Abstract
This study is devoted to the detailed in situ Raman spectroscopy investigation of propane C3H8 in laser-heated diamond anvil cells in the range of pressures from 3 to 22 GPa and temperatures from 900 to 3000 K. We show that propane, while being exposed to particular thermobaric conditions, could react, leading to the formation of hydrocarbons, both saturated and unsaturated as well as soot. Our results suggest that propane could be a precursor of heavy hydrocarbons and will produce more than just sooty material when subjected to extreme conditions. These results could clarify the issue of the presence of heavy hydrocarbons in the Earth’s upper mantle.
Subject terms: Applied physics, Chemical physics, Condensed-matter physics, Structure of solids and liquids, Organic chemistry, Physical chemistry
Introduction
Thermal and catalytic transformations of various hydrocarbon compounds at normal pressure have attracted significant attention in the field of petrochemistry. However, high-pressure chemistry of hydrocarbons as a science started to develop only recently, —primarily due to the unavailability of the specific equipment for the experiments. To date, only methane, the first member of the alkane homologous series, has been investigated when subjected to a wide range of pressures and temperatures1–4, because of its widespread occurrence in geological systems5–7 and well-known role in the atmosphere of the Solar System’s outer planets8,9. The behavior of other hydrocarbons, both unsaturated10,11 and saturated12,13, have been less widely investigated with the use of various high-pressure techniques.
The focus on the significance of methane’s high-pressure high-temperature behavior implies that the fate of higher hydrocarbons has been ignored. Though the high-pressure, high-temperature (HPHT) behavior of ethane has been investigated several times12,14, propane has only been studied at ambient temperatures15,16. Propane is the third most abundant hydrocarbon on Earth after methane and ethane. It has been detected in the atmosphere of outer planets17 and their satellites18, and is a typical product of HPHT hydrocarbon synthesis performed both for chemical and geological purposes19,20.
The relevance of the investigation of carbon-bearing compounds can be understood from the perspective of the growing evidence of the role of hydrocarbon compounds deep in the Earth’s interior, which could contribute to the global carbon cycle21,22. Unfortunately, even for methane, investigations into its behavior under conditions of high pressure have yielded inconclusive and mutually conflicting results.
Propane’s importance as a petrochemical feedstock led to detailed studies of its thermal transformations in the range of 500–900 °C in processes such as pyrolysis and thermal cracking23–25. By changing the basic conditions of the process, the content of hydrocarbon compounds complex systems could be varied from higher normal and isoalkanes, dienes, arenes, and alkenes to C1-C3 fractions. These thermal processes were only investigated in the diapason under relatively mild pressures because of the process goal—low pressures are favorable for the synthesis of low-molecular compounds, while higher pressures could cause secondary reactions, particularly, polymerization and condensation, to occur25.
Considering the previous information, this study deals with the HPHT study of propane under a pressure range of 2–22 GPa and a temperature range of ~900–3000 K.
Methods
Propane (Linde Gas Polska), with a purity of 99.99%, was used in the experimental procedure without any additional purification. In our experiment, propane was subjected to cooling by liquid nitrogen and subsequent cryogenic loading in symmetric BX-90-type diamond anvil cells (DACs) equipped with synthetic, CVD-type IIa diamonds with a culet size of 250 μm. The rhenium gaskets were indented to a thickness of 25 μm. Pressure chambers with a step (Fig. S1 in supplementary) were prepared in the gaskets by combination of laser ablation and drilling. Thin (~1–2 μm) gold foil act as heat absorber in experiments with laser heating.
The Raman spectra were excited using a He-Ne laser (632.8 nm excitation). Then the the acquisition of the spectra was made via the use of a LabRam spectrometer with a 2 cm−1 spectral resolution. If possible, the pressure was determined by a calibration of propane high-pressure behavior16, or else the pressure was determined by the first-order peak of the diamond. The uncertainties in the Raman peak positions were ±1 cm−1. Raman spectra were collected at several points of the heating areas to ensure that the transformations being investigated, actually occurred. The Raman spectra of propane and the products of the reaction were measured before and after heating under the required thermobaric conditions.
In some cases, the green Ar+-laser (514.5 nm) the LabRam spectrometer (2 cm−1 spectral resolution) was equipped with was also employed for the in situ analysis.
The laser heating of the samples was performed using a home-laboratory laser heating setup at the Bayreuth Geoinstitut26. This system could be described as transferable double-sided laser heating setup for diamond-anvil cells with the possibility of in situ temperature determination and precise heating of the samples inside a cell. Using high magnification and low working distance infinity corrected laser focusing objectives provided the opportunity of the laser beam size decrease less than 5 μm as well as achievement of the 320 times optical magnification.
