Abstract
Phosphoinositide 3-kinases (PI3Ks) are lipid kinases that regulate important intracellular signalling and vesicle trafficking events via the generation of 3-phosphoinositides. Comprising eight core isoforms across three classes, the PI3K family displays broad expression and function throughout mammalian tissues, and the (patho)physiological roles of these enzymes in the cardiovascular system present the PI3Ks as potential therapeutic targets in settings such as thrombosis, atherosclerosis and heart failure. This review will discuss the PI3K enzymes and their roles in cardiovascular physiology and disease, with a particular focus on platelet function and thrombosis. The current progress and future potential of targeting the PI3K enzymes for therapeutic benefit in cardiovascular disease will be considered, while the challenges of developing drugs against these master cellular regulators will be discussed.
Keywords: Cardiovascular disease, Thrombosis, Platelets, Phosphoinositide 3-kinase, PI3K, Phosphoinositides, Cellular signalling
Background
Cardiovascular diseases (CVDs) are a leading cause of mortality and morbidity worldwide [1]. Major causes of CVD-related deaths include ischaemic heart disease or stroke, for which arterial thrombosis is a key component [2]. Platelets play a critical role in arterial thrombosis, and antiplatelet therapy is therefore a frontline antithrombotic strategy. While platelets are essential for normal haemostasis, where localised thrombi stem bleeding and support repair at sites of vascular damage, excessive activation and accumulation of platelets in the vasculature may lead to blood vessel occlusion, which can result in myocardial infarction or stroke [3]. The role of platelets in disease can be more complex however, including contribution to inflammation, reperfusion injury, tumour progression and metastasis, and microbial infection, while metabolic conditions such as diabetes can lead to platelet hyperactivity [2, 4].
The cyclooxygenase (COX) inhibitor, aspirin (acetylsalicyclic acid), has served as a mainstay antiplatelet agent for decades, and acts via the inhibition of prostaglandin H2 generation, and thus prevention of the formation of the platelet activator, thromboxane A2 [5, 6]. Aspirin is often administered in combination with a drug targeting the major platelet G protein-coupled receptor for ADP, P2Y12, including thienopyridines such as clopidogrel and prasugrel, and the reversible cyclopentyl-triazolopyrimidine, ticagrelor [7, 8]. Other platelet receptors that represent current or potential targets for clinical intervention include the major integrin αIIbβ3 to prevent fibrinogen binding required for thrombus inter-platelet bridges (e.g. abciximab, eptifibatide), protease-activated receptor (PAR) 1 or 4 for thrombin signalling (e.g. vorapaxar and atopaxar), and the collagen receptor Glycoprotein VI (GPVI) [3, 5, 9, 10]. While existing antiplatelet drugs offer considerable value for patients, the risk of unwanted bleeding associated with these therapies remains a major limitation, while individuals may also show a poor response to existing agents due to the specific nature of their metabolism; individuals with a reduced-function cytochrome P450 CYP2C19 allele are unable to efficiently metabolise clopidogrel to its active metabolite, for example [11]. A need for novel antithrombotic targets and associated drugs therefore remains. One such potential target is the phosphoinositide 3-kinase (PI3K) family, which comprises a range of lipid kinases that catalyse phosphorylation of the inositol ring of phosphatidylinositol (PtdIns) and its associated phosphoinositides to generate 3-phosphorylated lipid regulators of cell function. These enzymes are commonly activated downstream of the key clinically-targeted platelet receptors discussed above, to support platelet activation and thrombus formation, and may therefore represent promising candidates for the prevention of thrombosis. The PI3K enzymes are also implicated in other cardiovascular settings, including angiogenesis, hypertension and heart failure. This review will provide an overview of the PI3K enzymes, cover their roles in cardiovascular disease with a particular focus on thrombosis, and discuss the potential, progress and challenges of targeting this family of proteins for therapeutic means.
