Abstract
BACKROUND:
CRISPR/Cpf1 is a class II, type V RNA-guided endonuclease that is distinct from the type II CRISPR/Cas9 nuclease, widely used for genome editing. Cpf1 is a smaller and simpler endonuclease than Cas9, overcoming some limitations of the CRISPR/Cas9 system. The applications of CRISPR to rodent embryos for the production of knock-out (KO) mice have been achieved mainly by microinjection, which requires heavily-equipped instruments with skillful hands. Here, we evaluated the genome editing efficiency between Cpf1/mRNA and Cpf1/ribonuclear protein (RNP) in mouse embryos, and established an easy, fast, and technically less demanding method to produce KO mice using electroporation of the Cfp1/RNP system.
METHODS:
The efficiency of electroporation-based delivery of AsCpf1/mRNA and AsCpf1/RNP to target exon 3 of leukemia inhibitory factor (Lif) into mouse zygotes was evaluated. Embryos that developed to the two-cell stage after zygote electroporation were transferred into the oviducts of surrogate mothers to produce AsCpf1-mediated LIF KO mice. The genome editing efficiency of blastocysts and pups was tested using the T7E1 assay and/or DNA sequencing. Congenital abnormalities and reproductive phenotypes in LIF KO mice produced by electroporation with AsCpf1/RNP were examined.
RESULTS:
Survival and two-cell development of electroporated zygotes were comparable between the AsCpf1/mRNA and AsCpf1/RNP groups, whereas genome editing efficiency was relatively higher in the AsCpf1/RNP group (13.3% vs 18.1% at blastocyst and 33.3% vs 45.5% at offspring), respectively. Two mouse lines with a frameshift mutation in exon 3 of the Lif gene were established from the AsCpf1/RNP group. All congenital abnormalities of LIF KO mice produced by AsCpf1/RNP electroporation were observed. AsCpf1-mediated LIF KO mice showed postnatal growth retardation and implantation failure, both of which are major phenotypes of LIF KO mice generated by conventional gene targeting.
CONCLUSION:
Electroporation of AsCpf1/RNP at the zygote stage is an efficient genome editing method to produce KO mice.
Keywords: CRISPR/AsCpf1, Electroporation, AsCpf1/RNP, Gene targeting, Embryo, LIF
Introduction
Mice with specific gene deletions are valuable tools to investigate the unique functions of genes. Traditional gene targeting procedures with microinjection of embryonic stem (ES) cells are complex and time-consuming [1]. In contrast, genome editing with engineered DNA nucleases induces site-directed gene modifications by direct injection of site-specific engineered nucleases into zygotes [2, 3]. Three kinds of engineered nucleases (ZFN, TALENs, and CRISPR) have been used for producing knock-out (KO) mice [4–6]. The bacterial type II CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats)/Cas system, which consists of the Cas9 DNA nuclease and guide RNA (gRNA) composed of crRNA (CRISPR RNA) and tracrRNA (trans-activating crRNA), has been recently developed as a genome editing method [7, 8]. In mouse zygotes, the CRISPR/Cas9 system is an efficient genome modification tool that generates KO mice. Mutations engineered using the CRISPR system are generated through site-specific double-strand break (DSB) repair by non-homologous end joining or homology directed repair [3]. There is a concern that CRISPR/Cas9 technology could cause the potential occurrence of off-target effects, compared to the use of Cas9 plasmid DNA. When Cas9 protein and gRNA as ribonucleoprotein (RNP) complexes are introduced into human cells such as fibroblasts and pluripotent stem cells, they efficiently induce site-specific mutations with reduced off-target effects [9, 10].
The type V CRISPR/Cpf1 RNA-guided endonucleases are distinct from the CRISPR/Cas9 nucleases. Cpf1 is a single RNA-guided endonuclease lacking tracrRNA, and it recognizes thymidine-rich protospacer adjacent motif (PAM) sequences (5′-TTTN-3′). Moreover, Cpf1 cleaves DNA via a staggered DNA DSB with a 4- or 5-nucleotide 5′ overhang. Among the Cpf1 family, Lachnospiraceae bacterium Cpf1 and Acidaminococcus sp. (As) Cpf1 exhibits robust nuclease activity in human cells [11]. It was previously reported that Cpf1 shows higher specificity with few or no off-target effects than Cas9 in mouse or human cell lines [12–14].
To generate gene KO animals using the CRISPR system, CRISPR components such as mRNA and RNPs containing target sequences were delivered into preimplantation embryos by microinjection or electroporation. Microinjection is a widely used technique for generating transgenic animals, but requires specialized equipment and skillful hands. However, electroporation is a very simple, easy-to-use and fast method that can deliver nucleotides or proteins into multiple embryos simultaneously. In most CRISPR studies, genetically engineered animals are generated by mRNA microinjection in mice, rats, and zebrafish [12, 15–20], or by RNP electroporation in mice [20, 21]. In the CRISPR/Cas9 system, electroporation of Cas9/RNP for gene targeting showed higher editing efficiency than Cas9/mRNA in mouse blastocysts [22]. In CRISPR/Cpf1 system, which exbibits higher specificity than CRISPR/Cas9, genome editing efficiency between mRNA and RNP systems to produce KO animals has not been clearly investigated. Here, we compared gene editing efficiency between Cpf1/mRNA and Cpf1/RNP electroporation into zygotes to disrupt a gene, leukemia inhibitory factor (LIF), that is critical for embryo implantation.
