Abstract
A few studies have explored the association between depression and sudden sensorineural hearing loss (SSNHL). This study was aimed to investigate the reciprocal relations between SSNHL and depression using a nationwide cohort of the Korean population. Subjects aged > 20 years from the Korean National Health Insurance Service-National Sample Cohort were enrolled from 2002 to 2013. In study I, a total of 60,178 depressed patients were matched 1:4 with 242,872 control I subjects. In study II, a total of 4,328 SSNHL patients were 1:4 matched with 17,312 control II subjects. They were matched for age, sex, income, and region of residence. The Charlson comorbidity index (CCI) was adjusted. Subgroup analysis was performed according to age and sex. The crude and adjusted CCI hazard ratios (HRs) of SSNHL in depressed patients (study I) and depression in SSNHL patients (study II) were analyzed using the stratified Cox proportional-hazard model. In study I, the depression group exhibited an elevated adjusted HR of SSNHL 1.16 times that of the control group (confidence interval [95% CI] = 1.02–1.31, P < 0.023). The middle-aged women subgroup demonstrated an increased risk of SSNHL within the depression group. In study II, the SSNHL group showed a higher adjusted HR of depression 1.29 times that of control II group (95% CI = 1.06–1.57, P = 0.010). The middle-aged women subgroup showed an elevated risk of depression in the SSNHL group. The risk of SSNHL was elevated in the depressed patients and the risk of depression was increased in the SSNHL patients.
Subject terms: Epidemiology, Risk factors
Introduction
Depression is a pathologic condition involving feelings of sadness and a loss of interest in activities that last at least two weeks. Several psychiatric diseases including major depressive disorders, dysthymic disorder and depressive disorder not otherwise specified are categorized as depressive disorder1. The lifetime prevalence of depression is as high as 20–25% in women and 7–12% in men in the general population2. In Korea, 9.6–17.3% and 2.8–3.6% of adults complain of depressive symptoms and depression, respectively3. The multiple factors of genetic or external factors were suggested to cause depression by evoking inflammation of the nervous system and subsequent vulnerability to social stress4. Cardiovascular and metabolic disorders could be risk factors for depression5–7. In addition, social isolation and loneliness were reported to elevate the risk of depressive symptoms8. Additionally, it was suggested that impaired social networks due to hearing loss might increase the risk of depression9.
Sensorineural hearing loss of ≥30 dB at 3 consecutive frequencies within 3 days of onset is defined as sudden sensorineural hearing loss (SSNHL). Approximately 27 per 100,000 people in the United States experience SSNHL annually10. The prevalence of SSNHL is 15 per 100,000 people in Korea11. The pathophysiologic causes of SSNHL have not yet been fully explained. Several etiologies associated with immunologic, ischemic, and endocrine factors have been proposed12–14. In addition to associations with chronic diseases such as hypertension, hypercholesteremia, and diabetes, recent studies have demonstrated a relation between SSNHL and depression15,16.
The impaired social communication could increase the risk of social isolation and depression. Especially, SSNHL patients could experience much frustration, because their hearing ability was normal before onset of SSNHL. Indeed several previous studies reported the high rate of depression in SSNHL patients16. A population cohort study reported the risk of depression following SSNHL16. On the other hands, the increased inflammation of nervous system and related comorbidities in depression patients could increase the risk of SSNHL. In line with this, one recent study reported the risk of SSNHL in depressed patients using a population-based cohort15. Thus, it could be inferred that the relation between depression and SSNHL might be bidirectional. The working hypothesis of the present study was that depression and SSNHL have reciprocal relations. No prior study investigated the bidirectional association between SSNHL and depression. We analyzed this relation using the merits of cohort data by time sequence.
Results
Study I
The time duration from index date to SSNHL was 49.1 months (SD = 35.2) in the depression group and 52.8 months (SD = 36.2) in the control group. Age, sex, income, and region of residence were matched between the depression and control groups (Table 1). The incidence of SSNHL was higher in the depression group (0.6% [371/60,718]) than in the control group (0.5% [1,160/242,872], P < 0.001). The CCI was different between the depression and control I groups (P < 0.001).
Table 1.