The PI3K family
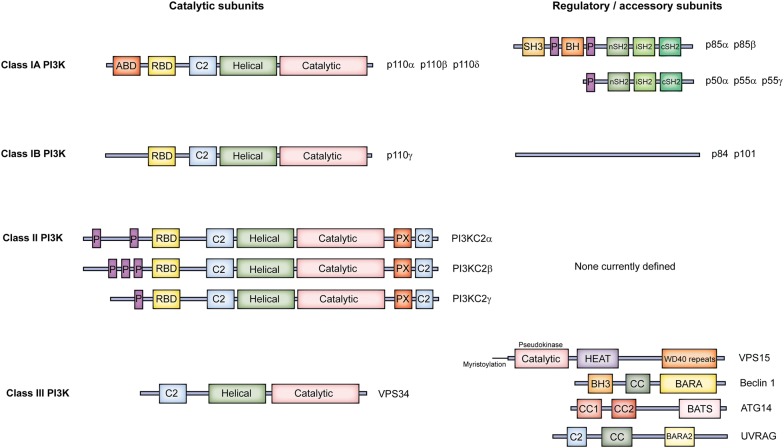
PI3Ks catalyse phosphorylation of the 3-OH moiety of the inositol ring of PtdIns and its related phosphoinositides using the γ-phosphate of ATP, and regulate various important aspects of cellular function to support organismal physiology [12]. Mechanistically, this predominantly occurs via the ability of the 3-phosphoinositide products to regulate the localisation and activity of varied repertoires of effector proteins [13]. Mammals have eight core PI3K isoforms (catalytic-regulatory subunit pairing diversity increases this number, discussed later), divided into three classes based on structural, catalytic and regulatory properties (Fig. 1) [14]. The Class I PI3K family is the best characterised, and much is known about its members’ ubiquitous roles in receptor-initiated signal transduction in multiple cardiovascular tissues, and their significance in disease. Although their characterisation has lagged behind that of the Class I PI3Ks, the Class II and Class III PI3K families have received increased attention in recent years, and details of their organismal roles are now coming to light.
Fig. 1.
Domain organisation of the PI3Ks. Class I, II and III PI3K catalytic subunits share a core region consisting of a C2 domain, helical domain and kinase domain, but differ in other regions of the protein. While the domain structure of the Class IA regulatory subunits is well defined, the Class IB regulatory subunits have no clearly-defined domain structure, while no regulatory subunits have been reported for the Class II PI3Ks. The Class III PI3K, VPS34, associates with a range of accessory proteins to form at least two distinct complexes
Class I PI3Ks
Class I PI3Ks are predominantly activated downstream of cell surface receptor stimulation to catalyse the phosphorylation of PtdIns(4,5)P2 to generate PtdIns(3,4,5)P3 [12]. These heterodimeric enzymes comprise a catalytic subunit, known as the p110, associated with a regulatory subunit [15]. The catalytic p110 subunit present in a Class I PI3K heterodimer defines the isoform nomenclature; i.e. PI3Kα, β, δ and γ isoforms contain p110α (PIK3CA gene), p110β (PIK3CB), p110δ (PIK3CD) or p110γ (PIK3CG), respectively. The Class I PI3K family is subdivided into Classes IA and IB, based on differing preferences of the catalytic subunits for regulatory partners (Fig. 1) [14]. The Class IA PI3K family comprises p110α, β and δ which can associate with the five regulatory subunits, p85α, p55α, p50α (PIK3R1 gene), p85β (PIK3R2) and p55γ (PIK3R3). In contrast, p110γ can associate with the p101 (PIK3R5) or p84 (PIK3R6) regulatory subunits to yield the Class IB PI3Ks (Fig. 2). Inter- and intra-subunit contacts allow regulation of the catalytic subunit to modulate the activity of these enzymes. For the Class IA PI3Ks, this includes important contacts between the N-terminal Src homology 2 (nSH2) and cSH2 domains of the p85 subunit and the regulatory arch within the kinase domain of the p110, although the cSH2 contact is lacking for p110α [14, 16, 17]. Although the structural composition of the Class IB PI3K regulatory subunits p101 and p84 remains more poorly defined than that of the Class IA regulatory subunits, these proteins appear to stabilise the C2-RBD (Ras-binding domain) linker and C2-helical linker of p110γ, respectively, while also stabilising p110γ’s helical domain [18, 19]. Activatory interactions between Class I PI3Ks and receptors or other proteins, discussed below, lead to a disruption of the inhibitory contacts within the PI3K heterodimers [14, 20]. Structural characterisation of these mechanisms not only advances understanding of fundamental Class I PI3K activation, but can also reveal the impact of mutations (e.g. oncogenic), and provides potential for novel approaches to drug design [20]. For example, building upon earlier structural studies [14], recent atomic level simulations have provided a detailed explanation of PI3Kα activation, revealing how loss of the interaction between the nSH2 of p85α and p110α initiates allosteric motions, with structural rearrangement reducing the distance between the ATP- and PtdIns(4,5)P2-binding sites in p110α to enable phosphoryl transfer [20]. Furthermore, observation of a deep cavity between the ATP- and substrate-binding sites in active PI3Kα suggests the potential for a novel approach to isoform-specific drug design [20], highlighting the value of structural studies of PI3K for drug discovery.
