carcinogenesis. Besides, the study about the association between iron and breast cancer risk and burden of disease provides a unique perspective for planning interventions and developing public health policies. Moreover, our findings indicated that preventive procedures and interventional methods for controlling concentration of iron in human are needed especially in those who are prone to developing cancer.
Limitations
This meta-analysis had several limitations that most of them were related to methodology. Some of these limitations include:
1. Absence of the same method for measuring variance.
2. Absence of data relation to habits and lifestyle of the population studied.
4. The screening methods varied and there was not a uniform standard method for measuring iron concentrations in different studies.
5. Some relevant articles were not available
In conclusion the present meta-analysis indicated a positive and straight association between iron concentration and risk of breast cancer (P=0.009). But because of high heterogeneity we recommend more accurate future studies.
Thus, Fe levels should be controlled in food sources, drugs, and etc. This study showed that the estimation of iron concentration in biological specimens has an important role in early detection of this disease. Our results also can be considered for debarment of breast cancer by controlling level of iron in the persons' diet.
Financial Disclosure:
There are no benefits in any form that have been or will be received from a commercial party related directly or indirectly to the subject of this article.
Acknowledgments
We would like to thank the Students Research Committee of Gorgan University of Medical Sciences.
Author Contributions
F.S, L.J and A.S contributed to the study design; F.K, L.J and M.S.G wrote the main manuscript text; K.S, F.K and F.S performed the data processing and statistical analyses.
References
- 1.Curigliano G, Burstein HJ, P Winer E, et al. De-escalating and escalating treatments for early-stage breast cancer: the St Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann Oncol. 2017;28:1700–12. doi: 10.1093/annonc/mdx308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu FC, Lin HT, Kuo CF, et al. Epidemiology and survival outcome of breast cancer in a nationwide study. Oncotarget. 2017;8:16939–50. doi: 10.18632/oncotarget.15207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akram M, Iqbal M, Daniyal M, Khan AU. Awareness and current knowledge of breast cancer. Biol Res. 2017;50 doi: 10.1186/s40659-017-0140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 5.Malvezzi M, Carioli G, Bertuccio P, et al. European cancer mortality predictions for the year 2016 with focus on leukaemias. Ann Oncol. 2016;27:725–31. doi: 10.1093/annonc/mdw022. [DOI] [PubMed] [Google Scholar]
- 6.Christopher PP, Appelbaum PS, Truong D, et al. Reducing therapeutic misconception: A randomized intervention trial in hypothetical clinical trials. PloS One. 2017;12:e0184224. doi: 10.1371/journal.pone.0184224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Visvanathan K, Chlebowski RT, Hurley P, et al. American society of clinical oncology clinical practice guideline update on the use of pharmacologic interventions including tamoxifen, raloxifene, and aromatase inhibition for breast cancer risk reduction. J Clin Oncol. 2009;27:3235–58. doi: 10.1200/JCO.2008.20.5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lichtenstein P, Holm NV, Verkasalo PK, et al. Environmental and heritable factors in the causation of cancer--analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000;343:78–85. doi: 10.1056/NEJM200007133430201. [DOI] [PubMed] [Google Scholar]
- 9.Jevtic M, Velicki R, Popovic M, et al. Dietary influence on breast cancer. J BUON. 2010;15:455–61. [PubMed] [Google Scholar]
- 10.Lappano R, Malaguarnera R, Belfiore A, Maggiolini M. Recent advances on the stimulatory effects of metals in breast cancer. Mol Cell Endocrinol. 2017;457:49–56. doi: 10.1016/j.mce.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 11.Sharma K, Mittal D, Kesarwani R, Kamboj V. Diagnostic and prognostic significance of serum and tissue trace elements in breast malignancy. Indian J Med Sci. 1994;48:227–32. [PubMed] [Google Scholar]
