Abstract
BACKGROUND:
Despite the many advantages of recombinant subunit vaccines, they have critical weaknesses that include a low efficacy for promoting cellular and humoral immune responses against antigens because of their poor immunogenicity, and a rapidly cleared properties as a result of proteolytic enzymes in the body. To circumvent these problems, we developed mannan-decorated inulin acetate microparticles (M-IA MPs) that functioned as carriers and adjuvants for immunization with the recombinant foot-and-mouth disease multi-epitope subunit vaccine (M5BT).
METHODS:
The M5BT-loaded M-IA MPs were obtained by a double-emulsion solvent-evaporation method. Their properties including morphology, size and release ability were determined by field emission scanning electron microscope, dynamic light-scattering spectrophotometer and spectrophotometer. To assess the immunization efficacy of the MPs, mice were immunized with MPs and their sera were analyzed by ELISA.
RESULTS:
The M-IA MPs obtained by a double-emulsion solvent-evaporation method were spherical and approximately 2–3 µm, and M5BT was encapsulated in the M-IA MPs. The M5BT-loaded M-IA MPs showed higher antigen-specific IgG, IgG1, IgG2a and anti-FMDV antibodies than the M5BT-loaded IA MPs and the Freund’s adjuvant as a control.
CONCLUSION:
The M-IA MPs showed a powerful and multifunctional polymeric system that combined two toll-like receptor agonists compared to the conventional adjuvant.
Keywords: Inulin acetate, Microparticle, Mannan, Food-and-mouth disease, Subunit vaccine
Introduction
Foot-and-mouth disease (FMD) is one of the major domestic animal disease agents; it belongs to the genus Aphthovirus of the family Picornaviridae and induces enormous economic shock to the livestock industries [1, 2]. The FMD virus (FMDV) has seven serotypes, namely, O, A, C, Asia-1, SAT-1, SAT-2, and SAT-3. In addition, many antigenic variants have been recognized within the same serotype, and some of these antigenic variants are an important influential factor for cross-protection [1].
Subunit vaccines, which consist of recombinant proteins produced in bacteria, have been suggested as an alternative that can solve problems experienced with inactivated vaccine, that is, the problem of distinguishing between infected and vaccinated animals (DIVA) and biosafety concerns during the cultivation of FMDV [3]. However, this strategy is vulnerable to mutations in the virus. In our previous study, to overcome antigenic variants of FMDV, a multi-epitope recombinant protein, M5BT, which consists of tandem repeats of five B cell epitopes derived from the GH loop of different FMDV variants and one T-cell epitope, as the novel FMDV subunit vaccine, was successfully designed, and anti-FMDV antibodies were also obtained in the serum from in vivo immunization experiments [4]. The advantages of the multi-epitope vaccine M5BT focused on the immune response for the B cell epitopes by eliminating unnecessary parts to overcome low immunogenicity of the recombinant protein vaccine and the expanded protective spectrum for the FMDV variants by introducing different B cell epitopes derived from different FMDV variants. Although the M5BT protein was designed to focus the immune responses for the B cell epitopes, the recombinant protein itself was not sufficient to induce the immune responses. Thus, the M5BT protein needs a vaccine carrier and adjuvant for an efficient induction of immune responses.
Inulin is one of the naturally occurring polysaccharides produced by many types of plants, including Jerusalem artichoke, chicory, dahlia, wheat, etc. [5]. Inulin consists of chain-terminating glucosyl moieties and a repetitive fructosyl moiety (GFn), which are linked by β (2–1) bonds. Because of the linkages, inulin is not digested by the digestive enzyme in animals [6]. Because some bacteria can use inulin as an energy source, researchers have studied inulin as a prebiotic [7]. Recently, inulin was reported as a novel TLR-4 agonist [8]. Thus, it has already been reported that the particulate form of inulin can act as an efficient adjuvant for vaccine [8].
Inulin acetate (IA) is an acetylated form of inulin that is produced by introducing an acetyl group to the inulin. The IA can be broken into particulates in water because the IA is self-assembled and is soluble in various organic solvents, including dichloromethane, which can be used to make the IA MPs in a double-emulsion method. Because the IA MPs functioned as a vaccine adjuvant when the antigen was encapsulated into the IA particles, the antigen-loaded IA MPs enhance the immune responses of the subunit vaccine by overcoming its low immunogenicity [8, 9]. The IA MPs function as an efficient adjuvant by enhancing the antigen uptake by immune cells such as macrophages and dendritic cells [10]. Additionally, the IA MPs can protect the protein vaccines loaded in the MPs from the proteolytic enzymes of the host and release the vaccines in a sustained manner known to induce long-term immune responses of the antigen [11, 12].
Mannan occurs in chains of up to several hundred mannoses that are added to fungal proteins via N- or O-linkages [13]. Mannan from cell wall of Saccharomyces cerevisiae is a polycarbohydrate that can be recognized for its pathogen-associated molecular patterns (PAMPs) by immune cells [14]. Therefore, IA MPs decorated with mannan as a TLR-4 have a synergistic effect, thereby recognizing immune cells and enhancing immune responses.
Here, we hypothesized that mannan-decorated IA MPs (M-IA MPs) loaded with subunit vaccine would enhance antigen-specific immune responses relative to a conventional adjuvant, Freund`s adjuvant, which was used as a control. To prove this hypothesis, a double-emulsion solvent-evaporation method was used to produce IA and M-IA MPs to encapsulate a recombinant vaccine M5BT. This approach showed that the M-IA MPs were formed in spheres of approximately 2–3 µm and exhibited sustained release. Furthermore, the in vivo immunization experiments showed that the M-IA MPs enhanced the immune responses of the host by eliciting the M5BT-specific antibodies and anti-FMDV antibodies. Our results will provide a new strategic insight for developing a dually functioned vaccine system as a safe and effective carrier and adjuvant for a recombinant subunit vaccine.
Materials and methods
Materials
Inulin from chicory (inulin), acetic anhydride, sodium acetate (NaOAc), dimethyl formamide (DMF), dimethyl sulfoxide-d6 (DMSO-d6), poly(vinyl alcohol) (PVA), mannan from Saccharomyces cerevisiae (mannan), Pluronic® F-127, dichloromethane (DCM), complete Freund’s adjuvant (CFA), incomplete Freund’s adjuvant (IFA), 3,3′,5,5′-tetramethylbenzidine (TMB), sulfuric acid, bovine serum albumin (BSA), fluorescein isothiocyanate isomer I (FITC) and Triton X-114 were purchased from Sigma Aldrich (St. Louis, MO, USA). Inulin, mannan and PVA dissolved in water were sterilized by filtering them using sterilized 0.45 µm filter syringe.
