Abstract
Background:
We first determined the efficacy of lesional injection of tonsil-derived MSCs (mesenchymal stem cells) for the treatment of 5-fluorouracil induced oral mucositis.
Methods:
Oral mucositis was induced in hamsters by administration of 5-fluorouracil (day 0, 2, 4) followed by mechanical trauma (day 1, 2, 4). The experimental groups included MT (mechanical trauma only), 5-FU + MT (mechanical trauma with 5-fluorouracil administration), TMSC (mechanical trauma with 5-fluorouracil administration, tonsil-derived mesenchymal stem cells injection), DEXA (mechanical trauma with 5-fluorouracil administration, dexamethasone injection), and saline (mechanical trauma with 5-fluorouracil administration, saline injection).
Results:
On day 10, gross and histologic analyses showed that nearly complete healing and epithelialization of the cheek mucosa of the TMSC group, whereas the other groups showed definite ulcerative lesions. Compared with the MT and DEXA groups, CD31 expression was greater in the TMSC group on days 10 and 14. Tendency towards a decrease in MMP2 expression with the time in the TMSC group was observed. In addition, the TMSC group showed higher expression of TGF-β, and NOX4 on day 10 compared with the other groups. Scratch assay demonstrated that the conditioned media harvested from tonsil-derived MSCs significantly increased migratory efficacy of NIH3T3 cells. Transwell assay showed that the preferential migration of tonsil-derived MSCs to the wound area.
Conclusion:
Intralesional administration of tonsil-derived MSCs may accelerate wound healing of 5-fluorouracil induced oral mucositis by upregulating neovascularization and effective wound contraction. In addition, tonsil-derived MSCs might contribute to oral ulcer regeneration via the stimulation of fibroblast proliferation and migration.
Electronic supplementary material
The online version of this article (10.1007/s13770-019-00226-7) contains supplementary material, which is available to authorized users.
Keywords: Stem cells, Tonsil, Oral mucositis, Wound healing, Mechanical trauma
Introduction
Oral mucositis (OM) is a severe and frequent adverse effect that occurs during chemotherapy or radiotherapy for cancer treatment [1, 2]. Clinically, it varies from an erythematous lesion with few symptoms to severe ulceration, and it may lead to oral pain, dysphagia, and reduced nutritional intake, which may decrease quality of life. The incidence of OM in patients who are treated with cytotoxic agents for cancer ranges from approximately 20% to 40% [2]. Chemotherapy and radiotherapy can interfere with the maturity, growth, and renewal rate of basal epithelial cells, causing changes to normal turnover and resulting in cell death [3]. These oral complications can result in malnutrition and delays or interruptions in treatment and can decrease disease remission and survival [2, 4]. There are limited evidence-based options for the prevention and treatment of OM [2, 4, 5]. Clinical interventions include two approaches: (1) prevention of OM and (2) management of symptoms, including pain control, nutritional support, and prevention and treatment of secondary infection [4–6]. Recently, biologically active factors, such as granulocyte-colony stimulating factor, keratinocyte growth factor, interleukin (IL)-11, and transforming growth factor (TGF)-β3, have been considered for their potential efficacy in preventing and/or treating OM [7–12]. Stem cells have received increased attention because of their potential for use in tissue engineering and clinical applications. Several studies reported that bone marrow-derived mesenchymal stem cells (MSCs) and adipose tissue-derived MSCs could improve cutaneous wound healing [13, 14]. The therapeutic efficacy of tonsil-derived MSCs (TMSCs) in cutaneous wound repair also has been suggested [15]. The human palatine tonsils are lymphoepithelial tissues that are located in the oropharynx and have been proposed as an alternative source of adult stem cells [16]. Recent studies have reported the identification and isolation of TMSCs from waste surgical tissue following tonsillectomies in relatively young donors (i.e., under 10 years old). Therefore, TMSCs offer several advantages, including superior proliferation and a shorter doubling time compared to bone marrow-derived MSCs [16, 17]. Furthermore, TMSCs have been reported to be beneficial for the treatment and prevention of various diseases, including liver fibrosis, peripheral nerve injury, allergic rhinitis, and osteoradionecrosis [18–21].
Various studies have shown the successful role of stem cell therapy in the treatment of oral mucosal lesions, such as oral ulcers (oral mucositis, pemphigus vulgaris) and premalignant conditions (oral submucous fibrosis, oral lichen planus) [22]. Zhang et al. [23] reported therapeutic effects of spheroid gingiva-derived MSCs (GMSCs) in a mouse model of OM induced by 5-fluorouracil (FU). However, this study discussed systemic injection of spheroid GMSCs, but did not focus on topical/lesional delivery.
In this study, we first determined the efficacy of lesional injection of TMSCs for the treatment of 5-FU-induced OM. To investigate the possible efficacy of TMSCs in mucosal healing, we created a uniform-sized OM model with a biopsy punch and injected TMSCs, saline, and dexamethasone into the submucosa around the mucosal defect. We then evaluated body weight and oral intake and performed gross inspection, histologic analysis, in vivo tracking of injected TMSC, and immunohistochemistry of the mucosal lesions, which allowed for an assessment of overall condition, healing, and inflammation. In vitro migration assay was also performed.
Materials and methods
Preparation and culture of TMSCs
We isolated and cultured the TMSCs, as previously described [16]. Briefly, tonsillar tissue was obtained from a ten-year-old girl who had undergone a tonsillectomy. Informed written consent was obtained from the legal guardians of all patients who participated in this study, and the study protocol was approved by the institutional review board of Hallym University Medical Center (2016-41). After surgery, the tonsillar tissue was chopped and digested in high-glucose (4500 mg/L) Dulbecco Modified Eagle Medium (DMEM) (Welgene Inc., Gyeongsan, Republic of Korea) that contained 210 U/mL collagenase type I (Invitrogen, Carlsbad, CA, USA) and 10 mg/mL DNase (Sigma Aldrich, St. Louis, MO, USA) for 30 min at 37 °C. Digested tissue was filtered through a wire mesh, and then the cells were washed twice in high-glucose DMEM/20% fetal bovine serum (FBS) (Welgene Inc., Gyeongsan, Republic of Korea) and once more in high-glucose DMEM/10% FBS. Adherent mononuclear cells were obtained with Ficolle-Paque (GE Healthcare, Little Chalfont, UK) density gradient centrifugation. Cells were plated at a density of 1 × 106 cells in a 100-mm culture dish in high-glucose DMEM, 10% FBS, 100 U/mL penicillin/streptomycin (Gibco, Grand Island, NY, USA). After 48 h, nonadherent cells were removed from the medium, and adherent mononuclear cells, the TMSCs, were replenished with new culture medium. All TMSCs used in this experiment were passage 5.
Oral mucositis model
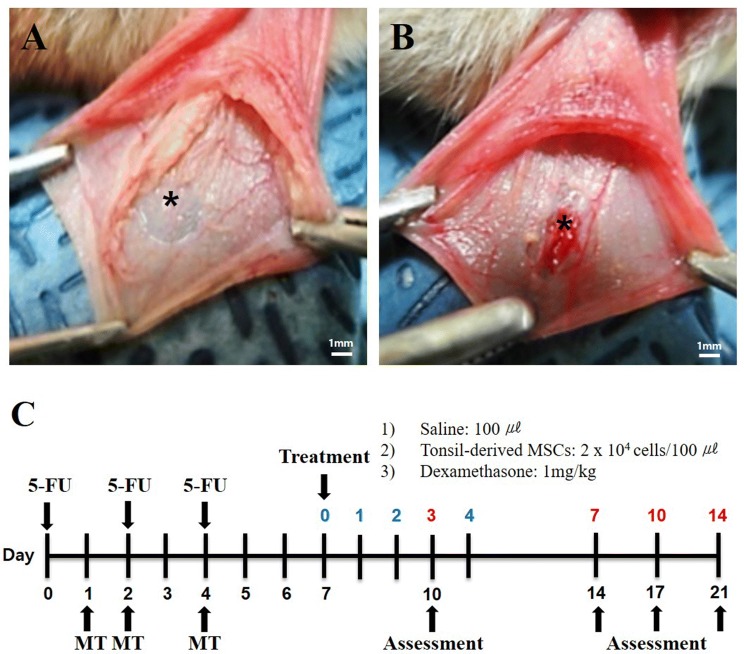
Male Syrian golden hamsters (Japan SLC, Nagoya, Japan), 7 weeks old and weighing 90 to 120 g, were used in this study. The animals were maintained at 23 ± 2 °C, 55 ± 10% humidity, and light from 08:00 a.m. to 08:00 p.m. This study was approved by the institutional review board of Hallym University (Hallym 2018-67), Chuncheon, Korea. Mucositis was induced by intraperitoneal injection of 60 mg/kg 5-FU on days 0, 2, and 4. The inner layer of the cheek pouch mucosa was removed with 8-mm biopsy punch, and the exposed outer layer of the cheek pouch mucosa was irritated by superficial scratching with the tip of an 18-gauge sterile needle (mechanical trauma, MT) by the same operator on day 1. On days 2 and 4, additional superficial scratches were performed within OM site made on day 1 (Fig. 1A, B).
