Abstract
The goal of this study was examining the effects of sodium alginate coating (SA) containing resveratrol (R) on enhancement rainbow trout fillets’ shelf-life. Treatments of the study were as follows: control, SA, SA-R 0.001% and SA-R 0.003%. Storage of the samples was done for 15 days at 4 °C. To analyze samples, 3-day intervals were used. Compared to the uncoated trout, the values of pH, peroxide and K were significantly lower in the coated samples (p < 0.05). R enhanced the impacts of alginate on extending the samples’ shelf life. Sensory analyses showed that R improved the sensory scores significantly (p < 0.05); besides, it did not show more changes on the sensory features and was invisible in the surface of samples. In the conclusion, R was suggested to be a strong alternative to synthetic antioxidants in refrigerated trout fillet in very low concentrations with many health benefits.
Keywords: Resveratrol, Sodium alginate coating, Rainbow trout fillet, Strong alternative, Synthetic antioxidants
Introduction
Rainbow trout (Oncorhynchus mykiss) is considered as a main comestible fish species worldwide. Recent approaches and studies have tried to enhance its production and processing quality (Volpe et al., 2015). Fish fillets can be spoiled quickly that can be correlated to its high levels of moisture, poly unsaturated fatty acids (PUFAs), simple nutrients (such as non-protein-nitrogen compounds) and pH; then, improving preservation techniques for prolonging their shelf-life appears to be very essential (Song et al., 2011). Some common approaches to enhance the shelf life of aquaculture products consist of freezing and cold storage. Nevertheless, they are not able to fully inhibit these products’ oxidative spoilages. Moreover, freezing, chilling, and super chilling methods can damage the tissue and different essential compounds including minerals, vitamins, and proteins of the fish fillet which make drop marketing of these products inevitable (Volpe et al., 2015). Besides, other techniques such as smoking, salting, irradiation or a combination of them can not completely inhibit oxidative reactions of fish fillets. This can also occur for the existence of numerous unsaturated fatty acids in fish, rapidly oxidizing in the presence of the environmental oxygen (Ojagh et al., 2010). Another common solution for the meat products’ preservation is synthetic preservative usage. Various synthetic antioxidants, such as butylated hydroxytoluene (BHT) butylated hydroxyanisole (BHA), tert-butylhydroxyquinone (TBHQ) and propyl gallate (PG) have been utilized in kinds of food products in order to stop lipid oxidation especially in the field of the preservation of manufactured meat. But, for their side effects like carcinogenicity and teratogenicity, the consumers complain about these synthetic preservatives (Kindleysides et al., 2012; Topuz et al., 2014).
Accordingly, food industries seek some solutions for solving this problem (Giatrakou and Savvaidis, 2012; Ojagh et al., 2010). Natural antioxidant additives such as various essential oils and herbal extracts are a new approach of food researchers for prolonging the shelf life of meat products. The major problems of their application in foods are organoleptic conversion of food, their potential toxicity, application costs, and vigorous aroma (Bazargani-Gilani et al., 2015; Petrou et al., 2012). A useful approach to minimize these problems can be isolating herbal products’ active ingredients for decreasing the required herbal product concentration in food models.
Resveratrol (trans-3,4,5-trihydroxystilbene), a phytoalexin compound and dietary supplement from stilbenes’ family is found in foodstuffs like dark chocolate, grapes, mulberries, red wine, green tea, leaves of edible plants, and peanuts with their known anti-inflammatory, antimicrobial, and antioxidant characteristics (Gerszon et al., 2014; Gulcin, 2010; Iuga et al., 2012). Low molecular weight secondary metabolites such as phytoalexins are yielded by the plants is a defense approach against diseases and infections. This defensive compound with its herbal origin is mostly verified to be safe for the food industry uses (GRAS) (FDA, 2010). As a strong antioxidant, resveratrol prevents platelet aggregation, increases high-density lipoprotein cholesterol and cardio-protecting factors, and promotes nitric oxide production. Also, some previous studies demonstrated that resveratrol is a chemo preventive, antiviral, anti-inflammatory, and neuroprotective factor (Gerszon et al., 2014; Gulcin, 2010; Surendran Nair et al., 2016).
Natural antioxidant activities have increased by their incorporation in many edible coatings and films in foods. Implant of food additives into films or coatings may raise antioxidant and antimicrobial properties of them; it may also reduce the leakage of water vapor, retarding the oxidation of the food lipid on which the films or coatings are utilized (Giatrakou and Savvaidis, 2012; Jeon et al., 2002; Ojagh et al., 2010). Alginate is a polymer of L-guluronic acid and D-mannuronic acid, a salt of alginic acid, as well as a GRAS substance, isolated from brown algae (FDA, 2010; Lu et al., 2009). Owning ideal colloidal activities, alginate develops hard gels and insoluble polymers by cross-linking with Ca+2 after treating with CaCl2 solution. Films which have biopolymer basis are able to preserve food freshness and prolong foods’ shelf life through microbial contamination inhibition, drop decreasing, cooking and weight loss, water barrier making, thawing, lipid and protein oxidation’s retarding or preventing that help keeping the foods’ organoleptic properties (Song et al., 2011; Yu et al., 2008).
Thus, this study sought to introduce resveratrol as a dietary supplement and a strong natural food preservative incorporated into edible coating of sodium alginate for the shelf life prolongation of the rainbow trout fillet, stored in the refrigerator.
Materials and methods
Materials
Alginate, glycerol, calcium chloride, butylated hydroxytoluene (BHT), standard adenosine diphosphate (ADP), adenosine triphosphate (ATP), hypoxanthine riboside (HXR), inosine monophosphate (IMP), adenosine monophosphate (AMP), and hypoxanthine (HX) were obtained from Sigma-Aldrich Chemie (Steinheim, Germany). Analytical grade methanol, chloroform, hexane-iso-propanol, sodium sulfate, ammonium thiocyanate, iron chlorid (II), sodium hydroxide, perchloric acid were purchased Merck (Darmstadt, Germany). Resveratrol (3,5,4′-trans-trihydroxystilbene) was procured from Sigma-Aldrich GmbH (Steinheim, Germany).
Preparation of fillet
Fresh-water trout (approximately 500 g) was taken alive to the lab after buying from a local fish farm, Rasht, Iran. Then, the fish were filleted after decapitating, gutting, and skinning by hands. They were finally washed via potable water in the lab. Two fillets were achieved in this method from each fish.