Materials and methods
Production of gRNAs and AsCpf1 mRNAs
All gRNA oligonucleotide templates (Lif gRNA1 5′-GGA GCC CTC TTC CCA TCA CCC CTG atc tac aag agt aga aat taC CCT ATA GTG AGT CGT ATT AAT TTC-3′; Lif gRNA2 5′- CTT ACT GCT GCT GGT TCT GCA CTG atc tac aag agt aga aat taC CCT ATA GTG AGT CGT ATT AAT TTC-3′) were synthesized from Macrogen Inc. (Seoul, Korea) and annealed to a short T7 priming sequence (5′-GAA ATT AAT ACG ACT CAC TAT AGG G-3′). Annealed T7-gRNA was used as the template for in vitro transcription using MEGAshortscript™ T7 High Yield Transcription kit (Invitrogen, San Diego, CA, USA). pTE4396 (for AsCpf1 mRNA; Addgene #74041) was linearized with BsmBI and XmaI and was used as a template for in vitro transcription using mMESSAGE mMACHINE® T7 Ultra Kit (Invitrogen, San Diego, CA, USA).
In vitro DNA cleavage assay
Alt-R™ AsCpf1 nuclease (500 ng; Integrated DNA Technologies [IDT]) was incubated with amplified DNA (200 ng), Lif_Forward (5′-GGA GGG ATG AGG CTA GAT GGT TG-3′), and Lif_Reverse (5′-TCT GTG CTT TGG CTA GGG TGT GG-3′) primers containing the target sequence and T7 transcribed gRNA (600 ng) in NEB buffer 3.1 in a reaction volume of 20 μl for 1 h at 37 °C. The reaction was terminated by the addition of proteinase K (40 μg) for 20 min at room temperature. Digested DNA was analyzed by electrophoresis on a 2% agarose gel.
T7E1 assay
The T7E1 assay was performed as previously described [23]. Briefly, mouse genomic DNA was extracted from tail biopsies. The region of DNA containing the nuclease target site was amplified using Lif_Forward 2 (5′-TAC CTT GCC TCT TAA TCC AGT GG-3′) and Lif_Reverse 2 (5′-TGA AGA GAG CAT TGG CGC TGC CA-3′) primers. The amplicons were denatured by heating and annealed to form heteroduplex DNA, which was then treated with 5 units of T7E1 (New England Biolabs, Ipswich, MA, USA) in NEB buffer 2.1 for 1 h at 37 °C, and analyzed using 2% agarose gel electrophoresis.
Sequencing analysis
Mouse genomic DNA containing the mutated site by AsCpf1 was amplified using Lif_Forward 2 and Lif_Reverse 2 primers. The amplicons were cloned into the T-vector using pMD20 (Takara Bio, Mountain View, CA, USA). Cloned products were sequenced using the M13R-pUC (-40) primer (5′- CAGGAAACAGCTATGAC-3′).
Animals
All mice used in this study were approved by the Institutional Animal Care and Use Committee (IACUC) of CHA University (IACUC approval No. IACUC170174). Mice were maintained and handled in a specific pathogen-free facility under temperature and light controlled conditions with light on for 12 h daily. Four-weeks-old C57BL/6 N and 8-weeks-old ICR mice were used as embryo donors and surrogate mothers, respectively.
Electroporation of zygote stage embryos
The experiment was performed as previously described by Hur et al. with some modifications [21]. Briefly, superovulation was induced in C57BL/6 N female mice at 4 weeks of age by intraperitoneal injection of pregnant mare serum gonadotropin (PMSG) (5 IU, Sigma–Aldrich, St. Louis, MO, USA) and human chorionic gonadotropin (hCG) hormone (5 IU, Sigma–Aldrich, St. Louis, MO, USA) at 48 h intervals. These mice were mated with proven fertile male mice, and zygotes were collected from the oviduct. Cumulus cells were removed from zygotes by exposure to 0.1% hyaluronidase in M2 (Sigma–Aldrich, St. Louis, MO, USA) media. For electroporation of zygotes, the glass chamber of a NEPA21 electroporator (NEPA GENE Co. Ltd. Chiba, Japan) was filled with 100 μl opti-MEM containing mRNAs of AsCpf1 (20 μg/100 μl) with gRNA (25 μg/100 μl) or RNP complexes of Alt-R™-AsCpf1 nuclease (10 μg/100 μl) with gRNA (25 μg/100 μl) reacted at room temperature for 30 min, respectively. Zygote stage embryos were placed in the glass chamber and electroporated. The embryos were cultured in microdrops of KSOM + AA containing d-glucose and phenol red (Millipore, Danvers, MA, USA) under mineral oil at 37 °C for 1 day in a humidified atmosphere consisting of 5% CO2 in air. Embryos that developed to the two-cell stage the next day were transferred into the oviducts of surrogate mothers on day 1 of pseudopregnancy.
Genotyping PCR
Mouse genomic DNA containing the AsCpf1 mutated site was amplified using Lif_Forward 3 (5′- CTT GCC TTT CCA GTC ACC CTC CG-3′) and Lif_Reverse 3 (5′- AGG GGT GAT GGG AAG AGG GCT C-3′) primers. The amplicons were analyzed using 4% MetaPhor™ agarose gel (LONZA, Basel, Switzerland) electrophoresis.
