Abstract
Background:
Cell-based therapies have been studied for articular cartilage regeneration. Articular cartilage defects have little treatments because articular cartilage was limited regenerative capacity. Damaged articular cartilage is difficult to obtain a successful therapeutic effect. In additionally these articular cartilage defects often cause osteoarthritis. Chondrocyte implantation is a widely available therapy used for regeneration of articular cartilage because this tissue has poor repair capacity after injury. Human nasal septum-drived chondrocytes (hNCs) from the septum show greater proliferation ability and chondrogenic capacity than human articular chondrocytes (hACs), even across different donors with different ages. Moreover, the chondrogenic properties of hNCs can be maintained after extensive culture expansion.
Methods:
In this study, 2 dimensional (2D) monolayer cultured hNCs (hNCs-2D) and 3 dimensional (3D) spheroids cultured hNCs (hNCs-3D) were examined for chondrogenic capacity in vitro by PCR and immunofluorescence staining for chondrogenic marker, cell survival during cultured and for cartilage regeneration ability in vivo in a rat osteochondral defect model.
Results:
hNCs-3D showed higher viability and more uniform morphology than 3D spheroids cultured hACs (hACs-3D) in culture. hNCs-3D also showed greater expression levels of the chondrocyte-specific marker Type II collagen (COL2A1) and sex-determining region Y (SRY)-box 9 (SOX9) than hNCs-2D. hNCs-3D also expressed chondrogenic markers in collagen. Specially, in the osteochondral defect model, implantation of hNCs-3D led to greater chondrogenic repair of focal cartilage defects in rats than implantation of hNCs-2D.
Conclusion:
These data suggest that hNCs-3D are valuable therapeutic agents for repair and regeneration of cartilage defects.
Keywords: Spheroid culture, Cartilage, Chondrocyte, Osteochondral defect model
Introduction
Autologous chondrocyte implantation (ACI) has been widely used for regeneration of articular cartilage defects [1]. Various drugs and surgical treatments have been developed to prevent cartilage defects, and hACs are used for treatment of ACI. In this cell-based technique, hACs are isolated from biopsies of healthy articular cartilage from patients undergoing arthroscopic surgery. However, hACs from isolated cartilage have disadvantageous characteristics that may influence the reproducibility of clinical results [2]. First, hACs exhibit limited redifferentiation ability after expansion in culture [3, 4]; in addition, hACs of aged donors exhibit decreased chondrogenic potential [5–8]. Finally, obtaining a biopsy causes injury to a healthy area of articular cartilage [9].
Some researchers have supported the use of hNCs from the septum as an alternative to overcome the disadvantages of hACs for ACI treatment and to potentially improve treatment outcomes. The use of hNCs instead of hACs could be advantageous [10]. When hNCs are exposed to different environments, they can react physically in a manner similar to that of hACs in vitro. In addition, for this treatment strategy, cartilage biopsies can be obtained with less invasive procedures than removal of tissue from a specific articular area, decreasing donor site morbidity due to biopsy [11]. Moreover, studies have shown that compared to hACs, hNCs has several other advantages; for example, hNCs are known to exhibit better cell proliferation and differentiation capacity than hACs, and the quality of the effects on isolated tissues have been shown to be less donor age-dependent for hNCs than for hACs [12, 13].
Long expansion cultures time and serial passaging increase dedifferentiation of chondrocytes [14]. Chondrocyte dedifferentiation has been shown to cause progressive loss of specific markers such as extracellular matrix (ECM) proteins, COL2A1, and aggrecan (ACAN). At the same time, chondrocyte dedifferentiation increases the levels of markers associated with the fibroblastic phenotype, such as type I collagen (COL1A1) [15]. Generally, chondrocyte dedifferentiation occurs during expansion culture [16]. Dedifferentiated chondrocytes cannot produce articular cartilage ECM; instead, they form fibro-like cartilage ECM. The fibro-like cartilage ECM of dedifferentiated chondrocytes cannot withstand the chemical and mechanical stresses of articular cartilage [17].
Some studies have presented methods to decelerate chondrocyte dedifferentiation, such as changing of culture medium supplements or cell densities or mimicking of the cartilage microenvironment with culture conditions. Several studies have reported successful redifferentiation of dedifferentiated chondrocytes through regulation of culture conditions. The results of these studies have shown that chondrocytes can recover the differentiated chondrocyte phenotype and the expression of chondrocyte marker molecules [18]. In addition, cell behavior in spheroid culture can differ from that in expansion culture [19]. Similarly, spheroid culture can be used to maintain the chondrocyte phenotype and support redifferentiation of dedifferentiated chondrocytes [19–21].
Several different types of natural scaffolds have been tested in experimental animals for articular cartilage repair [22, 23]. Spheroid culture is performed by embedding cells in scaffolds or collecting them into spheroids by centrifugation [24]. The major reason for embedding the cells in scaffolds during spheroid culture is to increase stability at implantation. Collagen scaffolds are the most popular because collagen is a constituent of articular cartilage. Collagen is used to build spheroid scaffolds and has been used in biocompatible scaffolds [25, 26]. Recently, the sizes of spheroid cultures have decreased because of advances in cartilage production processes [27–29]. The aim of this study was to develop an injectable therapeutic agent for articular cartilage defects. For this purpose, hACs and hNCs were isolated from individual tissues and expanded, and spheroid cultures of the cells were characterized and compared after 7 days. The characteristics of the two cell types were also compared at the RNA level. Specifically, we evaluated cell viability and the expression of chondrocyte-specific markers of expansion for spheroids cultured in a collagen mixture or expansion medium. The effects of the cells were confirmed after transplantation of the cells into osteochondral defect models.