Fig. 1.
Surgical procedure and flow chart of the study. A The inner layer of the cheek pouch mucosa was removed with an 8-mm biopsy punch. B The exposed outer layer of the cheek pouch mucosa was irritated by superficial scratching with the tip of an 18-gauge sterile needle. C Flow chart of the study design. 5-FU, 5-fluorouracil; MSC, mesenchymal stem cell; MT, mechanical trauma. *The asterisk indicates the oral mucosal lesion
Treatment group
We randomly divided the animals into five groups: (1) MT (mechanical trauma only, n = 6); (2) 5-FU + MT (mechanical trauma with 5-FU administration, oral mucositis model, n = 6); (3) TMSC (mechanical trauma with 5-FU administration, TMSC injection, n = 6); (4) DEXA (mechanical trauma with 5-FU administration, dexamethasone injection, n = 6); and (5) saline (mechanical trauma with 5-FU administration, saline injection, n = 6) (Fig. 1C). There were 3 animals in each experimental group. All groups received MT on the right and left (both sides) cheek pouch mucosa. Therefore, there were 6 samples in each experimental group.
TMSC group
Growth medium containing 2 × 104 TMSCs was mixed in at a ratio of 1:2 to the Matrigel® (MA, BD Biosciences, San Jose, CA, USA) to produce a final volume of 100 µl. TMSC-embedded MA was deposited into an Eppendorf tube and kept on ice to maintain sol status until injection. Then 100 µl TMSC-embedded MA was injected into the submucosa around the mucosa defect site on day 7 using 26-gauge sterile needle syringe.
DEXA group
Dexamethasone disodium phosphate (Ilsung Pharmaceuticals Co., Seoul, Republic of Korea, 5 mg/ml) was diluted to 1 mg/kg in a phosphate-buffered saline (PBS, pH 7.4) solution and injected into the submucosa around the mucosa defect site on day 7 using 26-gauge sterile needle syringe.
Saline group
Saline 100 µl was injected into the submucosa around the mucosa defect site on day 7 using 26-gauge sterile needle syringe.
Assessments
Body weight and oral intake
Body weight and oral intake of 15 animals in each group were measured daily to assess the animals’ condition.
Gross analysis
The cheek pouch mucosa was photographed to characterize the severity of mucositis. Severity was evaluated, and erythema, hyperemia, bleeding, epithelial ulcers, and abscess were given a score of 0–5 based on the previously described method [24]: 0, healthy mucosa and no evidence of erosion or vasodilation; (1) presence of erythema and no evidence of mucosal erosion; (2) severe erythema, vasodilatation, and superficial erosion; (3) presence of ulcers in one or more faces of the mucosa, affecting no more than 25% of the area, severe erythema, and vasodilatation; (4) ulcers in about 50% of the area of the jugal mucosa; (5) completely ulcerated jugal mucosa that made it impossible to expose the tissue.
Histologic analysis
Animals were euthanized in compliance with the Animal Experiment Guidelines of the Hallym University Medical Research Institute. Specimens were embedded in paraffin blocks and sectioned into 7-μm-thick slices. The slices were stained with hematoxylin–eosin (H&E).
In vivo tracking
PKH26 red fluorescent cells linker for cell membrane labeling (Sigma, St. Louis, MO, USA) was used to label the TMSCs. The TMSCs of 2 × 104 cells were treated with a mixed PKH26 solution and incubated for 5 min. Labeling was stopped by 1% BSA (Bovostar, Keilor east, Australia) and washed with 10% DMEM. In the TMSC group, TMSCs labeled with PKH26 were injected into the submucosa around the mucosa defect site on day 7 using 26-gauge sterile needle syringe. Oral mucosa tissues were harvested at 10, 14, 17 and 21 days and examined under a fluorescent microscopy (Leica, Wetzlar, Germany). For nuclear counter-staining, the sectioned oral mucosa tissues were incubated for 2 min with 10 μg/ml 4′6-diamidino-2-phenylindole (DAPI) (Invitrogen, Carlsbad, CA, USA) reagent in PBS. The cells were observed under a fluorescent microscopy (Carl Zeiss Microscopy GmbH, Zeiss, Germany) with 200-fold magnification.
Immunohistochemial analysis
The sectioned tissue slides were incubated in a tissue-drying oven for 40 min at 60 °C. The deparaffinized tissues were denatured with 10 mM sodium citrate buffer (pH 6.0) with a microwave and then blocked in a blocking buffer for 60 min. Primary antibodies were diluted and incubated for 16 h at 25 °C. Tissues were treated with 0.3% hydrogen peroxide in 10% PBS for 10 min. Primary antibodies were detected with horseradish peroxidase conjugated (HRP)-linked secondary antibody. Then HRP activity was detected with 3,3′-diaminobenzidine (DAB) substrate. Tissues were washed three times for 5 min each in PBS. All tissues were then counterstained with Mayer’s hematoxylin. Primary antibodies used were as follows: anti-TGF-β1 (1:100, Abcam, Cambridge, UK), mouse anti-CD31 (1:100, BD Pharmingen, Franklin Lakes, NJ, USA), anti-MMP2 (8B4) (1:100, Santa Cruz Biotechnology Inc., Dallas, TX, USA), and anti-NOX4 (1:500, Cusabio Technol LLC, Houston, TX, USA). Secondary antibodies used were as follows: goat anti-mouse IgG (1:500, Thermo Fisher Scientific Inc., Waltham, MA, USA) and goat anti-rabbit IgG, (1:500, Thermo Fisher Scientific Inc., Waltham, MA, USA). Oral mucosa tissues were obtained with light microscopy (Carl Zeiss Microscopy GmbH, Zeiss, Germany). Immune-positive cells were measured with the Image J program (ImageJ 1.49v, National institutes of health, Bethesda, MD, USA).
Scratch wound closure test
NIH3T3 mouse embryo fibroblast cells were obtained from Hallym University (Chuncheon, Korea). NIH3T3 cells were cultured in 10% FBS and DMEM containing 1% antibiotic–antimycotic solution at 37 °C in 5% CO2. NIH3T3 cells (2 × 104 cells/ml) were seeded in 6-well plates and grown to confluence about 90. Cells were wounded using 200 μl pipette tip. Wounded monolayers were washed three times with DPBS. Immediately after washing cells, cells were treated with FBS-free DMEM, 10% FBS/DMEM, conditioned medium (CM) (FBS-free DMEM) from the TMSCs, and CM (10% FBS/DMEM) from the TMSCs incubated for up to 6, 12, 18, and 24 h. Images were observed with optical light microscope (Nikon, ECLIPSE Ts2, Tokyo, Japan). Wound sizes were quantified using image J software (ImageJ 1.49v, National institutes of health, Bethesda, MD, USA).