Vector construction
Lif transcripts from wild-type (WT) and LIF mutated mice were amplified with Lif-ORF-Forward (5′- gga tcc ATG AAG GTC TTG GCC GCA GGG -3′) and Lif-ORF-Reverse (5′- gct agc GAA GGC CTG GAC CAC CAC AC -3′) primers. The two different amplicons were cloned into pCMV6-AC-IRES-GFP-puro at the BamHI and NheI site. The cloned plasmids were named pLIF-WT-FLAG and pLIF-KO-FLAG, respectively. The pLIF-WT-FLAG and pLIF-KO-FLAG plasmids were transfected into 293T cells using GenePORTER® 3000 (Genlantis, San Diego, CA, USA).
Western blotting analysis
Cells were lysed in 100 μl lysis buffer containing PRO-PREP Protein Extraction Solution (iNtRON Biotechnology, Seongnam, Gyeonggi, Korea) and 1 × phosphatase inhibitor (Roche Applied Science, Indianapolis, IN, USA). Lysates were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (10 μg/lane), transferred onto nitrocellulose membranes (Bio-Rad, Waltham, MA, USA), and blocked with 5% non-fat milk (Bio-Rad, Waltham, MA, USA) in 1 × TBS containing 0.1% Tween 20 (TBST). Membranes were incubated overnight at 4 °C with the following antibodies: FLAG (1:2000; Sigma–Aldrich, St. Louis, MO, USA) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 1:2000; Cell Signaling Technology, Danvers, MA, USA). Horseradish peroxidase-conjugated goat anti-rabbit and mouse IgG (Bio-Rad, Waltham, MA, USA) were diluted to 1:3000 in TBST with 5% milk and used as secondary antibodies (incubation for 1 h at room temperature). The signals were developed using the Clarity ECL Western Blotting Substrate Kit (Bio-Rad, Waltham, MA, USA) and detected using a ChemiDoc XRS + system with Image Lab software (version 4.0).
Statistical analysis
All values were plotted as mean ± standard deviation (SD). The data were analysed using student’s t test for statistical evaluation. Statistical significance was considered at p < 0.05.
Results and discussion
AsCpf1/gRNA RNP system for evaluating CRISPR activity in vitro
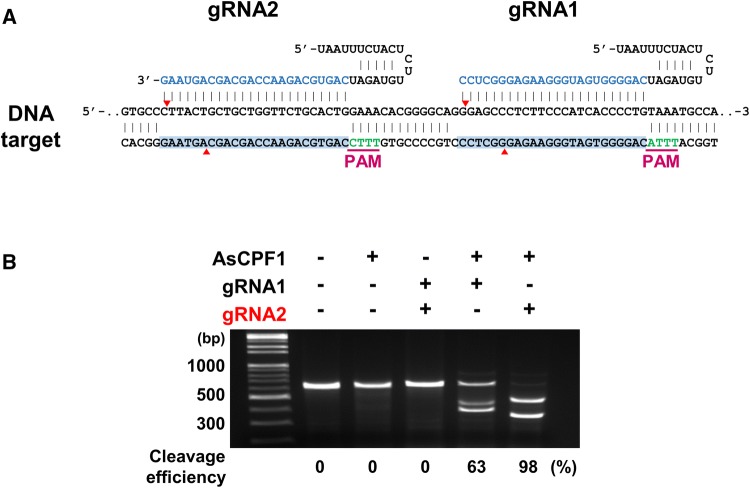
Engineered CRISPR/Cas9 nuclease is being developed as a versatile genome editing tool in various organisms, including mice. Recently, a new type V CRISPR/Cpf1 endonuclease, which is distinct from CRISPR/Cas9, has been shown to be efficient in genome editing [11]. We delivered AsCpf1/mRNA and AsCpf1/RNP by zygote electroporation to evaluate their genome editing efficiency in the mouse embryo. To examine not only the efficiency but also developmental safety of AsCpf1 electroporation, we designed different gRNA constructs at exon 3 of the Lif gene, which is closer to the N-terminus of the protein coding region (Fig. 1A). This site was targeted to produce LIF KO mice, and the developmental and reproductive phenotypes of AsCpf1-mediated LIF KO mice were examined. The mutations at this site were expected to produce a truncated LIF protein lacking over 180 amino acids from the C-terminal. To determine the cleavage efficiency of the two different Lif gRNAs (http://chopchop.cbu.uib.no), an in vitro cleavage assay was performed [24]. AsCpf1 or gRNAs alone did not cleave the linear DNA containing the Lif target sequence, but both the AsCpf1/gRNA1 and AsCpf1/gRNA2 complexes cleaved the target sequences in vitro (Fig. 1B). However, Lif gRNA2 showed greater efficiency, and was therefore used in subsequent experiments.
Fig. 1.