Materials and methods
Cell isolation and culture
hACs were obtained from the catholic university of Korea, Uijeongbu St. Mary’s Hospital. The experiments utilizing hACs were conducted in compliance with the Institutional Review Board of the Catholic Medical Center Clinical Research Coordinating Center (UC14CNSI0150) and after obtaining written informed consent from the donors themselves. hNCs were obtained from Seoul St. Mary’s Hospital. The experiments utilizing hNCs were also conducted in compliance with the Institutional Review Board of the Catholic Medical Center Clinical Research Coordinating Center (KC08TISS0341) and after obtaining written informed consent from the donors themselves. Harvested human nasal septum cartilage samples were sliced into 2-3 mm pieces in DPBS (Thermo Fisher Scientific, Waltham, MA, USA). The cartilage pieces were washed twice with medium and treated using Collagenase type 2 (Gibco-BRL, Grand Island, NY, USA) at 0.02 g/ml in medium overnight at 37 °C in incubator. The culture medium consisted of low-glucose DMEM (Gibco-BRL), supplemented with 1% antibiotic/antimycotic solution (Gibco-BRL) and 10% fetal bovine serum (Gibco-BRL). Each cell suspension was filtered through a 40 mm cell strainer (BD Falcon, San Jose, CA, USA), and the suspension was centrifuged to recover the cell spheroid. The supernatant was discarded, the spheroid was washed and resuspended in medium, and the cells were seeded in 100 mm culture dishes (Thermo Fisher Scientific).
Spheroid culture
Spheroid cultures were initiated by adding 1.2 × 106 hNCs or hACs in 1 ml of low-glucose DMEM (Gibco-BRL) supplemented with 10% fetal bovine serum (Gibco-BRL) with 1% antibiotic/antimycotic solution (Gibco-BRL) to each well of the micromolds (Prodizen, Gyeonggi-do, Korea). Cells were seeded onto each substrate and were subsequently cultured for 3 days to investigate cellular behavior on each substrate. Cell aggregation and spheroid formation with each method were observed daily under a microscope.
Cell viability and proliferation
The viability of the cells cultured in the concave molds was assessed using a live/dead assay kit (Invitrogen, Carlsbad, CA, USA). Briefly, 1.2 µl of calcein AM solution (Invitrogen) and 4 µl of ethidium homodimer-1 solution (Invitrogen) were dissolved in 1 ml of DPBS (Gibco-BRL), which was then added to the cells. The cells were then incubated at 37 °C for 20 min. The proliferation of the cells was analyzed after culture in 4-well slide dishes (Thermo Fisher Scientific) for 3 days. The cultured cells were incubated with BrdU antibodies (1:250, Abcam, Cambridge, UK) overnight. The cells were counterstained with DAPI (1:1000, Sigma-Aldrich Co., St. Louis, MO, USA) for 30 min. The stained cells were observed using a confocal laser scanning microscope (LSM 5 Exciter, Carl Zeiss, Oberkochen, Germany), and the resulting acquired images were analyzed using ZEN software (Carl Zeiss). To formulate a cell-laden collagen complex, we purchased collagen (Ubiosis, Seongnam, Korea) at a concentration of 30 mg/ml. hNCs-2D were detached from the tissue culture plate with 0.25% trypsin solution, and a cell suspension was prepared. The cell suspension was mixed with the collagen solution. The total cell concentration was 106 cells/ml. hNCs-3D were harvested from micromolds and mixed with the collagen solution.
Chondrocyte gene expression
We used PCR to analyze the expression of several chondrocyte-related genes (Table 1). The two types of human chondrocytes were cultured in 2D and 3D for harvesting of RNA. The samples were incubated with TRIzol™ (Thermo Fisher Scientific), and RNA was extracted. A RevertAid™ First Strand cDNA Synthesis Kit (Thermo Fisher Scientific) was used to synthesize cDNA. The normalized relative levels of the PCR products were determined for each target gene. The results were analyzed using a Gel Doc XR (Bio-Rad, Hercules, CA, USA).
Table 1.