Coating of fillet
Edible coating solution was prepared with sterile distilled water and 1.5% (w/v) alginate. It was then stirred at 70 °C and 1200 rpm within 30 min for obtaining complete dispersion of alginate. Beakers containing the solution were placed on a hotplate/magnetic stirrer. After adding glycerol, dissolving resveratrol (3,5,4′-trans-trihydroxystilbene) into sterile distilled water, it was added into the alginate solution. The obtained coating solution contained 1.5% glycerol, 1.5% algiante, singly or mixed with 0.001% and 0.003% R. Then, the fillet samples were divided into 4 groups randomly: one control (coated in sterile distilled water) and three experimental groups treated with the solutions of SA, SA-R 0.001%, and SA-R 0.003%. After a 2-min immersion in coating solutions of sterile distilled water, SA, SA-R 0.001% and SA-R 0.003%, each sample was fully drained, followed by another CaCl2 1.5% w/v immersion for 30 s to obtaining an improved crosslinking. Next, for making the edible coats, the fillet samples’ drain for 5 h was done at 10 °C. After aerobically-packing the samples in a polyethylene packages, they were stored at 4 °C for quality analyses. Then, their chemical and sensorial attributes were analyzed for assessing the total quality of the samples for 15 days and they were analyzed every 3 days during storage (Gulcin, 2010; Heydari et al., 2015; Song et al., 2011).
Physicochemical analysis
Determination of pH value
The pH value was measured, implementing a pH meter (PH-Meter E520, Metrohm Herisau, Bern, Switzerland). The fillets (5 g) were homogenized utilizing 25 mL of distilled water for 30 s. Then, homogenates were used to determine pH (Brannan, 2008).
Peroxide value determination
Fat from fillets was extracted with hexane-iso-propanol (3 + 2, v/v) containing 0.01% butylated hydroxytoluene, followed by washing with sodium sulfate solution (Hara and Radin, 1978). Based on International Dairy Federation (IDF), peroxide value (PV) was estimated (Shantha and Decker, 1994). The sample (extracted fat) (0.30 g) was vortexed by a glass tube for 2–4 s after mixing with 9.8 mL chloroform–methanol (3:7). After adding 0.05 mL of ammonium thiocyanate solution (10 mM), the sample was vortexed for 2–4 s. In next step, iron solution (II) (0.05 mL) was added to the mixture and was vortexed for 2–4 s again. Then, the samples were incubated at room temperature for 5 min. The mixture’s absorbance was read at 500 nm, using an UV–visible spectrophotometer (LKB Novaspec II; Pharmacia, Stockholm, Sweden). PV was reported as milliequivalents of O2 per kilogram of fat.
Determining K value
After some modifications, K value (i.e. the percentage of the ratio between Inosine + Hypoxanthine to the total ATP and its decomposed byproducts) was measured according to the method of Fan et al. (2008). After a 15-min centrifugation at 1500 g, 1 g of fish fillet was homogenized by 2 mL of 10% cold (4 °C) perchloric acid solution. After washing the sediment by 2 mL of cold 5% perchloric acid solution, it was centrifuged at 1500 g for 15 min again. The supernatants were all transferred into a centrifuge tube after repeating this process for 3 times and pH value was set to 6.4–6.5 via 1 mol/L NaOH. The white crystal was removed by centrifugation (1500 g, 15 min). After making up the supernatant to 10 mL via deionized water, it was filtered by a 0.45 membrane filter which was stored in − 20 °C for the next analyses.
Using HPLC (Knauer, Berlin, Germany), equipped with CAPCELL, pack ODS C18 column (4.0 × 100 mm, 3 μm) Adenosine-triphosphate (ATP) and its associated compounds were analyzed. The prepared sample (5 μl) was flowed at a speed of 1.2 ml/min, and the peak was observed at 254 nm. ATP and the amounts of its compounds were identified; then, referring to standard adenosine diphosphate (ADP), adenosine triphosphate (ATP), hypoxanthine riboside (HXR), inosine monophosphate (IMP), adenosine monophosphate (AMP), and hypoxanthine (HX), they were computed. K value was computed by the following equation:
Sensory analysis
Quality assessment of fish freshness in the industry and the inspection is the EU quality grading scheme, according to the Council Regulation (EC) No 2406/96 of November 26, 1996 (Anon, 1996). A group consisting of 10 (four female and six males between 26–46 years old from food hygiene and quality control department Master degree students) laboratory-trained and experienced judges in meat assessment conducted organoleptic analysis. All of these judges who assessed the sensory properties of fish fillets had participated in training meetings to learn about the sensory features of fish fillets. The panelists were especially trained in the principles of QIM evaluation (Martinsdóttir et al., 2001). The Quality Index Method (QIM) has been recommended for a European initiative regarding standardization and harmonization of sensory evaluation of fish. QIM-schemes have been developed for various common European fish specious. The panel had previously participated in QIM evaluations of cod, salmon, trout, plaice, flounder and dab. This training was conducted during 5 training sessions (each one 4 h) prior to the first trial (Nielsen and Hyldig, 2004).
Raw fish fillet
Four raw trout fillets were analyzed in white porcelain trays by panelists. The panelists were not informed about the experimental approach and the samples were blind-coded with 3-digit random numbers. Fresh fish fillet was concerned as the reference. The panelists assessed color, odor and general acceptability of those fillets. In case of reaching the scores of the sensory property below 3.0, fish fillets were rejected.
Cooked fish fillet
After (1.5%) salt addition, the fish fillets were steam-cooked by microwave oven (Solar DOM, LG, Seoul, Korea) for 20 min at 95–100 °C to go through sensory evaluation. The evaluations were performed under normal light and ambient temperature. Four steam cooked trout fillets were served in white porcelain trays. The panelist used water and piece of bread to clean their palate between samples. The panelists were not informed about the experimental approach and the samples were blind-coded with 3-digit random numbers. The panelists assessed color, odor, taste, and general acceptability of those cooked fillets. In case of reaching the scores of the sensory property below 4.0, fish fillets were rejected (Ojagh et al., 2010).
In order to determine the samples´ color or discoloration (1: extreme discoloration, 5: no discoloration), odor (1: extremely unacceptable/off-odors, 5: extremely desirable), taste (1: very poor, 5: excellent), and general acceptability (1: extremely unacceptable, 5: extremely desirable), a 5-point Hedonic scale was utilized.