CRISPR/AsCpf1 system for the confirmation of CRISPR activity in vitro. A Schematic diagram of gRNA targeting sites in exon 3 of the mouse Lif gene. The protospacer adjacent motif (PAM) sequence is shown in green. B The efficiency of targeted mutation with gRNA was determined by an in vitro DNA cleavage assay. Cleavage efficiency was assessed by band density after agarose gel electrophoresis
Relative higher efficiency of the AsCpf1/RNP system in editing the mouse genome during early embryogenesis in vitro
The application of CRISPR/Cpf1 to mammalian systems such as human cells and rodent embryos has been reported [12, 15–17, 21, 25]. So far, this system has been mainly applied with microinjection of gRNA with their cognate Cpf1 mRNA into zygotes [12, 17]. However, microinjection is another hurdle to bypass to efficiently achieve transgenesis in mice, because it requires experienced technician(s) with specialized equipment. We applied electroporation of the CRISPR/AsCpf1 system to mouse embryos and evaluated the efficiency of two different forms of AsCpf1/mRNA and AsCpf1/RNP on genome editing in preimplantation mouse embryos (Table 1). AsCpf1/mRNA and AsCpf1/RNP complexes with Lif gRNA2 were successfully delivered into zygotes by electroporation, and 71.6% (63/88) and 76.4% (107/140) of zygotes survived and developed to the two-cell stage, respectively. Some of these embryos were further cultured, and 68.1% (15/22) and 84.6% (22/26), respectively, of the two-cell embryos that survived after AsCpf1/mRNA and AsCpf1/RNP electroporation developed into blastocysts. PCR analysis followed by sequencing for each blastocyst showed that 13.3% (2/15) and 18.1% (4/22) of blastocysts developed from electroporated zygotes were edited at Lif exon 3 by AsCpf1/mRNA and AsCpf1/RNP, respectively. These results suggest that both AsCpf1/mRNA and AsCpf1/RNP electroporation are practicable for embryo gene targeting in mice, and AsCpf1/RNP has a relatively higher efficiency than AsCpf1/mRNA, although it did not reach statistical significance.
Table 1.
Summary of LIF mutants generated by AsCpf1/mRNA or AsCpf1/RNP electroporation with gRNA targeting the Lif site
| Target gene | Application | No. of examined embryos | No. of 2-cell embryos (%) | No. of blastocysts (%) | No. of offspring (%) | No. of mutants (%) (blastocyst or offspring) |
|---|---|---|---|---|---|---|
| Lif | mRNA | 33 | 22 (66.6) | 15 (68.1) | NA | 2 (13.3) |
| RNP | 31 | 26 (83.8) | 22 (84.6) | NA | 4 (18.1) | |
| mRNA | 55 | 41 (74.5) | NA | 12 (29.2) | 4 (33.3) | |
| RNP | 109 | 81 (74.3) | NA | 22 (27.1) | 10 (45.5) |
NA not available
In general, CRISPR/Cpf1 has a higher specificity for target sequence editing, but lower genome editing efficiency, than CRISPR/Cas9 [13]. To improve the editing potential of Cpf1, Wu et al. designed a gRNA transcription system by inserting a transfer RNA (tRNA) precursor sequence downstream of the gRNA for Cpf1. Genome editing using a gRNAtRNA system, showed significantly higher efficiency than did the original CRISPR/Cpf1 system, upon injection into embryos or via somatic cell nuclear transfer [18]. Moon et al. also developed engineered gRNA for highly efficient genome editing by Cpf1. The gRNA includes 20 base target sequence and a U4AU4 uridinylate (U)-rich 3′-overhang. The engineered U-rich gRNA showed highly efficient and specific genome editing by Cpf1 in human cells [26]. If these engineered gRNAs are used in vivo, CRISPR/Cpf1 system could potentially exhibit improved genome editing efficiency for the generation of gene KO animals.
Higher editing efficiency of AsCpf1/RNP electroporation at the zygote stage to produce live mutant pups with successful germline transmission
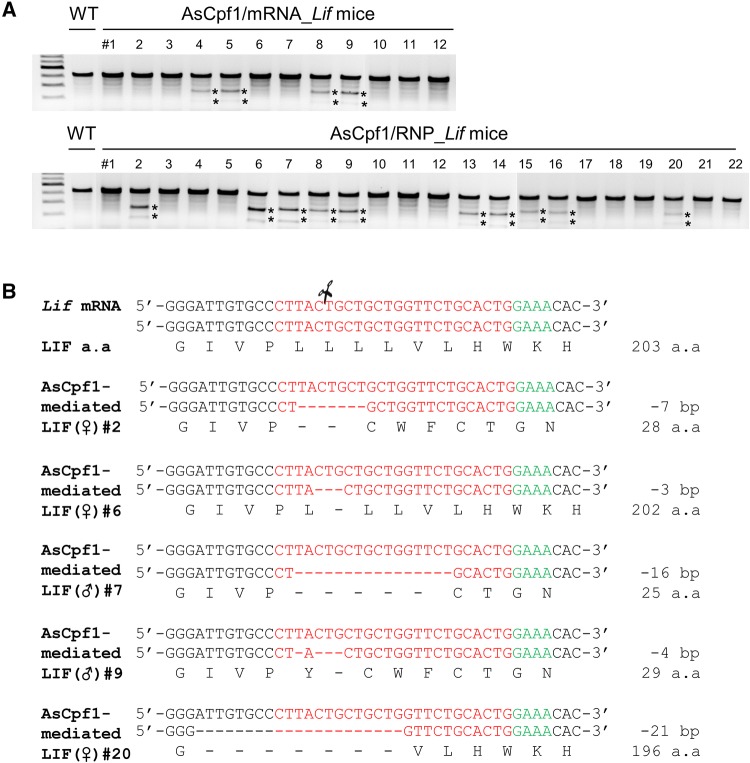
We investigated whether genome editing with AsCpf1/mRNA or AsCpf1/RNP using electroporation successfully produces live offspring from embryos that had undergone genome editing at the zygote stage in vitro. Embryos that survived after electroporation were transferred into the oviduct of surrogate mothers on day 1 of pseudopregnancy, and 29.2% (12/41) and 27.1% (22/81), respectively, of the transferred embryos in AsCpf1/mRNA and AsCpf1/RNP groups successfully completed gestation and birth (Table 1). T7E1-based genotyping analysis demonstrated that 33.3% (4/12) and 45.5% (10/22) of pups from AsCpf1/mRNA and AsCpf1/RNP embryos had genetic mutations (Table 1 and Fig. 2A), respectively. These results collectively suggest that electroporation delivery of AsCpf1/RNP provided a better chance to produce mutated live offspring. Sanger DNA sequencing analysis of five mice randomly selected from 10 AsCpf1/RNP-mediated mutants showed heterozygote deletions and defects ranging from 3 to 21 base pairs (bp) (Fig. 2B). It was previously reported that AsCpf1 mainly causes deletion mutations rather than insertion. The range of deletion mutation was relatively short from− 1 to− 16 bp deletion [13]. Among them, #2 (− 7 bp), #7 (− 16 bp), and #9 (− 4 bp) mouse lines were expected to produce truncated LIF protein due to frameshift mutations, resulting protein products of only 28, 25, and 29 amino acids, respectively. The #6 and #20 mouse lines were expected to produce LIF protein with deletions of 1 and 7 amino acid(s), respectively.