Heating of the sample is carried out by two YAG lasers (1064 nm central wavelength). For temperature measurements the thermal emission spectra of the heated area is guided into an IsoPlane SCT 320 spectrometer with a 1024 × 256 PI-MAX 4 camera. The temperature was determined by fitting the black body radiation spectra of the heated area in a given wavelength range (570–830 nm) to the Plank radiation function. Liquid and solid propane are optically transparent and do not absorb well at the central wavelength of the YAG laser. This means that it is important to find a way to heat the sample and eliminate the catalytic influence of the absorber that could appear because of the usage of noble metals such as Ir. For these reasons, gold foil was employed as the absorber of the laser radiation to dissipate heat to the sample. The Raman spectra were measured at the hot points, near the hot points (marked as “near” on the several spectra), as well in the cold sample areas to facilitate a deeper understanding of propane’s behavior.
Results and Discussion
3GPa
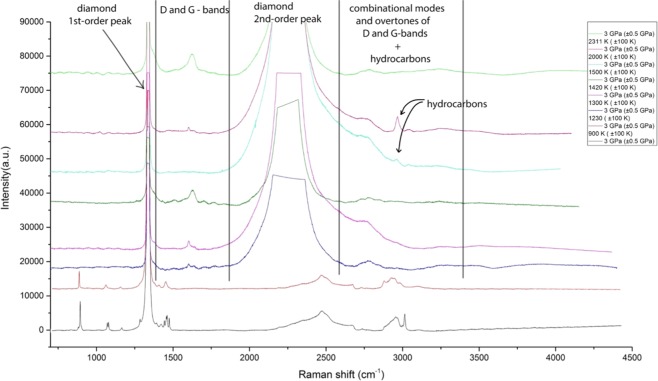
The main chemical transformation of propane at 3 GPa (Fig. 1) observed at the temperatures displayed by Fig. 1 is a reaction with the prevalent formation of a sooty material. This material has very similar Raman spectra to the typical black solid compound obtained during the thermal and catalytic petrochemical processes or as a by-product of combustion according to the reaction:
Figure 1.
Chemical transformations of propane at 3 GPa and T = 900–2300 K (±100 K). The reference peaks for graphite (soot) modes were taken from27,28, for C-H valence of hydrocarbon compounds2–4,12. The propane remained stable at 900 K. The spectra of untouched propane are in good correspondence with the previous experiments we carried out37.
During such processes, there is also the possibility of obtaining a graphite, which could be either disordered or highly ordered. However, the character of the presented spectra of the propane reaction products indicates the presence of disordered phases of graphite or soot27. The highly ordered Raman spectra of graphite exhibits only one band (first-order G-bands) at 1580 cm−1 at ambient temperature. On the contrary, the disordered structure of graphite has the presence of additional first-order bands (D-bands) at 1360 and 1620 cm−1 depending on the ambient conditions28. The bands in the region of 2800–3500 cm−1 could also correspond to the combinational models of D and G-bands26,27. The signals of the graphite and soot are hard to distinguish, however, there is evidence that the soot itself has broader peaks27,28. The spectra of propane under pressure of 3 GPa and at an ambient temperature of 900 K (±100 K) show no major changes in the display of any of the bands that are typical for propane16. The stability of propane at a temperature of ~900 K is in good correspondence with the earlier experiments of Kolesnikov et al.12 on methane and ethane, showing the same behavior of propane. The absence of the hydrogen peaks in the region of 500–800 cm−1 (the region was not shown on the graph) can be explained as being due to the high rate of hydrogen diffusion through the rhenium gasket or through the reaction products. The appearance of the intense peak at ~3000 cm−1 at temperatures of 1420 and 1500 K (±100 K) could be attributed to C-H vibrations of various aliphatic hydrocarbons due to the well-known radical reaction mechanism resulting in the formation of methyl and ethyl radicals. These radicals could subsequently react via various pathways leading to the formation of hydrocarbon compounds29:
Above mentioned reaction pathway may also explain why we do not observe pure hydrogen in the system. Hydrogen could be consumed in the reactions with other hydrocarbons:
6GPa
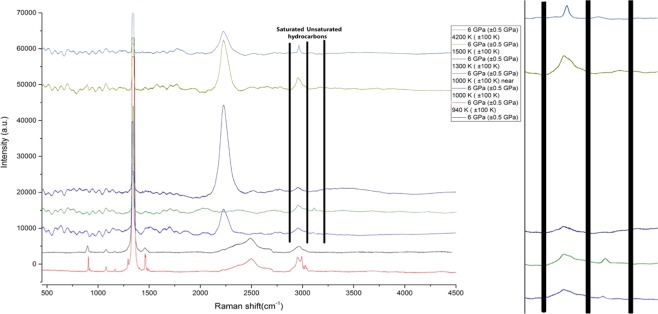
Heating at 940 K does not affect Raman spectra of propane (Fig. 2).
Figure 2.