Transwell assay
In vitro migration of TMSCs was evaluated using a 24-well plated and a transwell system (8.0 μm pore, Corning, Corning, NY, USA). For transwell assays, TMSCs (1x104 cells/ml) at 5 passages were seeded in the upper chamber and incubated at 37 °C in 5% CO2. The lower chambers were seeded with NIH3T3 cells (2 × 104 cells/ml) in the presence or absence of scratch. Cells treated with FBS-free DMEM and 10% FBS/DMEM were incubated. After a period of 24 and 48 h, medium was carefully removed from the transwell upper chambers. The transwell filter gently washed with DPBS and fixed with 4% formaldehyde for 2 min. 100% methanol was treated for 20 min and migrated cells were stained with crystal violet solution (Sigma, St. Louis, MO, USA) for 20 min. All the procedures has to be done at room temperature. The area of stained TMSCs was examined under a light microscope (Nikon, SMZ745T, Tokyo, Japan) with one-fold magnification. TMSCs migration area were quantified using image J software (ImageJ 1.49v, National institutes of health, Bethesda, MD, USA).
Statistical analysis
All data are presented as mean ± standard deviation. We performed statistical analysis of the experimental results with GraphPad Prism 6 (GraphPad Software, San Diego, CA, USA). A p value was generated with Student’s t test, with statistical significance set at *p < 0.05, **p < 0.01, and ***p < 0.005.
Results
Weight analysis and food intake
Mean body weight between different groups on distinct days is shown in Fig. 2A. Mean values for body weight on day 0 were 141.4 + 5.5 g in the MT group, 139.5 + 3.5 g in the 5-FU + MT group, 146.9 + 20.5 g in the saline group, 138.9 + 0.1 g in the TMSC group, and 145.6 + 16.4 g in the DEXA group; there was no significant difference between the groups. In addition, there was no significant change in body weight over time in all groups. Figure 2B shows the ratio of food intake for each measurement day to day 0. In all groups, food intake decreased by 40–60% on days 4–10 and days 11–14 compared with day 0. However, there was no statistical difference between the groups.
Fig. 2.
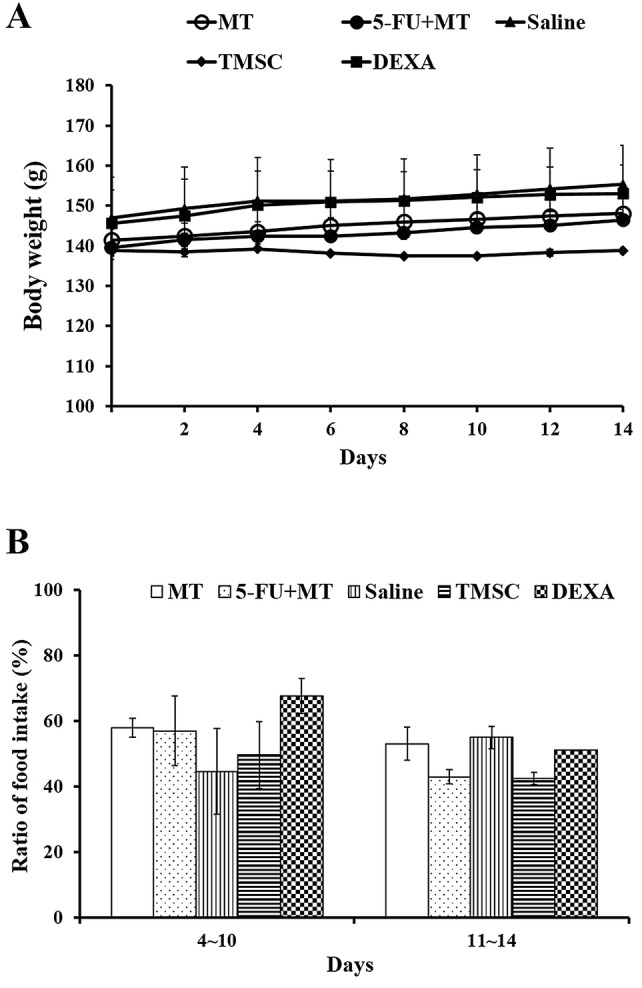
A, B Changes in body weight and food intake in hamsters. MT, mechanical trauma only; 5-FU + MT, mechanical trauma with 5-FU administration; TMSC, mechanical trauma with 5-FU administration, tonsil-derived MSC injection; DEXA, mechanical trauma with 5-FU administration, dexamethasone injection; saline, mechanical trauma with 5-FU administration, saline injection
Gross analysis
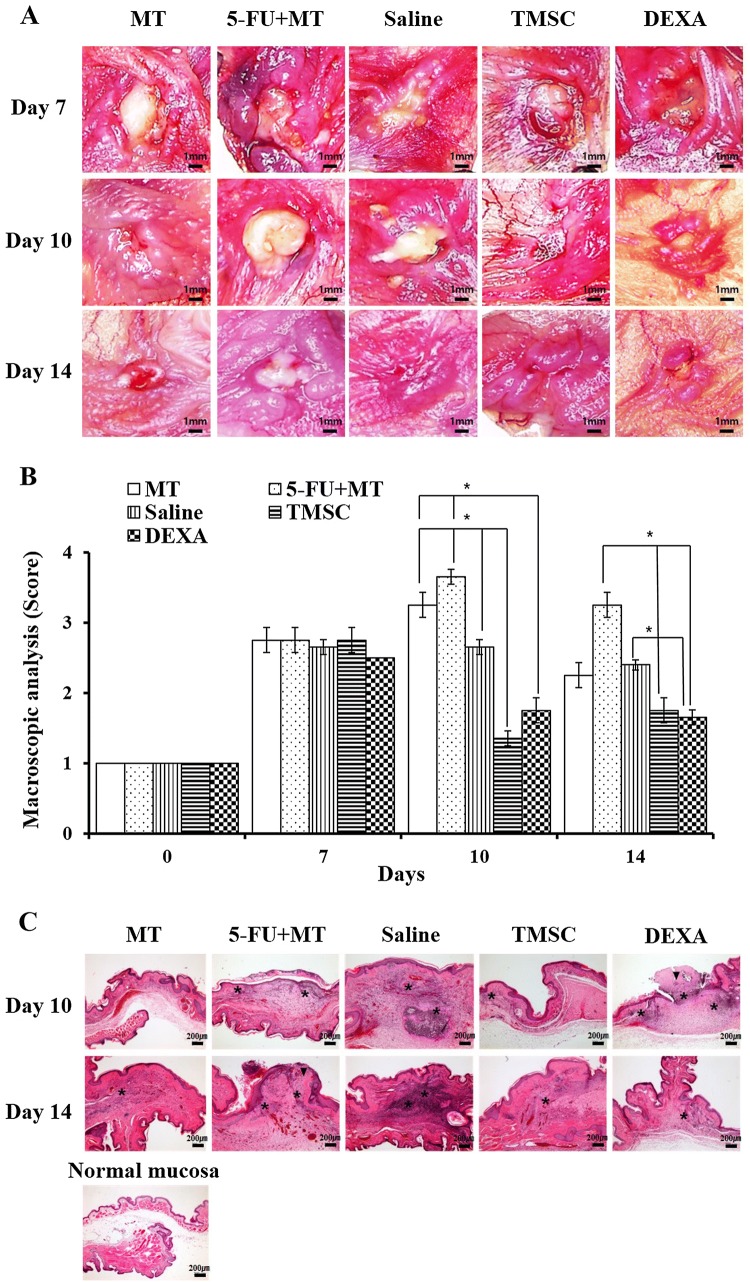
Grossly, the cheek pouches of the MT group showed mucosal irritation and ulceration on day 7. However, rapid and spontaneous healing was seen within 14 days. The 5-FU + MT group showed marked progressive mucositis, characterized by epithelial disruption and necrosis, on days 7 and 10. On day 14, the ulceration was slightly reduced; however, severe mucosal erythema and ulceration remained. The TMSC group showed nearly normal cheek mucosa, with minimal epithelial disruption and hyperemia, on day 10. However, the DEXA group showed an area of ulceration, with moderate hyperemia, on day 10. On day 14, epithelial disruption recovered in the TMSC group, and the DEXA group also showed reduced area of ulceration (Fig. 3A). Mean gross scores are shown in Fig. 3B. No significant differences in mean gross scores between the groups were seen on day 7. On day 10, gross scores of the TMSC and DEXA groups were significantly lower than those of the MT, 5-FU + MT, and saline groups (p < 0.05). Moreover, the TMSC group showed lower gross scores than the DEXA group. On day 14, the gross score of each group was decreased compared with day 10 The MT, TMSC, and DEXA groups had a lower gross score than the 5-FU + MT and saline groups. Gross scores of the TMSC and DEXA groups were significantly lower than those of the 5-FU + MT and saline groups (p < 0.05).