General Characteristics of Participants.
| Characteristics | Study I | Study II | ||||
|---|---|---|---|---|---|---|
| Depression (n, %) | Control I (n, %) | P-value | SSNHL (n, %) | Control II (n, %) | P-value | |
| Age (years old) | 1.000 | 1.000 | ||||
| 20–24 | 3,850 (6.3) | 15,400 (6.3) | 141 (3.3) | 564 (3.3) | ||
| 25–29 | 4,546 (7.5) | 18,184 (7.5) | 238 (5.5) | 952 (5.5) | ||
| 30–34 | 5,236 (8.6) | 20,944 (8.6) | 271 (6.3) | 1,084 (6.3) | ||
| 35–39 | 5,791 (9.5) | 23,164 (9.5) | 357 (8.3) | 1,428 (8.3) | ||
| 40–44 | 6,161 (10.1) | 24,644 (10.1) | 419 (9.7) | 1,676 (9.7) | ||
| 45–49 | 6,446 (10.6) | 25,784 (10.6) | 457 (10.6) | 1,828 (10.6) | ||
| 50–54 | 6,186 (10.2) | 24,744 (10.2) | 553 (12.8) | 2,212 (12.8) | ||
| 55–59 | 5,033 (8.3) | 20,132 (8.3) | 525 (12.1) | 2,100 (12.1) | ||
| 60–64 | 4,712 (7.8) | 18,848 (7.8) | 444 (10.3) | 1,776 (10.3) | ||
| 65–69 | 4,570 (7.5) | 18,280 (7.5) | 389 (9.0) | 1,556 (9.0) | ||
| 70–74 | 3,840 (6.3) | 15,360 (6.3) | 291 (6.7) | 1,164 (6.7) | ||
| 75–79 | 2,431 (4.0) | 9,724 (4.0) | 160 (3.7) | 640 (3.7) | ||
| 80–84 | 1,274 (2.1) | 5,096 (2.1) | 57 (1.3) | 228 (1.3) | ||
| 85+ | 642 (1.1) | 2,568 (1.1) | 26 (0.6) | 104 (0.6) | ||
| Sex | 1.000 | 1.000 | ||||
| Male | 20,705 (34.1) | 82,820 (34.1) | 1,976 (45.7) | 7,904 (45.7) | ||
| Female | 40,013 (65.9) | 160,052 (65.9) | 2,352 (54.3) | 9,408 (54.3) | ||
| Income | 1.000 | 1.000 | ||||
| 1 (lowest) | 9,521 (15.7) | 38,084 (15.7) | 637 (14.7) | 2,548 (14.7) | ||
| 2 | 8,962 (14.8) | 35,848 (14.8) | 559 (12.9) | 2,236 (12.9) | ||
| 3 | 10,448 (17.2) | 41,792 (17.2) | 755 (17.4) | 3,020 (17.4) | ||
| 4 | 13,063 (21.5) | 52,252 (21.5) | 967 (22.3) | 3,868 (22.3) | ||
| 5 (highest) | 18,724 (30.8) | 74,896 (30.8) | 1,410 (32.6) | 5,640 (32.6) | ||
| Region of residence | 1.000 | 1.000 | ||||
| Urban | 27,771 (45.7) | 111,084 (45.7) | 2,036 (47.0) | 8,144 (47.0) | ||
| Rural | 32,947 (54.3) | 131,788 (54.3) | 2,292 (53.0) | 9,168 (53.0) | ||
| CCI (score) | <0.001* | <0.001* | ||||
| 0 | 17,976 (29.6) | 104,740 (43.1) | 1,196 (27.6) | 7,416 (42.8) | ||
| 1 | 5,809 (9.6) | 25,616 (10.5) | 398 (9.2) | 1,616 (9.3) | ||
| ≥2 | 36,933 (60.8) | 112,516 (46.3) | 2,734 (63.2) | 8,280 (47.8) | ||
| SSNHL | 371 (0.6) | 1,160 (0.5) | <0.001* | 4,328 (100.0) | 0 (0.0) | <0.001* |
| Depression | 60,718 (100.0) | 0 (0.0) | <0.001* | 332 (7.7) | 993 (5.7) | <0.001* |
*Chi-square test, Significance at P Ch0.05
Abbreviation: CCI, Charlson Comorbidity Index; SSNHL, Sudden sensory neural hearing loss.
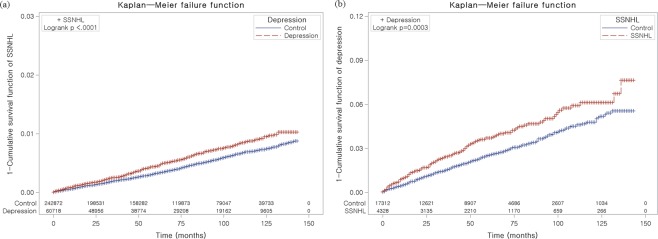
Depression increased the HR of SSNHL (adjusted HR = 1.16, 95% CI = 1.02–1.31; P = 0.023) (Table 2 and Fig. 1a). The depression with ≥ 4 times of treatments were also increased in SSNHL group compared to control I group (adjusted HR = 1.19, 95% CI = 1.04–1.36, P = 0.011, S1 Table).