Statistical analysis
Implementing different batches of fish samples, this study was replicated twice. Conducting all analyses in triplicate for every repetition (n = 2 × 3), the results’ mean values ± standard deviation (SD) were determined. Statistical analysis of data was provided via SPSS software (IBM SPSS statistics 21). All data underwent to the analysis of variance (ANOVA). Tukey’s test and Independent sample t test were utilized at p < 0.05 in order to compare the differences of mean values.
Results and discussion
Physicochemical analysis
PH value
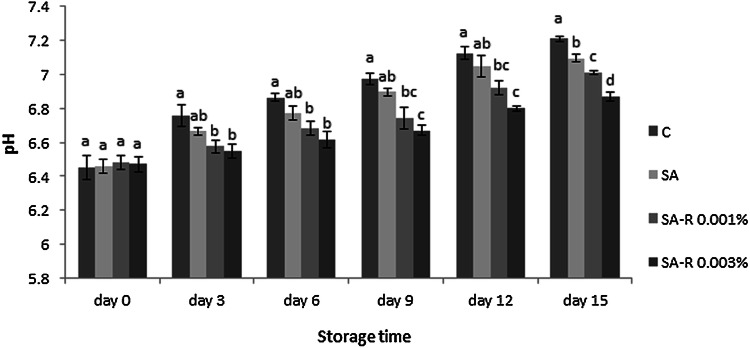
Variations of pH value in trout fillets stored at 4 °C are reflected in Fig. 1. Although, initial pH value of the fresh fish fillet (pH 6.45–6.48) consisted with the results of Hosseini et al. (2016) and Volpe et al. (2015), but was slightly more than the reported values by Chytiri et al. (2004). Observed differences in the results may stem from the amount of dissociation and dissolution of CO2 within the fish samples. In the post-mortem period, pH values of all samples increased; this result may be for the endogenous or microbial enzymes’ actions such as protease and lipase in the existence of oxygen which induce increased volatile bases (e.g. ammonia and trimethylamine) in long-term storage (Fan et al., 2008). Nitrogenous compounds’ decomposition increases pH, influencing the product’s quality during storage time besides negative affection of the sensorial properties like color, taste, odor, texture, and acceptability (Volpe et al., 2015). In this research, after storage time, control samples´ pH raised from 6.40 to 7.21; but, for the SA, SA-R 0.001% and SA-R 0.003% samples, the pH reached 7.09, 7.01 and 6.8, respectively in 15 days. These results were achieved because of the protective effect of SA edible coating against spoiling action of the oxygen, significantly enhanced by resveratrol specifically with a higher-dose treatment (p < 0.05). The lower pH value of other treatments (SA, SA-R 0.001% and SA-R 0.003%) could relate to the inhibition of microbial and endogenous proteases activities of trout fillet at different levels during the study ´s treatment, because these enzymes can break down food proteins to alkaline compounds that lead to pH increasing of foods. Fan et al. (2009) and Song et al. (2011) also got the same results about alginate coated silver carp and chitosan coated bream respectively. They reported that the used treatments have inhibited pH enhancement in studied fillets using the mentioned reasons.
Fig. 1.
pH values of refrigerated trout fillets during storage period. Treatments: control (C), sodium alginate (SA), sodium alginate containing resveratrol 0.001% (SA-R 0.001%), sodium alginate containing resveratrol 0.003% (SA-R 0.003%). Different letters represent a statistically significant difference (p < 0.05) for analysis days
Peroxide value
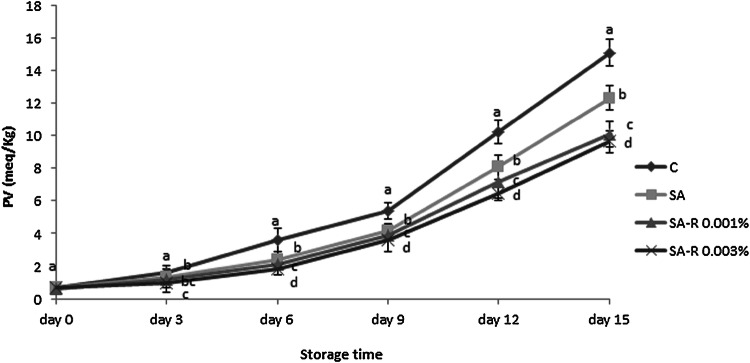
As one of the reasons for the spoilage of seafood and food, the rancidity of lipid oxidation can be mentioned (Connell, 1995). The peroxide value (PV) gauges the quantity of the hydroperoxides which are regarded as the auto-oxidation’s primary products (Kubow, 1992; Yanishlieva and Marinova, 2001). Figure 2 reflects peroxide values (PV) related to fish fillet samples during 15-day storage in the refrigerator. As seen in Fig. 2, PV of the control treatment rapidly increased compared to other treatments during 15 storage days. After 15 days, the PV of control samples reached from 0.69 to 15.7 meq peroxides/kg lipid; whereas, the PV of SA, SA-R 0.001% and SA-R 0.003% enhanced from 0.68 to 12.315, from 0.625 to 10.055, and from 0.655 to 9.62 meq peroxides/kg lipid after 15 storage days, respectively. In comparison to the control, all treatments reduced peroxide formation significantly during 15 days of storage (p < 0.05). Others demonstrated the inhibitory impacts of alginate edible coating against hydroperoxide formation in silver carp and bighead fillets after refrigerated storage (Heydari et al., 2015; Jalali et al., 2016). The edible coating shows a function like a barrier against water and oxygen permeability, retarding moisture retaining and oxidation reactions. This is the main mechanism used by different coatings such as alginate and chitosan for enhancing the quality and storage life (Bazargani-Gilani et al., 2015; Heydari et al., 2015; Jalali et al., 2016; Ojagh et al., 2010).
Fig. 2.
Peroxide values (meq O2/kg lipid) of refrigerated trout fillets during storage period. Treatments: control (C), sodium alginate (SA), sodium alginate containing resveratrol 0.001% (SA-R 0.001%), sodium alginate containing resveratrol 0.003% (SA-R 0.003%). Different letters represent a statistically significant difference (p < 0.05) for analysis days
Maximum decreased peroxide formation was found using SA-R 0.003% treatment. While the next ranks belonged to SA-R 0.001% and SA, respectively. This finding may be correlated to the R 0.003%’ s potent antioxidant activity. Gulcin (2010) observed more antioxidant activity of R 0.003% compared to the strong synthetic antioxidants including α-tocopherol, BHT (butylated hydroxytoluene), BHA (butylated hydroxyanisole), and trolox after different valid antioxidant tests (Gulcin, 2010).