Fig. 3.
A–C Gross appearance of a hamster cheek pouch, gross score and hematoxylin and eosin-stained sections on days 10 and 14. *p < 0.05. The asterisk indicates inflammatory cells. The arrowhead indicates the epithelial defect. × 10 (A), × 100 (C)
Histologic analysis
In the MT group, histologic findings on days 10 and 14 showed complete epithelialization, with normal connective tissue; however, on days 10 and 14, we observed epithelial defect, with infiltration of inflammatory cells and a hemorrhagic area with fibrosis in the 5-FU + MT and saline groups. The TMSC group showed complete epithelialization on day 10; however, we observed epithelial defect, with inflammatory cell infiltration and granulation formation, in the DEXA group on day 10. On day 14, the DEXA group showed complete epithelialization (Fig. 3C).
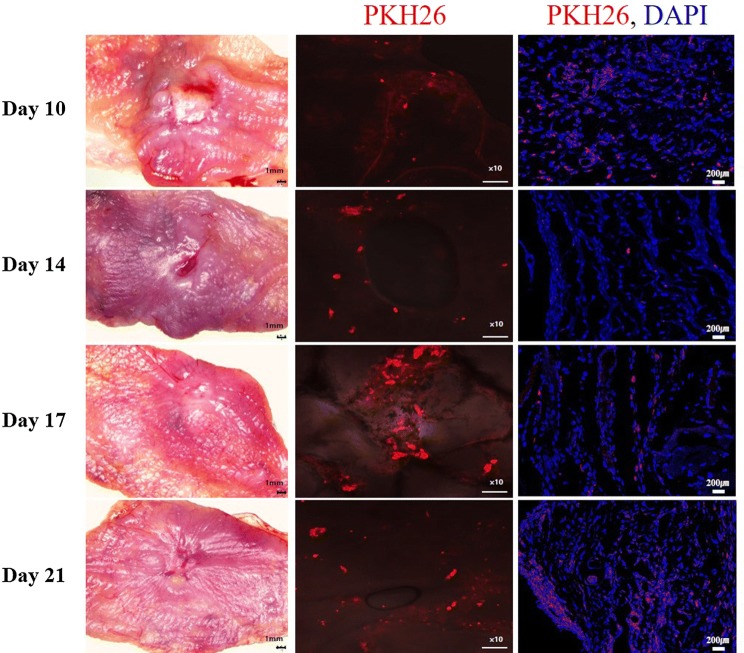
In vivo tracking of injected TMSCs
To evaluate the contributions of TMSCs in wound healing, we monitored the localization and migration of injected TMSCs labeled with PKH26. After harvesting cheek pouches, the fluorescence microscope analysis revealed a 2D image of the red fluorescence PKH26 dye gathered around the wound in 10 days (3 days after TMSC injection), and could still be detected until day 21 (14 days after TMSC injection) (Fig. 4). These results indicated that TMSCs were primarily localized to the wound area. In addition, 3D image of the fluorescence microscope on day 21 showed that TMSC-PKH26 cells evenly distributed around wound area (supplementary movie 1). In merge images, nuclei were stained with DAPI (blue). Localizations of the TMSC-PKH26 cells were consistent with DAPI staining (Fig. 4).
Fig. 4.
Fluorescent microscopy analysis of TMSCs labeled with PKH26. × 100 (PKH26), × 200 (PKH26, DAPI)
Immunohistochemistry for CD31, MMP2, TGF-β, and NOX4
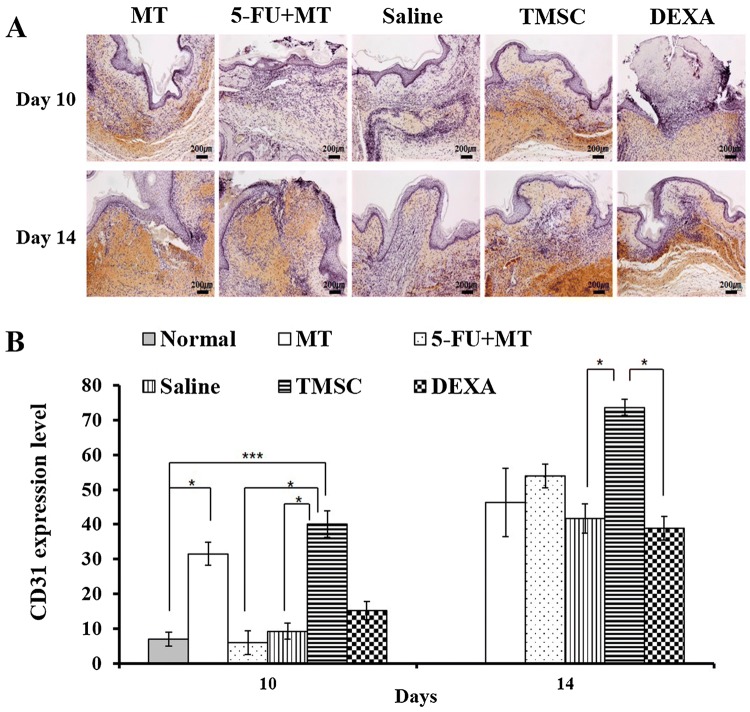
Wound angiogenesis was evaluated with CD31 staining. On day 10, the MT and TMSC groups showed greater CD31 expression compared with the other groups (5-FU + MT, saline, and DEXA). In the TMSC group, CD31 expression was significantly higher compared with the 5-FU + MT and saline groups (p < 0.05). In addition, CD31 expression in the TMSC group was higher than that in the MT group. The 5-FU + MT group showed significantly lower CD31 expression on day 10, whereas CD31 expression increased slightly between days 10 and 14. The DEXA group also showed higher CD31 expression than the 5-FU + MT and saline groups on day 10. However, it was lower than that of the MT group. On day 14, CD31 expression was increased in all groups compared with day 10. Furthermore, the TMSC group showed significantly greater CD31 expression compared with the DEXA group (p < 0.05) (Fig. 5).
Fig. 5.