Primer sequences used in PCR assays
| SOX9 | Forward | gcgacgtcatctccaacatc |
| Reverse | tggtcggtgtagtcgtactg | |
| ACAN | Forward | tccccaacagatgcttccat |
| Reverse | gaacatcattccactcgccc | |
| COL1A1 | Forward | caggctggtgtgatgggatt |
| Reverse | aaacctctctcgcctcttgc | |
| COL2A1 | Forward | cttaggcccgagagaagg |
| Reverse | gcgtaggaggtcatctgga |
Osteochondral defect model and treatment
Ten-week-old (~ 250 g) male Sprague–Dawley rats were anesthetized using Zoletil/Rompun (50 mg/kg and 5–10 mg/kg, respectively) administered by intraperitoneal (IP) injection in an IACUC-approved animal laboratory facility. After shaving the right knee of each rat and sterilizing it using povidone-iodine swabs, a medial parapatellar incision was made on the knee, and the patella was deflected laterally to expose the trochlear surface. A hole 1.5 mm in diameter and 1 mm deep was drilled into the center of the trochlea using a High-Speed Rotary Micromotor Kit (Seashin, Daegu, Korea) with a 1.5 mm trephine (Fine Science Tools, Foster City, CA, USA) to induce an osteochondral defect. The experimental animals were divided randomly into four groups: the normal group (n = 5), the collagen treatment group (n = 5), the collagen with hNCs treatment group (n = 5) and the collagen with hNCs-3D treatment group (n = 5). The animals were housed in pairs for the duration of the experiment. After defect creation, hNCs-2D or hNCs-3D encapsulated in collagen was implanted by injection using a 1 ml syringe (BD Biosciences, Franklin Lakes, NJ, USA) to achieve full defect filling. The gel usually hardens within 10 min, and hNCs-2D or hNCs-3D encapsulated in collagen was fitted in the defect site. At 4 and 8 weeks, the animals were double euthanized using CO2 gas and cervical dislocation, and the knees were harvested. The samples were fixed in 10% formalin for 24 h for analysis. The left knees (uninjured) were also harvested as animal-specific internal controls for observation.
Histological and immunohistochemical analyses
The fixed knees were decalcified in EDTA solution (DyneBIO, Seongnam, Korea) for 21 days (with replacement of the EDTA solution every 2 days), washed for 1 h in running deionized water and then washed in 10% formalin (Sigma-Aldrich Co.) for 30 min. The samples were serially dehydrated through ethanol/xylene and embedded in paraffin with the medial side of the knee facing down. The embedded samples were sectioned at 7 mm thickness. The sections on cover slides were rehydrated through xylene/ethanol/water and stained with Harris modified hematoxylin and eosin Y (H&E, Sigma Aldrich, St. Louis, MO, USA), Masson’s trichrome (Sigma Aldrich). Standard protocols were followed for all stains. Immunofluorescence (IF) staining was performed for COL1A1 (Abcam), COL2A1 (Abcam) and SOX9 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). The sections on cover slides were rehydrated through xylene, ethanol and water. The sections were then permeabilized with 0.1% Triton X-100 for 5 min, blocked with 10% normal goat serum (Invitrogen) for 30 min at room temperature, and stained with primary antibodies raised against COL1A1 (1:500, Abcam), COL2A1 (1:500, Abcam) and SOX9 (1:500, Santa Cruz Biotechnology, Inc.) in 1% BSA/PBS at 4 °C overnight. Secondary antibody detection was performed using Alexa Fluor 546-conjugated goat anti-rabbit IgG (Thermo Fisher) for 60 min at room temperature in the dark. After each staining step, the slides were washed 3 times with PBS for 5 min each. Nuclei were counterstained with DAPI (Thermo Fisher) for 5 min at room temperature in the dark.
Statistical analysis
All experiments contained 4 biological replicates (n = 4). The studies were repeated using hNCs derived from four donors. The data are presented as the mean ± SD or as the mean ± SEM from at least three independent experiments. All statistical analyses were performed in SPSS 13.0 (SPSS Inc., Chicago, IL) using one-way ANOVA with Fisher’s Least Significant Difference (LSD) post hoc test. Probability values less than 0.05 were considered to indicate statistical significance.
Results
ECM expression characterization of hNCs and hACs
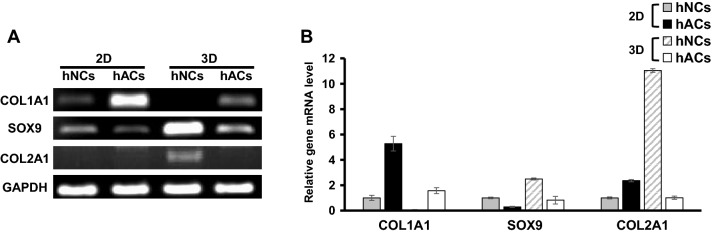
We first evaluated the ECM RNA expression of chondrocytes in 2D monolayer and 3D spheroid in vitro cultures after three passages. hNCs and hACs were isolated form individual tissues, and in vitro-cultured hNCs and hACs were analyzed for expression of ECM genes (Fig. 1). The hNCs-3D expressed higher levels of the COL2A1 and SOX9 genes than the other groups (Fig. 1A). COL1A1 levels were higher in the 2D monolayer cultured hACs (hACs-2D) than in the other groups. These differences were statistically significant (Fig. 1B).
Fig. 1.