- 12.Pasha Q, Malik SA, Iqbal J, Shaheen N, Shah MH. Comparative evaluation of trace metal distribution and correlation in human malignant and benign breast tissues. Biol Trace Elem Res. 2008;125:30–40. doi: 10.1007/s12011-008-8158-z. [DOI] [PubMed] [Google Scholar]
- 13.Zhai S, Yang L, Cui QC, et al. Tumor cellular proteasome inhibition and growth suppression by 8-hydroxyquinoline and clioquinol requires their capabilities to bind copper and transport copper into cells. J Biol Inorg Chem. 2010;15:259–69. doi: 10.1007/s00775-009-0594-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collins AR. Erratum-Oxidative DNA damage, antioxidants, and cancer. Bioessays. 1999;21:238–46. doi: 10.1002/(SICI)1521-1878(199903)21:3<238::AID-BIES8>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 15.Nasulewicz A, Mazur A, Opolski A. Role of copper in tumour angiogenesis--clinical implications. J Trace Elem Med Biol. 2004;18:1–8. doi: 10.1016/j.jtemb.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Rahman SS. The looming success in cancer vaccination. Adv Emerg Med. 2019;8:1–2. [Google Scholar]
- 17.Mulware SJ. Comparative trace elemental analysis in cancerous and noncancerous human tissues using PIXE. J Biophys. 2013;2013:192026. doi: 10.1155/2013/192026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Ebraheem A, Farquharson M, Ryan E. The evaluation of biologically important trace metals in liver, kidney and breast tissue. App Radiat Isot. 2009;67:470–4. doi: 10.1016/j.apradiso.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 19.Liehr JG, Jones J. Role of iron in estrogen-induced cancer. Cur Med Chem. 2001;8:839–49. doi: 10.2174/0929867013372931. [DOI] [PubMed] [Google Scholar]
- 20.Geraki K, Farquharson MJ, Bradley DA. Concentrations of Fe, Cu and Zn in breast tissue: a synchrotron XRF study. Phys Med Biol. 2002;47:2327–39. doi: 10.1088/0031-9155/47/13/310. [DOI] [PubMed] [Google Scholar]
- 21.Kuo HW, Chen SF, Wu CC, Chen DR, Lee JH. Serum and tissue trace elements in patients with breast cancer in Taiwan. Biol Trace Elem Res. 2002;89:1–11. doi: 10.1385/BTER:89:1:1. [DOI] [PubMed] [Google Scholar]
- 22.Joo NS, Kim SM, Jung YS, Kim KM. Hair iron and other minerals’ level in breast cancer patients. Biol Trace Elem Res. 2009;129:28–35. doi: 10.1007/s12011-008-8281-x. [DOI] [PubMed] [Google Scholar]
- 23.Rizk SL, Sky-Peck HH. Comparison between concentrations of trace elements in normal and neoplastic human breast tissue. Cancer Res. 1984;44:5390–4. [PubMed] [Google Scholar]
- 24.Drake EN, Sky-Peck HH. Discriminant analysis of trace element distribution in normal and malignant human tissues. Cancer Res. 1989;49:4210–5. [PubMed] [Google Scholar]
- 25.Singh V, Garg A. Trace element correlations in the blood of Indian women with breast cancer. Biol Trace Elem Res. 1998;64:237–45. doi: 10.1007/BF02783340. [DOI] [PubMed] [Google Scholar]
- 26.Ionescu JG, Novotny J, Stejskal V, et al. Increased levels of transition metals in breast cancer tissue. Neuro Endocrinol Lett. 2006;27:36–9. [PubMed] [Google Scholar]
- 27.Cui Y, Vogt S, Olson N, Glass AG, Rohan TE. Levels of zinc, selenium, calcium, and iron in benign breast tissue and risk of subsequent breast cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:1682–5. doi: 10.1158/1055-9965.EPI-07-0187. [DOI] [PubMed] [Google Scholar]
- 28.Ebrahim AM, Eltayeb MA, Shaat MK, et al. Study of selected trace elements in cancerous and non-cancerous human breast tissues from Sudanese subjects using instrumental neutron activation analysis. Sci Total Environ. 2007;383:52–8. doi: 10.1016/j.scitotenv.2007.04.047. [DOI] [PubMed] [Google Scholar]
- 29.Magalhaes T, Becker M, Carvalho M, Von Bohlen A. Study of Br, Zn, Cu and Fe concentrations in healthy and cancer breast tissues by TXRF. Spectrochimica Acta Part B: Atomic Spectroscopy. 2008;63:1473–9. [Google Scholar]
- 30.Arinola O, Charles-Davies M. Micronutrient levels in the plasma of Nigerian females with breast cancer. Afr J Biotechnol. 2008;7:1620–3. [Google Scholar]
- 31.Pasha Q, Malik SA, Shaheen N, Shah MH. Comparison of trace elements in the scalp hair of malignant and benign breast lesions versus healthy women. Biol Trace Elem Res. 2010;134:160–73. doi: 10.1007/s12011-009-8469-8. [DOI] [PubMed] [Google Scholar]
