Abstract
We use individual-based information on the behavior of wild female Japanese macaques in two consecutive years with different food availability (nut-rich vs. nut-poor) to test effects of dominance rank and nut fruiting on seed dispersal parameters. We predicted that social rank would affect dispersal (1) quantity, (2) quality, (3) species richness, and (4) percentage of berries in the diet in the nut-poor year, while these differences would disappear in the nut-rich year. We found seeds of nine fleshy-fruited plant species in the feces of the monkeys. The frequency of seed occurrence for two plant species (Viburnum dilatatum and Rosa multiflora) showed an interaction between dominance ranks and years; in the nut-poor year V. dilatatum seeds were more abundant among dominant females and R. multiflora among subordinates, while such inter-rank differences disappeared in the nut-rich year. Similarly, the intact ratio of V. dilatatum seeds was lower for dominants in the nut-poor year, while inter-rank variations disappeared in the nut-rich year. Finally, percentage of berries in diet and seed richness showed no inter-annual nor inter-rank variations. Our study highlights that differences in individuals’ social rank lead to within-group variation in seed dispersal services and that these differences are dependent on nut availability.
Subject terms: Behavioural ecology, Forest ecology
Introduction
Seed dispersal interactions that occur among animals and plants are inherently complex. Part of this complexity arises from the broad communities of interacting plant and animal species and the context-dependency of these interactions, such as temporal changes in food availability for animals1–4. Intraspecific variation within animal and plant populations is another key source of variability for seed dispersal outcomes5–7. Although the ecological effect of intraspecific variability has rarely been taken into account, it has been documented to influence seed dispersal outcomes across almost all frugivore groups, including insects8, other invertebrates9, fish10,11, reptiles12,13, birds14,15 and mammals16–19 and for plants as well5.
Sources of intraspecific variability within animal populations might result from sex differences, ontogenetic shifts in diet or behaviour, individual specialization or behavioral syndromes7. An individual’s social rank within a primate group determines its access to favored resources and can influence its handling behaviour and movement patterns20, which are important behaviours associated with seed dispersal21.
While social rank and food availability have been highlighted as independent sources of variation that influence seed dispersal outcomes2,3,7, it is likely that their impacts frequently interact. Variance in the spatial and temporal availability of fruit2,22–24 will influence competitive interactions within animal populations and are a potential key driver of intraspecific variability in the outcomes of seed dispersal. Hence, it is essential to develop an understanding of the joint impacts of food availability and social rank.
Japanese macaques (Macaca fuscata) are omnivorous primates endemic to the Japanese archipelago25,26. Among fruits, they prefer nuts to berries due to their higher fat contents27. Japanese macaques are also known to be important seed dispersers28,29. Intraspecific variation in seed dispersal is likely to be caused by high competition over food that triggers inter-rank differences in food access27,30,31 and feeding behaviour32. Severe competition over food resources (e.g., clumped distribution of food resources, and lower availability) forced subordinates to use non-preferred feeding patches, to process food faster, to increase the number of food items consumed, to extend feeding time, and to reduce the time allocated to rest and social activities30,32,33. The Japanese macaques inhabit a highly seasonal environment in which both temperature and resources fluctuate throughout the year34. In this environment, the masting of nuts, which are favored by the macaques, can influence the intensity of inter-rank interactions. On Kinkazan Island, where nuts of species such as Fagus crenata (Fagaceae) and Zelkova serrata (Ulmaceae) are preferred food items for the macaques, an inter-rank variation in feeding behavior between a nut-poor year (2004) and a masting nut-rich year (2005) has been reported. In 2004, only dominants obtained enough energy from nuts, while in 2005 all the group members could feed on nuts regardless of their social rank27. If inter-rank variation in feeding behavior is modified by variations in food availability, the characteristics of endozoochory shown by dominants and subordinates should also vary accordingly (e.g. inter-annually in the case of Kinkazan Island).
If individual differences are to have a strong impact on seed dispersal, they must impact the behaviours that drive seed dispersal outcomes7. Using data from macaques on Kinkazan over nut-poor and nut-rich years, we investigated whether key seed dispersal parameters35 differ across social-ranks (high, middle, and low). Specifically, we evaluated the parameters of 1) quantity, 2) quality, 3) species richness, and 4) proportion of berries, as a trait of dispersed propagules7. Because Japanese macaques generally move as a group, we did not consider effects on movement distances, another parameter of seed dispersal. We also determined the influence of nut-availability (rich and poor) on the behaviour of animals with different social ranks. We predicted that high competition for preferred foods (nuts) in low resource years would cause differences in the seed dispersal parameters measured among individuals of different social ranks. These differences should not be present in nut rich years.
Results
Seed dispersal quantity
During the study period, we collected 99 fresh fecal samples in total (n = 56 in 2004 and 43 in 2005), from which we identified 9,013 seeds from the following nine fleshy-fruit plant species: Viburnum dilatatum (Adoxaceae), Rosa multiflora (Rosaceae), Vitis flexuosa (Vitaceae), Cornus kousa (Cornaceae), Viscum album (Santalaceae), Pourthiaea villosa (Rosaceae), Ilex macropoda (Aquifoliaceae), Swida macrophylla (Cornaceae), and Malus tschonoskii (Rosaceae; Table 1). Seed appearance ratios for all species combined were 0.96 (54 out of 56) in 2004 and 0.91 (39 of 43) in 2005 and showed no significant variation between years and among dominance ranks (χ2 = 0.14, df = 5, p = 1.000). At the plant species level, however, there was significant inter-annual and inter-rank variation in the appearance ratios of R. multiflora (42 of 56 in 2004 vs. 6 of 43 in 2005; χ2 = 14.31, df = 5, p = 0.014) and V. flexuosa (1 of 56 in 2004 vs. 19 of 43 in 2005; χ2 = 18.68, df = 5, p = 0.002).
Table 1.