The mouse IFN-gamma ELISA kit and mouse IL-4 ELISA kit were purchased from KOMABIOTECH (Seoul, Korea). His bind resin was purchased from Novagen (Glendale, CA, USA). Tween 20 was purchased from Amresco Inc. (Solon, OH, USA). Phosphate buffered saline (PBS), fetal bovine serum (FBS) and Rosewell Park Memorial Institute (RPMI) 1640 medium were purchased from Thermo Scientific HyClone (Waltham, MA, USA). Ammonium-chloride-potassium (ACK) lysing buffer was purchased from Gibco-BRL (Burlington, Canada). Bicinchoninic acid (BCA) protein assay reagents (A and B) were purchased from Thermo Scientific Pierce (Rockford, IL, USA). HRP-labeled goat anti-mouse IgG, IgG1, and IgG2a were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Mice were purchased from Koatech, Co. Ltd. (Pyeongtaek, Korea). Inactivated FMD vaccine (ARRIAH-VAC) was kindly provided from the Jacob Pig Farm in Eumseong, Korea (Arriah, Vladimir, Russia).
Synthesis of IA
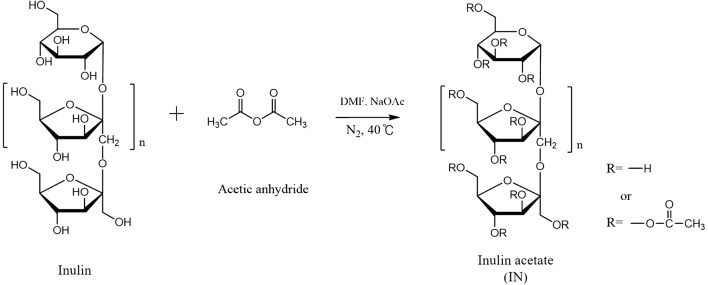
The synthesis of inulin acetate was carried out according to a previously reported method, with a little modification [12]. Briefly, 1 g of inulin was added to 5 ml of dimethyl formamide (DMF) with the addition of 0.2 ml of 5% acetic anhydride, and 5% (w/v) sodium acetate was used as a catalyst for the reaction. The acetylation reaction was carried out at 40 °C for 24 h under nitrogen. After 24 h, the IA was dialyzed against DMF for 24 h to remove free acetic acid and against distilled water to remove DMF and unreacted inulin. After dialysis, the IA was lyophilized and stored at− 20 °C until use. The conjugation of the acetyl group was confirmed by 600 MHz 1H NMR spectroscopy (AVANCE 600, Bruker, Germany), and the acetyl group content in the INAC was quantified.
Preparation of M5BT subunit vaccine
The preparation of subunit vaccine for the FMDV was performed by the previously reported method [4]. Briefly, E.coli BL21(DE3) with a gene encoding for M5BT protein was seeded and cultured overnight in LB medium supplemented with ampicillin. The seed culture was inoculated into LB medium with a 1:100 ratio and incubated at 37 °C with a constant shaking at 200 rpm until the optical density (O.D) 600 reached 0.5–0.6. After 4 h of 0.5 mM IPTG induction, the M5BT protein-producing cells were harvested by centrifugation at 6000 rpm for 10 min and washed with PBS two times. The cell pellets were resuspended in 20 ml of binding buffer (0.5 M NaCl, 5 mM imidazole, 20 mM Tris-HCl, pH 7.9) per 500 ml culture volume and were sonicated (10 s pulse on; 10 s pulse off) for 8 min with a blunt-end tip. The crude protein was harvested after centrifugation at 17,000 rpm for 15 min at 4 °C followed by filtration by 0.45 µm syringe filter. M5BT protein bearing six Histidine tags (his-tag) was purified using his-tag affinity chromatography. The crude protein solution was loaded onto His bind resin (3 ml of bed volume), equilibrated with 3(?) volume of binding buffer. The column was washed with washing buffer containing different concentrations of imidazole (5, 20, and 40 mM) to remove nonspecific protein. The M5BT was eluted with elution buffer (1 M imidazole, 20 mM Tris-HCl, pH 7.9). Each fraction was analyzed by SDS-PAGE to check the purification quality and purity of the protein. The purified protein was dialyzed against 5 L of distilled water at 4 °C for 24 h, with the water being changed three times to remove the salts in the elution buffer, which was followed by lyophilization. The resulting protein solution was treated with Triton X-114 to remove any remaining lipopolysaccharide (LPS) by a phase-separation technique.
Preparation and characterization of M5BT-loaded M-INAC MPs
Preparation of M5BT-loaded M-IA MPs
M5BT-loaded IA and M5BT-loaded M-IA MPs were prepared by a double-emulsion solvent-evaporation method [15, 16]. Briefly, 100 µl of M5BT solution (50 mg/ml) was mixed with 100 µl of 10% (w/v) Pluronic F-127 solution in an aqueous phase (W1). This aqueous-phase solution was emulsified with 5 ml of DCM as an oil phase (O) containing 100 mg of IA by using an ultrasonic processor (Sonics, Vibra cells™) (4 output watts) on ice for 1.5 min to form the 1st W1/O emulsion. This primary emulsion was then added dropwise into another aqueous (W2) phase (50 ml water) solution containing 0.75% (w/v) PVA solution with 0.25% mannan, with continuous homogenization at 13,000 rpm using Turrax, which resulted in the formation of the double emulsion (W1/O/W2). In the case of the M5BT-loaded IA MPs, a 1% PVA solution was used. The stirring was continued overnight for complete evaporation of the organic solvent. The resulting MPs were collected via centrifugation at 6000 rpm for 10 min at 4 °C. The pelleted M5BT-loaded IA or M5BT-loaded M-IA MPs were washed with distilled water and centrifuged. The final MPs were resuspended in 10 ml of distilled water and frozen by liquid nitrogen, followed by lyophilization under vacuum.