K value
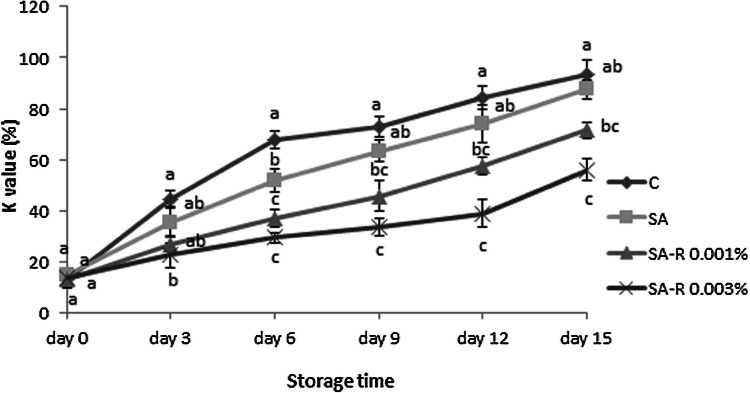
Nucleotides’ degradation in the muscle tissues till producing hypoxanthine by endogenous enzymes is a postmortem change of the fish body during-storage in the respective reactions that begin before bacterial spoilage (Manju et al., 2007). Following bacterial growth enhancement, the exogenous enzymes joined the endogenous ones. Computing those resulting compounds yields an index of fish freshness and quality, K value; it is estimated as the ratio (× 100) of nonphosphorylated ATP ´s decomposed products to the total decomposed products of ATP (Ocano-Higuera et al., 2011). Figure 3 demonstrates the K value changes for the rainbow trout fillet during storage period. Previous research reported that the rainbow trout flesh with a K value lower than 20% considered as very fresh, the values 20–50% were concerned as relatively fresh and values above 70% is named as undesirable (Gui et al., 2014). This study found the initial K value of 13.15–14.675% for the trout samples. Gui et al. (2014) and Shen et al. (2015) found the initial K values of all studied rainbow trout fillets to be 13.00–17.38%; thus, their reports agree with those of present research (Gui et al., 2014; Shen et al., 2015). As seen in Fig. 3, all samples of the studied groups especially control group showed a fast growth of K value on the 0–6th days of storage. Then, that growing rate became slower. The same slowing pattern was also seen in other studies on the silver carp (Fan et al., 2008), bream (Song et al., 2011) and rainbow trout (Gui et al., 2014; Shen et al., 2015). However, other fish species such as black skipjack (Mazorra-Manzano et al., 2000) and large yellow croaker (Li et al., 2012) showed a gradually growing K value during early storage; there was no change later and the rate of growth did not get faster. Those differences may stem from the type of fish species, initial pollution, and storage conditions.
Fig. 3.
K values (%) of refrigerated trout fillets during storage period. Treatments: Control (C), Sodium alginate (SA), Sodium alginate containing resveratrol 0.001% (SA-R 0.001%), Sodium alginate containing resveratrol 0.003% (SA-R 0.003%). Different letters represent a statistically significant difference (p < 0.05) for analysis days
In this study all treatments showed significantly lower K values (p < 0.05) than the control group at all storage period. The K values of the control (72.745%), SA (74.1%) and SA-R 0.001% (71.495%) samples were exceeded the permissible level maximum (70%) during the 9, 12 and 15th days, respectively, While, the K value of SA-R 0.003% samples never reached 70% during storage. There were significant differences (p < 0.05) among all groups after 9 days and until the end of the storage time. To inhibit the nucleotide decomposition, ranking of treatments were SA-R 0.003%, SA-R 0.001%, and AL, respectively. As seen in prior studies, decomposition of IMP to Inosine and Hypoxanthine can occur for 5-nucleotidase’s enzyme activity. Therefore, lower K value of the treatments can be linked to the inhibitory influence of edible coating with alginate basis on the 5-nucleotidase enzyme activity (Fan et al., 2009). Also, results proved more effectiveness of R-containing treatments such as SA-R 0.001% and especially SA-R 0.003% in inhibiting ATP degradation and retaining desired quality of trout fillets.
Sensory features
The sensory features changes in the raw and cooked trout during the entire storage are represented in Tables 1 and 2. Missed scores in the taste feature found in Table 2 are related to the during-storage off-flavor fillets. The odor, taste, color and general acceptability numbers decreased in (C, SA), (SA-R 0.001%), and (SA-R 0.003%) after 3, 6 and 9th days, respectively. Panelists recognized the taste, color, odor, and general acceptability of the control fillets as unacceptable on the 6th day.
Table 1.
Sensory attribute scores of raw refrigerated trout fillets
| Sensory attributes | Treatment1 | Storage period (days) | |||||
|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 12 | 15 | ||
| Color | C | 4.70 ± 0.42Aa2 | 3.80 ± 0.52Bc | 2.90 ± 0.62Cc | 2.00 ± 0.59Dc | 1.30 ± 0.31Ec | 1.00 ± 0.00Fd |
| SA | 4.70 ± 0.51Aa | 4.00 ± 0.42Bb | 3.70 ± 0.47BCbc | 3.40 ± 0.51Cb | 2.50 ± 0.42Db | 2.00 ± 0.51Dc | |
| SA-R 0.001% | 4.80 ± 0.48Aa | 4.60 ± 0.47ABab | 4.30 ± 0.48Bab | 4.00 ± 0.31Ba | 3.70 ± 0.48Cab | 3.20 ± 0.31Cb | |
| SA-R 0.003% | 5.00 ± 0.00Aa | 4.70 ± 0.52Aa | 4.60 ± 0.52ABa | 4.50 ± 0.66ABa | 4.00 ± 0.52Ba | 4.00 ± 0.52Ba | |
| Odor | C | 4.70 ± 0.52Aa | 3.50 ± 0.66Bb | 2.10 ± 0.56Cc | 1.50 ± 0.66Dc | 1.00 ± 0.00Ed | 1.00 ± 0.00Ed |
| SA | 4.70 ± 0.66Aa | 4.30 ± 0.51ABab | 4.00 ± 0.31Bb | 3.50 ± 0.66BCbc | 2.60 ± 0.42Cc | 1.80 ± 0.31Dc | |
| SA-R 0.001% | 5 ± 0.00Aa | 4.60 ± 0.42ABa | 4.20 ± 0.66ABab | 3.80 ± 0.52Bb | 3.50 ± 0.31Bb | 3.30 ± 0.42Cb | |
| SA-R 0.003% | 5 ± 0.00Aa | 4.60 ± 0.31Aa | 4.50 ± 0.42ABa | 4.50 ± 0.47ABa | 4.00 ± 0.51Ba | 4.00 ± 0.66Ba | |
| Overall | C | 4.80 ± 0.42Aa | 3.50 ± 0.48Bc | 2.60 ± 0.31Cc | 1.60 ± 0.42Dc | 1.00 ± 0.00Ed | 1.00 ± 0.00Ed |
| SA | 4.80 ± 0.67Aa | 4.50 ± 0.63Bb | 4.00 ± 0.51BCb | 3.50 ± 0.66Cb | 2.50 ± 0.31Dc | 1.90 ± 0.73Ec | |
| SA-R 0.001% | 4.90 ± 0.73Aa | 4.60 ± 0.56ABab | 4.30 ± 0.48Bab | 4.00 ± 0.67BCab | 3.60 ± 0.56Cb | 3.50 ± 0.63Db | |
| SA-R 0.003% | 5 ± 0.51Aa | 4.80 ± 0.31Aa | 4.50 ± 0.56Ba | 4.20 ± 0.31BCa | 4.00 ± 0.73Ca | 3.80 ± 0.51CDa | |
1Treatments: control (C), sodium alginate (SA), sodium alginate containing resveratrol 0.001% (SA-R 0.001%), sodium alginate containing resveratrol 0.003% (SA-R 0.003%)
2Means within the same row (A, B, C, D, E) and the same column (a, b, c, d) with different letters are significantly different (p < 0.05)
Table 2.