Obtained values and results of statistical tests on characteristics of seed dispersal by wild Japanese macaques on Kinkazan Island, northern Japan.
| Variables | Obtained values | Explanatory variables | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2004 | 2005 | Main effects | Interaction | |||||||||
| High (n = 12) | Middle (n = 19) | Low (n = 25) | High (n = 17) | Middle (n = 11) | Low (n = 15) | Rank | Year | Rank × Year | ||||
| a) Seed appearance ratio | ||||||||||||
| All species | 0.92 | 1.00 | 0.96 | 0.94 | 0.82 | 0.93 | χ2 = 0.00 | p = 1.000 | χ2 = 0.00 | p = 0.999 | χ2 = 0.14 | p = 1.000 |
| Viburnum dilatatum | 0.42 | 0.63 | 0.68 | 0.82 | 0.64 | 0.60 | χ2 = 0.01 | p = 0.996 | χ2 = 0.08 | p = 0.653 | χ2 = 12.06 | p = 0.944 |
| Rosa multiflora | 0.75 | 0.74 | 0.76 | 0.18 | 0.18 | 0.07 | χ2 = 0.32 | p = 0.852 | χ2 = 12.56 | p < 0.001*** | χ2 = 14.31 | p = 0.014* |
| Vitis flexuosa | 0.08 | 0.00 | 0.00 | 0.53 | 0.36 | 0.40 | χ2 = 3.26 | p = 0.196 | χ2 = 15.72 | p < 0.001*** | χ2 = 18.68 | p = 0.002** |
| Viscum album | 0.67 | 0.21 | 0.16 | 0.00 | 0.00 | 0.00 | χ2 = 5.16 | p = 0.076 | N/A | N/A | ||
| Cornus kousa | 0.00 | 0.00 | 0.00 | 0.12 | 0.09 | 0.20 | χ2 = 0.55 | p = 0.759 | N/A | N/A | ||
| Ilex macropoda | 0.25 | 0.21 | 0.12 | 0.00 | 0.00 | 0.00 | χ2 = 0.79 | p = 0.673 | N/A | N/A | ||
| Pourthiaea villosa | 0.08 | 0.05 | 0.04 | 0.29 | 0.09 | 0.13 | χ2 = 2.92 | p = 0.231 | χ2 = 2.34 | p = 0.105 | χ2 = 5.78 | p = 0.328 |
| Swida macrophylla | 0.00 | 0.00 | 0.00 | 0.06 | 0.09 | 0.07 | χ2 = 2.57 | p = 0.765 | N/A | N/A | ||
| Malus tschonoskii | 0.00 | 0.00 | 0.04 | 0.00 | 0.00 | 0.07 | χ2 = 2.87 | p = 0.239 | χ2 = 0.00 | p = 1.000 | χ2 = 3.16 | p = 0.675 |
| b) Number of seeds | ||||||||||||
| All species | 94.1 ± 188.4 | 100.1 ± 171.6 | 142.4 ± 231.1 | 89.4 ± 146.9 | 50.1 ± 53.1 | 28.7 ± 31.3 | z = 16.66 | p < 0.001*** | z = 23.00 | p < 0.001*** | z = −19.83 | p < 0.001*** |
| Viburnum dilatatum | 143.2 ± 248.7 | 25.8 ± 30.2 | 45.2 ± 52.1 | 75.5 ± 121.4 | 73.6 ± 47.1 | 35.4 ± 36.5 | z = −4.30 | p < 0.001*** | z = 2.35 | p = 0.019* | z = 3.90 | p < 0.001*** |
| Rosa multiflora | 34.2 ± 35.1 | 106.5 ± 190.7 | 145.9 ± 249.3 | 64.7 ± 71.2 | 6.5 ± 3.5 | 1.0 ± 0.0 | z = 11.90 | p < 0.001*** | z = 12.95 | p < 0.001*** | z = −11.47 | p < 0.001*** |
| Vitis flexuosa | 10.0 ± 0.0 | — | — | 14.0 ± 13.7 | 2.8 ± 3.0 | 13.7 ± 14.0 | z = 0.75 | p = 0.455 | N/A | N/A | ||
| Viscum album | 10.6 ± 10.8 | 18.3 ± 12.6 | 2.0 ± 1.2 | — | — | — | z = −2.17 | p = 0.030* | N/A | N/A | ||
| Cornus kousa | — | — | — | 11.5 ± 4.5 | 2.0 ± 0.0 | 7.0 ± 7.1 | z = 4.29 | p < 0.001*** | N/A | N/A | ||
| Ilex macropoda | 2.7 ± 2.4 | 6.3 ± 4.6 | 3.0 ± 2.8 | — | — | — | z = 0.92 | p = 0.357 | N/A | N/A | ||
| Pourthiaea villosa | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 10.0 ± 8.2 | 6.0 ± 0.0 | 1.0 ± 0.0 | N/A | N/A | N/A | |||
| Swida macrophylla | — | — | — | 4.0 ± 0.0 | 3.0 ± 0.0 | 1.0 ± 0.0 | N/A | N/A | N/A | |||
| Malus tschonoskii | — | — | 1.0 ± 0.0 | — | — | 8.0 ± 0.0 | N/A | N/A | N/A | |||
| c) Intact ratio | ||||||||||||
| All species | 0.85 ± 0.21 | 0.88 ± 0.18 | 0.94 ± 0.09 | 0.98 ± 0.06 | 1.00 ± 0.00 | 0.99 ± 0.02 | z = 6.31 | p < 0.001*** | z = 6.05 | p < 0.001*** | z = −6.19 | p < 0.001*** |
| Viburnum dilatatum | 0.83 ± 0.34 | 0.91 ± 0.28 | 0.97 ± 0.07 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | z = 3.66 | p < 0.001*** | z = 5.89 | p < 0.001*** | z = −3.45 | p < 0.001*** |
| Rosa multiflora | 0.86 ± 0.16 | 0.84 ± 0.20 | 0.91 ± 0.11 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | z = 0.19 | p = 0.847 | z = −0.10 | p = 0.922 | z = −0.12 | p = 0.904 |
| Vitis flexuosa | 1.00 ± 0.00 | − | − | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.01 | z = 0.02 | p = 0.987 | N/A | N/A | ||
| Viscum album | 0.91 ± 0.25 | 1.00 ± 0.00 | 0.88 ± 0.22 | − | − | − | z = −0.00 | p = 0.350 | N/A | N/A | ||
| Cornus kousa | — | — | — | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | z = 0.00 | p = 1.000 | N/A | N/A | ||
| Ilex macropoda | 0.94 ± 0.08 | 0.88 ± 0.22 | 1.00 ± 0.00 | — | — | — | z = 0.00 | p = 1.000 | N/A | N/A | ||
| Pourthiaea villosa | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 0.76 ± 0.15 | 1.00 ± 0.00 | 0.50 ± 0.50 | N/A | N/A | N/A | |||
| Swida macrophylla | — | — | — | 0.50 ± 0.00 | 0.00 ± 0.00 | 1.00 ± 0.00 | N/A | N/A | N/A | |||
| Malus tschonoskii | — | — | 1.00 ± 0.00 | — | — | 1.00 ± 0.00 | N/A | N/A | N/A | |||
| d) Seed species richness | 3.00 ± 1.00 | 2.53 ± 1.23 | 2.20 ± 1.06 | 2.41 ± 1.09 | 2.18 ± 1.03 | 1.87 ± 1.09 | z = −0.55 | p = 0.585 | z = −0.81 | p = 0.417 | z = 0.49 | p = 0.622 |
*p < 0.05, **p < 0.01, ***p < 0.001. We could not test the significance of number of seeds and intact ratio for Swida macrophylla, Malus tschonoskii, and Pourthiaea villosa due to their small sample sizes.