Characterization of hNCs and hACs. A Quantitative PCR analysis of type II collagen (COL2A1), sex-determining region Y (SRY)-box 9 (SOX9), Aggrecan (ACAN), and type I collagen (COL1A1). B The intensities of the PCR bands were quantified by densitometry. *Denotes significance versus individual glyceraldehyde-3-phosphate dehydrogenase (GAPDH) intensity
Proliferation and viability of hNCs and hACs
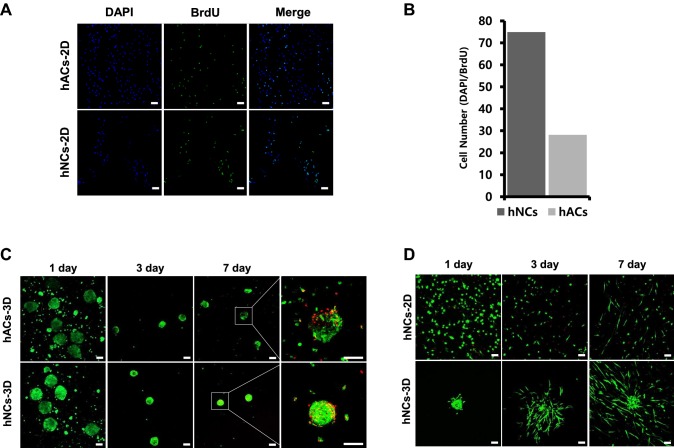
After isolation from cartilage, chondrocytes were expanded through monolayer culture. After three passages of in vitro culture, cell proliferation capacity was evaluated using BrdU staining. hNCs-2D showed higher proliferation rates than hACs-2D according to BrdU staining, and these results were confirmed by DAPI and BrdU quantification. Immunostaining of cells after BrdU administration revealed that hNCs-2D had approximately 1.5-fold greater proliferation rates than hACs-2D (Fig. 2A, B). The viability of the cells was confirmed by live/dead staining. After three passages, cells were cultured to form 3D spheroids. The 3D spheroids survived to the 7th day. Overall, hNCs formed tighter 3D spheroids than hACs (Fig. 2C). When we assessed the viability of hNCs in collagen, we were able to confirm that hNCs-2D and hNCs-3D survived for up to 7 days (Fig. 2D). Moreover, hNCs-2D showed growth, and hNCs-3D also showed internal growth. In the case of the hNCs-3D, it was confirmed that the cells spread and moved over time.
Fig. 2.
BrdU cell proliferation assay. A Confocal microscopic images of hNCs-2D and hACs-2D stained with BrdU dye in a cell proliferation assay. B DAPI and BrdU staining quantification. C Staining of live and dead hNCs-3D and hAC-3D in culture. D Staining of live/dead hNCs-2D, hNCs-3D in collagen. Scale bars: 100 μm
ECM expression characterization of hNCs in collagen
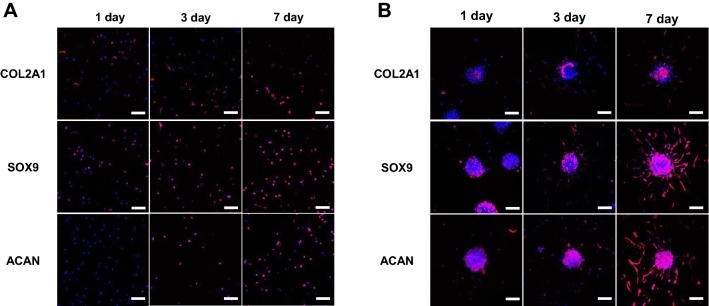
After confirming that the hNCs were viable in collagen, we confirmed that the expression patterns of ECM proteins were consistent with chondrocytes by immunostaining the cells with chondrocyte-specific antibodies. Immunostaining for COL2A1, ACAN and SOX9 showed that hNCs-2D in collagen continued to express the ECM proteins for 7 days (Fig. 3A). Encapsulation of the hNCs-2D in collagen as single cells increased the expression of the chondrocyte-specific proteins during the 7 days period. Similarly, compared to hNCs-2D, hNCs-3D in collagen exhibited increased ECM expression over the 7 days period (Fig. 3B).
Fig. 3.
Characterization of hNCs-2D and hNCs-3D in collagen. Images of hNCs stained with type II collagen (COL2A1), sex-determining region Y (SRY)-box 9 (SOX9) and Aggrecan (ACAN) antibodies are shown. A hNCs-2D in collagen. B hNCs-3D in collagen. Scale bars: 100 μm
Injection of the treatments into osteochondral defect models
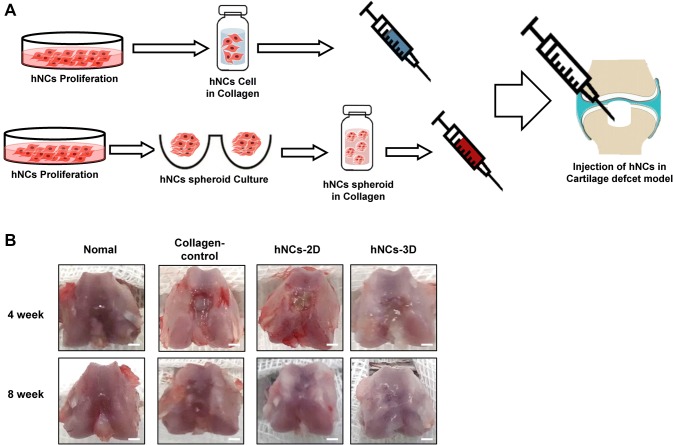
We investigated the therapeutic potential of hNCs for cartilage regeneration. Animal models were developed to evaluate the effects of encapsulated hNCs in collagen in vivo. The schema shows the design of the hNCs-2D and hNCs-3D in collagen (Fig. 4A). Articular cartilage defects were created by drilling holes 1.5 mm deep and 1 mm in diameter. hNCs-2D or hNCs-3D were implanted into the defects with collagen.
Fig. 4.