Determination of loading content and encapsulation efficiency
The loading content was determined as follows. The MPs (10 mg/ml) were dispersed in dimethyl sulfoxide (DMSO), and a clear solution was used for measurement with a spectrophotometer (NanoPhotomter™) of the protein concentration. The encapsulation efficiency of the M5BT into the MPs was determined by measuring the unloaded protein concentration in the supernatant during the double-emulsion method steps. The loading content and encapsulation efficiency were calculated using the following equations:
Observation of morphology and measurement of size of MPs
The surface topography was observed by field emission scanning electron microscope (FE-SEM) using a SUPRA 55VP-SEM (Carl Zeiss, Oberkochen, Germany). MPs were mounted on metal stubs with thin adhesive copper tape and coated with platinum under vacuum using coating chamber (CT 1500 HF, Oxford Instruments Oxfordshire, UK). Furthermore, the sizes of the MPs were measured with a dynamic light-scattering spectrophotometer (DLS-7000, Otsuka Electronics, Japan).
Confirmation of mannan decoration in the M-IA MPs
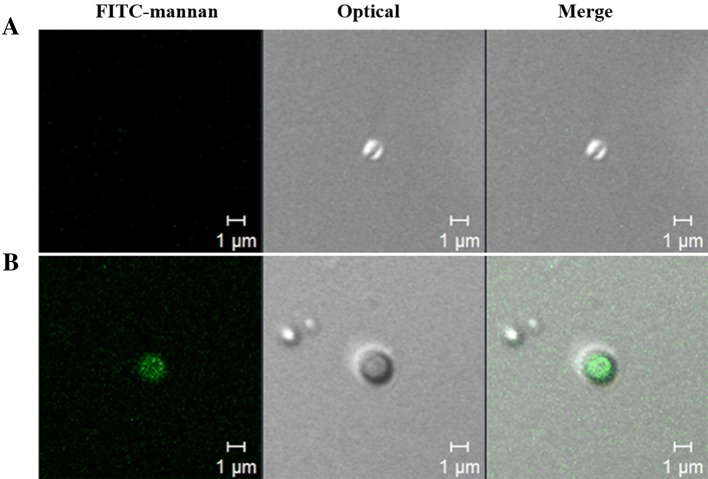
To confirm mannan decoration in the M-IA MPs, the FITC was conjugated with mannan. Briefly, mannan (0.1 g/ml) dissolved in distilled water was mixed with the FITC solution (5 mg/ml) dissolved in DMSO in a dark test tube, and the mixture was rotated for 4 h at room temperature. The final FITC-mannan conjugate was precipitated by dropping it into 8 volumes of ethanol, and the precipitate was washed 3 times with ethanol to remove free FITC after centrifugation at 16,000 rpm for 10 min. The FITC-mannan conjugate was used to decorate the IA MPs as mentioned above, and decoration of the FITC-mannan in IA MPs was confirmed by confocal laser scanning microscopy (Carl Zeiss LSM710, Oberkochen, Germany).
In vitro release test
The in vitro release of M5BT from the M5BT-loaded IA or M5BT-loaded M-IA MPs was determined as follows. The M5BT-loaded MPs (10 mg/ml) suspended in PBS (pH 7.4) were incubated at 37 °C with 100 rpm shaking. A 0.5 ml aliquot was withdrawn, and the same volume of PBS was supplemented at a predetermined time. The protein amount released from the MPs was measured using a spectrophotometer.
In vivo immunization in murine model
Schedule and groups of in vivo immunization
Five female BALB/c mice (6 weeks) were used for each group. Mice were raised in cages providing ad libitum access to feed and water in accordance with the guidelines for the care and use of laboratory animals (Seoul National University). All protocols were reviewed and approved by the Animal Care and Use Committee at Seoul National University (SNU-141201-1). The mice were immunized intramuscularly at days 0, 7 and 14 with 10 µg (0.125 µg/µl) of peptide emulsified in Complete Freund`s Adjuvant (CFA, priming) or Incomplete Freund`s Adjuvant (IFA, boosting), the MPs were resuspended in 80 µl of PBS, and inactivated FMD vaccine (iFMDV group) was used as a positive control. The mice were sacrificed on day 28. Blood samples were collected at 28 day from the intrapetrosal veins with a disposable syringe and delivered into a sterilized tube. Serum was separated by centrifugation at 7000 rpm for 3 min using serum separate tubes (BD microtainer, Franklin Lakes, NJ, USA).
FMDV serotype O specific antibody production
Antibodies against FMDV serotype O were detected in the serum samples by using a PrioCHECK® FMDV Type O ELISA Kit (Prionics, Schliere, Switzerland) according to the manufacturer’s instructions. Briefly, sera in 1:10 dilution were added to the wells, which were coated with inactivated FMDV serotype O antigen. After the incubation and washing steps, the mAb-HRPO conjugate was added to the plate and incubated at room temperature. Chromogen substrate (TMB) was added following the washing steps, and color development was stopped by adding the stop solution. The optical density (OD) was measured at the wavelength of 450 nm using a microplate reader (Infinite® 200 PRO, TECAN, Männedorf, Switzerland). The vaccination efficacy was presented as percentage inhibition (P.I) value, which was calculated by following equation:
Sera with P.I ≥ 50, which was interpreted as the presence of anti-FMDV serotype O Antibodies in test serum, were considered positive.
M5BT specific immunoglobulin detection by ELISA
The endpoint titer of anti-M5BT immunoglobulin was determined by ELISA, according to a previously reported method [4]. The M5BT antigen (0.1 µg in carbonate bicarbonate buffer, pH 9.6) was used to coat to the wells, and M5BT-coated plates were incubated at 37 °C for 2 h, followed by the blocking with PBS containing 1% BSA at room temperature (RT) for 1 h. Then, mouse sera were titrated in a 1:5 dilution starting from a 1:100 dilution in blocking buffer and added to M5BT-coated wells. For detection of M5BT-specific immunoglobulins, diluted goat anti-mouse immunoglobulin conjugated with HRP specific for IgG, IgG1 and IgG2a (1:2000 dilutions) was added to the wells, respectively, and incubated at RT for 1 h. Then, the plates were treated with TMB substrate solution followed by the addition of stop solution (0.16 M H2SO4) to stop the color development. The absorbance was measured at 450 nm using a microplate reader (Infinite® 200 PRO, TECAN, Switzerland). The endpoint titer of sera was calculated by Softmax® Pro v5.4.1 software (Molecular Devices, Inc., San Jose, CA, USA).
Secretory IL-4 and IFNγ in the supernatant of splenocyte culture
Spleens were extracted from immunized mice 28 day after euthanasia and meshed by physical homogenization. After treatment of ACK lysis buffer for 10 min on ice, cells were washed with RPMI 1640 by centrifugation at 1200 rpm for 5 min. Then, 5 × 106 cells were suspended in 1 ml of medium in the presence of M5BT antigen (20 µg) and cultured for 3 days at 37 °C in a CO2 incubator. The culture supernatants were stored at– 70 °C and used for measurement of cytokine levels by ELISA. Mouse IFNγ and IL-4 levels from splenocyte culture medium were measured using an ELISA kit according to the manufacturer’s instructions. The concentration of cytokines was determined by integrating the absorbance to the standard curve of each cytokine.