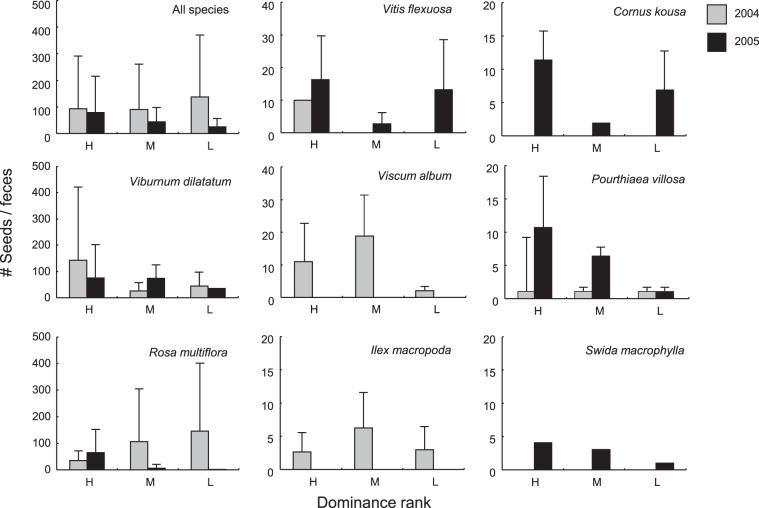
The total number (mean ± SD) of seeds per fecal sample ranged from 29 ± 31 (low rank individuals in 2005) to 142 ± 231 (low rank individuals in 2004; Table 1). Seeds of V. album and I. macropoda appeared only in 2004, while the seeds of C. kousa and S. macrophylla appeared only in 2005, likely due to inter-yearly differences in their availability (Fig. 1). The seeds of V. flexuosa and M. tschonoskii were found exclusively in the feces of specific dominance rank females (e.g. V. flexuosa appeared only in feces of high-rank females in 2004; Fig. 1). Our analyses were therefore restricted for these species. Further, for three species (P. villosa, S. macrophylla, and M. tschonoskii) we could not conduct statistical analyses due to small sample sizes.
Figure 1.
Inter-annual and inter-rank variation in mean (±SD) seed number per single feces collected in fall (October and November). H: high-rank, M: middle-rank, L: low-rank.
In the number of seeds per fecal sample we found a significant year-rank interaction for all species together, V. dilatatum, and R. multiflora (Table 1 and Fig. 1) – the number of seeds of all species together and V. dilatatum seeds were greater in feces of dominants (all species: z = −19.83, p < 0.001, V. dilatatum: z = 3.90, p < 0.001) while the number of R. multiflora seeds was larger in the feces of subordinates in 2004 (z = −11.47, p < 0.001) but not in 2005, when there were no inter-rank variations (Fig. 1). For V. album (z = −2.17, p = 0.030, H < M > L) and C. kousa (z = 4.30, p < 0.001, H > M < L), we also found inter-rank differences in seed numbers per fecal sample (Fig. 1).
Seed dispersal quality
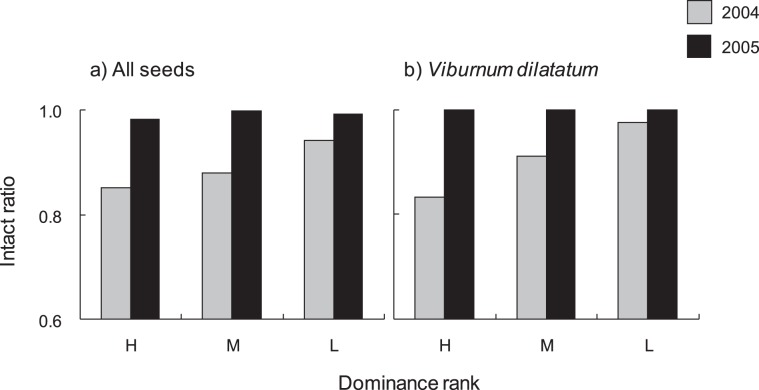
The seed intact ratio ranged from 0.85 to 1.00 (Table 1). The interaction between year and dominance rank was significant for the seeds of all species lumped together (z = −6.19, p < 0.001) and of V. dilatatum (z = −3.5, p < 0.001). In 2004, seed intact ratios were lower in dominants than in subordinates, while inter-rank variations disappeared in 2005 (Fig. 2).
Figure 2.
Inter-annual and inter-rank variation in mean intact ratio of seeds of the Japanese macaques for the (a) all plant species and (b) Viburnum dilatatum in fall (October and November). The intact ratio is obtained by dividing total number of intact seeds by total number of seeds in a given fecal sample. H: high-rank, M: middle-rank, L: low-rank.
Species richness
The mean seed species richness per fecal sample ranged from 1.9 to 3.0 species across dominance groups and years (Table 1). Neither year (z = −0.81, p = 0.417), dominance rank (z = −0.55, p = 0.585), nor their interaction (z = 0.49, p = 0.622) had any significant effect on seed species richness (Table 1).