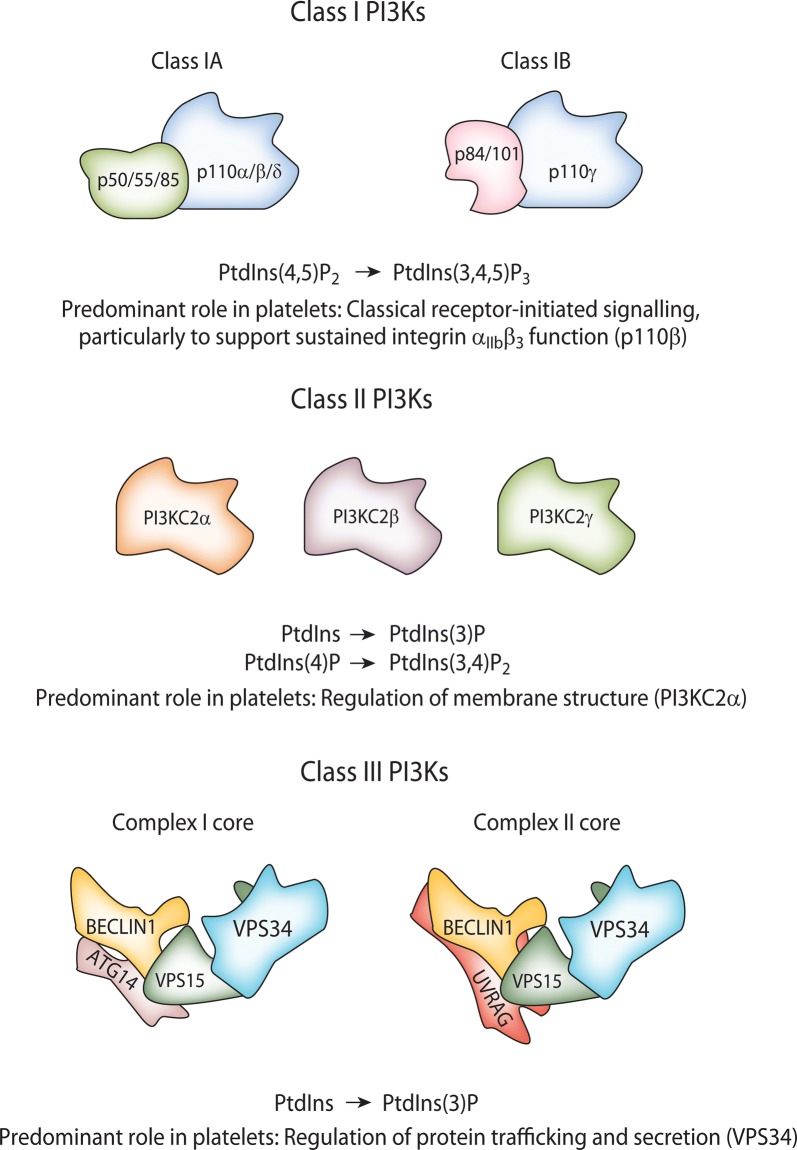
Fig. 2.