Sensory attribute scores of cooked refrigerated trout fillets
| Sensory attributes | Treatment1 | Storage period (days) | |||||
|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 12 | 15 | ||
| Taste | C | 4.90 ± 0.31Aa2 | 4.10 ± 0.31Bc | – | – | – | – |
| SA | 4.60 ± 0.51Aa | 4.40 ± 0.51Abc | 4.10 ± 0.31Ab | – | – | – | |
| SA-R 0.001% | 4.80 ± 0.42Aa | 4.80 ± 0.42Aab | 4.60 ± 0.69ABab | 4.10 ± 0.31BCb | 4.00 ± 0.00Cb | – | |
| SA-R 0.003% | 5.00 ± 0.00Aa | 4.90 ± 0.31Aa | 4.90 ± 0.31Aa | 4.70 ± 0.48ABa | 4.40 ± 0.51Ba | – | |
| Color | C | 4.70 ± 0.48Aa | 4.00 ± 0.00Bc | 3.50 ± 0.52Bc | 2.60 ± 0.69Cc | 1.40 ± 0.51Dc | 1.10 ± 0.31Dd |
| SA | 4.90 ± 0.31Aa | 4.30 ± 0.48ABbc | 4.00 ± 0.47BCbc | 3.50 ± 0.52Cb | 2.40 ± 0.51Db | 1.80 ± 0.42Dc | |
| SA-R 0.001% | 4.90 ± 0.31Aa | 4.70 ± 0.48Aab | 4.50 ± 0.52ABab | 4.30 ± 0.48ABa | 4.00 ± 0.66BCa | 3.40 ± 0.51Cb | |
| SA-R 0.003% | 5.00 ± 0.00Aa | 4.80 ± 0.42ABa | 4.80 ± 0.42ABa | 4.60 ± 0.51ABa | 4.30 ± 0.48BCa | 4.00 ± 0.47Ca | |
| Odor | C | 4.90 ± 0.31Aa | 4.00 ± 0.47Bb | 3.00 ± 0.66Cc | 2.10 ± 0.73Dc | 1.20 ± 0.42Ec | 1.10 ± 0.31Ed |
| SA | 4.80 ± 0.42Aa | 4.50 ± 0.52ABab | 4.00 ± 0.66Bb | 3.00 ± 0.66Cb | 2.10 ± 0.56Db | 1.70 ± 0.48Dc | |
| SA-R 0.001% | 4.90 ± 0.31Aa | 4.70 ± 0.48ABa | 4.60 ± 0.51ABab | 4.20 ± 0.78ABa | 4.00 ± 0.47Ba | 3.10 ± 0.56Cb | |
| SA-R 0.003% | 4.90 ± 0.31Aa | 4.80 ± 0.42Aa | 4.80 ± 0.42Aa | 4.60 ± 0.69ABa | 4.40 ± 0.51ABa | 4.00 ± 0.47Ba | |
| Overall | C | 4.70 ± 0.48Aa | 4.00 ± 0.66Bb | 3.20 ± 0.42Cc | 2.40 ± 0.51Dc | 1.40 ± 0.69Ec | 1.00 ± 0.00Ed |
| SA | 4.90 ± 0.31Aa | 4.30 ± 0.67ABab | 4.00 ± 0.66BCb | 3.30 ± 0.67Cb | 2.20 ± 0.63Db | 1.70 ± 0.67Dc | |
| SA-R 0.001% | 4.80 ± 0.42Aa | 4.70 ± 0.48Aa | 4.50 ± 0.52Aab | 4.30 ± 0.48Aa | 4.10 ± 0.73Aa | 3.10 ± 0.56Bb | |
| SA-R 0.003% | 4.90 ± 0.31Aa | 4.80 ± 0.42Aa | 4.70 ± 0.48Aa | 4.60 ± 0.51ABa | 4.40 ± 0.51ABa | 4.10 ± 0.31Ba | |
1Treatments: control (C), sodium alginate (SA), sodium alginate containing resveratrol 0.001% (SA-R 0.001%), sodium alginate containing resveratrol 0.003% (SA-R 0.003%)
2Means within the same row (A, B, C, D, E) and the same column (a, b, c, d) with different letters are significantly different (p < 0.05)
These findings consisted with the chemical findings of samples (Table 3 (A–C)). In fact, the sensory scores have been reduced by increasing the physicochemical values (such as pH, pv and k value) during storage period. They may be the result of protein decomposition, producing lipid oxidation, and non-protein nitrogen degradation products like hypoxanthine, ketone, trimethylamine, aldehyde, ammonia, and dimethylamine which lead to the off-odor and off-flavor that induce the low score for these samples (Bazargani-Gilani et al., 2015; Ojagh et al., 2010; Tingting et al., 2012). In Tables 1 and 2, significant differences are found (p < 0.05) among all treatments compared to the control samples during the storage period. Control, SA, SA-R 0.001%, and SA-R 0.003% groups gained acceptable scores in taste, color, odor and general acceptability on the 3, 6, 12 and 15th days of the storage period. Antioxidant and gas barrier impacts by coating could be the causes decreasing the oxidation and enhancing the shelf life of the fish along with quality preservation. Results of this study agreed with those of Lu et al. (2009) on the shelf life prolongation of northern snakehead (Channa argus) flesh with alginate coating until 7th day of storage (Lu et al., 2009). Song et al. (2011) also found that sodium alginate coating increased the refrigerated bream (Megalobrama amblycephala) shelf life (Song et al., 2011). Combining carboxyl methyl cellulose (CMC) and sodium alginate in another research prolonged the silver carp fillet shelf life until the 8th day of refrigerated storage (Jalali et al., 2016). R addition to alginate coating significantly improved all sensory features such as color, taste, odor and overall acceptability of the rainbow trout fillet in all storage periods (p < 0.05). R combined with SA prolonged the sample shelf life until 15 days based on the sensory analysis; whereas, it didn’t have any detrimental effects on the sensory features such as color, taste, and odor, being imperceptible in the rainbow trout fillet surface.