Traits of dispersed propagules (proportion of berries)
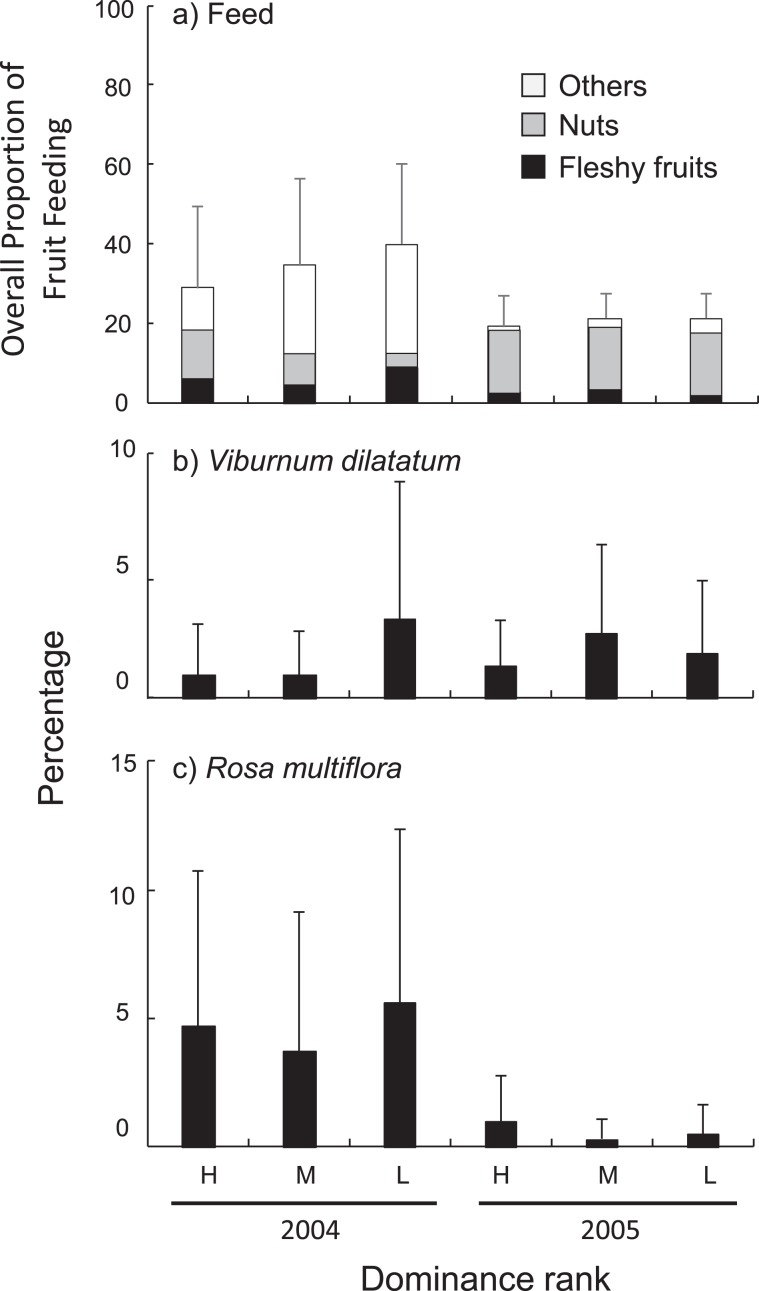
The percentage of total feeding (across all items) differed significantly between years (2004 > 2005; z = −4.92, p < 0.001) and among dominance ranks (high < middle < low; high vs. middle: z = −2.62, p = 0.009; high vs low: z = −2.17, p = 0.030; Fig. 3a). The percentage of nut feeding also varied between years (2004 < 2005; z = 3.67, p < 0.001) and among dominance ranks (high vs. middle: z = −0.24, p = 0.814; high vs low: z = −2.11, p = 0.035; Fig. 3a). The percentage of fleshy-fruit feeding, however, did not vary between years (z = −0.19, p = 0.848, Fig. 3a) nor among dominance ranks (high vs. middle: z = −0.23, p = 0.821; high vs low: z = 1.65, p = 0.098). We conducted the analyses for two fleshy-fruit species: for V. dilatatum we found no significant difference between years (z = 0.81, p = 0.419, Fig. 3b) and among dominance ranks (high vs middle: z = −0.04, p = 0.972; high vs low: z = 1.77, p = 0.077); for R. multiflora, we found a significant year-rank interaction (high rank in 2004 vs. low in 2005: z = −2.00, p = 0.045; and high in 2004 vs. middle in 2005: z = −2.51, p = 0.012). The percentage of R. multiflora feeding by low-ranking females was higher than by high and middle-ranking females in 2004, while such inter-rank differences disappeared in 2005 (Fig. 3c).
Figure 3.
Inter-annual and inter-rank difference in percentage of (a) feeding (filled part and grey part represent fleshy fruits and nuts, respectively) of the adult female Japanese macaques on Kinkazan Island, northern Japan. For the fleshy-fruit feeding, we also show percentage of the main two species (b) Viburnum dilatatum and (c) Rosa multiflora. H: high-rank, M: middle-rank, L: low-rank.