Table 2.
Crude and adjusted hazard ratios (95% confidence interval) of SSNHL in depression (study I) and depression in SSNHL (study II).
| Characteristics | HRs | |||
|---|---|---|---|---|
| Crude† | P-value | Adjusted†‡ | P-value | |
| Study I | ||||
| Depression | 1.29 (1.14–1.47) | <0.001* | 1.16 (1.02–1.31) | 0.023* |
| Control | 1.00 | 1.00 | ||
| Study II | ||||
| SSNHL | 1.42 (1.17–1.72) | <0.001* | 1.29 (1.06–1.57) | 0.010* |
| Control | 1.00 | 1.00 | ||
*Cox-proportional hazard regression model, Significance at P Co0.05.
†Stratified model for age, sex, income, and region of residence.
‡Adjusted model for Charlson Comorbidity Index.
Figure 1.
Kaplan-Meier survival analysis. (a) The cumulative rate of SSNHL was higher in the depression group than in the control I group; (b) The group with SSNHL demonstrated a higher cumulative rate of depression than the control group.
In the subgroup analysis according to age and sex, middle-aged women showed high HRs for SSNHL (adjusted HR = 1.36, 95% CI = 1.10–1.68, P = 0.005) (Table 3). The crude and adjusted HRs did not reach statistical significance in either the young or old men/women.
Table 3.
Subgroup analysis of crude and adjusted hazard ratios (95% confidence interval) of sudden sensory neural hearing loss in depression according to age and sex in study I.
| Characteristics | HRs of SSNHL | |||
|---|---|---|---|---|
| Crude† | P-value | Adjusted†‡ | P-value | |
| Young men (20–39 years old, n = 33,400) | ||||
| Depression | 1.17 (0.71–1.93) | 0.531 | 0.99 (0.60–1.64) | 0.967 |
| Control I | 1.00 | 1.00 | ||
| Young women (20–39 years old, n = 63,715) | ||||
| Depression | 1.20 (0.85–1.70) | 0.311 | 1.08 (0.76–1.53) | 0.688 |
| Control I | 1.00 | 1.00 | ||
| Middle aged men (40–59 years old, n = 40,510) | ||||
| Depression | 1.39 (1.00–1.92) | 0.047* | 1.22 (0.88–1.70) | 0.229 |
| Control I | 1.00 | 1.00 | ||
| Middle aged women (40–59 years old, n = 78,620) | ||||
| Depression | 1.54 (1.25–1.90) | <0.001* | 1.36 (1.10–1.68) | 0.005* |
| Control I | 1.00 | 1.00 | ||
| Old men (60 + years old, n = 29,615) | ||||
| Depression | 1.05 (0.69–1.58) | 0.832 | 0.92 (0.61–1.40) | 0.709 |
| Control I | 1.00 | 1.00 | ||
| Old women (60 + years old, n = 57,730) | ||||
| Depression | 1.24 (0.86–1.47) | 0.386 | 1.03 (0.79–1.34) | 0.836 |
| Control I | 1.00 | 1.00 | ||
*Cox-proportional hazard regression model, Significance at P Co0.05.
†Stratified model for age, sex, income, and region of residence.
‡Adjusted model for Charlson Comorbidity Index.
Study II
The time duration from index date to depression was 34.9 months (SD = 31.6) in the SSNHL group and 39.3 months (SD = 31.0) in the control group. Age, sex, income, and region of residence were matched between the SSNHL and control groups (Table 1). The incidence of depression was higher in the SSNHL group (7.7% [332/4,328]) than in the control group (5.7% [993/17,312], P < 0.001). The CCI was different between the SSNHL and control I groups (P < 0.001).
SSNHL increased the HR of depression (adjusted HR = 1.29, 95% CI = 1.06–1.57; P = 0.010) (Table 2 and Fig. 1b). The SSNHL were also increased in depression with ≥ 4 times of treatments group compared to control II group (adjusted HR = 1.34, 95% CI = 1.08–1.68, P = 0.009, S1 Table).
In the subgroup analysis according to age and sex, middle-aged women showed high HRs for depression (adjusted HR = 1.46, 95% CI = 1.02–2.09, P = 0.042) (Table 4). The crude and adjusted HRs did not reach statistical significance in other age and sex subgroups.
Table 4.