Fig. 2.
CRISPR/AsCpf1 system for the confirmation of CRISPR activity in vivo. A Characterization of offspring obtained after AsCpf1/mRNA eletroporation, and characterization of offspring obtained after AsCpf1/RNP electroporation. T7E1 assays were used to confirm the genotype. * indicates the digested fragments by T7E1. B DNA sequences of five AsCpf1/RNP-mediated LIF mutant mice obtained by Sanger sequencing. The PAM sequences are highlighted in green; the targeting sequences are shown in red; deletions are indicated with (-)
Efficient germline transmission of mutations is required to establish genetically modified mouse models. To examine the germline transmission of AsCpf1-mediated gene mutations, AsCpf1/RNP-mediated LIF mutant founders #2, #7, and #9 were mated with fertile males, and the genotypes of their pups (F1) were evaluated by PCR. Among them, the #2 (7 deletions) and #7 (16 deletions) mutant alleles were successfully transmitted to F1 pups (Fig. 3A), whereas we failed to validate germline transmission in #9 mutant alleles in 31 F1 pups (data not shown). When F1 LIF (+/−7) and (+/−16) females were mated with F1 (+/−7) and (+/−16) males, respectively, they produced (−7/−7) and (−16/−16) homozygous mutant mice with the expected Mendelian ratio (Fig. 3B). Their mutated sequences were validated by Sanger sequencing (data not shown). We did not observe any distinct congenital malformations or morphological defects in live pups born from AsCpf1/RNP electroporation followed by embryo transfer (data not shown). Our results suggest that AsCfp1/RNP electroporation can produce viable gene mutated mouse lines with germline transmission.
Fig. 3.
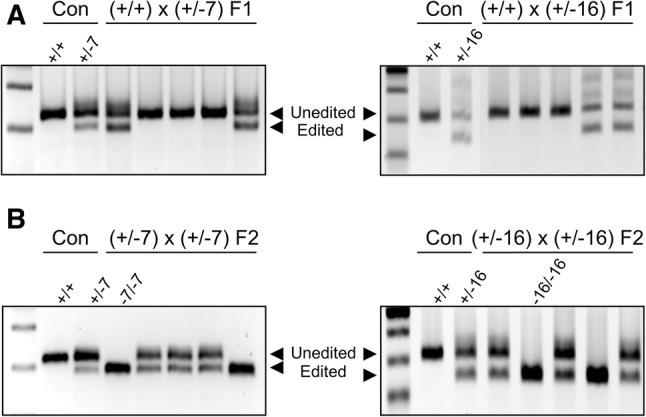
Genotyping of LIF KO mice by AsCpf1/RNP electroporation. (A) Representative genotyping PCR results of AsCpf1-mediated LIF F1 mice indicate successful germline transmission. (B) AsCpf1-mediated LIF KO mouse lines were established by mating heterozygous F1 females with F1 males
Phenotype characterization of LIF KO mice generated by AsCpf1/RNP electroporation
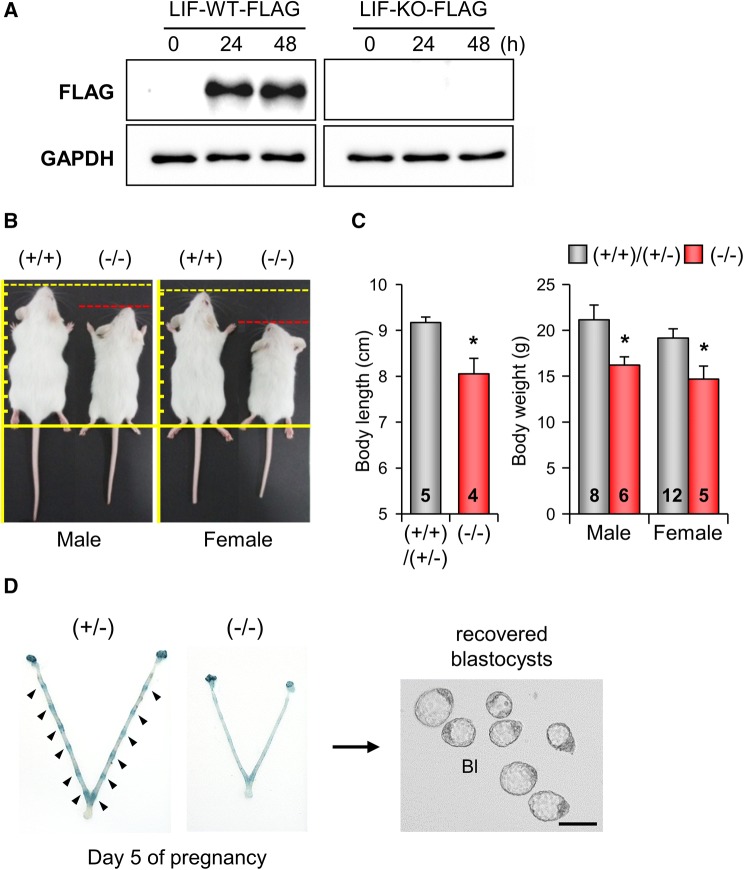
Before we characterized the phenotypes of LIF KO mice produced by AsCpf1/RNP electroporation, we validated the absence of functional LIF protein in our AsCpf1-mediated LIF KO mice. mRNA transcripts of LIF WT (+/+) and LIF KO mice (−7/−7) were cloned into pCMV-AC-IRES-GFP-Puro (pLIF-WT-FLAG and pLIF-KO-FLAG), respectively. These plasmid vectors were transfected into 293T cells, and LIF expression was evaluated by Western blot 24 and 48 h after transfection. C-terminal FLAG-tagged LIF protein was detected in cells transfected with LIF-WT-FLAG but not LIF-KO-FLAG (Fig. 4A). This result suggests that genetic mutation induced by electroporation of AsCpf1 RNPs for Lif exon 3 results in the absence of a functional LIF protein.
Fig. 4.