A, B Immunohistochemistry for CD31 on days 10 and 14. The TMSC group showed significantly higher CD31 expression compared with the 5-FU + MT and saline groups on day 10. The TMSC group showed significantly greater CD31 expression than the DEXA group on day 14. *p<0.05, ***p<0.005. × 200
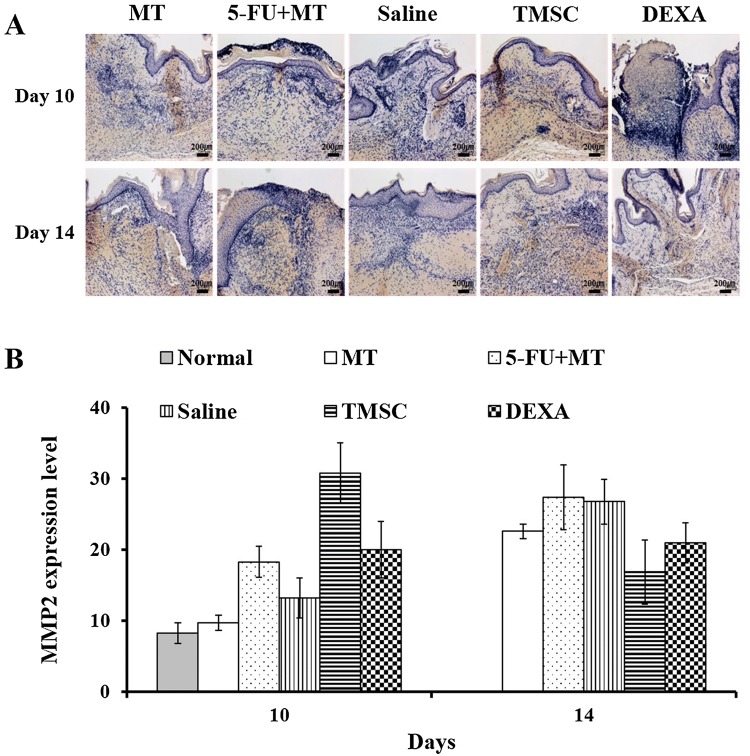
MMP2 expression was evaluated as an indicator of changes in wound healing. Prolonged high-MMP expression destroys local growth factors and impairs wound healing [25]. The TMSC group showed the highest MMP2 expression on day 10, and expression decreased between days 10 and 14. In addition, the DEXA group showed higher MMP2 expression than the 5-FU + MT group. On day 14, MMP2 expression in the TMSC group was the lowest compared with other groups. Early high expression of MMP2 in the TMSC group indicated that effective wound healing process in oral ulcer. In addition, decrease of MMP2 in the TMSC group on day 14 represents completion of wound healing process. However, there was no significant difference between the groups on days 10 and 14 (Fig. 6).
Fig. 6.
A, B Immunohistochemistry for MMP2 on days 10 and 14. There was no significant difference between the different groups on days 10 and 14. × 200
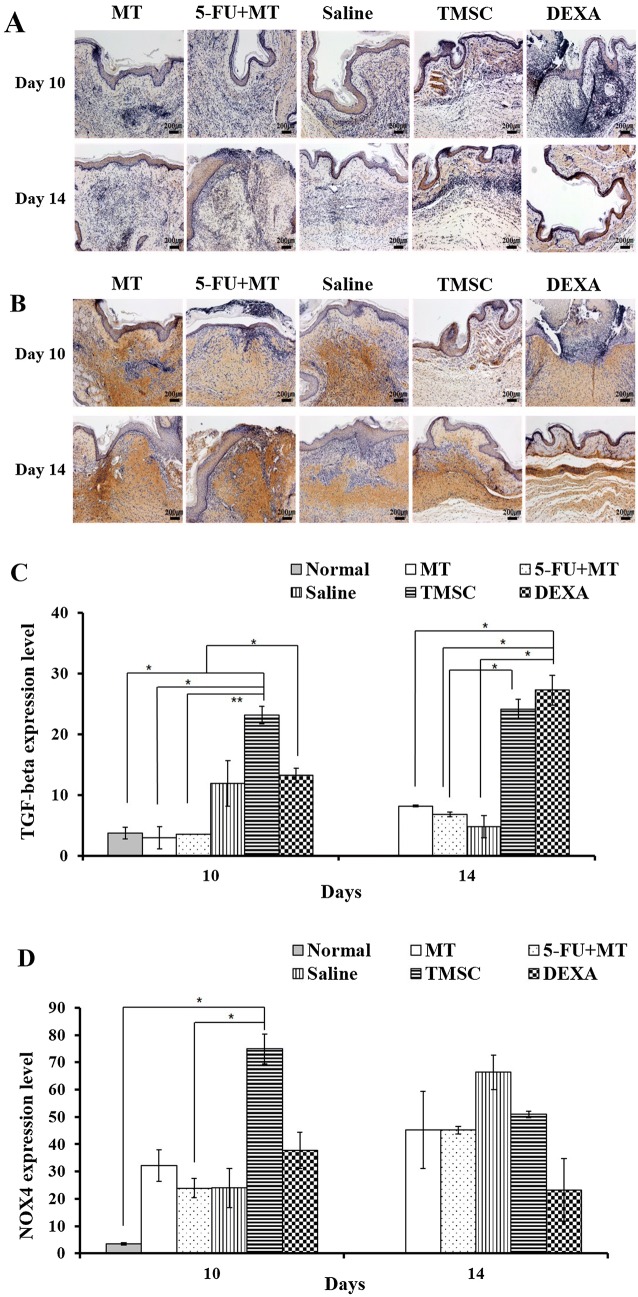
Wound contraction was evaluated with TGF-β and NOX4. In the TMSC group, staining with TGF-β showed strong expression in the epithelium and submucosa on day 10 (Fig. 7). Expression was increased significantly compared with the other groups (normal mucosa, MT, and 5-FU + MT), particularly the 5-FU + MT group (p < 0.01). On day 14, TGF-β expression in the TMSC and DEXA groups was stronger than that in the MT, 5-FU + MT, and saline groups. Expression was significantly higher than that in the 5-FU + MT group (p < 0.05) (Fig. 7C). On day 10, the TMSC group showed higher NOX4 expression than the other groups, and expression was significantly greater than that in the normal mucosa and 5-FU + MT groups (p < 0.05). However, no significant difference was found between groups on day 14 (Fig. 7B, D).
Fig. 7.
A–D Immunohistochemistry for TGF-β and NOX4 on days 10 and 14. On day 10, the TMSC group showed the highest TGF-β and NOX4 expression. *p < 0.05, **p < 0.01. × 200
Migration assay
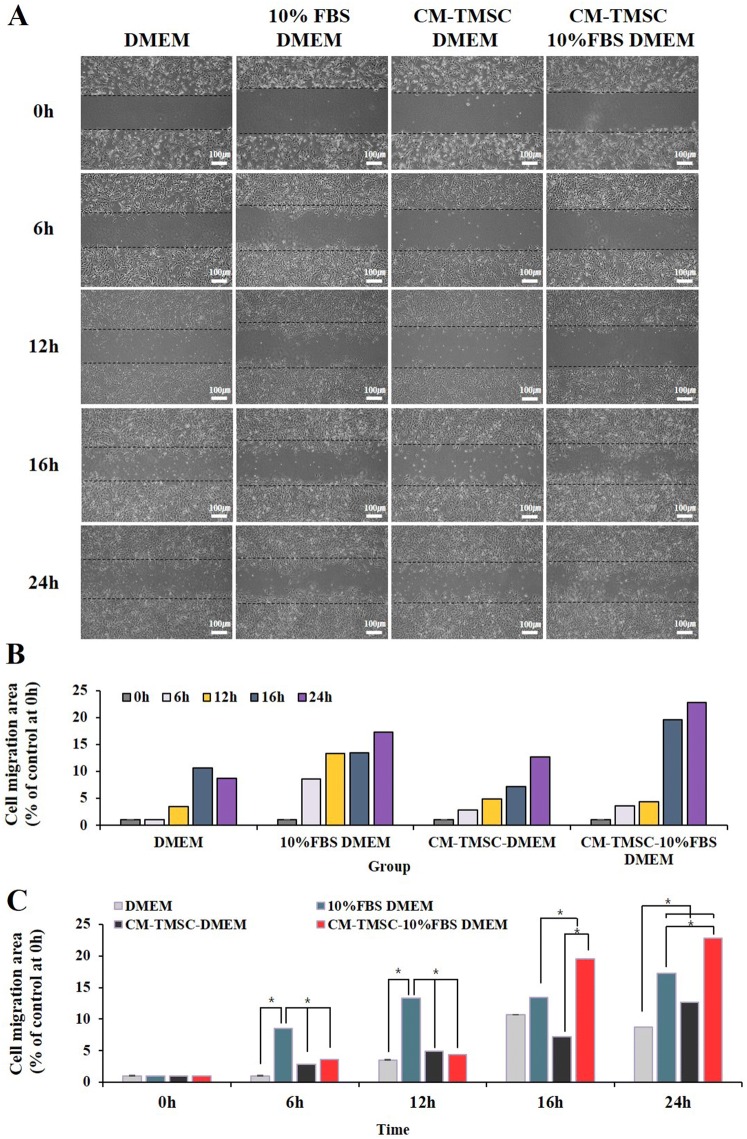
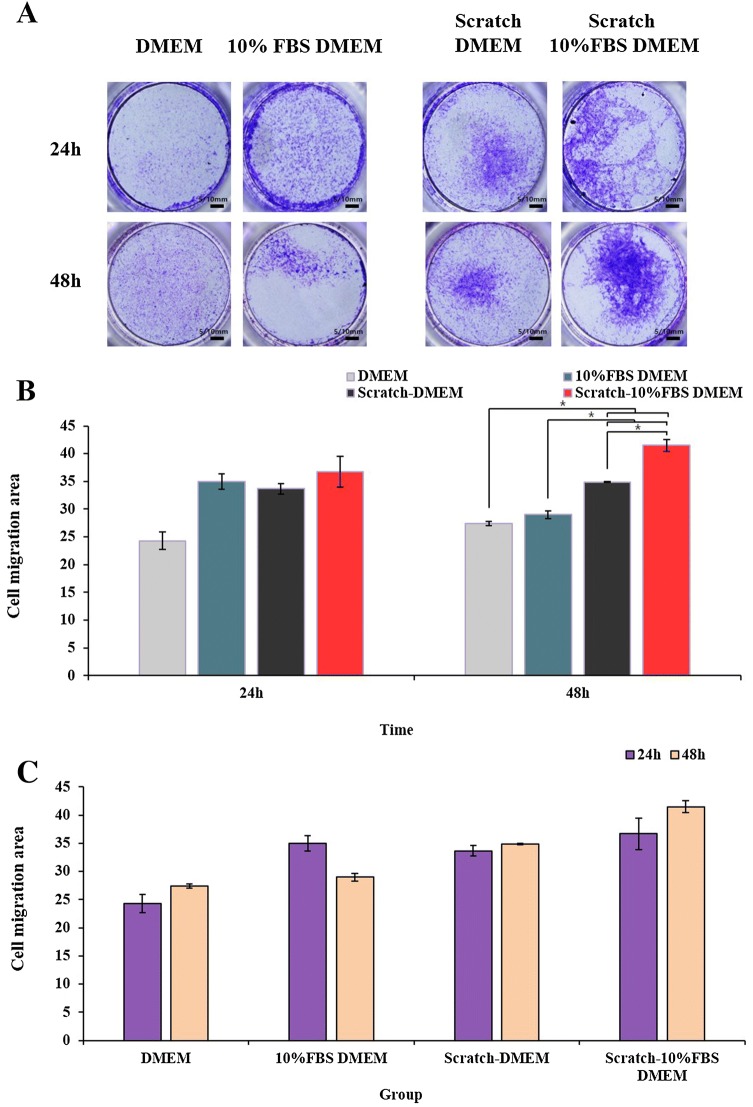
To examine wound healing potency of the conditioned media (CM) harvested from TMSCs, we measured NIH3T3 fibroblast migration using a scratch wound assay. Images of scratch wound on NIH3T3 fibroblast monolayers were captured 6, 12, 16 and 24 h after scratching. The results of scratch wound healing assay displayed that NIH3T3 cells migration into the scratch wound area was accelerated in culture with CM-TMSCs-10% FBS/DMEM compared with other culture conditions (DMEM, 10% FBS/DMEM) (Fig. 8). Transwell assay was performed to evaluate direct migration of TMSCs towards a NIH3T3 fibroblast in the presence or absence of scratch. The results showed that transwell migration of TMSCs was the most prominent with scratch-10% FBS/DMEM compared with other conditions (DMEM, scratch-DMEM, 10% FBS/DMEM) (Fig. 9). The transwell migration assay results were consistent with the in vivo tracking results which indicated that TMSCs were primarily localized to the wound area.
Fig. 8.
A–C Scratch wound healing assay. Migration of NIH3T3 cells treated with DMEM, CM-TMSC-DMEM, 10% FBS DMEM, CM-TMSC-10% FBS/DMEM was analyzed by the scratch wound healing assay. *p < 0.05. CM, conditioned media
Fig. 9.
A–C Transwell assay was performed to evaluate direct migration of TMSCs towards a NIH3T3 fibroblast in the presence or absence of scratch. *p < 0.05