Discussion
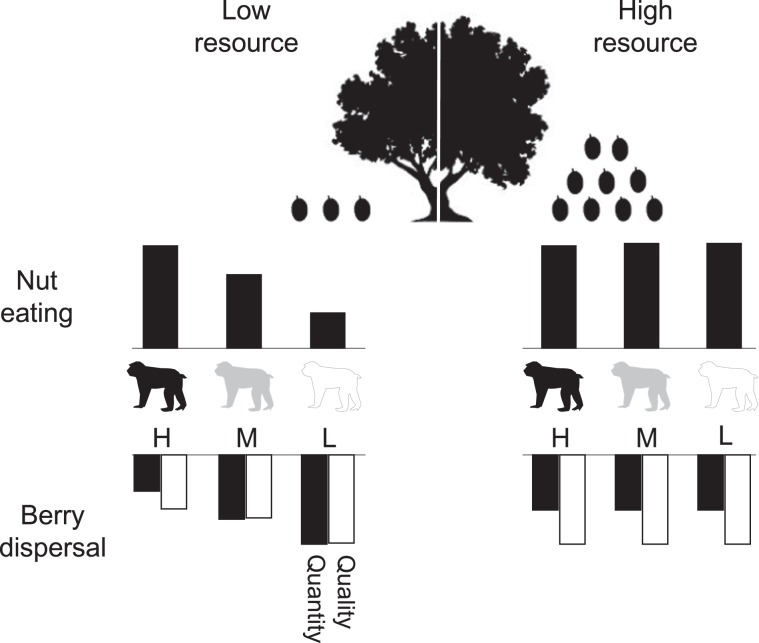
Seed dispersal services performed by Japanese macaques were related to resource abundance, which differentially influenced the roles played by individuals of different social ranks. In years when resource abundance was low, high-ranking individuals dominated preferred resources (nuts), which forced low ranking individuals to consume, and disperse, berries. More berry seeds were also chewed up by the high ranking individuals during this year, probably to maximize the nutritional value as has been observed in other monkey species16,36. Low ranking individuals were higher quality seed dispersers as they had lower rates of seed mastication, perhaps as a result of having to swallow foods quickly to avoid conflict27,33. Conversely, in the nut-rich year, these differences among individuals of different social ranks disappeared, and the overall impact on seed dispersal was slightly reduced in quantitative terms (eating fewer berries) but increased in qualitative terms (due to lower rates of seed mastication for berries). Hence, Japanese macaque’s seed dispersal behaviour was impacted by both social rank and nut availability as well as the interaction between them (Fig. 4).
Figure 4.
A schematic chart of the effects of social rank and nut fruiting (nut-poor year in 2004 and nut-rich year in 2005) on (1) nut eating, (2) quantitative and (3) qualitative parameters of berry dispersal, based on results of this study. H: high-rank, M: middle-rank, and L: low-rank. Images were drawn by FLOP DESIGN (Masashi Kato).
Two conditions are necessary for individual variation within populations to exert a strong influence on seed dispersal7. First, the differences among individuals should have a strong influence on seed dispersal outcomes. Among the four seed dispersal parameters we measured, we found three significant intraspecific differences (quality, quantity, and propagule-identity) in the nut poor year. Low and middle ranking individuals were more effective dispersers than high ranking individuals, because they dispersed a higher quantity of seeds, and were also higher quality dispersers, due to a reduced rate of seed mastication and a higher consumption of berries. However, individuals of different ranks showed no difference in species richness dispersed. While this intraspecific variation was not found in the high resource year, masting events in northern Japan happen around every 5–7 years37,38, and in most years there is competition over preferred resources27,29. Hence, intraspecific differences among individuals of different social ranks probably occur in most years for the Japanese macaques.
Troop size is another variable that can influence seed dispersal outcomes. The troop size (N = 35) of the studied group is average for this species39. However, in other populations, particularly on the mainland, larger troops sometimes occur and this will increase the number of individuals in each social rank and potentially vary the ratio of individuals across ranks. The relative contribution of specific ranked animals on dispersal could be much greater when the ratio of high to low ranking is different.
Under scenarios of anthropogenic disturbance, this intraspecific variation we observed could have important consequences for seed dispersal at the population level for Japanese macaques40,41. Group sizes can be reduced due to direct mortality (e.g., conflict with farmers42), or due to habitat or resource change which causes large groups to either split or adjust to the reduction in resources43. Under these scenarios, competition for resources may be reduced among social ranks, or there may be fewer low-ranking individuals, negatively shifting the population averages for most seed dispersal parameters we measured. Competition for resources may also be reduced under scenarios of provisioning by humans because it increases resource abundance, further reducing differences among social ranks41,44. Conversely, a population increase – perhaps due to more individuals being forced into a smaller area – would increase competition and the differences in seed dispersal outcomes across social ranks. Overall this could lead to a higher population-level seed dispersal effectiveness.
The importance of density-dependence is well established in the seed dispersal literature, mainly focusing on the effects of temporal changes in food availability2,3,45. We and recent studies7,46 have found that the temporal availability of fruit influences frugivore choices, which in turn causes intraspecific variation in seed dispersal parameters. Intraspecific variability in both plants and animals does not simply add noise to systems, but alters dispersal processes and patterns with consequences for plant demography, communities, evolution and responses to anthropogenic changes; by reducing this variability to an average value, important details on the seed dispersal capacity and resilience of animals has been lost5–7,46. Individuals within a population respond to fruit abundance, which might alter the extent and direction of intraspecific variation within populations. Hence, more research into understanding these inter-relationships are required, particularly as the source of context-change is increasingly a form of anthropogenic disturbance5,41.
So far, the detailed intra-group data that can provide a wealth of information on social interactions and behavior, has been collected by primatologists, as it is difficult or impossible to collect similar data for many other animal groups47. This information could be used to explore the relationships between intraspecific variation and context dependency in seed dispersal across a broader range of taxa and habitats. Because context-dependency can alter competitive relationships under a variety of scenarios20 and, potentially, in any animal taxa, it is likely that many other sources of intraspecific variation can also be context-dependent; for example, differences in dispersal due to body size or sex could be a result of dominance-hierarchies in some cases. Japanese macaques are known to leave the natal troop and remain as solitary individuals or move into different troops48. This means that the interspecific differences in seed dispersal that we describe could be more extreme if males were considered. It is of particular urgency to explore the influence that disturbance contexts can have on intra-specific variability and seed dispersal effectiveness.
Methods
Ethics statement
Our field study did not involve endangered or protected animal and plant species. The research methodology complied with protocols approved by the guidelines (Guide for the Care and Use of Laboratory Primates, Third Edition) of the Primate Research Institute, Kyoto University, Japan, and the legal requirements of Japan.
Study site and subjects
Kinkazan Island (38.3°N, 141.6°E) is located 700 m off the Oshika Peninsula, in northern Japan. The total area of the island is ca. 9.6 km2, and the highest peak is 450 m a.s.l. The monthly mean air temperature on the island ranges from 2.5 °C in February to 22.3 °C in August. Deciduous forests of Fagus crenata (Fagaceae) dominate the higher elevations (>150 m), whereas a mixture of deciduous forests of Carpinus spp. (C. tschonoskii and C. laxiflora, Betulaceae) and coniferous forests of Abies firma (Pinaceae) cover the lower elevations (<150 m) on the island23,27.