Statistical analysis
The statistical significance was analyzed by t-test or one-way analysis of variance (ANOVA) and post hoc Tukey multiple comparison test using GraphPad PRISM software (GraphPad Software, Inc.). The results are shown as the mean ± standard deviation (SD). All statistical significance is expressed by *p < 0.05, **p < 0.01, and ***p < 0.001.
Results
Preparation and characterization of IA
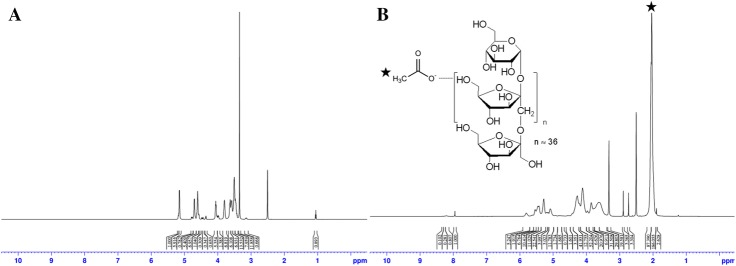
IA was synthesized by the conjugation of acetyl anhydride with inulin at 40 °C for 24 h using sodium acetate as a catalyst as shown in Fig. 1. The conjugation of the acetyl groups with inulin was confirmed and calculated by 600 MHz 1H-NMR spectroscopy as shown in Fig. 2. The peak of three hydrogens from acetyl groups (-COCH3) was identified at 2 ppm (integration value: 87.390) in the NMR spectra of IA, whereas no peak is present at 2 ppm in that of inulin. Furthermore, the peak of hydrogen of the hydroxyl group of the sixth carbon in the inulin was identified at 3.8 ppm (integration value: 6.911). Therefore, the degree of acetylation in the IA was 80.8 mol%.
Fig. 1.
Chemical reaction scheme for synthesis of inulin acetate
Fig. 2.
1H 600 MHz NMR spectra of A inulin and B inulin acetate. The asterisk indicates three hydrogens in an acetyl group with 2 ppm
Characteristics of M5BT-loaded and M5BT-loaded M-IA MPs
The morphology and size of MPs
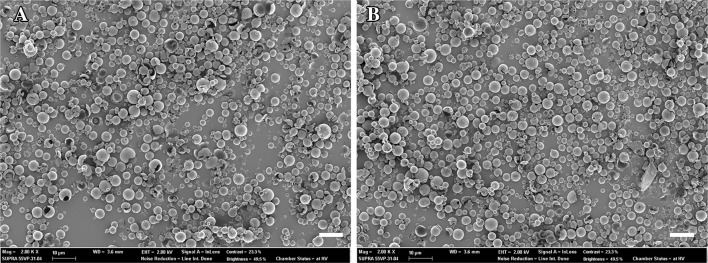
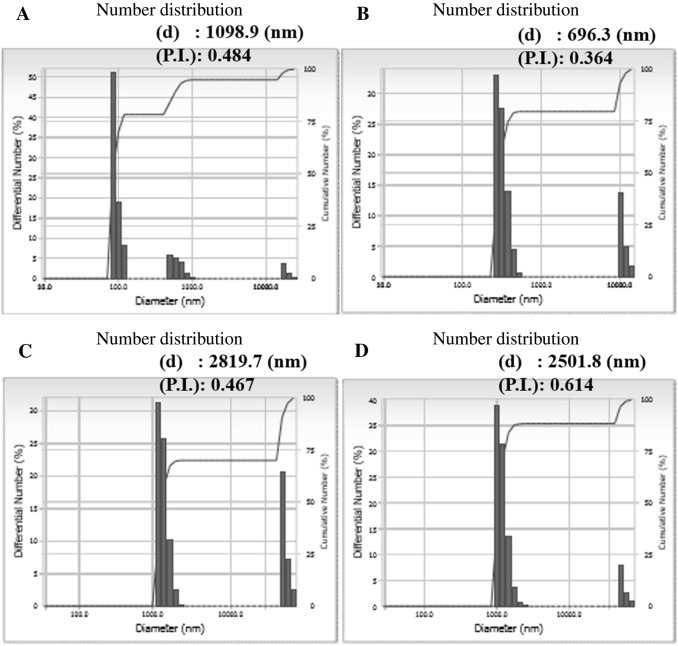
M5BT-loaded IA and M5BT-loaded M-IA MPs were prepared by the double-emulsion evaporation method. The characteristics of the M5BT-loaded IA and M-IA MPs are summarized in Table 1. M5BT-loaded IA and M-IA MPs showed loading contents of 2.47 ± 0.624 and 3.25 ± 0.25 wt%, respectively, with an encapsulation efficiency of 49.45 ± 4.73 and 64.97 ± 5.01 wt%, respectively. The microparticle morphologies of the MPs were observed by FE-SEM, which revealed the spherical microparticles shown in Fig. 3. The average particle sizes of the M5BT-loaded IA and M-IA MPs were 2.82 and 2.50 µm, respectively (Fig. 4C and D). The data showed that the sizes of the MPs increased by loading the M5BT because the sizes of the IA and M-IA MPs, except for those that were self-aggregated, were 1.37 ± 0.21 and 1.20 ± 0.21 µm, respectively, although small amounts of MPs larger than 10 µm in diameter were observed.
Table 1.
Loading characteristics of antigen-loaded MPs
| Microparticles | Loading content (wt%) | Encapsulation efficiency (wt%) | Size distribution (µm) |
|---|---|---|---|
| IA MPs | 2.47 ± 0.24 | 49.45 ± 4.73 | 2.82 |
| M-IA MPs | 3.25 ± 0.25 | 64.97 ± 5.01 | 2.50 |
IA MPs, inulin acetate microparticles; M-IA MPs, mannan-decorated inulin acetate microparticles
Fig. 3.
Analysis of the morphology of MPs by FE-SEM. A M5BT-loaded IA MPs and B M5BT-loaded M-IA MPs (Magnification 2000 X and scale bar (white bar): 10 µm)
Fig. 4.