- 32.Feng JF, Lu L, Zeng P, et al. Serum total oxidant/antioxidant status and trace element levels in breast cancer patients. Int J Clin Oncol. 2012;17:575–83. doi: 10.1007/s10147-011-0327-y. [DOI] [PubMed] [Google Scholar]
- 33.Silva MP, Soave DF, Ribeiro-Silva A, Poletti ME. Trace elements as tumor biomarkers and prognostic factors in breast cancer: a study through energy dispersive x-ray fluorescence. BMC Res Notes. 2012;5:194. doi: 10.1186/1756-0500-5-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rehman S, Husnain SM. A probable risk factor of female breast cancer: study on benign and malignant breast tissue samples. Biol Trace Elem Res. 2014;157:24–9. doi: 10.1007/s12011-013-9865-7. [DOI] [PubMed] [Google Scholar]
- 35.Pavithra V, Sathisha TG, Kasturi K, et al. Serum levels of metal ions in female patients with breast cancer. J Clin Diag Res. 2015;9:BC25–27. doi: 10.7860/JCDR/2015/11627.5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karki K, Pande D, Negi R, et al. Correlation of serum toll like receptor 9 and trace elements with lipid peroxidation in the patients of breast diseases. J Trace Elem Med Biol. 2015;30:11–6. doi: 10.1016/j.jtemb.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 37.Romaniuk AM, Lyndin M, Moskalenko RA, Hortynska OM, Lyndina YM. The role of heavy metal salts in pathological biomineralization of breast cancer tissue. Adv Clin Exp Med. 2016;25:907–10. doi: 10.17219/acem/34472. [DOI] [PubMed] [Google Scholar]
- 38.Quintana Pacheco DA, Sookthai D, Graf ME, et al. Iron status in relation to cancer risk and mortality: Findings from a population‐based prospective study. Int J Cancer. 2018;143:561–9. doi: 10.1002/ijc.31384. [DOI] [PubMed] [Google Scholar]
- 39.Ionescu JG, Novotny J, Stejskal V, et al. Increased levels of transition metals in breast cancer tissue. Neuro Endocrinol Lett. 2006;27:36–9. [PubMed] [Google Scholar]
- 40.Jablonska E, Socha K, Reszka E, et al. Cadmium, arsenic, selenium and iron–implications for tumor progression in breast cancer. Environ Toxicol Pharmacol. 2017;53:151–7. doi: 10.1016/j.etap.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 41.Lamy PJ, Durigova A, Jacot W. Iron homeostasis and anemia markers in early breast cancer. Clin Chim Acta. 2014;434:34–40. doi: 10.1016/j.cca.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 42.Marques O, da Silva BM, Porto G, Lopes C. Iron homeostasis in breast cancer. Cancer Lett. 2014;347:1–14. doi: 10.1016/j.canlet.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 43.Chua AC, Knuiman MW, Trinder D, Divitini ML, Olynyk JK. Higher concentrations of serum iron and transferrin saturation but not serum ferritin are associated with cancer outcomes. Am J Clin Nutr. 2016;104:736–42. doi: 10.3945/ajcn.115.129411. [DOI] [PubMed] [Google Scholar]
- 44.Orlandi R, De Bortoli M, Ciniselli C, et al. Hepcidin and ferritin blood level as noninvasive tools for predicting breast cancer. Ann Oncol. 2013;25:352–7. doi: 10.1093/annonc/mdt490. [DOI] [PubMed] [Google Scholar]
- 45.Pizzamiglio S, De Bortoli M, Taverna E, et al. Expression of iron-related proteins differentiate non-cancerous and cancerous breast tumors. Int J Mol Sci. 2017;18:E410. doi: 10.3390/ijms18020410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kwok JC, Richardson DR. The iron metabolism of neoplastic cells: alterations that facilitate proliferation? Crit Rev Oncol Hematol. 2002;42:65–78. doi: 10.1016/s1040-8428(01)00213-x. [DOI] [PubMed] [Google Scholar]
- 47.Wen CP, Lee JH, Tai YP, et al. High serum iron is associated with increased cancer risk. Cancer Res. 2014;74:6589–97. doi: 10.1158/0008-5472.CAN-14-0360. [DOI] [PubMed] [Google Scholar]
- 48.Gaur A, Collins H, Wulaningsih W, et al. Iron metabolism and risk of cancer in the Swedish AMORIS study. Cancer Causes Control. 2013;24:1393–402. doi: 10.1007/s10552-013-0219-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baader SL, Bruchelt G, Carmine TC, et al. Ascorbic-acid-mediated iron release from cellular ferritin and its relation to the formation of DNA strand breaks in neuroblastoma cells. J Cancer Res Clin Oncol. 1994;120:415–21. doi: 10.1007/BF01240141. [DOI] [PubMed] [Google Scholar]
- 50.Okada S. Iron‐induced tissue damage and cancer: the role of reactive oxygen species‐free radicals. Pathol Int. 1996;46:311–32. doi: 10.1111/j.1440-1827.1996.tb03617.x. [DOI] [PubMed] [Google Scholar]