Subgroup analysis of crude and adjusted hazard ratios (95% confidence interval) of depression in sudden sensory neural hearing loss according to age and sex in study II.
| Characteristics | HRs of depression | |||
|---|---|---|---|---|
| Crude† | P-value | Adjusted†‡ | P-value | |
| Young men (20–39 years old, n = 2,315) | ||||
| SSNHL | 1.46 (0.52–4.04) | 0.520 | 1.43 (0.51–4.06) | 0.498 |
| Control II | 1.00 | 1.00 | ||
| Young women (20–39 years old, n = 2,720) | ||||
| SSNHL | 1.45 (0.85–2.48) | 0.176 | 1.35 (0.78–2.31) | 0.284 |
| Control II | 1.00 | 1.00 | ||
| Middle aged men (40–59 years old, n = 4,590) | ||||
| SSNHL | 1.45 (0.87–2.43) | 0.152 | 1.27 (0.76–2.13) | 0.362 |
| Control II | 1.00 | 1.00 | ||
| Middle aged women (40–59 years old, n = 5,180) | ||||
| SSNHL | 1.55 (1.08–2.23) | 0.017* | 1.46 (1.02–2.09) | 0.042* |
| Control II | 1.00 | 1.00 | ||
| Old men (60 + years old, n = 2,975) | ||||
| SSNHL | 1.51 (0.90–2.52) | 0.119 | 1.32 (0.79–2.22) | 0.295 |
| Control II | 1.00 | 1.00 | ||
| Old women (60 + years old, n = 3,860) | ||||
| SSNHL | 1.23 (0.86–1.78) | 0.263 | 1.21 (0.78–1.62) | 0.544 |
| Control II | 1.00 | 1.00 | ||
*Cox-proportional hazard regression model, Significance at P Co0.05.
*Cox-proportional hazard regression model, Significance at P Co0.05
†Stratified model for age, sex, income, and region of residence.
‡Adjusted model for Charlson Comorbidity Index.
Discussion
Depression increased the risk of SSNHL (adjusted HR = 1.16, 95% CI = 1.02–1.31). On the other hand, SSNHL patients demonstrated an increased risk of depression (adjusted HR = 1.29, 95% CI = 1.06–1.57). In the subgroup analysis according to age and sex, these reciprocal associations between depression and SSNHL were consistent in the middle-aged women group. There was no study investigating the bidirectional association between depression and SSNHL in a single study, according to our knowledge. Moreover, few previous studies have explored the risk of SSNHL in depression according to age and sex.
In accordance with the present results, several researchers have demonstrated an association between depression and SSNHL15,16. However, most of the previous studies focused on the incidence of depressive symptoms or depression in SSNHL patients. The risk of depression was 2.17 times higher in SSNHL patients than in control participants in a retrospective cohort study ([95% CI] = 1.51–3.08, P < 0.001)16. Similarly, depressive symptoms were 2.04 times more likely to be present in SSNHL patients who have not yet recovered from SSNHL than in patients who recovered from SSNHL17. On the other hand, an elevated risk of SSNHL was reported in depressed patients. A prior study demonstrated an HR of 1.46 for SSNHL after adjustments for age, sex, income, region of residence, diabetes, hypertension, chronic kidney disease, and hyperlipidemia (95% CI = 1.10–1.93)15. The present study added to the previous findings by delineating the bidirectional association between SSNHL and depression. In addition, this study used multiple inclusion criteria of ICD-10 codes, pure tone audiometric examinations and treatment histories.
The impairment of auditory recognition function in depressed patients might contribute to the development of SSNHL. Decreased auditory processing could be linked to the onset of SSNHL in patients with depression. Auditory processing requires both peripheral auditory perception and central auditory processing. Thus, the impaired auditory processing and speech recognition might influence hearing function. For instance, impaired cognitive function was found to be related to hearing loss in previous studies18,19. Multiple cognitive functions, including executive function, verbal memory, and attention, are impaired in depressed patients20. Moreover, reduced activity, lack of drive and concern, and inability to plan could reduce the auditory input and processing of central auditory pathways21.
On the other hand, the decreased quality of life following SSNHL could increase the risk of depression. The stress from sudden hearing loss and accompanied symptoms of tinnitus or vertigo could impair the quality of life and influence the onset of depression. It was reported that annoying tinnitus and vertigo following SSNHL were the negative predictive factors for quality of life22. The impact of SSNHL on poor quality of life was highest in the aspect of mental functioning23. Another retrospective study demonstrated that the risk of depressive symptoms were higher in SSNHL patients who did not recover from SSNHL or complained of tinnitus17. In addition, un-recovered hearing loss resulted in the impairments in communication and social relationships, which was reported to be associated with cognitive impairment and depression24. In terms of neural function, hearing loss decreases the activities of the central auditory systems, which in turn increases the cognitive loads and disturb the emotional relativities25.