Overview of the PI3K complexes and their activities. Class I PI3Ks form heterodimers comprised of a catalytic subunit and a regulatory subunit, although free Class IA regulatory subunits also exist in cells. In vivo, Class I PI3Ks 3-phosphorylate PtdIns(4,5)P2 to generate PtdIns(3,4,5)P3. Class II PI3Ks appear to function as independent catalytic subunits, and are currently considered to 3-phosphorylate PtdIns and PtdIns(4)P in vivo to yield PtdIns(3)P and PtdIns(3,4)P2, respectively. However, Class II PI3K-deficient mouse models suggest PI3KC2α may only act to regulate a housekeeping pool of PtdIns(3)P in platelets, while PI3KC2β has no clear functional role, and PI3KC2γ is not expressed in this cell type. Class III PI3K, VPS34, associates with various proteins to form at least two known complexes, although it is likely that other associations and complexes exist. Class III PI3K phosphorylates PtdIns to generate PtdIns(3)P. Class I, II and III PI3Ks all play roles in platelet thrombus formation, and the predominant role of each is detailed
Studies to uncover the isoform-specific roles of the Class I PI3Ks have understandably focussed primarily on the catalytic p110 subunits using mouse gene targeting and small molecule catalytic inhibitors, while the identity and significance of the regulatory subunit in any given Class I PI3K heterodimer and context is often overlooked. Interestingly, recent work has demonstrated selectivity of Class IA PI3K composition in vivo, with p85α showing preferential association with p110δ [15], while Class IB PI3K family p110γ-p84 and p110γ-p101 heterodimers can fulfil distinct functional roles [21, 22]. In addition, p110-free p85 regulatory subunits exist within cells and can modulate PI3K activation, including a tumour suppressor role [15, 23, 24]. Specificity of heterodimer formation and function, and the presence of p110-free regulatory subunits, thus likely afford a currently underappreciated level of complexity and regulation to Class I PI3K function, which warrants further investigation and may offer new avenues for greater isoform and/or functional selectivity in drug design.
Mechanistically, selectivity of activation and function is known to be afforded to this enzyme family by the ability of the Class I PI3K isoforms to be differentially regulated by distinct sets of protein–protein interactions. The SH2 domains of the regulatory subunits of the Class IA PI3Ks allow interaction with phosphorylated tyrosines (often within the context of a ‘YXXM motif’) present on receptors or adaptor proteins [15]. In contrast, the Class IB PI3K regulatory subunits, p84 and p101, lack these SH2 domains but, like the catalytic subunit p110γ, can interact with Gβγ subunits to permit activation of PI3Kγ downstream of G protein-coupled receptors (GPCRs) [18, 25–27]. Class IA PI3Kβ can also be activated via a direct interaction of Gβγ subunits with p110β [28, 29], making it uniquely poised to respond to both (receptor) tyrosine kinase- and GPCR-directed signalling. Furthermore, PI3Kβ is unique among the Class I PI3Ks in its ability to respond to small GTPases, receiving activatory input via direct interaction with RHO-family RAC and CDC42 proteins [30] and RAB5 [31], while PI3Kα, PI3Kδ and PI3Kγ interact with RAS-family GTPases [32–34]. It is important to note however that, despite these isoform-specific properties, the interconnectivity of signalling events in cells, whereby different PI3Ks may be activated both directly and indirectly by overlapping and cross-talking sets of receptors and upstream regulators, can make it considerably challenging to dissect isoform-specific activities, as does potential isoform synergy and redundancy. Despite this, it would seem that PI3Kβ is well-suited to serve as a hub to receive and integrate signals from multiple inputs [35, 36], which may explain the dominance of this Class I PI3K isoform in platelets, where coincidence signalling is critical for cellular function [37].