Analysis of dynamic light scattering for determining the diameter of the MPs. A IA MPs, B M-IA MPs, C M5BT-loaded IA MPs and D M5BT-loaded M-IA MPs. (d) means average diameter of MPs and (P.I.) is polydispersity Index
In vitro release behavior of M5BT from M5BT-loaded IA and M5BT-loaded M-INAC MPs
To investigate the antigen release behavior in physiological conditions, the in vitro release study was conducted at PBS (pH 7.4). As results, the release of the M5BT from M5BT-loaded M-IA MPs was faster than that of M5BT-loaded IA MPs (Fig. 5). Furthermore, 45.1 ± 3.45 wt% of M5BT was released from the M5BT-loaded M-IA MPs within 24 h, whereas 27.55 ± 3.23 wt% of M5BT was released from M5BT-loaded INAC MPs within 24 h. More than 90 wt% of M5BT was released from M-IA MPs within 6 days, whereas less than 55 wt% of M5BT was released from the INAC MPs within 7 days due to the hydrophilic property of the decorated mannan.
Fig. 5.
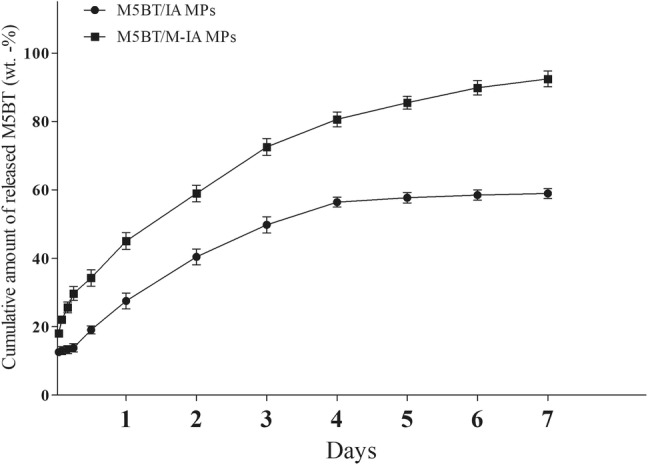
In vitro release profile of the M5BT protein from M5BT loaded IA and M-IA MPs at serum pH. MPs (10 mg/ml) were suspended in PBS (pH 7.4). The protein concentration was measured by micro BCA assay. All values represent the mean ± SD (n = 3)
Confirmation of mannan-decoration in M-IA MPs
To confirm the mannan-decoration on the surface of the IA MPs, the FITC-mannan was used to decorate the IA MPs, and these FITC-mannan-decorated IA MPs were visualized by CLSM (Fig. 6). The confocal images indicated that FITC-mannan successfully decorated the surface of the IA MPs.
Fig. 6.
Confirmation of mannan-decoration of INAC MPs by CLSM. FITC-labeled mannan (FITC-mannan) was used to decorate the IA MPs. A M-IA MPs and B FITC-M-IA MPs
In vivo immunization in murine model
FMDV serotype O specific antibody production
Because we hypothesized that the mannan-decorated IA MPs would enhance antigen-specific immune response by synergistic effect on the TLR pathway relative to non-modified IA MPs, we conducted an in vivo immunization study in mice. The immunization schedules and vaccine doses are summarized in Table 2. To assess the immunization efficacy of the MPs, the levels of the FMDV serotype O specific antibody in sera from immunized mice was determined by FMDV SP ELISA with an FMDV serotype O structure protein-coated plate as shown in Fig. 7. The vaccination efficacy was presented as percentage inhibition (PI), with neutralizing antibody being produced when the PI was more than 50.
Table 2.
Immunization groups and vaccine dose used in the in vivo immunization study
| Groups | No. mice | Vaccine dose/total volume | Total no. injection | Adjuvant | Annotation |
|---|---|---|---|---|---|
| Untreated | 5 | – | 3 | – | Untreated |
| iFMDV | 5 | 80 µl | 3 | – | iFMDV |
| M5BT | 5 | 10 µg/80 µl | 3 | – | M5BT |
| M5BT (CFA) | 5 | 10 µg/80 µl | 3 | CFA/IFA | CFA |
| M5BT/IA MP | 5 | 10 µg/80 µl | 3 | – | IA |
| M5BT/M-IA MPs | 5 | 10 µg/80 µl | 3 | M-IA |
iFMDV, inactivated foot-and-mouth disease vaccine; M5BT, a multi-epitope recombinant protein which consists of tandem repeats of five B-cell epitopes derived from the GH loop of different FMDV variants and one T-cell epitope; CFA, Complete Freund’s Adjuvant; IA, inulin acetate; M-IA, mannan-decorated inulin acetate
Fig. 7.
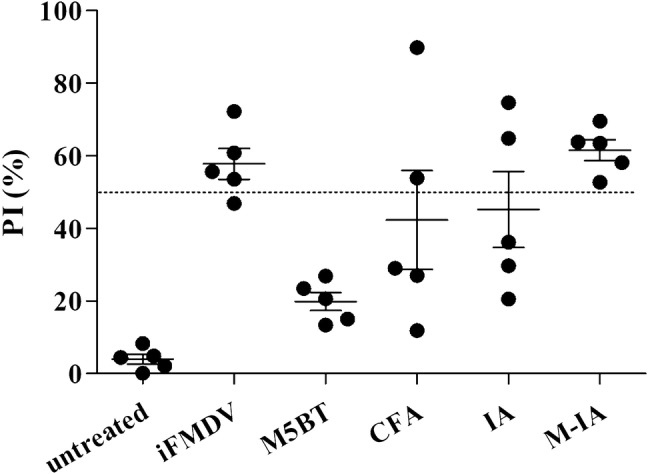
FMDV serotype O-specific antibody assay using SP competition ELISA. Test sera were collected at week 4 postimmunization. PI (%) means the percent inhibition. Each symbol represents the value for an individual animal. The dotted line describes 50 PI (%). Horizontal lines describe the mean value for each group of animals
The results indicate that group immunized with M5BT delivered by M-IA MPs showed a PI value greater than 50 in all mice. In addition, the anti-FMDV antibody production level was similar to both the M-IA MPs and iFMDV as a commercial vaccine. The M-IA MPs elicited a higher level of FMDV antibodies than the IA MPs and CFA group as a commercial adjuvant.