The undetected common pathologic causes, such as cardiovascular, endocrine, and autoimmune disorders, could mediate both depression and SSNHL. Moreover, the inflammatory cytokines C-reactive protein and interleukin-6 are elevated in depressed patients26. Increased peripheral inflammation could induce atherosclerosis and hamper the central nervous system, including the auditory nervous system. Diabetes could result in atherosclerotic changes and abnormal hormonal control. Hyperglycemia and high cortisol levels increase the risk of depression27. Indeed, the incidence of depression is 2–3 times higher in diabetic patients than in nondiabetic patients28. Moreover, the association between depression and diabetic control is bidirectional27. Depression increases the risk of hypoglycemia and hyperglycemia in diabetes patients and vice versa. Likewise, autoimmune disorders have been suggested to have a reciprocal relation with depression29. Because cardiovascular diseases, diabetes, and autoimmune disorders are also risk factors for SSNHL, the risk of SSNHL in depression could be attributed to these shared risk factors30.
Among the age and sex subgroups, the middle-aged female groups showed an increased risk of SSNHL among the depressed patients and increased risk of depression in SSNHL patients in this study. The high proportion of social activities and working positions in the middle-aged population compared to that in other age groups could increase the impact of depression on SSNHL in this age group. The sudden hearing loss and subsequent impaired communication might create more challenges and discomfort in the subjects with social activities and working positions. On the other hand, the low incidence of SSNHL in the young adult population could mitigate the association between depression and SSNHL in the present data. In the older population, causative factors other than depression, such as chronic diseases, could influence the development of SSNHL. A previous study reported that the association between depression and SSNHL was high in the younger age group (<60 years)16.
This study has several virtues in addition to the bidirectional analyses of the association between depression and SSNHL. The present study used a nationwide population cohort that was validated in a previous study31. All of Korea was included in the NHIS data, thus there were no missing participants. Control groups were randomly selected and matched for age, sex, income, and region of residence. Income and region of residence were matched and adjusted because socioeconomic status was one of the important determinants of medical care accessibility. Income was precisely investigated using the Korean NHIS data. In addition, possible confounders were comprehensively adjusted using CCI. For the accuracy of diagnoses, depression was evaluated ≥2 times by physicians, and SSNHL was classified based on the examination of pure tone audiometry and steroid treatment. However, the interpretation of the present results should involve the consideration of some limitations. Although numerous confounders were matched and adjusted in this study, there were still potential confounding factors, including stress factors, diet, physical activity, smoking and body mass index, that were not considered. The severity of depression and the treatment history of the depression patients could not be assessed using the NHIS data. To evaluate the association between depression and SSNHL, the depression patients with ≥4 times of treatment were analyzed for the reciprocal relation with SSNHL (S1 Table). The depression patients with ≥4 times of treatment also showed bidirectional association with SSNHL. Similarly, the severity and the prognosis of SSNHL were heterogeneous in the present study.
In conclusion, depression increased the risk of SSNHL in the adult. On the other hands, SSNHL increased the rate of depression in the adult population.
Materials and Methods
Study population and data collection
This study was approved by the ethics committee of Hallym University (2017-I102). The Institutional Review Board of Hallym University exempted written informed consent. All methods were performed in accordance with the guidelines and regulations of the ethics committee of Hallym University.
This national cohort study relied on data from the Korean Health Insurance Review and Assessment Service-National Sample Cohort (HIRA-NSC). The details of these data can be found in prior studies32,33.
Participant selection
Out of 1,125,691 cases with 114,369,638 medical claim codes, we included participants who were diagnosed with depression. Depression was diagnosed based on the ICD-10 codes F31 (bipolar affective disorder) through F39 (unspecified mood disorder) between 2002 and 2013. Among them, the participants who visited clinics for depression ≥2 times (n = 68,019) following our previous studies32,34.
SSNHL was indicated by a diagnosis with the ICD-10 code H912. Among individuals with SSNHL, we selected participants who underwent audiometry exams (claim codes: E6931-E6937, F6341-F6348) and were prescribed steroids. Between 2002 and 2013, 5,244 of the SSNHL participants were included following our previous studies35,36.