Schematic of the osteochondral defect model. A Overview of the preparation of cells for injectable treatments. B Macroscopic findings of the femur at 4 and 8 weeks after operation. Scale bars: 1 mm
Implantation of hNCs and hNC-3D spheroids into cartilage defect models
To examine the therapeutic effects of these strategies for cartilage regeneration in an osteochondral defect model, hNCs-2D, hNCs-3D and collagen were implanted into osteochondral defect model rats (Fig. 5A). After 8 weeks, H&E staining showed that the hNCs-2D and hNCs-3D had infiltrated into the defect area (Fig. 5A). hNCs-3D infiltrated tissues exhibited articular-like surfaces. The hNCs-3D surfaces were smooth compared to the hNCs-2D and collagen group surfaces (Fig. 5B).
Fig. 5.
In vivo transplantation of hNCs-2D, hNCs-3D into osteochondral defect models. A H&E staining of cartilage repair tissue. B Masson’s trichrome staining for proteoglycan visualization in cartilage repair tissue. Scale bars: 250 μm
Discussion
The pathogenesis of OA is complex. Various types of stress are believed to stimulate articular chondrocyte metabolism, providing a mechanism for cartilage to adapt to physiological needs [30]. There are various contributing factors for OA, including genetic predisposition, cumulative cartilage load, and genetic changes in chondrocytes [31–33]. Changes in chondrocyte metabolism caused by the disease weaken the structure of cartilage. Additionally, imbalance between cartilage synthesis and degradation due to various stimuli, such as injury and stress, can cause OA by inducing tissue collapse [34]. Normal articular cartilage is composed of ECM proteins such as COL2A1 and ACAN. This composition contributes to the stiffness of cartilage tissue [35]. Chondrocytes are the only differentiated cells of articular cartilage and are responsible for the maintenance and production of the ECM [36]. However, articular chondrocytes are not suitable for treatment because they do not normally express their phenotype in culture. Most donors have experienced arthritis. Pathological changes in cartilage tissues can negatively affect chondrocytes [37]. Additionally, chondrocytes lose their phenotype during the culture process; dedifferentiation happens, and the cells change shape [38]. These changes make chondrocytes difficult to use for treatment purposes. The stability of the chondrocyte phenotype depends on the shape and the density of the cells [39]. Chondrocytes in high densities maintain their phenotype [40]. The differentiation ability of chondrocytes is important for cartilage tissue reconstruction. Dedifferentiated chondrocytes do not produce hyaline cartilage and thus cannot heal articular cartilage damage [41]. The aim of this study was to develop an injectable treatment for OA. For this purpose, hNCs and hACs were isolated from individual tissues. The characteristics and RNA expression of the cell types were compared, and the findings were confirmed by transplantation of the cells into osteochondral defect models.
Spheroid culture was first described in 1998 [42]. Traditionally, spheroid culture methods involve the aggregation of 1–5 × 105 mesenchymal stem cells (MSCs) in chondrogenic differentiation medium. However, one issue associated with spheroid culture is the steep diffusion gradient caused by the large diameter of each spheroid (2–3 mm). As a result, necrotic spheroid cores are sometimes observed [43]. This issue can be addressed by reducing the sizes of the spheroids. Reductions in spheroid diameter enhance metabolite and molecule diffusion, enable greater homogeneity and reduce the size to an injectable scale [44]. Generally, spheroid culture methods allow MSCs to differentiate for 14–28 days [45]. Our primary focus was to identify the early chondrogenic potential of cells grown with spheroid culture methods without differentiation of hNCs. Chondrocyte differentiation is more likely to occur at later time points than at earlier time points during culture. We decided to culture the spheroids for 7 days. Experiments were performed using hACs as controls. It was expected that the spheroids cultured by both 3D spheroid culture methods would express chondrogenic markers such as COL2A1, ACAN and SOX9 but would express relatively low levels of dedifferentiation genes. The 2D culture method was compared with the spheroid culture methods. We indeed found expression of the abovementioned chondrogenic markers. In RNA analysis, hNCs-2D showed higher levels of SOX9 and lower levels of COL1A1 than hACs-2D (Fig. 1A). COL2A1 levels were similarly expressed between the cell types in 2D culture. As expected, the 3D spheroids showed higher RNA levels than the 2D cultured cells. The 3D spheroids exhibited lower COL1A1 RNA levels but higher SOX9 and COL2A1 RNA levels than the 2D cultured cells. This finding suggests that the 3D spheroid culture method supports a more chondrocyte phenotype at the RNA level than 2D culture and that hNCs show therapeutic potential for articular cartilage.
Cell proliferative capacity was evaluated through BrdU staining (Fig. 2A). Cell proliferation is an important characteristic with regards to procurement of cells for treatment. Cells with sufficiently high proliferation rates can be provided to a patient stably as a remedy, enabling the patient to receive adequate treatment. Additionally, a suitable cell supply is economically beneficial for a patient. The cell proliferative capacity was confirmed by assessing the number of proliferating cells compared to the total number of cells through BrdU and DAPI staining. hNCs-2D had higher proliferation rates than hACs-2D (Fig. 2B). Constant size of spheroids during 3D culture is an important feature for injectable drugs. Traditionally, cartilage spheroid culture methods have attempted to aggregate large numbers of cells into a single spheroid and differentiate them into cartilage. These spheroids form sharp radial diffusion gradients and form necrotic cores. To alleviate this issue, we reduced the diameters of the spheroids. This adjustment eliminated the necrotic cores and facilitated metabolite and signaling molecule diffusion. In general, smaller spheroids can more easily pass through needles and can be developed as injectable treatments. Cell viability was confirmed in the 3D cultures through live and dead cell staining. In 3D culture, it was confirmed that hNCs-3D formed tighter spheroids than hACs-3D (Fig. 2C). hNCs are more advantageous than hACs in terms of supply and stability in 3D culture. We assessed the ability of the cells to grow and survive in collagen (Fig. 2D), and hNCs were able to grow, migrate and survive in collagen.