Characterization of LIF KO mice by AsCpf1/RNP electroporation. A Western blot analysis for functional LIF protein of LIF WT and LIF KO mice. No LIF-KO-FLAG fusion protein was detected. B, C Phenotype of LIF KO mice by AsCpf1/RNP electroporation. As shown in LIF KO mice produced by conventional gene targeting, AsCpf1-mediated LIF KO mice displayed a significantly decreased body length and weight compared to control mice. The numbers within the graph bars indicate the number of examined mice. D AsCpf1-mediated LIF KO female mice showed complete failure of embryo implantation and produced no pups. Scale bar: 100 μm. *p < 0.05
LIF is a member of the interleukin (IL)-6 family that is expressed in various embryonic and adult tissues [27, 28] with particularly high levels in the uterus [29]. LIF is expressed in uterine glands by estrogen specifically on day 4 of pregnancy, and is critical for regulating uterine receptivity and embryo implantation [30–32]. Two independent groups produced LIF KO mice using conventional gene targeting methods [33, 34]. We and other groups have previously investigated the phenotypes of LIF KO mice produced by conventional gene targeting. These LIF KO mice exhibited growth retardation and female sterility [32, 33]. LIF KO female mice can produce fertile oocytes with successful embryo development to blastocysts, but the blastocysts cannot implant in the LIF-deficient uterus [33, 34]. Following these findings, there have been numerous reports that LIF is spatiotemporally induced in the endometrium during the reproductive cycle, possibly for embryo implantation, in many species including humans [35–37]. To compare the functional consequences of conventional LIF KO and AsCpf1-mediated LIF KO mice, we investigated postnatal growth and embryo implantation in AsCpf1-mediated LIF KO mice. AsCpf1-mediated LIF KO mice (−7/−7 and −16/−16) had postnatal growth retardation with significantly decreased body weights, as previously reported (Fig. 4B, C) [38]. When AsCpf1-mediated LIF KO (−7/−7 and −16/−16) female mice were mated naturally with fertile wild-type males, we failed to detect any implantation sites on day 5 of pregnancy, and unimplanted blastocysts were recovered from AsCpf1-mediated LIF KO (−7/−7 and −16/−16) female mice (Fig. 4D and Table 2), as previously reported [33]. AsCpf1-mediated LIF KO (−7/−7; −16/−16) female mice showed complete failure of embryo implantation and subsequently produced no pups. Collectively, our results suggest that CRISPR/AsCpf1 electroporation is an easy, fast, and technically less demanding method to achieve genome editing in mouse embryos, and AsCpf1/RNP offers a better probability of producing KO mice.
Table 2.
Embryo implantation in AsCpf1-mediated LIF KO female mice
| Genotype | Pregnant mice [n] | Mice with IS [n] | ISs [n] | Recovered blastocysts [n] |
|---|---|---|---|---|
| (±) | 4 | 4 | 11.3 ± 4.3 | 0 |
| (-/-) | 4 | 0 | 0 | 7.0 ± 0 |
Acknowledgements
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2019R1A6A1A03032888) and from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (Grant No. HI17C1133).