Study I
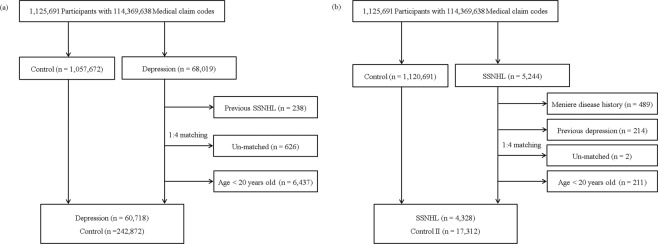
In study I, the depressed patients and the control participants without history of depression were 1:4 matched 1:4. The control participants were extracted from the mother population (n = 1,057,672) and were matched for age, group, sex, income group, and region of residence as previously described32,33. The 238 participants who diagnosed SSNHL before the history of depression were excluded in the depression group. The 626 participants who had not enough matched control participants were excluded. The average period of follow-up was almost comparable between the depression group (71.9 months, standard deviation [SD] = 43.5) and the control group (73.2 months, SD = 43.3). The participants under 20 years of age were excluded because the prevalence of SSNHL in the young population was as low as 2.6% of whole age SSNHL patients, and the characteristics of SSNHL were different in these age groups (n = 6,437)37. Finally, the 60,718 depressed participants and 242,872 control participants were enrolled (Fig. 2). The occurrence of SSNHL in both depression and control I groups were analyzed. The SSNHL patients who had history of Meniere’s disease were excepted.
Figure 2.
Schematic illustration of the participant selection process used in the present study. (a) Out of a total of 1,125,691 participants, 60,718 depressed participants were matched with 242,872 control participants according to age, group, sex, income group, and region of residence. (a) Out of a total of 1,125,691 participants, 4,328 SSNHL participants were matched with 17,312 control participants according to age, group, sex, income group, and region of residence.
Study II
SSNHL patients and control II participants who were not diagnosed were 1:4 matched. Matching processes was similar with those of study I. The 489 participants with Meniere’s disease and 214 participants with history of depression were excluded. Two SSNHL patients were excluded due to the lack of control participants. The 211 participants with less than 20 years old were excluded. The average period of follow-up was almost comparable between SSNHL (55.8 months, SD = 38.4) and control groups (56.4 months, SD = 38.4). Lastly, the 4,328 SSNHL patients and 17,312 control II participants (Fig. 2b). The occurrence of depression in both SSNHL and control II participants were analyzed.
Variables
Age, income, and region of residence were classified as previous studies38. The age groups were classified using 5-year intervals. The income groups were classified as 5 classes (class 1 [lowest income] − 5 [highest income]). The region of residence was divided into urban areas and rural areas.
The past medical history of each participant was evaluated using ICD-10 codes. The Charlson comorbidity index (CCI) was used as previous studies39.
Statistical analyses
The demographic factors and other variables were compared between the depression and control groups using a chi-square test.
The hazard ratio (HR) of SSNHL for depression in study I, and the HR of depression for SSNHL in study II were analyzed using a stratified Cox proportional-hazards model. Crude and adjusted (CCI) models were calculated. The 95% confidence interval (CI) was evaluated. These analyses were stratified forage, sex, income, and region of residence. The Kaplan-Meier curve and Log rank test was conducted.
The participants were sub grouped according to age and sex (20–39 years of age, 40–59 years of age, 60 + years of age; men and women) because we thought that these associations may show different relations in the subgroups.
Two-tailed analyses were performed. The statistical significance was defined as P-values less than 0.05. The SPSS v. 21.0 (IBM, Armonk, NY, USA) and SAS version 9.4 (SAS Institute Inc. Cary, NC, USA) were used.
Supplementary information
Acknowledgements
This work was supported in part by a research grant (NRF-2018-R1D1A1A02085328 and NRF-2016M3A9E8941669) from the National Research Foundation (NRF) of Korea. The funders had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author contributions
conceptualization, H.G.C.; methodology, H.G.C., C.H.L. and C.M.; formal analysis, B.P. and D.J.O.; writing—original draft preparation, H.G.C. and S.Y.K.; writing—review and editing, H.G.C. and S.Y.K.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-58547-w.