The hNCs expressed characteristic chondrocyte markers when cultured in collagen (Fig. 3). In addition, it was confirmed that the hNCs expressed chondrocyte markers even when they were mixed with collagen after 2D culture and were subsequently grown in 3D culture (Fig. 3A). In particular, chondrocyte marker expression was higher in 3D spheroids cultured in collagen than in cells subjected to 2D culture in collagen. These results were similar to those of the RNA analysis. The ability of the cells to be used as therapeutic agents for cartilage regeneration was evaluated by filling cartilage defects with a mixture of hNCs and collagen.
We studied the therapeutic potential of hNCs implantation for the treatment of articular cartilage defects. To confirm the efficient cartilage regeneration potential of hNCs, we established a osteochondral defect model in rats by drilling holes to a depth of 2 mm and with a diameter of 2 mm (Fig. 4A). At 4 and 8 weeks after transplantation, some cartilage tissues still exhibited defects in each group (Fig. 4B).
Through various types of staining, we confirmed the ability of the hNCs-3D to regenerate cartilage (Fig. 5). We identified hyaline cartilage-specific metachromasia and ECM accumulation through these staining methods.
Our data demonstrate that hNC transplantation promotes cartilage regeneration in rats with osteochondral defects. In particular, cartilage regeneration occurred more clearly in models transplantation with hNCs-3D than the hNCs-2D. These results were also confirmed 8 weeks after transplantation in the hNCs-3D group. Overall, our findings suggest that hNCs-3D have several properties that make them well suited for regenerative medicine targeting osteochondral diseases.
Acknowledgements
This work was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Ministry of Science & ICT (NRF-2019M3E5D5064110, NRF-2019M3A9H2032424), Institute for Information & communications Technology Promotion (IITP) grant funded by the Korea government (MSIP) (No.2017-0-00953), and the Korea Health Industry Development Institute funded by the Ministry of Health and Welfare (HI14C3228). This work was also supported by the Institute of Clinical Medicine Research of Bucheon St. Mary’s Hospital, Research Fund, 2018. The sponsors had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Compliance with ethical standards
Conflict of interest
The authors have no financial conflicts of interest.
Ethical statement
The study protocol was approved by the institutional review board of the Catholic Medical Center Clinical Research Coordinating Center (IRB No. KC08TISS0341, UC14CNSI0150). Informed consent was confirmed (or waived) by the IRB. IACUC and Department of Laboratory Animal (DOLA) in Catholic University of Korea, Songeui Campus accredited the Korea Excellence Animal laboratory Facility from Korea Food and Drug Administration in 2017 and acquired AAALAC International full accreditation in 2018. All of surgical interventions and presurgical and postsurgical animal care were provided in accordance with the Laboratory Animals Welfare Act, the Guide for the Care and Use of Laboratory Animals and the Guidelines and Policies for Rodent Survival Surgery provided by the IACUC (Institutional Animal Care and Use Committee) in school of medicine, The Catholic University of Korea. (Approval Number: CUMS-2017-0040-04).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 2.Demoor M, Ollitrault D, Gomez-Leduc T, Bouyoucef M, Hervieu M, Fabre H, et al. Cartilage tissue engineering: molecular control of chondrocyte differentiation for proper cartilage matrix reconstruction. Biochim Biophys Acta. 2014;1840:2414–2440. doi: 10.1016/j.bbagen.2014.02.030. [DOI] [PubMed] [Google Scholar]
- 3.Darling EM, Athanasiou KA. Rapid phenotypic changes in passaged articular chondrocyte subpopulations. J Orthop Res. 2005;23:425–432. doi: 10.1016/j.orthres.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 4.Holtzer H, Abbott J, Lash J, Holtzer S. The loss of phenotypic traits by differentiated cells in vitro, I. dedifferentiation of cartilage cells. Proc Natl Acad Sci U S A. 1960;46:1533–1542. doi: 10.1073/pnas.46.12.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kempson GE. Relationship between the tensile properties of articular cartilage from the human knee and age. Ann Rheum Dis. 1982;41:508–511. doi: 10.1136/ard.41.5.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kempson GE. Age-related changes in the tensile properties of human articular cartilage: a comparative study between the femoral head of the hip joint and the talus of the ankle joint. Biochim Biophys Acta. 1991;1075:223–230. doi: 10.1016/0304-4165(91)90270-q. [DOI] [PubMed] [Google Scholar]