Behavioral observation and fecal sample collection took place in two months in both 2004 and 2005. During this period, approximately 200–250 Japanese macaques belonging to six troops inhabited the island49. Our study subjects were the adult (>5 yr) females of one of these troops called Troop A. This troop has been habituated to observation at close proximity (<10 m) by researchers since 1982. During the study period, the size of Troop A varied from 29 to 39 individuals, including 2–5 adult males, 14–17 adult females, 8–9 juveniles (1–5 yr), and 1–12 infants (<1 yr), and we had good knowledge of the maternal kinship and dominance ranks of the 17 adult females27.
Fecal sample collection and analysis
During the study period, we collected fresh fecal samples from each focal animal during our observations. Each sample was rinsed through 0.5 mm sieves under fresh water. We picked up all seeds from the fecal samples, identified seeds to the species level, and counted the number of seeds of each species. Seed identification was based on our previous work29,50.
We analyzed seed data by year (2004 vs. 2005) and the individual macaque dominance rank (high, middle, or low rank, based on our previous observation). We did not analyze data by month because fruit did not vary in abundance during each year’s two-month window50. We set several seed dispersal parameters based on previous studies29,51. First we calculated the frequency of seed occurrence, which was defined as the number of fecal samples containing seeds, for each year and dominance rank of macaques for each plant species. Since we did not collect fecal samples in equal quantities across the two years and dominance rank of macaques, we calculated seed appearance ratios (the number of dispersal events divided by the number of fecal samples examined). Secondarily, we calculated seed appearance ratios for each year and dominance rank of macaques, for all plant species combined, and for each species separately. For each fecal sample in which seeds appeared, we then recorded the number of seeds in the sample as the quantitative index of seed dispersal. We also recorded the seed intact ratio (the ratio of seeds with no apparent physical damage after gut passage) as another qualitative index of the efficacy of seed dispersal29, and the seed species richness as an index of plant diversity.
Frugivory
Here we use data collected from early October to late November in 2004 (38 days) and 2005 (33 days), which is a subset of our previous study27. The total observation time was 479 hr (276 hr in 2004 and 223 hr in 2005). We recorded activity patterns every 1-minute using instantaneous sampling (3–5 hours per single session). We classified activity into feeding (including picking up, processing, and chewing at one location) and others (moving, resting, social grooming, and others such as drinking, fighting, and alarm calling). If the focal animals were feeding, we classified food items into fleshy fruits, nuts, or others (leaves, herbaceous plants, fungi, animal materials, soil and unidentified materials). Details of the observation are published in a previous study27. For each food category, we defined the percentage of feeding on it as the percentage of instantaneous sampling points consuming the food item relative to all sampling points associated with feeding. In this study, we treated the percentage of the fleshy fruit in the diet as an index of dispersal propagules7.
Statistical analysis
We performed chi-square tests for independence to examine inter-annual and inter-rank difference in the seed appearance ratios for each fleshy fruit species separately, and for all the species combined. To evaluate both inter-annual and inter-rank differences in the number of seeds within single fecal samples we employed generalized linear mixed models (GLMM) with count data (with the Poisson error distribution). We included the identity of each animal as a random effect in this and following GLMM models. Only fecal samples containing seeds were included in this analysis. The response variable was the total number of seeds found in each fecal sample, and explanatory variables were year, dominance ranks, and their interaction. In order to test the inter-annual and inter-rank differences in the seed intact ratio, we fitted another GLMM with the negative binomial error family, due to overdispersion of the seed intact ratio. The response variable in this case was the number of intact seeds in each fecal sample (the overall number of seeds was treated as an offset term). To analyze the seed species richness in fecal samples, we again ran a GLMM (Poisson error family) with the number of seed species as the response variable and the fixed effects were the same as those in the first and second GLMM models. Finally, to examine the effects of year, dominance rank of macaques, and their interaction on the percentage of the total feeding, nut feeding, and fleshy fruit feeding, we used another GLMM with the negative binomial error family. The response variable in this case was time spent on a given activity (represented by the number of instantaneous samples). The overall number of instantaneous samples was treated as an offset term). We performed the GLMMs using the “glmmML”, “aod”, and “nlmn” packages in the statistical software R.3.3.252. The significance levels of these analyses in this study were set α = 0.05.