Isoform-selective physiological roles for the Class I PI3Ks have emerged over the years, with PI3Kα holding a key role in embryonic development and angiogenesis [38–40], growth factor signalling [41], and insulin signalling and metabolism [42, 43], among other processes. The other broadly expressed Class I isoform, PI3Kβ, holds a key role in male fertility [44, 45], autophagy [46], immune complex-mediated neutrophil activation [35, 47], and platelet function [48], as discussed later. The functional significance of PI3Kδ and PI3Kγ is most apparent in cells of the myeloid and lymphoid lineages where these isoforms show highest expression, and includes the regulation of various aspects of inflammation and immunity, including B cell development, T cell differentiation and neutrophil migration [49–53]. While differential expression may offer broad explanations for some isoform-selective roles, in most scenarios several isoforms are present to some extent, and the specific details and context of the activatory stimulus often define the relative contribution of the different Class I PI3Ks in any given setting.
PtdIns(3,4,5)P3 generated by Class I PI3Ks serves to dictate cell function via the recruitment and regulation of various effector proteins. These effectors span a range of functional protein classes, including protein kinases and other enzymes, signalling adaptors, and regulators of small GTPases, thus allowing Class I PI3Ks to initiate and contribute to diverse signalling pathways inside cells [13]. PtdIns(3,4,5)P3 (and PtdIns(3,4)P2) effectors commonly possess a subtype of the pleckstrin homology (PH) domain, which interacts with the phosphorylated headgroup of this phosphoinositide via a conserved network of basic residues, although other protein domains can also interact with PtdIns(3,4,5)P3, including the DHR-1 domain of DOCK-family GEFs and the PX domain of sorting nexins [54–57]. While AKT (also known as Protein Kinase B) is the best characterised PtdIns(3,4,5)P3 effector and has received considerable attention in platelets [58], a range of others have key roles in this cell type, including RASA3 [59, 60], DAPP1 [13] and ELMO1 [61]. In highly dynamic cells like platelets, which utilise major cytoskeletal reorganisation events and protein trafficking during activation and thrombus formation, the ability of Class I PI3Ks to control small GTPases via a range of PtdIns(3,4,5)P3-regulated GAPs and GEFs is particularly interesting and warrants further investigation. Furthermore, the apparent ability of RAC and CDC42, which are key regulators of platelet function [62], to integrate both upstream (via interactions with the RBD of p110β) and downstream (via PtdIns(3,4,5)P3-regulated GEFs and GAPS) of PI3K suggests an intricate and tightly feedback-regulated signalling pathway in this cell type. PtdIns(3,4)P2 may support PtdIns(3,4,5)P3 signalling and can activate a subset of the same effectors, while it may also drive specific signalling, depending on the cell type and stimulatory context [63, 64]. The strength and duration of signalling is highly dependent on phosphatase activity, with 5′ phosphatases such as SHIP1 and SHIP2 dephosphorylating PtdIns(3,4,5)P3 to PtdIns(3,4)P2, while PTEN dephosphorylates PtdIns(3,4,5)P3 (and also PtdIns(3,4)P2) at the 3′ position of the inositol ring to yield PtdIns(4,5)P2 (and PtdIns(4)P) [65, 66]. In addition to the ability of Class I PI3Ks to regulate cellular behaviour via their catalytic activity, they can also contribute to cellular function via non-catalytic protein–protein interactions, commonly referred to as ‘scaffolding’ roles [67]. This explains divergence between animal studies with mice either lacking expression of Class I PI3K catalytic subunits, or expressing mutant kinase-dead forms, and is also an important consideration for therapeutic targeting using small molecules that may only inhibit the PI3Ks’ catalytic activity.