Discussion
The current study showed for the first time that human TMSCs can accelerate wound healing in the 5-FU-induced OM hamster model. Bone marrow- and adipose-derived MSCs have been shown to promote the healing of oral ulcers [22]. Zhang et al. [23] used spheroid gingiva-derived MSCs (GMSCs) in the 5-FU-induced OM mouse model and found that GMSCs decreased the severity and incidence of ulceration compared with the untreated group.
TMSCs have been reported to be beneficial for wound repair in a skin defect model by contributing to immunoregulation and regeneration [15]. The current study showed that the TMSC group had lower gross scores than the 5-FU + MT and DEXA groups on day 10. Histologic results also showed complete epithelialization, with minor inflammation in the TMSC group on day 10, whereas the other groups showed epithelial defect, infiltration of many inflammatory cells, and hyperemia. Dexamethasone is an anti-inflammatory agent that is widely used in clinical practice and has shown to have beneficial preventive effects on 5-FU-induced OM in the hamster [24]. The current study showed that the TMSC group had faster wound healing compared with the DEXA group. The TMSC group showed nearly completely healing of the OM on day 10; however, the DEXA group showed remaining OM. Because OM affects quality of life and successful completion of chemotherapy, the use of TMSCs seems to be a promising option for OM treatment.
Angiogenesis is essential for tissue regeneration as well as wound repair, and we investigated whether TMSC injection could improve neovascularization during wound healing. The TMSC and MT groups showed higher CD31 expression compared with the other groups (5-FU + MT, saline, and DEXA) on day 10. Compared with the MT and DEXA groups, neovascularization was greater in the TMSC group on days 10 and 14. The result of CD31 staining was correlated with the gross result. These results indicate that TMSCs can improve wound repair by promoting neovascularization, and TMSC-mediated angiogenesis seems superior to angiogenesis induced by normal mucositis. In addition, enhanced neovascularization could accelerate wound healing in 5-FU-induced OM.
A pathophysiologic model for OM proposed by Sonis has five phases: initiation, upregulation, activation, signal amplification, ulceration, and healing [26]. Several signaling pathways, including the NFκB pathway, participate in the pathophysiology of OM. Because NFκB is the gatekeeper for the inflammatory pathway, it enhances the proinflammatory cytokines, such as TNF-α, IL-6, and IL-1β. It also induces the expression of COX-2, TGF-β, and MMPs [4, 24, 25]. The role of MMPs is to destroy dead protein debris as a natural physiologic mechanism to clean the ulcer and create a favorable ground for cell growth. However, unfortunately, prolonged high-MMPs also destroy the cell matrix protein, which is essential for cell growth and ulcer healing. Therefore, specific MMP inhibition seems effective to treat OM induced by chemotherapy and/or radiotherapy [24, 27]. Nonetheless, balance of MMPs and their natural inhibitors (tissue inhibitors of metalloproteinase, TIMP) is essential for effective wound healing [25]. MMP2 and MMP9 double knockout mice have been shown delayed primary wound healing compared with wild type mice [28]. MMP2 and MMP9 are involved in the initial inflammatory response and remodeling of the extracellular matrix [29], and MMP2 has been suggested as an indicator of wound stage and changes induced by therapeutic interventions [25].