Chemical transformations of propane at 6 GPa and T = 940–1600 K (±100 K). The reference peaks for C-H vibrations of saturated hydrocarbon compounds were taken from2–4,12, and for unsaturated hydrocarbons from30,32. The strong fluorescence in the region of the hydrocarbon footprint is explained by the presence of complex hydrocarbon systems having a mixed structure. The formation of ultradispersive diamonds could also affect the spectra. The propane remained stable at 940 K. The spectra of pristine propane are in good correspondence with the previous experiments that we carried out37. On the right side of the figure there is the magnified region of the C-H valence vibrations of the saturated and unsaturated hydrocarbons.
The spectra collected after heating at higher temperatures characterized by presence of bands at ~3100–3200 cm−1 (may be attributed to formation of unsaturated compounds2–4,12), and peak at ~3000 cm−1 (probably due to saturated hydrocarbon(s)). It is impossible to attribute these peaks to an individual compound or group of compounds due to high fluorescence in C-C stretching region or due to formation of ultradisperse diamonds30. We hypothesize that due to complicated thermal mechanisms of propane transformations the products of polymerization or aromatization were obtained, for instance, via allyl-radical reaction:

It is important to notice, that in the works of Kolesnikov et al.31,32 the formation of unsaturated hydrocarbons wasn’t reported. Propane is heavier than methane by mass, and thus propane can easier decompose and produce larger number of hydrocarbon compounds.
8GPa
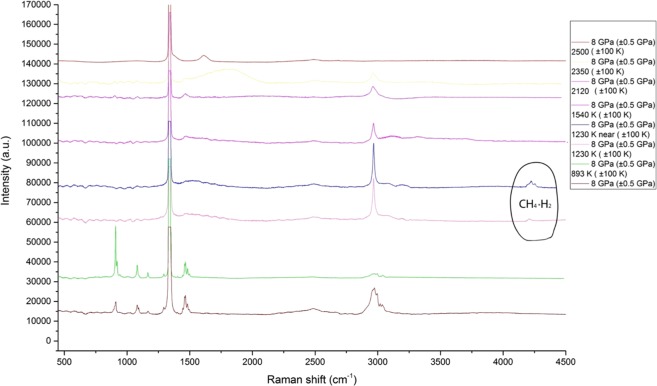
With the increase of the pressure to 8 GPa only methane could be identified among all of the hydrocarbons along with the formation of complex compounds with hydrogen at 1230 K (Fig. 3). These are formed due to the escape of hydrogen from the reacting system and the consequent formation of Van Der Waals bonds33.
Figure 3.
Chemical transformations of propane at 8 GPa and T = 893–2500 K (±100 K). The reference peaks for C-H valence of saturated hydrocarbon compounds were taken from2–4,12, for unsaturated hydrocarbons, they were obtained from30,32, and for graphite (soot) modes, they were taken from27,28. The strong fluorescence in the region of the hydrocarbon footprint is explained by the presence of complex hydrocarbon systems of mixed structure. The possible formation of ultradispersive diamonds could also affect the spectra. The propane remained stable at 893 K. The spectra of untouched propane are in good correspondence with the previous experiments carried out by us37. The complex methane-hydrogen compounds reference peaks were obtained from33.
Unfortunately, the region of the hydrocarbon footprint lacks the characteristic peaks overlapped by the fluorescence. However, the C-H of aliphatic and unsaturated fragments in the region of valence vibrations suggests the presence of various hydrocarbon compounds. With the increase of the temperature up to 2000 K and higher, the formation of graphite-like systems could be seen with the total disappearance of C-H and C-C vibrations at 2350 K.
Another possible mechanism of hydrocarbon generation as well as hydrogen could be the interaction of various forms of carbon and hydrocarbons. There is evidence that C-H fluids could be in contact with carbon34 in the Earth’s mantle, which could lead to certain chemical reactions of hydrogenation. For example, the hydrogen generation in our case could be explained not only by thermal decomposition of hydrocarbons, but by the catalytic effect of the carbon in the form of graphite or soot35,36.
11 and 14 GPa
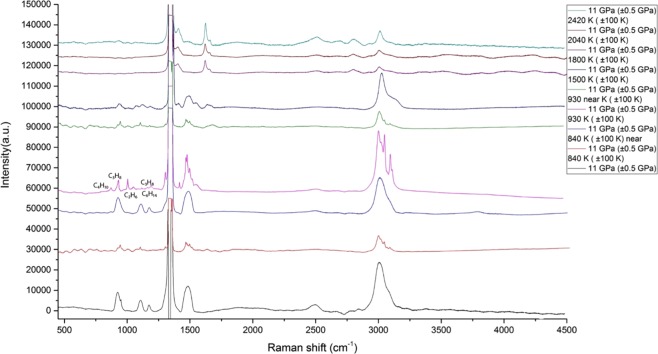
The reference peaks for C-H valence of saturated hydrocarbon compounds were obtained from2–4,12, for unsaturated hydrocarbons from31,32, for graphite (soot) modes, they were obtained from27,28. The reference peaks for C-C stretching and C-C bending of hydrocarbons were obtained for ethane from12, for propane from12,37, for n-butane from12,37, for n-pentane from38, and for n-hexane from38. The propane remained stable at temperatures of 840 K and lower. The spectra of untouched propane are in good correspondence with the previous experiments we carried out37.