References
- 1.Fiest KM, et al. Systematic review and assessment of validated case definitions for depression in administrative data. BMC Psychiatry. 2014;14:289. doi: 10.1186/s12888-014-0289-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang J, et al. Prevalence of depression and depressive symptoms among outpatients: a systematic review and meta-analysis. BMJ open. 2017;7:e017173. doi: 10.1136/bmjopen-2017-017173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seo J, Choi B, Kim S, Lee H, Oh D. The relationship between multiple chronic diseases and depressive symptoms among middle-aged and elderly populations: results of a 2009 korean community health survey of 156,747 participants. BMC Public. Health. 2017;17:844. doi: 10.1186/s12889-017-4798-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Menard C, et al. Social stress induces neurovascular pathology promoting depression. Nat. Neurosci. 2017;20:1752–1760. doi: 10.1038/s41593-017-0010-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huffman JC, Celano CM, Beach SR, Motiwala SR, Januzzi JL. Depression and cardiac disease: epidemiology, mechanisms, and diagnosis. Cardiovasc. Psychiatry Neurol. 2013;2013:695925. doi: 10.1155/2013/695925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quinn Meghan E., Grant Kathryn E., Adam Emma K. Negative cognitive style and cortisol recovery accentuate the relationship between life stress and depressive symptoms. Stress. 2017;21(2):119–127. doi: 10.1080/10253890.2017.1414800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Webb M, et al. The association between depressive symptoms and insulin resistance, inflammation and adiposity in men and women. PLoS One. 2017;12:e0187448. doi: 10.1371/journal.pone.0187448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ge L, Yap CW, Ong R, Heng BH. Social isolation, loneliness and their relationships with depressive symptoms: A population-based study. PLoS one. 2017;12:e0182145. doi: 10.1371/journal.pone.0182145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawrence, B. J. et al. Hearing Loss and Depression in Older Adults: A Systematic Review and Meta-analysis. The Gerontologist, 10.1093/geront/gnz009 (2019). [DOI] [PubMed]
- 10.Alexander TH, Harris JP. Incidence of sudden sensorineural hearing loss. Otology & neurotology: official publication of the American Otological. Soc., Am. Neurotology Society [and] Eur. Acad. Otology Neurotology. 2013;34:1586–1589. doi: 10.1097/MAO.0000000000000222. [DOI] [PubMed] [Google Scholar]
- 11.Kim Jong-Yeup, Hong Jee Young, Kim Dong-Kyu. Association of Sudden Sensorineural Hearing Loss With Risk of Cardiocerebrovascular Disease. JAMA Otolaryngology–Head & Neck Surgery. 2018;144(2):129. doi: 10.1001/jamaoto.2017.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rossini BAA, Penido NO, Munhoz MSL, Bogaz EA, Curi RS. Sudden Sensorioneural Hearing Loss and Autoimmune Systemic Diseases. Int. Arch. Otorhinolaryngol. 2017;21:213–223. doi: 10.1055/s-0036-1586162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim C, et al. Ischemia as a potential etiologic factor in idiopathic unilateral sudden sensorineural hearing loss: Analysis of posterior circulation arteries. Hear. Res. 2016;331:144–151. doi: 10.1016/j.heares.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Lin CF, Lee KJ, Yu SS, Lin YS. Effect of comorbid diabetes and hypercholesterolemia on the prognosis of idiopathic sudden sensorineural hearing loss. Laryngoscope. 2016;126:142–149. doi: 10.1002/lary.25333. [DOI] [PubMed] [Google Scholar]
- 15.Lin CS, et al. Increased risk of sudden sensorineural hearing loss in patients with depressive disorders: population-based cohort study. J. laryngology otology. 2016;130:42–49. doi: 10.1017/S0022215115002960. [DOI] [PubMed] [Google Scholar]
- 16.Tseng CC, et al. Risk of depressive disorders following sudden sensorineural hearing loss: A nationwide population-based retrospective cohort study. J. Affect. Disord. 2016;197:94–99. doi: 10.1016/j.jad.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 17.Chen J, Liang J, Ou J, Cai W. Mental health in adults with sudden sensorineural hearing loss: an assessment of depressive symptoms and its correlates. J. Psychosom. Res. 2013;75:72–74. doi: 10.1016/j.jpsychores.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Lin VYW, Black SEL. Deafness and Dementia: Challenges and Opportunities. Otol. Neurotol. 2017;38:e237–e239. doi: 10.1097/MAO.0000000000001408. [DOI] [PubMed] [Google Scholar]
- 19.Martini A, Castiglione A, Bovo R, Vallesi A, Gabelli C. Aging, cognitive load, dementia and hearing loss. Audiology neuro-otology. 2014;19(Suppl 1):2–5. doi: 10.1159/000371593. [DOI] [PubMed] [Google Scholar]