Table 3.
(A–B) Correlation values between physicochemical properties (pH, K and p values) and sensory attribute (overall) of trout fillets during storage
| pH0 | pH3 | pH6 | pH9 | pH12 | pH15 | |
|---|---|---|---|---|---|---|
| (A) | ||||||
| Overall 0 | ||||||
| Pearson correlation | 0.000 | 0.333 | 0.406 | 0.314 | 0.393 | 0.510 |
| Sig. (2-tailed) | 1.000 | 0.421 | 0.318 | 0.449 | 0.335 | 0.196 |
| Overall 3 | ||||||
| Pearson correlation | − 0.119 | − 0.060 | − 0.141 | − 0.238 | − 0.166 | − 0.185 |
| Sig. (2-tailed) | 0.779 | 0.888 | 0.738 | 0.570 | 0.694 | 0.661 |
| Overall 6 | ||||||
| Pearson correlation | 0.229 | − 0.852** | − 0.865** | − 0.923** | − 0.795* | − 0.878** |
| Sig. (2-tailed) | 0.585 | 0.007 | 0.006 | 0.001 | 0.018 | 0.004 |
| Overall 9 | ||||||
| Pearson correlation | 0.237 | − 0.912** | − 0.954** | − 0.964** | − 0.967** | − 0.989** |
| Sig. (2-tailed) | 0.572 | 0.002 | 0.000 | 0.000 | 0.000 | 0.000 |
| Overall 12 | ||||||
| Pearson correlation | 0.404 | − 0.902** | − 0.879** | − 0.897** | − 0.825* | − 0.820* |
| Sig. (2-tailed) | 0.321 | 0.002 | 0.004 | 0.003 | 0.012 | 0.013 |
| Overall 15 | ||||||
| Pearson correlation | 0.249 | − 0.877** | − 0.905** | − 0.876** | − 0.947** | − 0.916** |
| Sig. (2-tailed) | 0.553 | 0.004 | 0.002 | 0.004 | 0.000 | 0.001 |
| PV0 | PV3 | PV6 | PV9 | PV12 | PV15 | |
|---|---|---|---|---|---|---|
| (B) | ||||||
| Overall 0 | ||||||
| Pearson correlation | − 0.109 | 0.477 | 0.555 | 0.556 | 0.505 | 0.369 |
| Sig. (2-tailed) | 0.797 | 0.232 | 0.153 | 0.152 | 0.202 | 0.369 |
| Overall 3 | ||||||
| Pearson correlation | 0.049 | − 0.230 | − 0.065 | − 0.016 | − 0.116 | − 0.225 |
| Sig. (2-tailed) | 0.908 | 0.583 | 0.878 | 0.969 | 0.784 | 0.592 |
| Overall 6 | ||||||
| Pearson correlation | − 0.379 | − 0.898** | − 0.809* | − 0.813* | − 0.849** | − 0.878** |
| Sig. (2-tailed) | 0.355 | 0.002 | 0.015 | 0.014 | 0.008 | 0.004 |
| Overall 9 | ||||||
| Pearson correlation | − 0.391 | − 0.986** | − 0.918** | − 0.931** | − 0.965** | − 0.961** |
| Sig. (2-tailed) | 0.338 | 0.000 | 0.001 | 0.001 | 0.000 | 0.000 |
| Overall 12 | ||||||
| Pearson correlation | − 0.558 | − 0.871** | − 0.877** | − 0.885** | − 0.902** | − 0.952** |
| Sig. (2-tailed) | 0.150 | 0.005 | 0.004 | 0.003 | 0.002 | 0.000 |
| Overall 15 | ||||||
| Pearson correlation | − 0.422 | − 0.916** | − 0.931** | − 0.941** | − 0.960** | − 0.951** |
| Sig. (2-tailed) | 0.298 | 0.001 | 0.001 | 0.000 | 0.000 | 0.000 |
| K0 | K3 | K6 | K9 | K12 | K15 | |
|---|---|---|---|---|---|---|
| (C) | ||||||
| Overall 0 | ||||||
| Pearson correlation | − 0.251 | 0.366 | 0.397 | 0.345 | 0.406 | 0.355 |
| Sig. (2-tailed) | 0.548 | 0.373 | 0.330 | 0.403 | 0.319 | 0.388 |
| Overall 3 | ||||||
| Pearson correlation | − 0.438 | − 0.174 | − 0.328 | − 0.283 | − 0.232 | − 0.205 |
| Sig. (2-tailed) | 0.277 | 0.681 | 0.428 | 0.497 | 0.580 | 0.627 |
| Overall 6 | ||||||
| Pearson correlation | 0.197 | − 0.818* | − 0.897** | − 0.902** | − 0.857** | − 0.831* |
| Sig. (2-tailed) | 0.640 | 0.013 | 0.003 | 0.002 | 0.007 | 0.011 |
| Overall 9 | ||||||
| Pearson correlation | 0.009 | − 0.923** | − 0.977** | − 0.972** | − 0.968** | − 0.961** |
| Sig. (2-tailed) | 0.983 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 |
| Overall 12 | ||||||
| Pearson correlation | 0.119 | − 0.814* | − 0.927** | − 0.874** | − 0.790* | − 0.828* |
| Sig. (2-tailed) | 0.779 | 0.014 | 0.001 | 0.005 | 0.020 | 0.011 |
| Overall 15 | ||||||
| Pearson correlation | − 0.130 | − 0.908** | − 0.933** | − 0.891** | − 0.894** | − 0.896** |
| Sig. (2-tailed) | 0.759 | 0.002 | 0.001 | 0.003 | 0.003 | 0.003 |
*Correlation is significant at the 0.05 level (2-tailed)
**Correlation is significant at the 0.01 level (2-tailed)
In conclusion, resveratrol decreased and retarded the physicochemical reactions. It also enhanced the rainbow trout fillet’s shelf life in combination with edible coating of sodium alginate at refrigerator. Resveratrol, which was recently introduced as a natural antioxidant candidate for various foods, unlike other herbal preservatives such as different kinds of extracts and essential oils, exhibited strong antioxidant activities at very low concentrations (0.001% and 0.003%). It also led to the stable and unchanged sensory properties such as odor, color, flavor, and general acceptability in the used food models. Moreover, herbal preservatives’ very low concentrations in food can minimize potential toxicity, allergenicity, or other plausible side effects and the expenses of utilized natural materials in foods. As a result, regarding the consumers´ requirement for natural preservatives, resveratrol, as a useful natural antioxidant without changing the sensory properties of the food at low concentrations, can be implemented in rainbow trout fillets or other meat products. However, R’s effects on the other meat products demand further researches. Utilizing other packaging or coating types are also suggested for the long-term storage of those new products.