The formation of C1-C6 hydrocarbons at 11 and 14 GPa (Figs. 4 and 5 respectively) starting from 900 K corresponds with the previous results for methane of Kolesnikov12 and is in good agreement with the results from simulation experiments2. The spectra of 11 and 14 GPa have a main, obvious difference—the presence of hydrogen. The absence of hydrogen at 11 GPa is because of hydrogen diffusion or secondary reactions of hydrocarbons or graphite. The C-C vibrations of n-butane37, n-hexane39, and n-pentane38 were detected in the spectra. In the case of the n-pentane and n-hexane, they were never detected in such types of experiments. This result proposes the complicated condensation mechanism that has a radical character, as in the case of industrial processes of pyrolysis. These series of reactions could be described with a classic radical-polymerization mechanism, because of a certain regularity in the decreasing intensity of the hydrocarbon peaks with the consequent increase in the molecular mass. By modifying the reaction of12, it is reasonable to assume that we obtain the following result:
Figure 4.
Chemical transformations of propane at 11 GPa and T = 840–2420 K (±100 K).
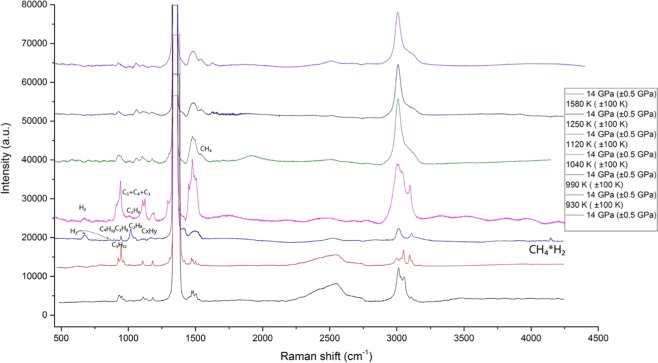
Figure 5.
Chemical transformations of propane at 14 GPa and T = 930–1580 K (±100 K). The reference peaks for C-H valence of saturated hydrocarbon compounds were obtained from2–4,12, for unsaturated compounds the reference peaks were obtained from31,32, and for graphite (soot) modes, the reference peaks were obtained from27,28. The reference peaks for C-C stretching and C-C bending of hydrocarbons were obtained for ethane from12, the peaks for propane were obtained from12,37, the peaks for n-butane from12,37, the peaks for n-pentane from39, and the peaks for n-hexane were obtained from38. The propane remained stable at 930 K. The spectra of untouched propane are in good correspondence with the previous experiments we carried out37. The hydrogen vibrational modes were investigated in those experiments33.
The most important fact that was observed during the experiments at 14 GPa is the contemporaneous presence of hydrogen, graphite, and other hydrocarbons during the laser heating, which could be interpreted as the equilibrium state. However, with the pressure increase it is hard to distinguish the particular hydrocarbon signal. After 1500 K only graphite and C-H valence vibrations could be seen.
17 GPa and 22 GPa
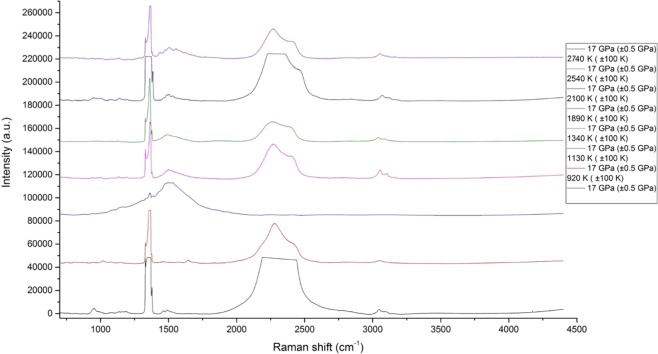
At 17 and 22 GPa the Raman bands of hydrocarbons become less distinguished with the overlap of the C-C bending region by graphite frequencies with the presence of unidentified C-H vibrations of saturated hydrocarbons in the region of 3000 cm−1 (Figs. 6 and 7).
Figure 6.
Chemical transformations of propane at 17 GPa and T = 920–3100 K (±100 K). The reference peaks for C-H valence of saturated hydrocarbon compounds were obtained from2–4,12, the corresponding reference peaks for graphite (soot) modes were obtained from27,28.