M5BT specific immunoglobulin detection by ELISA
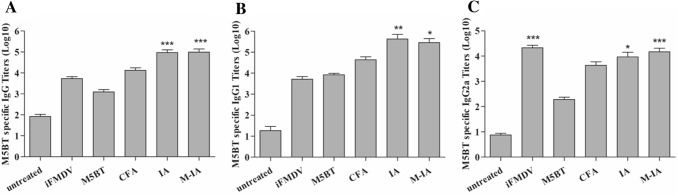
To assess the systemic humoral immune response after immunization with M5BT-loaded M-IA MPs, anti-M5BT serum IgG levels in sera from immunized mice were analyzed by ELISA, as shown in Fig. 8A. Among the immunized groups, anti-M5BT IgG titer was increased in all adjuvant groups (iFMDV, CFA, IA MP, and M-IA MPs) compared to that of the M5BT-only group. Furthermore, the M5BT-loaded M-IA MPs groups elicited significantly higher IgG titers, suggesting an immune-cell-activating effect of the mannan-decorated IA MPs relative to the CFA group. Furthermore, the anti-M5BT IgG2a and IgG1 titers were analyzed to investigate the induction of the type 1 helper T-cell (Th1) and type 2 helper T-cell (Th2) responses [17]. The anti-M5BT IgG1 titer was the highest in the M5BT-loaded IA MPs, although no significant difference existed in the anti-M5BT IgG1 between the M5BT-loaded IA MPs and M5BT-loaded M-IA MPs groups (Fig. 8B). The anti-M5BT IgG2a titers of the mice immunized with M5BT-loaded M-IA MPs and iFMDV were significantly higher than that of untreated, M5BT only, and CFA group (Fig. 8C).
Fig. 8.
M5BT-specific IgG and IgG subtype immune responses at 4 weeks postimmunization. The anti-M5BT serum immunoglobulin titers were measured using blocking ELISA. Note that the endpoint titers of the result are represented on a log scale on the y-axis. A Anti-M5BT total IgG titers, B IgG1 titers and C IgG2a titers. All values represent the mean ± SD (n = 5). Significant differences were compared to the untreated group. *: p < 0.05; **: p < 0.01; ***: p < 0.001, one-way ANOVA
Secretory IL-4 and IFNγ in supernatant of splenocyte culture
To assess the immunization efficacy in the mannan-decorated carrier, splenocytes were isolated from immunized mice and cultured in vitro for 3 days with antigen restimulation. The IFNγ and IL-4 levels of the splenocyte culture medium were analyzed by ELISA, as shown in Fig. 9. The results were represented as the relative cytokine levels of antigen-stimulated splenocytes compared to that of untreated controls. As a result, the M5BT-loaded M-IA MPs induced higher IFNγ and IL-4 levels than the other treatments.
Fig. 9.
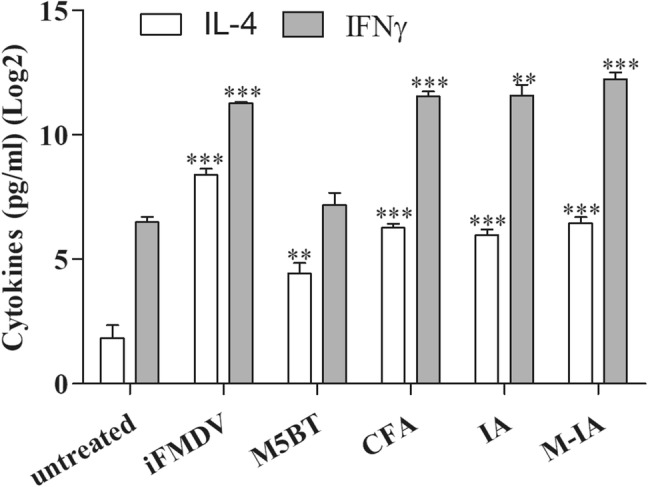
In vitro released cytokines in the splenocyte culture supernatant of vaccinated animals when restimulated with antigen. The white bar is IL-4, and the gray bar is IFNγ. Relative A IFNγ and B IL-4 levels. All values represent the mean ± SD (n = 5). Significant differences were compared to the untreated group. **: p < 0.01; ***: p < 0.001, one-way ANOVA
Discussion
A vaccine is a method for improving immunity for the prevention of specific diseases [18]. Classical vaccines are composed of attenuated or inactivated microorganisms or viruses [19]. Although such vaccines have successfully prevented some diseases, a new-generation vaccine, such as recombinant protein vaccine or designed vaccine, needs to be developed to meet demands for safe and cost-effective vaccines [20, 21]. However, the recombinant protein vaccines have some weaknesses, such as low immunogenicity and rapid clearance in the host [22, 23]. Therefore, the development of an adjuvant and a carrier for delivery is needed to overcome the weaknesses of the recombinant protein vaccines.
Adjuvants are compounds that are able to enhance the immune response against co-delivered antigens. Many adjuvants such as Freund`s adjuvant, cholera toxin, aluminum salts and CpG ODN, have already been used, although these adjuvants need to overcome their host-induced toxicity problem [24, 25]. Thus, safe and effective adjuvants are required to improve the immune responses of recombinant protein vaccine. Therefore, we developed M-IA MPs as a new polymer vaccine carrier with adjuvanting ability to protect against the host`s enzymatic degradation and enhance the immune responses of the used vaccine.
Inulin from the plant polysaccharide has been recently reported as TLR-4 agonist [8]. Because inulin is soluble in water, however, the MPs are not formed, and the vaccines cannot be loaded in the MPs. Therefore, IA was prepared by substituting hydroxyl groups to the acetyl ones in the inulin [12]. The acetylated inulin is not soluble in water due to weak hydrogen bonding between the hydroxyl groups of inulin and the water molecules when compared to the polymer inulin precursor. Thus, the acetylated inulin can be used to form MPs by the double-emulsion evaporation method. When manufacturing MPs using this method, a specific ligand such as mannan can be easily introduced into the MPs to enhance the carrier`s function [16] because the mannan also can function as a stabilizer because of the amphiphilic property of the mannan.
We introduced mannan as PAMP in IA to obtain a synergistic effect as a specific ligand. Mannan can target the pathogen recognition receptors (PRRs) and mannose receptors (MRs) of APCs [14]. We confirmed the decoration of the IA MPs with mannan as shown in Fig. 6. In our previous study, the MPs coated with mannan showed greater uptake by the Raw264.7 cells than shown by the MPs alone [16]. In addition, the M-IA MPs were spherical, ranging in diameter from 1 to 2 µm. Those MPs less than 5 µm in diameter can be taken up through phagocytosis by antigen-presenting cells (APCs) such as macrophages and dendritic cells, thereby playing a crucial role in initiating the innate immune response. The MPs of these sizes can be internalized into the APCs [26, 27].