Compliance with ethical standards
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical statement
All mice used in this study were approved by the Institutional Animal Care and Use Committee (IACUC) of CHA University (IACUC approval No. IACUC170174).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yeon Sun Kim and Gyeong Ryeong Kim are equally contributed to this work.
Contributor Information
Haengseok Song, Email: hssong@cha.ac.kr.
Hye-Ryun Kim, Email: hrkim@cha.ac.kr.
References
- 1.Capecchi MR. Gene targeting in mice: functional analysis of the mammalian genome for the twenty-first century. Nat Rev Genet. 2005;6:507–512. doi: 10.1038/nrg1619. [DOI] [PubMed] [Google Scholar]
- 2.Sung YH, Baek IJ, Kim DH, Jeon J, Lee J, Lee K, et al. Knockout mice created by TALEN-mediated gene targeting. Nat Biotechnol. 2013;31:23–24. doi: 10.1038/nbt.2477. [DOI] [PubMed] [Google Scholar]
- 3.Wang H, Yang H, Shivalila CS, Dawlaty MM, Cheng AW, Zhang F, et al. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell. 2013;153:910–918. doi: 10.1016/j.cell.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wefers B, Ortiz O, Wurst W, Kühn R. Generation of targeted mouse mutants by embryo microinjection of TALENs. Methods. 2014;69:94–101. doi: 10.1016/j.ymeth.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Chen YG, Forsberg MH, Khaja S, Ciecko AE, Hessner MJ, Geurts AM. Gene targeting in NOD mouse embryos using zinc-finger nucleases. Diabetes. 2014;63:68–74. doi: 10.2337/db13-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang H, Wang H, Shivalila CS, Cheng AW, Shi L, Jaenisch R. One-step generation of mice carrying reporter and conditional alleles by CRISPR/Cas-mediated genome engineering. Cell. 2013;154:1370–1379. doi: 10.1016/j.cell.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wiedenheft B, Sternberg SH, Doudna JA. RNA-guided genetic silencing systems in bacteria and archaea. Nature. 2012;482:331–338. doi: 10.1038/nature10886. [DOI] [PubMed] [Google Scholar]
- 8.Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, et al. RNA-guided human genome engineering via Cas9. Science. 2013;339:823–826. doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim S, Kim D, Cho SW, Kim J, Kim JS. Highly efficient RNA-guided genome editing in human cells via delivery of purified Cas9 ribonucleoproteins. Genome Res. 2014;24:1012–1019. doi: 10.1101/gr.171322.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang X, Potter J, Kumar S, Zou Y, Quintanilla R, Sridharan M, et al. Rapid and highly efficient mammalian cell engineering via Cas9 protein transfection. J Biotechnol. 2015;208:44–53. doi: 10.1016/j.jbiotec.2015.04.024. [DOI] [PubMed] [Google Scholar]
- 11.Zetsche B, Gootenberg JS, Abudayyeh OO, Slaymaker IM, Makarova KS, Essletzbichler P, et al. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR–Cas system. Cell. 2015;163:759–771. doi: 10.1016/j.cell.2015.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim Y, Cheong SA, Lee JG, Lee SW, Lee MS, Baek IJ, et al. Generation of knockout mice by Cpf1-mediated gene targeting. Nat Biotechnol. 2016;34:808–810. doi: 10.1038/nbt.3614. [DOI] [PubMed] [Google Scholar]
- 13.Kim D, Kim J, Hur JK, Been KW, Yoon SH, Kim JS. Genome-wide analysis reveals specificities of Cpf1 endonucleases in human cells. Nat Biotechnol. 2016;34:863–868. doi: 10.1038/nbt.3609. [DOI] [PubMed] [Google Scholar]
- 14.Zetsche B, Heidenreich M, Mohanraju P, Fedorova I, Kneppers J, DeGennaro EM, et al. Multiplex gene editing by CRISPR–Cpf1 using a single crRNA array. Nat Biotechnol. 2017;35:31–34. doi: 10.1038/nbt.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watkins-Chow DE, Varshney GK, Garrett LJ, Chen Z, Jimenez EA, Rivas C, et al. Highly efficient Cpf1-mediated gene targeting in mice following high concentration pronuclear injection. G3 (Bethesda) 2017;7:719–722. doi: 10.1534/g3.116.038091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teng F, Cui T, Feng G, Guo L, Xu K, Gao Q, et al. Repurposing CRISPR–Cas12b for mammalian genome engineering. Cell Discov. 2018;4:63. doi: 10.1038/s41421-018-0069-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JG, Ha CH, Yoon B, Cheong SA, Kim G, Lee DJ, et al. Knockout rat models mimicking human atherosclerosis created by Cpf1-mediated gene targeting. Sci Rep. 2019;9:2628. doi: 10.1038/s41598-019-38732-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu H, Liu Q, Shi H, Xie J, Zhang Q, Ouyang Z, et al. Engineering CRISPR/Cpf1 with tRNA promotes genome editing capability in mammalian systems. Cell Mol Life Sci. 2018;75:3593–3607. doi: 10.1007/s00018-018-2810-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moreno-Mateos MA, Fernandez JP, Rouet R, Vejnar CE, Lane MA, Mis E, et al. CRISPR–Cpf1 mediates efficient homology-directed repair and temperature-controlled genome editing. Nat Commun. 2017;8:2024. doi: 10.1038/s41467-017-01836-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen S, Lee B, Lee AY, Modzelewski AJ, He L. Highly efficient mouse genome editing by CRISPR ribonucleoprotein electroporation of zygotes. J Biol Chem. 2016;291:14457–14467. doi: 10.1074/jbc.M116.733154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hur JK, Kim K, Been KW, Baek G, Ye S, Hur JW, et al. Targeted mutagenesis in mice by electroporation of Cpf1 ribonucleoproteins. Nat Biotechnol. 2016;34:807–808. doi: 10.1038/nbt.3596. [DOI] [PubMed] [Google Scholar]