Figure 7.
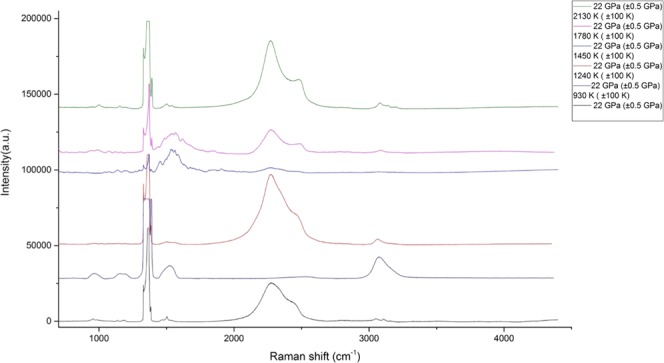
Chemical transformations of propane at 22 GPa and T = 930–2100 K (±100 K). The reference peaks for C-H valence of saturated hydrocarbon compounds were obtained from2–4,12, the reference peaks for graphite (soot) modes were taken from27,28.
Summary of The Results
The Table 1 and Fig. 8 summarize observations described above. Our experiments demonstrate that at pressure and temperature conditions relevant for wide range of depth within the Earth pure propane (without any catalyst) can transform in to different hydrocarbons, both saturated and unsaturated. Under these conditions propane is reacting via two simultaneous and competing pathways – (1) the growing of the hydrocarbon chain via condensation or polymerization mechanisms with the formation of higher hydrocarbons and (2) destruction via the cleavage of C-C and C-H bonds with the formation of lighter hydrocarbons and also graphite (or sooty material). Our observations suggest that propane, if subducted in to the mantle, undergoes complex transformations and may be source of more complex organic compounds.The issue of presence of heavy hydrocarbon compounds in the Earth’s mantle was thoroughly described and examined in these works40,41.
Table 1.
Overview of the experiments carried out during the investigation.
| Pressure | Temperature, K (±100 K) | Products |
|---|---|---|
| 3 GPa | 298 | C3H8 |
| 900 | ||
| 1230 | C | |
| 1300 | ||
| 1420 | ||
| 1500 | Saturated hydrocarbons, C | |
| 2000 | ||
| 2311 | С | |
| 6 GPa | 298 | C3H8 |
| 940 | ||
| 1000 | unsaturated and saturated hydrocarbons | |
| 1200 | ||
| 1300 | ||
| 1500 | ||
| 1600 | ||
| 8 GPa | 298 | C3H8 |
| 893 | ||
| 1230 | C, unsaturated and saturated hydrocarbons, H2 | |
| 1230 near | ||
| 1540 | ||
| 2120 | C, saturated hydrocarbons | |
| 2350 | ||
| 2500 | C | |
| 11 GPa | 298 | C3H8 |
| 840 | ||
| 840 near | ||
| 930 near | ||
| 930 | C, CH4, C4H10, C2H6, C3H8, saturated hydrocarbons, C6H14 | |
| 1500 | C, saturated hydrocarbons, CH4 | |
| 1800 | ||
| 2040 | C, saturated hydrocarbons | |
| 2420 | ||
| 14 GPa | 298 | C3H8 |
| 930 | ||
| 990 | H2, CH4, C4H10, C2H6, C3H8, saturated hydrocarbons, C5H12 | |
| 1040 | ||
| 1120 | CH4, saturated hydrocarbons, C | |
| 1250 | ||
| 1580 | ||
| 17 GPa | 298 | C3H8 |
| 920 | Hydrocarbons, С | |
| 1130 | ||
| 1340 | ||
| 1890 | ||
| 2100 | ||
| 2540 | ||
| 2740 | ||
| 22 GPa | 298 | C3H8 |
| 930 | Hydrocarbons | |
| 1240 | Hydrocarbons, С | |
| 1450 | ||
| 1780 | ||
| 2130 |
Figure 8.