In this study, the TMSC group showed the highest MMP2 expression on day 10, although the difference was not statistically significant. In addition, the DEXA group showed greater MMP2 expression than the 5-FU + MT group. On day 14, the TMSC and DEXA groups showed lower MMP2 expression than the 5-FU + MT group, and the TMSC group had lower MMP2 expression than the DEXA group. Although the results were not statistically significant, tendency towards a decrease in MMP2 expression with the time in the TMSC and the DEXA group indicated that effective wound improvement in oral ulcer. On the other hand, the trend of increasing MMP2 in the other groups suggests delayed wound healing process. However, further study is needed to clarify the mechanistic roles of MMP2 and other MMPs in wound healing.
Wound repair is a dynamic and complex process. Wound contraction is an essential step to guarantee rapid and effective wound closure, although excessive wound contraction can lead to disfigurement and scar formation [30, 31]. Cellular contraction of specialized contractile fibroblast-myofibroblasts-constitutes is a key element of effective wound contraction. Fibroblast-to-myofibroblast differentiation occurs primarily in response to several cytokines, mainly TGF-β1 [30, 31]. A previous study suggested the potential efficacy of reducing 5-FU-induced OM associated with topical TGF-β application [11]. In addition, NOX4 has been reported to mediate TGF-β1-induced myofibroblast differentiation of cardiac and pulmonary fibrosis [31–33]. Until now, studies of NOX4 focused mainly on fibrosis; however, Lévigne et al. [31] reported that NOX4 is required for effective wound contraction. In this study, the results for expression of TGF-β and NOX4 were similar. On day 10, the TMSC group showed the highest expression of TGF-β and NOX4, which indicated effective wound contraction of OM of the hamster cheek mucosa.
The present study found that the beneficial efficacy of CM harvested from TMSCs. Scratch assay demonstrated that the CM-TMSCs-10%FBS/DMEM had significantly increased migratory efficacy of NIH3T3 cells. In addition, transwell assay showed that the preferential migration of TMSCs to the wound area. Taken together, these results proposed that TMSCs may participate directly and indirectly in wound closure through the augmentation of fibroblast proliferation and migration. Shin et al. [15] also reported that the TMSCs support dermal regeneration via the stimulation of fibroblast proliferation ad migration. Furthermore, our study demonstrated CM derived from TMSCs also accelerated oral ulcer healing. Several studies have shown that MSCs secrete a variety of cytokines and growth factors which are known to enhance wound healing [34].
To generate the OM hamster model, we modified a previous established protocol from Horii et al. [2]: (1) we used a biopsy punch to create uniformly sized mucosal ulcers; (2) we increased 5-FU injection and MT counts (from 2 to 3); and (3) we provided treatment on day 7. Several studies reported that MT (light scratch) was performed with a needle within a 100-mm2 area. However, we believed that this procedure would not generate uniformly sized ulcers. Furthermore, we observed that the most severe oral ulcer occurred on day 7 of our modified protocol (unpublished preliminary data). Taken together, we could effectively generate the OM hamster model in this study. In addition, we suggest that it is better to provide treatment on day 7 to evaluate treatment efficacy on 5-FU-induced OM.
In the process of generating OM hamster model, we hypothesized that the body weight and food intake of the 5-FU + MT group may decreased compared with the other groups because of ulcer pain. However, significant weight loss and reduction of food intake between the groups were not noted throughout the experimental period. These results could be related to the major roles of hamster cheek pouches (HCPs). HCPs are bilateral invagination of the oral mucosa and can be easily be everted. Therefore, HCPs are one of the most well-characterized animal tumor models (oral carcinogenesis), as well as oral mucositis model [35]. Major role of HCPs is storing food and bedding. Therefore, it is possible that the food did not cause significant pain in the animal since HCPs mucosa is not the area where directly contact with food during feeding. Sonis et al. [36] also reported that they could not observe significant weight loss in their 5-FU induced OM hamster model.
To the best of our knowledge, this is the first study to use TMSCs to treat 5-FU-induced OM. We observed rapid wound healing activity of TMSCs in a 5-FU-induced OM hamster model. We could not observe TMSCs injection related adverse events in this study. There are several studies that investigated intra-articular MSCs treatment safety in osteoarthritis, and the most common adverse events were pain and swelling after injection which could be simply resolved by conservative treatments [37].
In conclusion, lesional administration of TMSCs could accelerate wound healing in 5-FU-induced OM through induced angiogenesis and effective wound contraction. In addition, TMSCs might contribute to oral ulcer regeneration via the stimulation of fibroblast proliferation and migration. However, further investigations are needed, including long term follow up period with large sample size, and in vitro study to examine related adverse effects and the molecular mechanisms involved in the accelerated wound healing effects of TMSCs.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Ministry of Science, Information and Communication Technologies and Future Planning of the Korea government (NRF-2017M3A9E8033206); the National Research Foundation of Korea (NRF) grant funded by the Korea government (NRF-2017R1D1A1B04034145); the Hallym University Research Fund.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
Informed written consent was obtained from the legal guardians of all patients who participated in this study, and the study protocol was approved by the institutional review board of Hallym University Medical Center (2016-41). The animal studies were performed after receiving approval of the Institutional review board of Hallym University (Hallym 2018-67), Chuncheon, Republic of Korea.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Elting LS, Cooksley C, Chambers M, Cantor SB, Manzullo E, Rubenstein EB. The burdens of cancer therapy: clinical and economic outcomes of chemotherapy-induced mucositis. Cancer. 2003;98:1531–1539. doi: 10.1002/cncr.11671. [DOI] [PubMed] [Google Scholar]
- 2.Horii K, Kanayama T, Miyamoto H, Kohgo T, Tsuchimochi T, Shigetomi T, et al. Platelet-rich fibrin has a healing effect on chemotherapy-induced mucositis in hamsters. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117:445–453. doi: 10.1016/j.oooo.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Tanideh N, Tavakoli P, Saghiri MA, Garcia-Godoy F, Amanat D, Tadbir AA, et al. Healing acceleration in hamsters of oral mucositis induced by 5-fluorouracil with topical Calendula officinalis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115:332–338. doi: 10.1016/j.oooo.2012.08.450. [DOI] [PubMed] [Google Scholar]
- 4.Biswal BM. Current trends in the management of oral mucositis related to cancer treatment. Malays J Med Sci. 2008;15:4–13. [PMC free article] [PubMed] [Google Scholar]
- 5.Lalla RV, Sonis ST, Peterson DE. Management of oral mucositis in patients who have cancer. Dent Clin North Am. 2008;52:61–77. doi: 10.1016/j.cden.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Beek MT, Laheij AM, Raber-Durlacher JE, von dem Borne PA, Wolterbeek R, van der Blij-de Brouwer CS, et al. Viral loads and antiviral resistance of herpesviruses and oral ulcerations in hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2012;47:1222–1228. doi: 10.1038/bmt.2012.2. [DOI] [PubMed] [Google Scholar]