Acknowledgements
We thank the members of the Kinkazan Deer Research Group, especially Drs. Seiki Takatsuki and Masato Minami, and Nobumasa Ohnishi for their cooperation with fecal sample collection; Kaede Sato, Hiroshi Ebihara, Tomomi Ochiai, Natsuko Noguchi, Takahumi Tatewaki, and Tayo Harasaki for their assistance with fecal sample analyses; Dr. Kosei Izawa and his colleagues for their support during fieldwork on Kinkazan Island; Drs. Sindhu Radhakrishna and Asmita Sengupta for comments to early draft of the manuscript. This study was supported by a Grant-in-Aid (No. 23780160) and the bilateral research program (between Japan and India) from the Japan Society for the Promotion of Science (DST/INT/JSPS/P-250/2017) and the Corporative Research Fund of the Primate Research Institute, Kyoto University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author contributions
Y.T. conceived and designed the experiments. Y.T. performed the observation. Y.T. and A.C.A. analyzed the data. Y.T., K.R.M., A.C.A. wrote the manuscript; S.P. and S.K. provided editorial advice.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kitamura S, et al. Interactions between fleshy fruits and frugivores in a tropical seasonal forest in Thailand. Oecologia. 2002;133:559–572. doi: 10.1007/s00442-002-1073-7. [DOI] [PubMed] [Google Scholar]
- 2.Carlo TA. Interspecific neighbors change seed dispersal pattern of an avian-dispersed plant. Ecology. 2005;86:2440–2449. doi: 10.1890/04-1479. [DOI] [Google Scholar]
- 3.Prasad S, Sukumar R. Context-dependency of a complex fruit-frugivore mutualism: temporal variation in crop size and neighborhood effects. Oikos. 2010;119:514–523. doi: 10.1111/j.1600-0706.2009.17971.x. [DOI] [Google Scholar]
- 4.Donatti CI, et al. Analysis of a hyper-diverse seed dispersal network: modularity and underlying mechanisms. Ecol. Let. 2011;14:773–781. doi: 10.1111/j.1461-0248.2011.01639.x. [DOI] [PubMed] [Google Scholar]
- 5.Snell RS, et al. Consequences of intraspecific variation in seed dispersal for plant demography, communities, evolution, and global change. AoB Plants. 2019;11:plz016. doi: 10.1093/aobpla/plz016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.González-Varo JP, Traveset A. The labile limits of forbidden interactions. Trend. Ecol. Evol. 2016;31:700–710. doi: 10.1016/j.tree.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Zwolak R. How intraspecific variation in seed-dispersing animals matters for plants. Biol. Rev. 2018;93:897–913. doi: 10.1111/brv.12377. [DOI] [PubMed] [Google Scholar]
- 8.Larsen H, Burns KC. Seed dispersal effectiveness increases with body size in New Zealand alpine weta (Deinacrida connectens) Aust. Ecol. 2012;37:800–806. doi: 10.1111/j.1442-9993.2011.02340.x. [DOI] [Google Scholar]
- 9.Calvino-Cancela M, Rubido-Bara M. Effects of seed passage through slugs on germination. Plant Ecol. 2012;213:663–673. doi: 10.1007/s11258-012-0030-8. [DOI] [Google Scholar]
- 10.Anderson JY, Nuttle T, Saldaña Rojas JS, Pendergast TH, Flecker AS. Extremely long-distance seed dispersal by an overfished Amazonian frugivore. Proc. Royal Soc. B. 2011;278:3329–3335. doi: 10.1098/rspb.2011.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Correa SB, et al. Overfishing disrupts an ancient mutualism between frugivorous fishes and plants in Neotropical wetlands. Biol. Cons. 2015;191:159–167. doi: 10.1016/j.biocon.2015.06.019. [DOI] [Google Scholar]
- 12.Herrel A, Vanhooydonck B, Joachim R, Irschick DJ. Frugivory in polychrotid lizards: effects of body size. Oecologia. 2004;140:160–168. doi: 10.1007/s00442-004-1558-7. [DOI] [PubMed] [Google Scholar]
- 13.Tulipani DC, Lipcius RN. Evidence of eelgrass (Zostera marina) seed dispersal by northern diamondback terrapin (Malaclemys terrapin terrapin) in lower Chesapeake Bay. PLoS ONE. 2014;9:e103346. doi: 10.1371/journal.pone.0103346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jung RE. Individual variation in fruit choice by American robins (Turdus migratorius) The Auk. 1992;109:98–111. doi: 10.2307/4088270. [DOI] [Google Scholar]
- 15.Krijger CL, Opdam M, Théry M, Bongers F. Courtship behaviour of manakins and seed bank composition in a French Guianan rain forest. J. Trop. Ecol. 1997;13:631–636. doi: 10.1017/S0266467400010774. [DOI] [Google Scholar]
- 16.Kaplin BA, Moermond TC. Variation in seed handling by two species of forest monkeys in Rwanda. Am. J. Primatol. 1998;45:83–101. doi: 10.1002/(SICI)1098-2345(1998)45:1<83::AID-AJP7>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 17.Clarke MF, Kramer DL. Scatter-hoarding by a larder-hoarding rodent: intraspecific variation in the hoarding behaviour of the eastern chipmunk, Tamias striatus. Anim. Behav. 1994;48:299–308. doi: 10.1006/anbe.1994.1243. [DOI] [Google Scholar]
- 18.Dochtermann NA, Jenkins SH. Behavioural syndromes in Merriam’s kangaroo rats (Dipodomys merriami): a test of competing hypotheses. Proc. Royal Soc. B. 2007;274:2343–2349. doi: 10.1098/rspb.2007.0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jadeja S, Prasad S, Quader S, Isvaran K. Antelope mating strategies facilitate invasion of grasslands by a woody weed. Oikos. 2013;122:1441–1452. [Google Scholar]
- 20.Vogel ER, Munch SB, Janson CH. Understanding escalated aggression over food resources in white-faced capuchin monkeys. Anim. Behav. 2007;74:71–80. doi: 10.1016/j.anbehav.2007.02.003. [DOI] [Google Scholar]
- 21.Schupp EW. Quantity, quality and the effectiveness of seed dispersal by animals. Vegetatio. 1993;107:15–29. [Google Scholar]
- 22.Curran LM, Leighton M. Vertebrate responses to spatiotemporal variation in seed production of mast-fruiting Dipterocarpaceae. Ecol. Monogr. 2000;70:101–128. doi: 10.1890/0012-9615(2000)070[0101:VRTSVI]2.0.CO;2. [DOI] [Google Scholar]
- 23.Tsuji Y, Fujita S, Sugiura H, Saito C, Takatsuki S. Long-term variation in fruiting and the food habits of wild Japanese macaques on Kinkazan Island, northern Japan. Am. J. Primatol. 2006;68:1068–1080. doi: 10.1002/ajp.20307. [DOI] [PubMed] [Google Scholar]
- 24.Suwanvecho U, et al. High interannual variation in the diet of a tropical forest frugivore (Hylobates lar) Biotropica. 2017;50:346–356. doi: 10.1111/btp.12525. [DOI] [Google Scholar]
- 25.Tsuji, Y. Regional, temporal, and inter-individual variation in the feeding ecology of Japanese macaques in The Japanese macaques (eds. Nakagawa, N., Nakamichi, M. & Sugiura, H.) 95–123 (Springer, 2010).
- 26.Tsuji Y, Ito TY, Wada K, Watanabe K. Spatial patterns in the diet of the Japanese macaque Macaca fuscata and their environmental determinants. Mamm. Rev. 2015;45:227–238. doi: 10.1111/mam.12045. [DOI] [Google Scholar]
- 27.Tsuji Y, Takatsuki S. Inter-annual variation in nut abundance is related to agonistic interactions of foraging female Japanese macaques (Macaca fuscata) Int. J. Primatol. 2012;33:489–512. doi: 10.1007/s10764-012-9589-0. [DOI] [Google Scholar]
- 28.Otani, T. Seed dispersal by Japanese macaques in The Japanese macaques (Eds Nakagawa, N., Nakamichi, M. & Sugiura, H.) 129–142 (Springer, 2010).