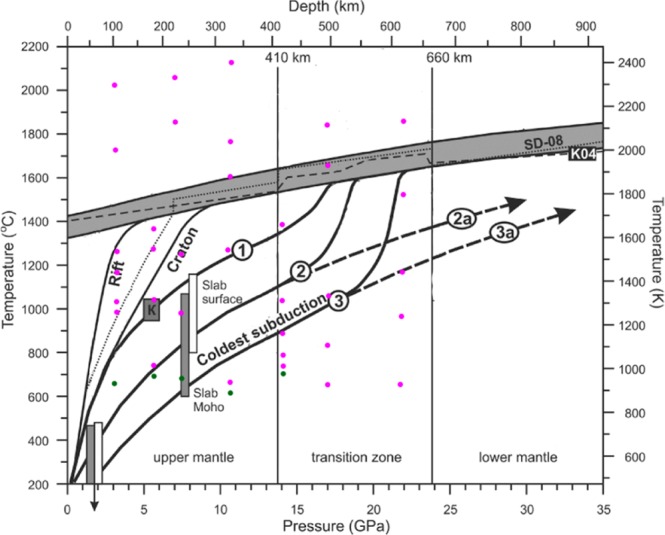
PT-range of hydrocarbon formation from propane in laser heating DAC experiments in comparison with mantle PT-profiles provided in the diagram taken from42. The pink dots represent the chemical transformations of propane, while the green – its stability.The gray field is the range of mantle adiabats with potential temperatures 1315–1415 °C. The dashed line represents the K04–1400 °C adiabat43. The dotted line depicts the average mantle thermal model44. 1- hottest subduction, 2- medium subduction, 3 – coldest subduction stagnant in the transition zone and penetrating into the lower mantle (2a and 3a)45,46.
Conclusion
The observations of propane chemical transformations under a wide range of high pressures and temperatures that are also present in the Earth’s mantle and in subduction environments, provides an insight into the fate of the carbon-bearing fluids fate deep in the Earth’s interior. The thermodynamic stability of propane under the pressures of 3–14 GPa and temperatures less than 900 K have been shown. At temperatures greater than 900 K, over a full range of pressures, propane transformation led to the formation of complex hydrocarbon systems and soot. At pressures of 11 and 14 GPa it was possible to identify the product mixture which includes light hydrocarbons, methane, and ethane and heavy hydrocarbons such as n-butane, n-pentane, and n-hexane.
We also have shown that the formation of heavier alkanes from propane at temperatures in the range of ~1000–2000 K and under pressures from 6 to 22 GPa is possible without any catalysts, and corresponds to the reactions leading to the formations of similar compounds occurring at depths of more than 130 km beneath the Earth’s surface.
Supplementary information
Acknowledgements
We thank A. Kurnosov for valuable information, fresh ideas, and comments during the experiments. We are thankful to A. Audetat for the preparation of the gaskets’ ablated areas. We also appreciate the recommendations of Lidong Dai from Institute of Geochemistry (Chinese Academy of Sciences) during the review of the paper. This research was supported by grants from the Sloan Foundation through the Deep Carbon Observatory, Global education programs. Open access funding provided by Royal Institute of Technology.
Author contributions
D.K., K.V. and L.D. designed the experiments. K.V. provided the idea of study. D.K., L.D., S.K., E.K. and T.F. participated in the conducting of the experiments. D.K. and S.K. did all of the preparations for the high-pressure experiments. T.F. and D.K. did the laser heating of the sample. E.K. and D.K. performed the cryogenic loading of propane. Collection and analysis of Raman spectra were made by D.K. D.K. wrote the text of the paper with essential advices from L.D. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-58520-7.
References
- 1.Goncharov A, et al. Structure of methane and ethane at high pressure. Acta Crystallogr. Sect. A Found. Adv. 2014;70:C757–C757. doi: 10.1107/S2053273314092420. [DOI] [Google Scholar]
- 2.Ancilotto F, Chiarotti GL, Scandolo S, Tosatti E. Dissociation of methane into hydrocarbons at extreme (planetary) pressure and temperature. Sci. 1997;275:1288–1290. doi: 10.1126/science.275.5304.1288. [DOI] [PubMed] [Google Scholar]
- 3.Gao, G. et al. Dissociation of methane under high pressure. J. Chem. Phys. 133 (2010). [DOI] [PubMed]
- 4.Culler TS, Schiferl D. New chemical reactions in methane at high temperatures and pressures. J. Phys. Chem. 1993;97:703–706. doi: 10.1021/j100105a028. [DOI] [Google Scholar]
- 5.Etiope G, Ciotoli G, Schwietzke S, Schoell M. Gridded maps of geological methane emissions and their isotopic signature. Earth Syst. Sci. Data. 2019;11:1–22. doi: 10.5194/essd-11-1-2019. [DOI] [Google Scholar]
- 6.Etiope G, Klusman RW. Geologic emissions of methane to the atmosphere. Chemosphere. 2002;49:777–789. doi: 10.1016/S0045-6535(02)00380-6. [DOI] [PubMed] [Google Scholar]
- 7.Morner N, Etiope G. Carbon degassing from the lithosphere. Glob. Planet. Change. 2002;33:185–203. doi: 10.1016/S0921-8181(02)00070-X. [DOI] [Google Scholar]
- 8.Munsell, K., Smith, H. & Harvey, Samantha (13 November 2007). Neptune overview. Solar System Exploration. NASA. Archived from the original on 3 March 2008. Retrieved 20 February 2008.
- 9.Thunnissen, D P., Guernsey, C. S., Baker, R. S., Miyake, R. N. Advanced Space Storable Propellants for Outer Planet Exploration. American Institute of Aeronautics and Astronautics (4–0799), 28 (2004).