- 7.Koepp H, Eger W, Muehleman C, Valdellon A, Buckwalter JA, Kuettner KE, et al. Prevalence of articular cartilage degeneration in the ankle and knee joints of human organ donors. J Orthop Sci. 1999;4:407–412. doi: 10.1007/s007760050123. [DOI] [PubMed] [Google Scholar]
- 8.Verzijl N, DeGroot J, Bank RA, Bayliss MT, Bijlsma JW, Lafeber FP, et al. Age-related accumulation of the advanced glycation endproduct pentosidine in human articular cartilage aggrecan: the use of pentosidine levels as a quantitative measure of protein turnover. Matrix Biol. 2001;20:409–417. doi: 10.1016/s0945-053x(01)00158-5. [DOI] [PubMed] [Google Scholar]
- 9.Matricali GA, Dereymaeker GP, Luyten FP. Donor site morbidity after articular cartilage repair procedures: a review. Acta Orthop Belg. 2010;76:669–674. [PubMed] [Google Scholar]
- 10.van Osch GJ, Marijnissen WJ, van der Veen SW, Verwoerd-Verhoef HL. The potency of culture-expanded nasal septum chondrocytes for tissue engineering of cartilage. Am J Rhinol. 2001;15:187–192. doi: 10.2500/105065801779954166. [DOI] [PubMed] [Google Scholar]
- 11.Candrian C, Vonwil D, Barbero A, Bonacina E, Miot S, Farhadi J, et al. Engineered cartilage generated by nasal chondrocytes is responsive to physical forces resembling joint loading. Arthritis Rheum. 2008;58:197–208. doi: 10.1002/art.23155. [DOI] [PubMed] [Google Scholar]
- 12.Kafienah W, Jakob M, Démarteau O, Frazer A, Barker MD, Martin I, et al. Three-dimensional tissue engineering of hyaline cartilage: comparison of adult nasal and articular chondrocytes. Tissue Eng. 2002;8:817–826. doi: 10.1089/10763270260424178. [DOI] [PubMed] [Google Scholar]
- 13.Rotter N, Bonassar LJ, Tobias G, Lebl M, Roy AK, Vacanti CA. Age dependence of biochemical and biomechanical properties of tissue-engineered human septal cartilage. Biomaterials. 2002;23:3087–3094. doi: 10.1016/s0142-9612(02)00031-5. [DOI] [PubMed] [Google Scholar]
- 14.Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30:215–224. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- 15.Ma B, Leijten JC, Wu L, Kip M, van Blitterswijk CA, Post JN, et al. Gene expression profiling of dedifferentiated human articular chondrocytes in monolayer culture. Osteoarthritis Cartilage. 2013;21:599–603. doi: 10.1016/j.joca.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 16.Lin Z, Fitzgerald JB, Xu J, Willers C, Wood D, Grodzinsky AJ, et al. Gene expression profiles of human chondrocytes during passaged monolayer cultivation. J Orthop Res. 2008;26:1230–1237. doi: 10.1002/jor.20523. [DOI] [PubMed] [Google Scholar]
- 17.Schnabel M, Marlovits S, Eckhoff G, Fichtel I, Gotzen L, Vécsei V, et al. Dedifferentiation-associated changes in morphology and gene expression in primary human articular chondrocytes in cell culture. Osteoarthritis Cartilage. 2002;10:62–70. doi: 10.1053/joca.2001.0482. [DOI] [PubMed] [Google Scholar]
- 18.Haudenschild DR, McPherson JM, Tubo R, Binette F. Differential expression of multiple genes during articular chondrocyte redifferentiation. Anat Rec. 2001;263:91–98. doi: 10.1002/ar.1079. [DOI] [PubMed] [Google Scholar]
- 19.Baker BM, Chen CS. Deconstructing the third dimension – how 3D culture microenvironments alter cellular cues. J Cell Sci. 2012;125:3015–3024. doi: 10.1242/jcs.079509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caron MM, Emans PJ, Coolsen MM, Voss L, Surtel DA, Cremers A, et al. Redifferentiation of dedifferentiated human articular chondrocytes: comparison of 2D and 3D cultures. Osteoarthritis Cartilage. 2012;20:1170–1178. doi: 10.1016/j.joca.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 21.Choi BG, Park MH, Cho SH, Joo MK, Oh HJ, Kim EH, et al. In situ thermal gelling polypeptide for chondrocytes 3D culture. Biomaterials. 2010;31:9266–9272. doi: 10.1016/j.biomaterials.2010.08.067. [DOI] [PubMed] [Google Scholar]
- 22.Getgood A, Bhullar T, Rushton N. Current concepts in articular cartilage repair. Orthop Trauma. 2009;23:189–200. [Google Scholar]
- 23.Minas T. A primer in cartilage repair. J Bone Joint Surg Br. 2012;94:141–146. doi: 10.1302/0301-620X.94B11.30679. [DOI] [PubMed] [Google Scholar]
- 24.Anderer U, Libera J. In vitro engineering of human autogenous cartilage. J Bone Miner Res. 2002;17:1420–1429. doi: 10.1359/jbmr.2002.17.8.1420. [DOI] [PubMed] [Google Scholar]
- 25.Aigner T, Stöve J. Collagens–major component of the physiological cartilage matrix, major target of cartilage degeneration, major tool in cartilage repair. Adv Drug Deliv Rev. 2003;55:1569–1593. doi: 10.1016/j.addr.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 26.Li SW, Prockop DJ, Helminen H, Fässler R, Lapveteläinen T, Kiraly K, et al. Transgenic mice with targeted inactivation of the Col2 alpha 1 gene for collagen II develop a skeleton with membranous and periosteal bone but no endochondral bone. Genes Dev. 1995;9:2821–2830. doi: 10.1101/gad.9.22.2821. [DOI] [PubMed] [Google Scholar]