Acknowledgements
Many thanks for all who financially supported this work.
Compliance with ethical standards
Conflict of interests
Based on the author’ declaration, there is no conflict of interests in terms of the publication of this paper.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Anon. 1996. Council Regulation (EC) No. 2406/96 of 26. November 1996 laying down common marketing standards for certain fishery product, OJEC. No. L334: 1-14 (1996)
- Bazargani-Gilani B, Aliakbarlu J, Tajik H. Effect of pomegranate juice dipping and chitosan coating enriched with Zataria multiflora Boiss essential oil on the shelf-life of chicken meat during refrigerated storage. Innov. Food Sci. Emerg. Technol. 2015;29:280–287. doi: 10.1016/j.ifset.2015.04.007. [DOI] [Google Scholar]
- Brannan RG. Effect of grape seed extract on physicochemical properties of ground, salted, chicken thigh meat during refrigerated storage at different relative humidity levels. J. food sci. 2008;73:C36–C40. doi: 10.1111/j.1750-3841.2007.00588.x. [DOI] [PubMed] [Google Scholar]
- Chytiri S, Chouliara I, Savvaidis IN, Kontominas MG. Microbiological, chemical and sensory assessment of iced whole and filleted aquacultured rainbow trout. Food Microbiol. 2004;21:157–165. doi: 10.1016/S0740-0020(03)00059-5. [DOI] [Google Scholar]
- Connell J. Quality deterioration and extrinsic quality defects in raw material. Control of Fish Quality, Fishing News Books Ltd. Surrey, England. 31-35 (1995)
- Fan W, Chi Y, Zhang S. The use of a tea polyphenol dip to extend the shelf life of silver carp (Hypophthalmicthys molitrix) during storage in ice. Food Chem. 2008;108:148–153. doi: 10.1016/j.foodchem.2007.10.057. [DOI] [Google Scholar]
- Fan W, Sun J, Chen Y, Qiu J, Zhang Y, Chi Y. Effects of chitosan coating on quality and shelf life of silver carp during frozen storage. Food Chem. 2009;115:66–70. doi: 10.1016/j.foodchem.2008.11.060. [DOI] [Google Scholar]
- FDA. Fda.gov. In: http://www.fda.gov/ucm/groups/fdagov-public/@fdagov-foods-gen/documents/document/ucm269540.pdf. Accessed Nov 9 2015 (2010)
- Gerszon J, Rodacka A, Puchała M. Antioxidant properties of resveratrol and its protective effects in neurodegenerative diseases. In: Advances in Cell Biology, p. 97 (2014)
- Giatrakou V, Savvaidis I. Bioactive packaging technologies with chitosan as a natural preservative agent for extended shelf-life food products. In: Arvanitoyannis I, editor. Modified atmosphere and active packaging technologies. 1. Boca Raton: Taylor & Francis; 2012. pp. 685–730. [Google Scholar]
- Gui M, Zhao B, Song J, Zhang Z, Peng Z, Li P. Paraplantaricin L-ZB1, a novel bacteriocin and its application as a biopreservative agent on quality and shelf life of rainbow trout fillets stored at 4 degrees c. Appl. Biochem. Biotechnol. 2014;174:2295–2306. doi: 10.1007/s12010-014-1160-3. [DOI] [PubMed] [Google Scholar]
- Gulcin İ. Antioxidant properties of resveratrol: a structure–activity insight. Innov. Food Sci. Emerg. Technol. 2010;11:210–218. doi: 10.1016/j.ifset.2009.07.002. [DOI] [Google Scholar]
- Hara A, Radin NS. Lipid extraction of tissues with a low-toxicity solvent. Anal. Biochem. 1978;90:420–426. doi: 10.1016/0003-2697(78)90046-5. [DOI] [PubMed] [Google Scholar]
- Heydari R, Bavandi S, Javadian SR. Effect of sodium alginate coating enriched with horsemint (Mentha longifolia) essential oil on the quality of bighead carp fillets during storage at 4 degrees C. Food Sci. Nutr. 2015;3:188–194. doi: 10.1002/fsn3.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini SF, Rezaei M, Zandi M, Ghavi FF. Effect of fish gelatin coating enriched with oregano essential oil on the quality of refrigerated rainbow trout fillet. J. Aquat. Food Prod. Technol. 2016;25:835–842. doi: 10.1080/10498850.2014.943917. [DOI] [Google Scholar]
- Iuga C, Alvarez-Idaboy JR, Russo N. Antioxidant activity of trans-resveratrol toward hydroxyl and hydroperoxyl radicals: a quantum chemical and computational kinetics study. J. Org. Chem. 2012;77:3868–3877. doi: 10.1021/jo3002134. [DOI] [PubMed] [Google Scholar]
- Jalali N, Ariiai P, Fattahi E. Effect of alginate/carboxyl methyl cellulose composite coating incorporated with clove essential oil on the quality of silver carp fillet and Escherichia coli O157:H7 inhibition during refrigerated storage. J. Food Sci. Technol. 2016;53:757–765. doi: 10.1007/s13197-015-2060-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon YJ, Kamil JY, Shahidi F. Chitosan as an edible invisible film for quality preservation of herring and atlantic cod. J. Agric. Food Chem. 2002;50:5167–5178. doi: 10.1021/jf011693l. [DOI] [PubMed] [Google Scholar]
- Kindleysides S, Quek SY, Miller MR. Inhibition of fish oil oxidation and the radical scavenging activity of New Zealand seaweed extracts. Food Chem. 2012;133:1624–1631. doi: 10.1016/j.foodchem.2012.02.068. [DOI] [Google Scholar]