- 20.Bortolato B, et al. Cognitive remission: a novel objective for the treatment of major depression? BMC Med. 2016;14:9. doi: 10.1186/s12916-016-0560-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomas P., Hazif Thomas C., Billon R., Peix R., Faugeron P., Clément J.-P. Dépression et syndrome frontal : quels risques pour la personne âgée ? L'Encéphale. 2009;35(4):361–369. doi: 10.1016/j.encep.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 22.Carlsson PI, Hall M, Lind KJ, Danermark B. Quality of life, psychosocial consequences, and audiological rehabilitation after sudden sensorineural hearing loss. Int. J. audiology. 2011;50:139–144. doi: 10.3109/14992027.2010.533705. [DOI] [PubMed] [Google Scholar]
- 23.Sano H, Okamoto M, Ohhashi K, Iwasaki S, Ogawa K. Quality of life reported by patients with idiopathic sudden sensorineural hearing loss. Otology neurotology: Off. Publ. Am. Otological Soc., Am. Neurotology Society [and] Eur. Acad. Otology Neurotology. 2013;34:36–40. doi: 10.1097/MAO.0b013e318278540e. [DOI] [PubMed] [Google Scholar]
- 24.Dawes P, et al. Hearing loss and cognition: the role of hearing AIDS, social isolation and depression. PLoS one. 2015;10:e0119616. doi: 10.1371/journal.pone.0119616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rutherford BR, Brewster K, Golub JS, Kim AH, Roose SP. Sensation and Psychiatry: Linking Age-Related Hearing Loss to Late-Life Depression and Cognitive Decline. Am. J. psychiatry. 2018;175:215–224. doi: 10.1176/appi.ajp.2017.17040423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valkanova V, Ebmeier KP, Allan CL. CRP, IL-6 and depression: a systematic review and meta-analysis of longitudinal studies. J. Affect. Disord. 2013;150:736–744. doi: 10.1016/j.jad.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Gilsanz Paola, Karter Andrew J., Beeri Michal Schnaider, Quesenberry Charles P., Whitmer Rachel A. The Bidirectional Association Between Depression and Severe Hypoglycemic and Hyperglycemic Events in Type 1 Diabetes. Diabetes Care. 2017;41(3):446–452. doi: 10.2337/dc17-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laiteerapong N, et al. Correlates of quality of life in older adults with diabetes: the diabetes & aging study. Diabetes Care. 2011;34:1749–1753. doi: 10.2337/dc10-2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Euesden J, Danese A, Lewis CM, Maughan B. A bidirectional relationship between depression and the autoimmune disorders - New perspectives from the National Child Development Study. PLoS One. 2017;12:e0173015. doi: 10.1371/journal.pone.0173015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aimoni C, et al. Diabetes, cardiovascular risk factors and idiopathic sudden sensorineural hearing loss: a case-control study. Audiol. Neurootol. 2010;15:111–115. doi: 10.1159/000231636. [DOI] [PubMed] [Google Scholar]
- 31.Lee J, Lee JS, Park SH, Shin SA, Kim K. Cohort Profile: The National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int. J. Epidemiol. 2016 doi: 10.1093/ije/dyv319. [DOI] [PubMed] [Google Scholar]
- 32.Kim SY, et al. Bidirectional association between gastroesophageal reflux disease and depression: Two different nested case-control studies using a national sample cohort. Sci. Rep. 2018;8:11748. doi: 10.1038/s41598-018-29629-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim SY, Lim JS, Kong IG, Choi HG. Hearing impairment and the risk of neurodegenerative dementia: A longitudinal follow-up study using a national sample cohort. Sci. Rep. 2018;8:15266. doi: 10.1038/s41598-018-33325-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim So Young, Kim Mi Kyung, Lim Jae-Sung, Kong Il Gyu, Choi Hyo Geun. Migraine increases the proportion of sudden sensorineural hearing loss: A longitudinal follow-up study. Auris Nasus Larynx. 2019;46(3):353–359. doi: 10.1016/j.anl.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 35.Kim SY, Lim JS, Sim S, Choi HG. Sudden Sensorineural Hearing Loss Predicts Ischemic Stroke: a Longitudinal Follow-Up Study. Otology neurotology: Off. Publ. Am. Otological Soc., Am. Neurotology Society [and] Eur. Acad. Otology Neurotology. 2018;39:964–969. doi: 10.1097/MAO.0000000000001902. [DOI] [PubMed] [Google Scholar]
- 36.Kim SY, Kong IG, Lim H, Choi HG. Increased Risk of Sudden Sensory Neural Hearing Loss in Osteoporosis: A Longitudinal Follow-Up Study. J. Clin. Endocrinol. Metab. 2018;103:3103–3109. doi: 10.1210/jc.2018-00717. [DOI] [PubMed] [Google Scholar]
- 37.Kitoh R, et al. Nationwide epidemiological survey of idiopathic sudden sensorineural hearing loss in Japan. Acta oto-laryngologica. 2017;137:S8–S16. doi: 10.1080/00016489.2017.1297537. [DOI] [PubMed] [Google Scholar]
- 38.Kim SY, Min C, Oh DJ, Choi HG. Tobacco Smoking and Alcohol Consumption Are Related to Benign Parotid Tumor: A Nested Case-Control Study Using a National Health Screening Cohort. Clin. Exp. Otorhinolaryngol. 2019;12:412–419. doi: 10.21053/ceo.2018.01774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim SY, Min C, Oh DJ, Lim JS, Choi HG. Bidirectional association between asthma and migraines in adults: Two longitudinal follow-up studies. Sci. Rep. 2019;9:18343. doi: 10.1038/s41598-019-54972-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.