- 51.McCord JM. Effects of positive iron status at a cellular level. Nutr Rev. 1996;54:85–8. doi: 10.1111/j.1753-4887.1996.tb03876.x. [DOI] [PubMed] [Google Scholar]
- 52.Weinberg ED. Iron loading and disease surveillance. Emerg Infect Dis. 1999;5:346–52. doi: 10.3201/eid0503.990305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Norrby K, Mattsby‐Baltzer I, Innocenti M, Tuneberg S. Orally administered bovine lactoferrin systemically inhibits VEGF165‐mediated angiogenesis in the rat. Int J Cancer. 2001;91:236–40. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1024>3.3.co;2-k. [DOI] [PubMed] [Google Scholar]
- 54.Ionescu JG. Heavy metal accumulation in malignant tumours as basis for a new integrative therapy model. Anti-Aging Ther. 2006;9:189–201. [Google Scholar]
- 55.Ionescu JG, Novotny J, Stejskal V, et al. Breast tumours strongly accumulate transition metals. Maedica J Clin Med. 2007;2:5–9. [Google Scholar]
- 56.Lieu PT, Heiskala M, Peterson PA, Yang Y. The roles of iron in health and disease. Mol Aspects Med. 2001;22:1–87. doi: 10.1016/s0098-2997(00)00006-6. [DOI] [PubMed] [Google Scholar]
- 57.Cunzhi H, Jiexian J, Xianwen Z, et al. Serum and tissue levels of six trace elements and copper/zinc ratio in patients with cervical cancer and uterine myoma. Biol Trace Elem Res. 2003;94:113–22. doi: 10.1385/BTER:94:2:113. [DOI] [PubMed] [Google Scholar]
- 58.Kucharzewski M, Braziewicz J, Majewska U, Góźdź S. Iron concentrations in intestinal cancer tissue and in colon and rectum polyps. Biol Trace Elem Res. 2003;95:19–28. doi: 10.1385/BTER:95:1:19. [DOI] [PubMed] [Google Scholar]
- 59.Reddy SB, Charles MJ, Raju GN, et al. Trace elemental analysis of cancer-afflicted intestine by PIXE technique. Biol Trace Elem Res. 2004;102:265–81. doi: 10.1385/bter:102:1-3:265. [DOI] [PubMed] [Google Scholar]
- 60.Garg AN, Weginwar RG, Sagdeo V. Minor and trace elemental contents of cancerous breast tissue measured by instrumental and radiochemical neutron activation analysis. Nuclear Analytical Methods in the Life Sciences: Springer. 1990;pp:485–96. doi: 10.1007/BF02992704. [DOI] [PubMed] [Google Scholar]
- 61.Garg AN, Singh V, Weginwar RG, Sagdeo V. An elemental correlation study in cancerous and normal breast tissue with successive clinical stages by neutron activation analysis. Biol Trace Elem Res. 1994;46:185–202. doi: 10.1007/BF02789296. [DOI] [PubMed] [Google Scholar]
- 62.Abraham BK, Justenhoven C, Pesch B, et al. Investigation of genetic variants of genes of the hemochromatosis pathway and their role in breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1102–7. doi: 10.1158/1055-9965.EPI-05-0013. [DOI] [PubMed] [Google Scholar]
- 63.Ferrucci LM, Cross AJ, Graubard BI, et al. Intake of meat, meat mutagens, and iron and the risk of breast cancer in the prostate, lung, colorectal, and ovarian cancer screening trial. Br J Cancer. 2009;101:178–8. doi: 10.1038/sj.bjc.6605118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Inoue‐Choi M, Sinha R, Gierach GL, Ward MH. Red and processed meat, nitrite, and heme iron intakes and postmenopausal breast cancer risk in the NIH‐AARP Diet and Health Study. Int J Cancer. 2016;138:1609–18. doi: 10.1002/ijc.29901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Diallo A, Deschasaux M, Partula V, et al. Dietary iron intake and breast cancer risk: modulation by an antioxidant supplementation. Oncotarget. 2016;7:79008–16. doi: 10.18632/oncotarget.12592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Farvid MS, Cho E, Chen WY, Eliassen AH, Willett WC. Adolescent meat intake and breast cancer risk. Int J Cancer. 2015;136:1909–20. doi: 10.1002/ijc.29218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kabat GC, Cross AJ, Park Y, et al. Intakes of dietary iron and heme-iron and risk of postmenopausal breast cancer in the National Institutes of Health–AARP Diet and Health Study. Am J Clin Nutr. 2010;92:1478–83. doi: 10.3945/ajcn.2010.29753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kabat GC, Miller AB, Jain M, Rohan TE. Dietary iron and heme iron intake and risk of breast cancer: a prospective cohort study. Cancer Epidemiol Biomarkers Prev. 2007;16:1306–8. doi: 10.1158/1055-9965.EPI-07-0086. [DOI] [PubMed] [Google Scholar]