- 10.Citroni M, Ceppatelli M, Bini R, Schettino V. High-pressure reactivity of propene. J. Chem. Phys. 2005;123(19):194510. doi: 10.1063/1.2109947. [DOI] [PubMed] [Google Scholar]
- 11.Cansell F, Fabre D, Petitet JP. Phase transitions and chemical transformations of benzene up to 550 °C and 30 GPa. J. Chem. Phys. 1993;99:7300. doi: 10.1063/1.465711. [DOI] [Google Scholar]
- 12.Kolesnikov A, Kutcherov VG, Goncharov AF. Methane-derived hydrocarbons produced under upper-mantle conditions. Nat. Geosci. 2009;2:566. doi: 10.1038/ngeo591. [DOI] [Google Scholar]
- 13.Lobanov SS, et al. Carbon precipitation from heavy hydrocarbon fluid in deep planetary interiors. Nat. Commun. 2013;4:1–7. doi: 10.1038/ncomms3446. [DOI] [PubMed] [Google Scholar]
- 14.Shimizu H, Shimazaki I, Sasaki S. High-Pressure Raman Study of Liquid and Molecular Crystal Ethane Up to 8 {GPa} Jpn. J. Appl. Phys. 1989;28:1632–1635. doi: 10.1143/JJAP.28.1632. [DOI] [Google Scholar]
- 15.Podsiadło M, Olejniczak A, Katrusiak A. Why Propane? J. Phys. Chem. C. 2013;117:4759–4763. doi: 10.1021/jp311747m. [DOI] [Google Scholar]
- 16.Kudryavtsev D, et al. Raman and IR Spectroscopy Studies on Propane at Pressures of Up to 40 GPa. J. Phys. Chem. A. 2017;121:6004–6011. doi: 10.1021/acs.jpca.7b05492. [DOI] [PubMed] [Google Scholar]
- 17.Greathouse TK, et al. The first detection of propane on Saturn. Icarus. 2006;181:266–271. doi: 10.1016/j.icarus.2005.09.016. [DOI] [Google Scholar]
- 18.Roe, H. G., Greathouse, T. K., Richter, M. J. & Lacy, J. H. Propane on Titan. Astrophys. J. 597, L65–L68 (2003).
- 19.Mukhina, E., Kolesnikov, A. & Kutcherov, V. The lower pT limit of deep hydrocarbon synthesis by CaCO3 aqueous reduction. Sci. Rep. 7 (2017). [DOI] [PMC free article] [PubMed]
- 20.Kutcherov VG, Bendeliani NA, Alekseev VA, Kenney JF. Synthesis of hydrocarbons from minerals at pressures up to 5 GPa. Dokl. Phys. Chem. 2002;387:328–330. doi: 10.1023/A:1021758915693. [DOI] [Google Scholar]
- 21.Wilson M. Where do Carbon Atoms Reside within Earth’s Mantle? Phys. Today. 2003;56(10):21–22. doi: 10.1063/1.1628990. [DOI] [Google Scholar]
- 22. Does Earth’s Core Host a Deep Carbon Reservoir? Deep Carbon Observatory. Retrieved 2019-03-09 (14 April 2015).
- 23.Kershenbaum LS, Martin JJ. Kinetics of the nonisothermal pyrolysis of propane. AIChE J. 1967;13:148–155. doi: 10.1002/aic.690130127. [DOI] [Google Scholar]
- 24.Khan RU, et al. Pyrolysis of propane under vacuum carburizing conditions: An experimental and modeling study. J. Anal. Appl. Pyrolysis. 2008;81:148–156. doi: 10.1016/j.jaap.2007.09.012. [DOI] [Google Scholar]
- 25.Buekens AG, Froment GF. Thermal Cracking of Propane. Kinetics and Product Distributions. Ind. Eng. Chem. Process. Des. Dev. 1968;7:435–447. doi: 10.1021/i260027a022. [DOI] [Google Scholar]
- 26.Fedotenko T, et al. Laser heating setup for diamond anvil cells for in situ synchrotron and in house high and ultra-high pressure studies. Rev. Sci. Instrum. 2019;90:104501. doi: 10.1063/1.5117786. [DOI] [Google Scholar]
- 27.Blank, V. D. et al. Pressure-Induced Transformation of Graphite and Diamond to Onions. Crystals 2018, 8, 68.].