Recombinant proteins loaded in the MPs were also protected from enzymatic degradation. The sustained release properties of the MPs protected protein from enzymatic degradation and maintained immune responses for a long time as shown in Fig. 5. In our previous study, the recombinant protein used to prevent FMD was easily degraded by endogenous protease during cell extract at 4 °C [4]. On the other hand, the M-IA MPs protected the M5BT protein from enzymatic degradation due to loading of the protein inside the MPs. As shown in Fig. 5, more than 90 wt% of M5BT was released from the M-IA MPs within 7 days, whereas less than 55 wt% of M5BT was released from the IA MPs. The results indicate that the mannan-decoration of the M5BT-loaded IA MPs affected the fast release of M5BT from the M5BT-loaded M-IA MPs due to the hydrophilic property of the mannan.
In the immunization assay, the M-IA MPs and iFMDV group produced anti-FMDV antibody in their serum, as shown in Fig. 7. All individuals in the M-IA MPs and iFMDV groups recorded PI (%) over 50, suggesting an efficient immune response ability to the FMDV because a PI (%) greater than 50 indicates enough production of neutralizing antibodies to achieve protection against FMDV. The production of the IgG and IgG subtypes between the IA and M-IA MPs groups are similar, as shown in Fig. 8. Although the results of the M5BT-specific antibodies indicate that the IA MPs are sufficient as an adjuvant, the result of the anti-FMDV antibodies demonstrated that the M-IA MPs worked more efficiently than the IA MPs in the mechanism of producing antibodies. Furthermore, the IgG1 and IgG2a were analyzed as indicators of Th2 or Th1 bias in the immune response [28] to better understand the characteristics of the immune responses induced by the M-IA MPs. While the Th1 response induced was higher than the Th2 response induced in the iFMDV group, the Th2 response induced was higher than the Th1 response induced in the CFA, IA MPs and M-INAC MPs groups. The immune response increased in the vaccine groups with microparticulates compared with the native antigen and CFA group. In addition, we confirmed the immune memory via reactivated splenocytes [29]. As shown in Fig. 9, the cytokines were highly expressed in the splenocytes of the iFMDV, CFA and vaccine groups with microparticulates through reactivation of the M5BT protein relative to reactivation of the native protein group. The results suggest that M5BT-loaded M-IA MPs enhance immune responses through adjuvant carrier abilities and protect M5BT protein from enzymatic degradation. These effects are not confined to M5BT protein. M-IA MPs can be applied to other diverse protein vaccines and reinforce their stability and immunogenicity, as they can encapsulate all proteins soluble in aqueous phase. Moreover, as M-IA MPs stimulate Th1 responses, they also can be applied to vaccines which target diseases that need cellular immune responses including African swine fever [30].
Because the FMDV infects through respiratory aerosols, the induction of mucosal immune responses is important. To induce the mucosal immune responses in mucosal-associated lymphoid tissue (MALT), the delivery carrier system is required to protect protein vaccines from degradation by harsh environments in mucosal systems [31]. Although no effect was induced by the oral administration of naked M5BT protein (data not shown) due to the instability of the M5BT protein, oral vaccination using M5BT-loaded mucoadhesive cellulose acetate phthalate microparticles elicited mucosal IgA [32]. In a follow-up study, we are also preparing a nasal vaccination assay for mice, which will use M-IA MPs. Ultimately, vaccination will be administered via the nasal route, which, like the results of this study, is also expected to be effective.
Although the recombinant protein vaccine will be a next-generation vaccine, the vaccine requires an appropriate carrier to protect clearance by enzymatic degradation in the host and an adjuvant to overcome its low-immunogenicity. In this study, mannan-decorated IA MPs that were successfully prepared and delivered to the MR on APCs were evaluated in vitro and in vivo. Vaccination with M5BT-loaded M-IA MPs in mice induced enhanced serum IgG and anti-FMDV antibody responses compare to a commercial adjuvant. This indicated that vaccination of subunit vaccine via M5BT-loaded M-IA MPs effectively delivered the vaccine to the APCs and elicited humoral immune responses. This finding represents a step forward in the development of a promising subunit vaccine in the livestock industries.
Acknowledgements
This works was founded by the Ministry of Science, ICT and Future Planning (Project No. 2016R1D1A1B03933491). The first authors were supported by Brain Korea 21 Plus program.
Compliance with ethical standards
Conflicts of interest
The authors have no financial conflicts of interest.
Ethical statement
Mouse in vivo experiment were carried out following the policy and regulations for the care and use of laboratory animal (Laboratory animal center, Seoul National University, Korea). All protocols were reviewed and approved by the Animal Care and Use Committee at Seoul National University (SNU-141201-1).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
So-Yeon Yoon and Sang-Kee Kang are contributed equally to this work.
Contributor Information
Chong-Su Cho, Email: chocs@snu.ac.kr.
Yun-Jaie Choi, Email: cyjcow@snu.ac.kr.
References
- 1.Jamal SM, Belsham GJ. Foot-and-mouth disease: past, present and future. Vet Res. 2013;44:116. doi: 10.1186/1297-9716-44-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sobrino F, Domingo E. Foot and mouth disease: current perspectives. Boca Raton: CRC Press; 2019. [Google Scholar]
- 3.Rodriguez LL, Grubman MJ. Foot and mouth disease virus vaccines. Vaccine. 2009;27:D90–D94. doi: 10.1016/j.vaccine.2009.08.039. [DOI] [PubMed] [Google Scholar]
- 4.Lee HB, Piao DC, Lee JY, Choi JY, Bok JD, Cho CS, et al. Artificially designed recombinant protein composed of multiple epitopes of foot-and-mouth disease virus as a vaccine candidate. Microb Cell Fact. 2017;16:33. doi: 10.1186/s12934-017-0648-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberfroid MB. Inulin-type fructans: functional food ingredients. J Nutr. 2007;137:2493S–2502S. doi: 10.1093/jn/137.11.2493S. [DOI] [PubMed] [Google Scholar]
- 6.Barclay T, Ginic-Markovic M, Cooper P, Petrovsky N. Inulin-a versatile polysaccharide with multiple pharmaceutical and food chemical uses. J Excip Food Chem. 2010;1:27–50. [Google Scholar]
- 7.Gibson GR. Dietary modulation of the human gut microflora using the prebiotics oligofructose and inulin. J Nutr. 1999;129:1438S–41. doi: 10.1093/jn/129.7.1438S. [DOI] [PubMed] [Google Scholar]
- 8.Kumar S, Tummala H. Development of soluble inulin microparticles as a potent and safe vaccine adjuvant and delivery system. Mol Pharm. 2013;10:1845–1853. doi: 10.1021/mp3006374. [DOI] [PubMed] [Google Scholar]
- 9.Tummala H, Kumar S (2013). Inulin and inulin acetate formulations. US Patent US20130195930A1, 21 Jan 2013.