- 27.Langenbach F, Berr K, Naujoks C, Hassel A, Hentschel M, Depprich R, et al. Generation and differentiation of microtissues from multipotent precursor cells for use in tissue engineering. Nat Protoc. 2011;6:1726–1735. doi: 10.1038/nprot.2011.394. [DOI] [PubMed] [Google Scholar]
- 28.Markway BD, Tan GK, Brooke G, Hudson JE, Cooper-White JJ, Doran MR. Enhanced chondrogenic differentiation of human bone marrow-derived mesenchymal stem cells in low oxygen environment micropellet cultures. Cell Transplant. 2010;19:29–42. doi: 10.3727/096368909X478560. [DOI] [PubMed] [Google Scholar]
- 29.Babur BK, Ghanavi P, Levett P, Lott WB, Klein T, Cooper-White JJ, et al. The interplay between chondrocyte redifferentiation pellet size and oxygen concentration. PLoS ONE. 2013;8:e58865. doi: 10.1371/journal.pone.0058865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hering TM. Regulation of chondrocyte gene expression. Front Biosci. 1999;4:D743–D761. doi: 10.2741/hering. [DOI] [PubMed] [Google Scholar]
- 31.Lotz M, Blanco FJ, von Kempis J, Dudler J, Maier R, Villiger PM, et al. Cytokine regulation of chondrocyte functions. J Rheumatol Suppl. 1995;43:104–108. [PubMed] [Google Scholar]
- 32.Church V, Nohno T, Linker C, Marcelle C, Francis-West P. Wnt regulation of chondrocyte differentiation. J Cell Sci. 2002;115:4809–4818. doi: 10.1242/jcs.00152. [DOI] [PubMed] [Google Scholar]
- 33.Long F, Zhang XM, Karp S, Yang Y, McMahon AP. Genetic manipulation of hedgehog signaling in the endochondral skeleton reveals a direct role in the regulation of chondrocyte proliferation. Development. 2001;128:5099–5108. doi: 10.1242/dev.128.24.5099. [DOI] [PubMed] [Google Scholar]
- 34.Anderson DD, Chubinskaya S, Guilak F, Martin JA, Oegema TR, Olson SA, et al. Post-traumatic osteoarthritis: improved understanding and opportunities for early intervention. J Orthop Res. 2011;29:802–809. doi: 10.1002/jor.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buckwalter JA, Mankin HJ. Articular cartilage: tissue design and chondrocyte-matrix interactions. Instr Course Lect. 1998;47:477–486. [PubMed] [Google Scholar]
- 36.Loeser RF. Chondrocyte integrin expression and function. Biorheology. 2000;37:109–116. [PubMed] [Google Scholar]
- 37.Lane Smith R, Trindade MC, Ikenoue T, Mohtai M, Das P, Carter DR, et al. Effects of shear stress on articular chondrocyte metabolism. Biorheology. 2000;37:95–107. [PubMed] [Google Scholar]
- 38.Schulze-Tanzil G. Activation and dedifferentiation of chondrocytes: implications in cartilage injury and repair. Ann Anat. 2009;191:325–338. doi: 10.1016/j.aanat.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 39.von der Mark K, Gauss V, von der Mark H, Müller P. Relationship between cell shape and type of collagen synthesised as chondrocytes lose their cartilage phenotype in culture. Nature. 1977;267:531–532. doi: 10.1038/267531a0. [DOI] [PubMed] [Google Scholar]
- 40.van Kampen GP, Veldhuijzen JP, Kuijer R, van de Stadt RJ, Schipper CA. Cartilage response to mechanical force in high-density chondrocyte cultures. Arthritis Rheum. 1985;28:419–424. doi: 10.1002/art.1780280410. [DOI] [PubMed] [Google Scholar]
- 41.Vavken P, Samartzis D. Effectiveness of autologous chondrocyte implantation in cartilage repair of the knee: a systematic review of controlled trials. Osteoarthritis Cartilage. 2010;18:857–863. doi: 10.1016/j.joca.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 42.Kunz-Schughart LA, Kreutz M, Knuechel R. Multicellular spheroids: a three-dimensional in vitro culture system to study tumour biology. Int J Exp Pathol. 1998;79:1–23. doi: 10.1046/j.1365-2613.1998.00051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carlsson J, Stålnacke CG, Acker H, Haji-Karim M, Nilsson S, Larsson B. The influence of oxygen on viability and proliferation in cellular spheroids. Int J Radiat Oncol Biol Phys. 1979;5:2011–2020. doi: 10.1016/0360-3016(79)90953-2. [DOI] [PubMed] [Google Scholar]
- 44.Futrega K, Palmer JS, Kinney M, Lott WB, Ungrin MD, Zandstra PW, et al. The microwell-mesh: a novel device and protocol for the high throughput manufacturing of cartilage microtissues. Biomaterials. 2015;62:1–12. doi: 10.1016/j.biomaterials.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 45.Winter A, Breit S, Parsch D, Benz K, Steck E, Hauner H, et al. Cartilage-like gene expression in differentiated human stem cell spheroids: a comparison of bone marrow-derived and adipose tissue-derived stromal cells. Arthritis Rheum. 2003;48:418–429. doi: 10.1002/art.10767. [DOI] [PubMed] [Google Scholar]