- 28.Sadezky A, Muckenhuber H, Grothe H, Niessner R, Pöschl U. Raman microspectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information. Carbon N. Y. 2005;43:1731–1742. doi: 10.1016/j.carbon.2005.02.018. [DOI] [Google Scholar]
- 29.Safrik I, Strausz P. The thermal decomposition of hydrocarbons: Part 1. n-alkanes (C ≥ 5) Res. Chem. Intermed. 1996;22:275–310. doi: 10.1163/156856796X00458. [DOI] [Google Scholar]
- 30.Zerr A, Serghiou G, Boehler R, Ross M. Decomposition of alkanes at high pressures and temperatures. High. Press. Res. 2006;26(1):23–32. doi: 10.1080/08957950600608931. [DOI] [Google Scholar]
- 31.Benedetti LR. Dissociation of CH4 at High Pressures and Temperatures: Diamond Formation in Giant Planet Interiors? Sci. 1999;286(5437):100–102. doi: 10.1126/science.286.5437.100. [DOI] [PubMed] [Google Scholar]
- 32.Jin-Yang C, Lu-Jiang J, Jun-Ping D, Hai-Fei Z. In Situ Raman Spectroscopy Study on Dissociation of Methane at High Temperatures and at High Pressures. Chin. Phys. Lett. 2008;25:780–782. doi: 10.1088/0256-307X/25/2/116. [DOI] [Google Scholar]
- 33.Somayazulu MS, Hemley RJ, Goncharov AF, Mao HK, Finger LW. High-pressure compounds in the methane-hydrogen system: X-ray, infrared and Raman studies on CH4(H2)2. Eur. J. Solid. State Inorg. Chem. 1997;34:705–713. [Google Scholar]
- 34.Holloway JR. Graphite-CH4 -H2O-CO2 equilibria at low-grade metamorphic conditions. Geol. 1984;12:455–458. doi: 10.1130/0091-7613(1984)12<455:GEALMC>2.0.CO;2. [DOI] [Google Scholar]
- 35.Huang L, Santiso EE, Nardelli MB, Gubbins KE. Catalytic role of carbons in methane decomposition for CO- and CO2-free hydrogen generation. J. Chem. Phys. 2008;128:214702–214709. doi: 10.1063/1.2931456. [DOI] [PubMed] [Google Scholar]
- 36.Sharma A, Cody GD, Hemley RJ. In situ diamond-anvil cell observations of methanogenesis at high pressures and temperatures. Energ. Fuel. 2009;23:5571–5579. doi: 10.1021/ef9006017. [DOI] [Google Scholar]
- 37.Kudryavtsev DA, Kutcherov VG, Dubrovinsky LS. Raman high-pressure study of butane isomers up to 40 GPa. AIP Adv. 2018;8:115104. doi: 10.1063/1.5049481. [DOI] [Google Scholar]
- 38.Kavitha G, Narayana C. Pressure-Induced Structural Transition in n-Pentane: A Raman Study. J. Phys. Chem. 2007;B 111:7003–7008. doi: 10.1021/jp068285a. [DOI] [PubMed] [Google Scholar]
- 39.Kavitha G, Narayana C. Raman Spectroscopic Investigations of Pressure-Induced Phase Transitions in n-Hexane. J. Phys. Chem. 2007;B 111:14130–14135. doi: 10.1021/jp075188o. [DOI] [PubMed] [Google Scholar]
- 40.Kolesnikov AY, Saul JM, Kutcherov VG. Chemistry of Hydrocarbons Under Extreme Thermobaric Conditions. ChemistrySelect. 2017;2:1336–1352. doi: 10.1002/slct.201601123. [DOI] [Google Scholar]
- 41.Sonin VM, et al. Synthesis of heavy hydrocarbons under P-T conditions of the Earth’s upper mantle. Dokl. Earth Sci. 2014;454:32–36. doi: 10.1134/S1028334X1401005X. [DOI] [Google Scholar]
- 42.Karato Shun-Ichiro. Physics and Chemistry of the Deep Earth. Chichester, UK: John Wiley & Sons, Ltd; 2013. Rheological Properties of Minerals and Rocks; pp. 94–144. [Google Scholar]
- 43.Katsura T, Yoneda A, Yamazaki D, Yoshino T, Ito E. Adiabatic temperature profile in the mantle. Phys. Earth Planet. 2010;Inter. 183:212–218. doi: 10.1016/j.pepi.2010.07.001. [DOI] [Google Scholar]
- 44.Lewandowski M. Physics of the Earth by Frank D. Stacey and Paul M. Davis, fourth edition. Pure Appl. Geophys. 2009;166(12):2091–2092. doi: 10.1007/s00024-009-0534-x. [DOI] [Google Scholar]
- 45.E Van Keken, P., Kiefer, B., Peacock, S. & van Keken, P. High-resolution models of subduction zones: Implications for mineral dehydration reactions and the transport of water into the deep mantle. Geochem. Geophys. Geosystems - Geochem Geophys Geosyst 3 (2002).
- 46.M. Syracuse, E. et al. The global range of subduction zone thermal models. Phys. Earth Planet. Inter. 183 (2010).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.