- 7.Mitsuhashi H, Suemaru K, Li B, Cui R, Araki H. Evaluation of topical external medicine for 5-fluorouracil-induced oral mucositis in hamsters. Eur J Pharmacol. 2006;551:152–155. doi: 10.1016/j.ejphar.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Saarilahti K, Kajanti M, Joensuu T, Kouri M, Joensuu H. Comparison of granulocyte-macrophage colony-stimulating factor and sucralfate mouthwashes in the prevention of radiation-induced mucositis: a double-blind prospective randomized phase III study. Int J Radiat Oncol Biol Phys. 2002;54:479–485. doi: 10.1016/S0360-3016(02)02935-8. [DOI] [PubMed] [Google Scholar]
- 9.Spielberger R, Stiff P, Bensinger W, Gentile T, Weisdorf D, Kewalramani T, et al. Palifermin for oral mucositis after intensive therapy for hematologic cancers. N Engl J Med. 2004;351:2590–2598. doi: 10.1056/NEJMoa040125. [DOI] [PubMed] [Google Scholar]
- 10.Sonis ST, Van Vugt AG, McDonald J, Dotoli E, Schwertschlag U, Szklut P, et al. Mitigating effects of interleukin 11 on consecutive courses of 5-fluorouracil-induced ulcerative mucositis in hamsters. Cytokine. 1997;9:605–612. doi: 10.1006/cyto.1997.0208. [DOI] [PubMed] [Google Scholar]
- 11.Sonis ST, Van Vugt AG, Brien JPO, Muska AD, Bruskin AM, Rose A, et al. Transforming growth factor-β3 mediated cycling and attenuation of 5-fluorouracil mucositis modulation induced of cell oral. Oral Oncol. 1997;33:47–54. doi: 10.1016/S0964-1955(96)00043-7. [DOI] [PubMed] [Google Scholar]
- 12.Blijlevens N, Sonis S. Palifermin (recombinant keratinocyte growth factor-1): A pleiotropic growth factor with multiple biological activities in preventing chemotherapy- and radiotherapy-induced mucositis. Ann Oncol. 2007;18:817–826. doi: 10.1093/annonc/mdl332. [DOI] [PubMed] [Google Scholar]
- 13.Kim WS, Park BS, Sung JH, Yang JM, Park SB, Kwak SJ, et al. Wound healing effect of adipose-derived stem cells: a critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci. 2007;48:15–24. doi: 10.1016/j.jdermsci.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 14.Fathke C, Wilson L, Hutter J, Kapoor V, Smith A, Hocking AIF, et al. Contribution of bone marrow-derived cells to skin: collagen deposition and wound repair. Stem Cells. 2004;22:812–822. doi: 10.1634/stemcells.22-5-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shin SC, Seo Y, Park HY, Jung DW, Shin TH, Son H, et al. Regenerative potential of tonsil mesenchymal stem cells on surgical cutaneous defect. Cell Death Dis. 2018;9:183. doi: 10.1038/s41419-017-0248-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryu KH, Cho KA, Park HS, Kim JY, Woo SY, Jo I, et al. Tonsil-derived mesenchymal stem cells: evaluation of biological, immunological and genetic factors for successful banking. Cytotherapy. 2012;14:1193–1202. doi: 10.3109/14653249.2012.706708. [DOI] [PubMed] [Google Scholar]
- 17.Cho KA, Lee HJ, Jeong H, Kim M, Jung SY, Park HS, et al. Tonsil-derived stem cells as a new source of adult stem cells. World J Stem Cells. 2019;11:506–518. doi: 10.4252/wjsc.v11.i8.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jung N, Park S, Choi Y, Park JW, Hong YB, Park HH, et al. Tonsil-derived mesenchymal stem cells differentiate into a schwann cell phenotype and promote peripheral nerve regeneration. Int J Mol Sci. 2016;17:E1867. doi: 10.3390/ijms17111867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park HS, Lee J, Kim JW, Kim HY, Jung SY, Lee SM, et al. Preventive effects of tonsil-derived mesenchymal stem cells on osteoradionecrosis in a rat model. Head Neck. 2018;40:526–535. doi: 10.1002/hed.25004. [DOI] [PubMed] [Google Scholar]
- 20.Park M, Kim YH, Woo SY, Lee HJ, Yu Y, Kim HS, et al. Tonsil-derived mesenchymal stem cells ameliorate CCl4-induced liver fibrosis in mice via autophagy activation. Sci Rep. 2015;5:8616. doi: 10.1038/srep08616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Samivel R, Kim EH, Chung YJ, Mo JH. Immunomodulatory effect of tonsil-derived mesenchymal stem cells in a mouse model of allergic rhinitis. Am J Rhinol Allergy. 2015;29:262–267. doi: 10.2500/ajra.2015.29.4216. [DOI] [PubMed] [Google Scholar]
- 22.Suma GN, Arora MP, Lakhanpal M. Stem cell therapy: a novel treatment approach for oral mucosal lesions. J Pharm Bioallied Sci. 2015;7:2–8. doi: 10.4103/0975-7406.149809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Q, Nguyen AL, Shi S, Hill C, Wilder-Smith P, Krasieva TB, et al. Three-dimensional spheroid culture of human gingiva-derived mesenchymal stem cells enhances mitigation of chemotherapy-induced oral mucositis. Stem Cells Dev. 2012;21:937–947. doi: 10.1089/scd.2011.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ribeiro SB, de Araújo AA, Araújo Júnior RF, Brito GAC, Leitão RC, Barbosa MM, et al. Protective effect of dexamethasone on 5-FU induced oral mucositis in hamsters. PLoS One. 2017;12:e0186511. doi: 10.1371/journal.pone.0186511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karim RB, Brito BL, Dutrieux RP, Lassance FP, Hage JJ. MMP-2 assessment as an indicator of wound healing: a feasibility study. Adv Skin Wound Care. 2006;19:324–327. doi: 10.1097/00129334-200607000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Sonis ST. The pathobiology of mucositis. Nat Rev Cancer. 2004;4:277–284. doi: 10.1038/nrc1318. [DOI] [PubMed] [Google Scholar]
- 27.Shrivastava R, Deshmukh S. A new therapeutic approach to treat oral mucositis using specific MMP blockers in an osmotically active solution. J Cancer Res Treat. 2013;1:4–11. [Google Scholar]
- 28.Hingorani DV, Lippert CN, Crisp JL, Savariar EN, Hasselmann JPC, Kuo C, et al. Impact of MMP-2 and MMP-9 enzyme activity on wound healing, tumor growth and RACPP cleavage. PLoS One. 2018;13:e0198464. doi: 10.1371/journal.pone.0198464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Asimakidou M, Oikonomou L, Filipopoulos A, Tsikopoulos G, Petropoulos AS. Regulation of matrix metalloproteinase-2 and -9 during healing of dermal wounds after incision using radiofrequency energy in neonatal and adult rats. Hippokratia. 2017;21:85–92. [PMC free article] [PubMed] [Google Scholar]
- 30.Hinz B. Masters and servants of the force: the role of matrix adhesions in myofibroblast force perception and transmission. Eur J Cell Biol. 2006;85:175–181. doi: 10.1016/j.ejcb.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 31.Lévigne D, Modarressi A, Krause KH, Pittet-Cuénod B. NADPH oxidase 4 deficiency leads to impaired wound repair and reduced dityrosine-crosslinking, but does not affect myofibroblast formation. Free Radic Biol Med. 2016;96:374–384. doi: 10.1016/j.freeradbiomed.2016.04.194. [DOI] [PubMed] [Google Scholar]
- 32.Cucoranu I, Clempus R, Dikalova A, Phelan PJ, Ariyan S, Dikalov S, et al. NAD(P)H oxidase 4 mediates transforming growth factor-β1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res. 2005;97:900–907. doi: 10.1161/01.RES.0000187457.24338.3D. [DOI] [PubMed] [Google Scholar]
- 33.Hecker L, Vittal R, Jones T, Jagirdar R, Luckhardt TR, Horowitz JC, et al. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med. 2009;15:1077–1081. doi: 10.1038/nm.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sagaradze G, Grigorieva O, Nimiritsky P, Basalova N, Kalinina N, Akopyan Z, et al. Conditioned medium from human mesenchymal stromal cells: towards the clinical translation. Int J Mol Sci. 2019;20:E1656. doi: 10.3390/ijms20071656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gimenez-conti IB, Slaga TJ. The hamster cheek pouch carcinogenesis model. J Cell Biochem Suppl. 1993;17F:83–90. doi: 10.1002/jcb.240531012. [DOI] [PubMed] [Google Scholar]
- 36.Sonis ST, Tracey C, Shklar G, Jenson J, Florine D. An animal model for mucositis induced by cancer chemotherapy. Oral Surg Oral Med Oral Pathol. 1990;69:437–443. doi: 10.1016/0030-4220(90)90376-4. [DOI] [PubMed] [Google Scholar]
- 37.Jevotovsky DS, Alfonso AR, Einhorn TA, Chiu ES. Osteoarthritis and stem cell therapy in humans: a systematic review. Osteoarthr Cartilage. 2018;26:711–729. doi: 10.1016/j.joca.2018.02.906. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.