- 29.Tsuji Y, Sato K, Sato Y. The role of Japanese macaques (Macaca fuscata) as endozoochorous seed dispersers on Kinkazan Island, northern Japan. Mammal. Biol. 2011;76:525–533. doi: 10.1016/j.mambio.2011.01.001. [DOI] [Google Scholar]
- 30.Saito C. Dominance and feeding success in female Japanese macaques, Macaca fuscata: effects of food patch size and inter-patch distance. Anim. Behav. 1996;51:967–980. doi: 10.1006/anbe.1996.0100. [DOI] [Google Scholar]
- 31.Kazahari N, Agetsuma N. Mechanisms determining relationships between feeding group size and foraging success in food patch use by Japanese macaques Macaca fuscata. Behaviour. 2010;147:1481–1500. doi: 10.1163/000579510X521573. [DOI] [Google Scholar]
- 32.Soumah AG, Yokota N. Female rank and feeding strategies in a free-ranging provisioned troop of Japanese macaques. Folia Primatol. 1991;57:191–200. doi: 10.1159/000156586. [DOI] [Google Scholar]
- 33.Mori A. Rank and age-related feeding strategy observed through field experiments in the Koshima group of Japanese macaques. Primates. 1995;36:11–26. doi: 10.1007/BF02381912. [DOI] [Google Scholar]
- 34.Tsuji Y, Takatsuki S. Effects of yearly change in nut fruiting on autumn home range use of Japanese macaques on Kinkazan Island, northern Japan. Int. J. Primatol. 2009;30:169–181. doi: 10.1007/s10764-009-9336-3. [DOI] [Google Scholar]
- 35.Schupp EW, Jordano P, Gómez JM. Seed dispersal effectiveness revisited: a conceptual review. New Phytol. 2010;188:333–353. doi: 10.1111/j.1469-8137.2010.03402.x. [DOI] [PubMed] [Google Scholar]
- 36.Stevenson PA. Seed dispersal by woolly monkeys (Lagothrix lagothricha) at Tinigua National Park, Colombia: dispersal distance, germination rates, and dispersal quantity. Am. J. Primatol. 2000;50:275–289. doi: 10.1002/(SICI)1098-2345(200004)50:4<275::AID-AJP4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 37.Yasaka M, Terazawa K, Koyama H, Kon H. Masting behavior of Fagus crenata in northern Japan: spatial synchrony and pre-dispersal seed predation. For. Ecol. Manag. 2003;184:277–284. doi: 10.1016/S0378-1127(03)00157-9. [DOI] [Google Scholar]
- 38.Suzuki W, Osumi K, Masaki T. Mast seeding and its spatial scale in Fagus crenata in northern Japan. For. Ecol. Manag. 2005;205:105–116. doi: 10.1016/j.foreco.2004.10.050. [DOI] [Google Scholar]
- 39.Yamagiwa J, Hill DA. Intraspecific variation in the social organization of Japanese macaques: past and present scope of field studies in natural habitats. Primates. 1998;39:257–273. doi: 10.1007/BF02573076. [DOI] [Google Scholar]
- 40.McConkey KR, O’Farrill G. Cryptic function loss in animal populations. Trend. Ecol. Evol. 2015;30:182–189. doi: 10.1016/j.tree.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 41.McConkey KR, O’Farrill G. Loss of seed dispersal before the loss of seed dispersers. Biol. Cons. 2016;201:38–49. doi: 10.1016/j.biocon.2016.06.024. [DOI] [Google Scholar]
- 42.Ikeda H. Population changes and ranging behaviour of wild Japanese monkeys at Mt. Kawaradake in Kyushu, Japan. Primates. 1982;23:338–347. doi: 10.1007/BF02381318. [DOI] [Google Scholar]
- 43.Yamagiwa J. Socio-sexual factors of troop fission in wild Japanese monkeys (Macaca fuscata yakui) on Yakushima Island, Japan. Primates. 1995;26:105–120. doi: 10.1007/BF02382011. [DOI] [Google Scholar]
- 44.Sengupta A, McConkey KR, Radhakrishna S. Primates, provisioning and plants: impacts of human cultural behaviours on primate ecological functions. PLoS ONE. 2015;10:e0140961. doi: 10.1371/journal.pone.0140961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perea R, Delibes M, Polko M, Suarez-Esteban A, Fedriani JM. Context-dependent fruit–frugivore interactions: partner identities and spatio-temporal variations. Oikos. 2013;122:943–951. doi: 10.1111/j.1600-0706.2012.20940.x. [DOI] [Google Scholar]
- 46.Pires LP, Melo C. Individual-resource networks reveal distinct fruit preferences of selective individuals from a generalist population of the Helmeted Manakin. Ibis. 2019 doi: 10.1111/ibi.12794. [DOI] [Google Scholar]
- 47.Strier, K. Primate behavioral ecology fifth edition (Routledge 2015).
- 48.Kawazoe T. Association patterns and affiliative relationships outside a troop in wild male Japanese macaques, Macaca fuscata, during the non-mating season. Behaviour. 2016;153:69–89. doi: 10.1163/1568539X-00003325. [DOI] [Google Scholar]
- 49.Izawa, K. Research on wild Japanese macaques (Dobutsusha, 2009).
- 50.Tsuji Y. Inter-annual variation in characteristics of endozoochory by wild Japanese macaques. PLoS ONE. 2014;9:e108155. doi: 10.1371/journal.pone.0108155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kunz BK, Linsenmair KE. The role of the olive baboon (Papio anubis, Cercopithecidae) as seed disperser in a savanna-forest mosaic of West Africa. J. Trop. Ecol. 2008;24:235–246. doi: 10.1017/S0266467408005014. [DOI] [Google Scholar]
- 52.R Development Core Team. R: a language and environment for statistical computing. Version 3.3.2., R Foundation for Statistical Computing, Vienna, Austria (2016).