- Kubow S. Routes of formation and toxic consequences of lipid oxidation products in foods. Free Radic. Biol. Med. 1992;12:63–81. doi: 10.1016/0891-5849(92)90059-P. [DOI] [PubMed] [Google Scholar]
- Li T, Hu W, Li J, Zhang X, Zhu J, Li X. Coating effects of tea polyphenol and rosemary extract combined with chitosan on the storage quality of large yellow croaker (Pseudosciaena crocea) Food Control. 2012;25:101–106. doi: 10.1016/j.foodcont.2011.10.029. [DOI] [Google Scholar]
- Lu F, Liu D, Ye X, Wei Y, Liu F. Alginate-calcium coating incorporating nisin and EDTA maintains the quality of fresh northern snakehead (Channa argus) fillets stored at 4 & #xB0;C. J. Sci. Food Agric. 2009;89:848–854. doi: 10.1002/jsfa.3523. [DOI] [Google Scholar]
- Manju S, Srinivasagopal T, Jose L, Ravishankar C, Ashokkumar K. Nucleotide degradation of sodium acetate and potassium sorbate dip treated and vacuum packed Black Pomfret (Parastromateus niger) and Pearlspot (Etroplus suratensis) during chill storage. Food Chem. 2007;102:699–706. doi: 10.1016/j.foodchem.2006.06.059. [DOI] [Google Scholar]
- Martinsdóttir E, Sveinsdóttir K, Luten J, Schelvis-Smith R, Hyldig G. Sensory evaluation of fish freshness. Reference manual for the fish sector. Ijmuiden: QIM Eurofish. www.qim-eurofish.com (2001)
- Mazorra-Manzano MA, Pacheco-Aguilar R, Diaz-Rojas EI, Lugo-Sanchez ME. Postmortem Changes in Black Skipjack Muscle During Storage in Ice. J. Food Sci. 2000;65:774–779. doi: 10.1111/j.1365-2621.2000.tb13585.x. [DOI] [Google Scholar]
- Nielsen D, Hyldig G. Influence of handling procedures and biological factors on the QIM evaluation of whole herring (Clupea harengus L.). Food Res. Int. 37: 975-983 (2004)
- Ocano-Higuera VM, Maeda-Martínez AN, Marquez-Ríos E, Canizales-Rodríguez DF, Castillo-Yáñez FJ, Ruíz-Bustos E, Graciano-Verdugo AZ, Plascencia-Jatomea M. Freshness assessment of ray fish stored in ice by biochemical, chemical and physical methods. Food Chem. 2011;125:49–54. doi: 10.1016/j.foodchem.2010.08.034. [DOI] [Google Scholar]
- Ojagh SM, Rezaei M, Razavi SH, Hosseini SMH. Effect of chitosan coatings enriched with cinnamon oil on the quality of refrigerated rainbow trout. Food Chem. 2010;120:193–198. doi: 10.1016/j.foodchem.2009.10.006. [DOI] [Google Scholar]
- Petrou S, Tsiraki M, Giatrakou V, Savvaidis IN. Chitosan dipping or oregano oil treatments, singly or combined on modified atmosphere packaged chicken breast meat. Int. J. Food Microbiol. 2012;156:264–271. doi: 10.1016/j.ijfoodmicro.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Shantha NC, Decker EA. Rapid, sensitive, iron-based spectrophotometric methods for determination of perorlride values of food lipids. J. AOAC Int. 1994;77:421–424. doi: 10.1093/jaoac/77.2.421. [DOI] [PubMed] [Google Scholar]
- Shen S, Jiang Y, Liu X, Luo Y, Gao L. Quality assessment of rainbow trout (Oncorhynchus mykiss) fillets during super chilling and chilled storage. J. Food Sci. Technol. 2015;52:5204–5211. doi: 10.1007/s13197-014-1539-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Liu L, Shen H, You J, Luo Y. Effect of sodium alginate-based edible coating containing different anti-oxidants on quality and shelf life of refrigerated bream (Megalobrama amblycephala) Food Control. 2011;22:608–615. doi: 10.1016/j.foodcont.2010.10.012. [DOI] [Google Scholar]
- Surendran Nair M, Lau P, Belskie K, Fancher S, Chen CH, Karumathil DP, Yin HB, Liu Y, Ma F, Upadhyaya I, Upadhyay A, Mancini R, Venkitanarayanan K. Potentiating the heat inactivation of Escherichia coli O157:h7 in ground beef patties by natural antimicrobials. Front. Microbiol. 2016;7:15–22. doi: 10.3389/fmicb.2016.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingting LI, Wenzhong HU, Jianrong LI, Xuguang ZH, Junli ZHU, Xuepeng LI. Coating effects of tea polyphenol and rosemary extract combined with chitosan on the storage quality of large yellow croaker (Pseudosciaena crocea) Food Control. 2012;25:101–106. doi: 10.1016/j.foodcont.2011.10.029. [DOI] [Google Scholar]
- Topuz OK, Yerlikaya P, Ucak I, Gumus B, Büyükbenli HA. Effects of olive oil and olive oil–pomegranate juice sauces on chemical, oxidative and sensorial quality of marinated anchovy. Food Chem. 2014;154:63–70. doi: 10.1016/j.foodchem.2013.12.103. [DOI] [PubMed] [Google Scholar]
- Volpe MG, Siano F, Paolucci M, Sacco A, Sorrentino A, Malinconico M, Varricchio E. Active edible coating effectiveness in shelf-life enhancement of trout (Oncorhynchus mykiss) fillets. LWT Food Sci. Technol. 2015;60:615–622. doi: 10.1016/j.lwt.2014.08.048. [DOI] [Google Scholar]
- Yanishlieva NV, Marinova EM. Stabilisation of edible oils with natural antioxidants. Eur. J. Lipid Sci. Technol. 2001;103:752–767. doi: 10.1002/1438-9312(200111)103:11<752::AID-EJLT752>3.0.CO;2-0. [DOI] [Google Scholar]
- Yu XL, Li XB, Xu XL, Zhou GH. Coating with sodium alginate and its effects on the functional properties and structure of frozen pork. J. Muscle Foods. 2008;19:333–351. doi: 10.1111/j.1745-4573.2008.00113.x. [DOI] [Google Scholar]