- 22.Wang W, Kutny PM, Byers SL, Longstaff CJ, DaCosta MJ, Pang C, et al. Delivery of Cas9 protein into mouse zygotes through a series of electroporation dramatically increases the efficiency of model creation. J Genet Genomics. 2016;43:319–327. doi: 10.1016/j.jgg.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin Y, Cradick TJ, Bao G. Designing and testing the activities of TAL effector nucleases. In: Storici F, editor. Gene Correction. Methods in Molecular Biology (Methods and Protocols) Totowa, NJ: Humana Press; 2014. pp. 203–219. [DOI] [PubMed] [Google Scholar]
- 24.Labun K, Montague TG, Gagnon JA, Thyme SB, Valen E. CHOPCHOP v2: a web tool for the next generation of CRISPR genome engineering. Nucleic Acids Res. 2016;44:W272–W276. doi: 10.1093/nar/gkw398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kissling L, Monfort A, Swarts DC, Wutz A, Jinek M. Preparation and electroporation of Cas12a/Cpf1-guide RNA complexes for introducing large gene deletions in mouse embryonic stem cells. Methods Enzymol. 2019;616:241–263. doi: 10.1016/bs.mie.2018.10.028. [DOI] [PubMed] [Google Scholar]
- 26.Bin Moon S, Lee JM, Kang JG, Lee NE, Ha DI, Kim DY, et al. Highly efficient genome editing by CRISPR–Cpf1 using CRISPR RNA with a uridinylate-rich 3′-overhang. Nat Commun. 2018;9:3651. doi: 10.1038/s41467-018-06129-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hilton DJ, Gough NM. Leukemia inhibitory factor: a biological perspective. J Cell Biochem. 1991;46:21–26. doi: 10.1002/jcb.240460105. [DOI] [PubMed] [Google Scholar]
- 28.Schäfer-Somi S. Cytokines during early pregnancy of mammals: a review. Anim Reprod Sci. 2003;75:73–94. doi: 10.1016/S0378-4320(02)00222-1. [DOI] [PubMed] [Google Scholar]
- 29.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Müller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/bj20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhatt H, Brunet LJ, Stewart CL. Uterine expression of leukemia inhibitory factor coincides with the onset of blastocyst implantation. Proc Natl Acad Sci U S A. 1991;88:11408–11412. doi: 10.1073/pnas.88.24.11408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosario GX, Stewart CL. The multifaceted actions of leukaemia inhibitory factor in mediating uterine receptivity and embryo implantation. Am J Reprod Immunol. 2016;75:246–255. doi: 10.1111/aji.12474. [DOI] [PubMed] [Google Scholar]
- 32.Song H, Lim H, Das SK, Paria BC, Dey SK. Dysregulation of EGF family of growth factors and COX-2 in the uterus during the preattachment and attachment reactions of the blastocyst with the luminal epithelium correlates with implantation failure in LIF-deficient mice. Mol Endocrinol. 2000;14:1147–1161. doi: 10.1210/mend.14.8.0498. [DOI] [PubMed] [Google Scholar]
- 33.Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Köntgen F, et al. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992;359:76–79. doi: 10.1038/359076a0. [DOI] [PubMed] [Google Scholar]
- 34.Escary JL, Perreau J, Duménil D, Ezine S, Brûlet P. Leukaemia inhibitory factor is necessary for maintenance of haematopoietic stem cells and thymocyte stimulation. Nature. 1993;363:361–364. doi: 10.1038/363361a0. [DOI] [PubMed] [Google Scholar]
- 35.Charnock-Jones DS, Sharkey AM, Fenwick P, Smith SK. Leukaemia inhibitory factor mRNA concentration peaks in human endometrium at the time of implantation and the blastocyst contains mRNA for the receptor at this time. J Reprod Fertil. 1994;101:421–426. doi: 10.1530/jrf.0.1010421. [DOI] [PubMed] [Google Scholar]
- 36.Chen JR, Cheng JG, Shatzer T, Sewell L, Hernandez L, Stewart CL. Leukemia inhibitory factor can substitute for nidatory estrogen and is essential to inducing a receptive uterus for implantation but is not essential for subsequent embryogenesis. Endocrinology. 2000;141:4365–4372. doi: 10.1210/endo.141.12.7855. [DOI] [PubMed] [Google Scholar]
- 37.Kimber SJ. Leukaemia inhibitory factor in implantation and uterine biology. Reproduction. 2005;130:131–145. doi: 10.1530/rep.1.00304. [DOI] [PubMed] [Google Scholar]
- 38.Stewart CL. Leukaemia inhibitory factor and the regulation of pre-implantation development of the mammalian embryo. Mol Reprod Dev. 1994;39:233–238. doi: 10.1002/mrd.1080390217. [DOI] [PubMed] [Google Scholar]