- 10.Kumar S, Kesharwani SS, Kuppast B, Rajput M, Bakkari MA, Tummala H. Discovery of inulin acetate as a novel immune-active polymer and vaccine adjuvant: synthesis, material characterization, and biological evaluation as a toll-like receptor-4 agonist. J Mater Chem B. 2016;4:7950–7960. doi: 10.1039/C6TB02181F. [DOI] [PubMed] [Google Scholar]
- 11.Robert P, García P, Reyes N, Chávez J, Santos J. Acetylated starch and inulin as encapsulating agents of gallic acid and their release behaviour in a hydrophilic system. Food Chem. 2012;134:1–8. doi: 10.1016/j.foodchem.2012.02.019. [DOI] [Google Scholar]
- 12.Wu XY, Lee PI. Preparation and characterization of inulin ester microspheres as drug carriers. J Appl Polym Sci. 2000;77:833–840. doi: 10.1002/(SICI)1097-4628(20000725)77:4<833::AID-APP17>3.0.CO;2-4. [DOI] [Google Scholar]
- 13.Levitz SM. Innate recognition of fungal cell walls. PLoS Pathog. 2010;6:e1000758. doi: 10.1371/journal.ppat.1000758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van de Veerdonk FL, Marijnissen RJ, Kullberg BJ, Koenen HJ, Cheng SC, Joosten I, et al. The macrophage mannose receptor induces IL-17 in response to Candida albicans. Cell Host Microbe. 2009;5:329–340. doi: 10.1016/j.chom.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Sander JS, Studart AR. Multiwalled functional colloidosomes made small and in large quantities via bulk emulsification. Soft Matter. 2014;10:60–68. doi: 10.1039/C3SM51900G. [DOI] [PubMed] [Google Scholar]
- 16.Li HS, Shin MK, Singh B, Maharjan S, Park TE, Kang SK, et al. Nasal immunization with mannan-decorated mucoadhesive HPMCP microspheres containing ApxIIA toxin induces protective immunity against challenge infection with Actinobacillus pleuropneumoiae in mice. J Control Release. 2016;233:114–125. doi: 10.1016/j.jconrel.2016.05.032. [DOI] [PubMed] [Google Scholar]
- 17.Taniuchi I. CD4 helper and CD8 cytotoxic T cell differentiation. Annu Rev Immunol. 2018;36:579–601. doi: 10.1146/annurev-immunol-042617-053411. [DOI] [PubMed] [Google Scholar]
- 18.Zhao G, Chandrudu S, Skwarczynski M, Toth I. The application of self-assembled nanostructures in peptide-based subunit vaccine development. Eur Polym J. 2017;93:670–681. doi: 10.1016/j.eurpolymj.2017.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burke CJ, Hsu TA, Volkin DB. Formulation, stability, and delivery of live attenuated vaccines for human use. Crit Rev Ther Drug Carrier Syst. 1999;16:1–83. [PubMed] [Google Scholar]
- 20.Jana S, Deb JK. Retracted article: strategies for efficient production of heterologous proteins in Escherichia coli. Appl Microbiol Biotechnol. 2005;67:289–298. doi: 10.1007/s00253-004-1814-0. [DOI] [PubMed] [Google Scholar]
- 21.Stevens RC. Design of high-throughput methods of protein production for structural biology. Structure. 2000;8:R177–R185. doi: 10.1016/S0969-2126(00)00193-3. [DOI] [PubMed] [Google Scholar]
- 22.Skwarczynski M, Toth I. Peptide-based synthetic vaccines. Chem Sci. 2016;7:842–854. doi: 10.1039/C5SC03892H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Azmi F, Ahmad Fuaad AA, Skwarczynski M, Toth I. Recent progress in adjuvant discovery for peptide-based subunit vaccines. Hum Vaccines Immunother. 2014;10:778–796. doi: 10.4161/hv.27332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oleszycka E, Lavelle EC. Immunomodulatory properties of the vaccine adjuvant alum. Curr Opin Immunol. 2014;28:1–5. doi: 10.1016/j.coi.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 25.Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33:492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eldridge JH, Hammond CJ, Meulbroek JA, Staas JK, Gilley RM, Tice TR. Controlled vaccine release in the gut-associated lymphoid tissues. I. Orally administered biodegradable microspheres target the peyer’s patches. J Control Release. 1990;11:205–214. doi: 10.1016/0168-3659(90)90133-E. [DOI] [Google Scholar]
- 27.Katare YK, Muthukumaran T, Panda AK. Influence of particle size, antigen load, dose and additional adjuvant on the immune response from antigen loaded PLA microparticles. Int J Pharm. 2005;301:149–160. doi: 10.1016/j.ijpharm.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 28.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–93. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 29.Shin MH, Min DY. Production of interferon-y and interleukin-4 by splenocytes in mice infected with Paragonimus westermani. Korean J Parasitol. 1998;34:185–189. doi: 10.3347/kjp.1996.34.3.185. [DOI] [PubMed] [Google Scholar]
- 30.Oura CA, Denyer MS, Takamatsu H, Parkhouse RM. In vivo depletion of CD8+ T lymphocytes abrogates protective immunity to African swine fever virus. J Gen Virol. 2005;86:2445–2450. doi: 10.1099/vir.0.81038-0. [DOI] [PubMed] [Google Scholar]
- 31.Singh B, Jiang T, Kim YK, Kang SK, Choi YJ, Cho CS. Release and cytokine production of BmpB from BmpB-loaded pH-sensitive and mucoadhesive thiolated eudragit microspheres. J Nanosci Nanotechnol. 2015;15:606–610. doi: 10.1166/jnn.2015.8781. [DOI] [PubMed] [Google Scholar]
- 32.Lee HB, Yoon SY, Singh B, Oh SH. Oral immunization of FMDV Vaccine using pH-Sensitive and mucoadhesive thiolated cellulose acetate phthalate microparticles. Tissue Eng Regen Med. 2017;15:1–11. doi: 10.1007/s13770-017-0082-x. [DOI] [PMC free article] [PubMed] [Google Scholar]