Class I PI3Ks in platelet function and thrombosis
Human platelets express all Class I PI3K isoforms, with PI3Kβ expressed at the highest level followed by PI3Kγ > PI3Kα > PI3Kδ [68]. Of these, PI3Kβ has been revealed as the most important Class I isoform in platelet function and thrombosis, as demonstrated by the use of different mouse models and pharmacological approaches [69–74]. Deletion of p110β in megakaryocytes/platelets, or expression of a catalytically-inactive form, resulted in impaired platelet activation downstream of the collagen receptor GPVI and integrin αIIbβ3, whereas the contribution of PI3Kβ to thrombin-mediated platelet activation was more modest [69, 71]. Interestingly, agonist-mediated production of PtdIns(3,4,5)P3 and downstream phosphorylation of AKT was largely impaired, demonstrating that PI3Kβ is the dominant isoform in raising intracellular PtdIns(3,4,5)P3 upon platelet activation [69, 71]. p110β-deficient conditional mice also showed impaired clot retraction and impaired in vivo thrombosis following FeCl(3) injury [71]. Although thrombus growth under physiological shear rate was not affected in p110β-deficient mice, at higher shear rates the formed thrombi showed enhanced embolization both ex vivo and in vivo [74]. Similar findings were obtained in ex vivo experiments using human blood treated with the PI3Kβ inhibitor AZD6482 [74]. This effect could be rescued by GSK3 inhibitors, suggesting that impaired activation of the AKT/GSK3 pathway may underlie thrombus instability [75]. Pharmacological approaches using selective p110β inhibitors such as TGX-221 and AZD6482 support the importance of PI3Kβ in platelet activation and thrombus formation, both in mouse and human studies [70, 72, 75–77].
Interestingly, the Gi-coupled ADP receptor P2Y12 promotes PI3Kβ activation upon platelet stimulation, and supports platelet function by maintaining RAP1B activation, integrin αIIbβ3 activation and aggregate stability, as well as promoting TxA2 formation through the ERK1/2 pathway [59, 73, 77]. In addition, P2Y12 signalling to PI3Kβ contributes to thrombin-mediated Ca2+ mobilisation and procoagulant activity [78]. Platelet primers such as thrombopoietin (TPO) can also synergistically increase PAR1-mediated RAP1B activation, integrin αIIbβ3 activation and α-granule secretion, which was largely prevented by PI3Kβ inhibitors [79]. Furthermore, PI3Kβ contributes to the potentiation of platelet function by anti-phospholipid antibodies [80]. Indeed, multiple signalling inputs from different receptors, including P2Y12 and receptors that prime platelet function, can combine in their ability to activate PI3Kβ, thereby further promoting platelet function. The mechanisms by which PI3Kβ and other isoforms modulate platelet function have not been completely elucidated but are likely to involve signalling molecules regulated downstream of PtdIns(3,4,5)P3 and/or PtdIns(3,4)P2, including RASA3 [59, 60], DAPP1 [13], ELMO1 [61] and AKT/GSK3 [74, 81, 82].
The PI3Kγ isoform has also been shown to support platelet activation downstream of the ADP receptor P2Y12. The aggregation response to ADP, but not collagen and thrombin, was significantly reduced in platelets deficient in PI3Kγ [83]. Furthermore, these mice were protected against ADP-dependent thromboembolic vascular occlusion [83]. Interestingly, both PI3Kγ and PI3Kβ are required for maintaining integrin αIIbβ3 activation and platelet aggregate stability, as determined in aggregation and ex vivo and in vivo thrombosis models [73, 84], with the contribution of PI3Kγ potentially mediated through a non-catalytic signalling mechanism [84]. Furthermore, dual activation of PI3Kγ and PI3Kβ underlies the ability of TPO to enhance platelet function through the ERK1/2/TxA2 pathway [79].
PI3Kα has a more subtle role in platelet function, with initial studies using the PI3Kα selective inhibitor, PIK75, reporting a role for PI3Kα in IGF1-mediated enhancement of platelet function [85, 86]. Furthermore, inhibitor studies showed that PI3Kα may also contribute to GPVI-mediated platelet function [86, 87]. Two groups, including our own, subsequently generated a mouse model where p110α has been selectively deleted in the megakaryocytic lineage [88]. Combining genetic and pharmacological approaches, including careful titration of PI3Kα and β inhibitors, we revealed that both PI3Kα and PI3Kβ contribute to IGF1-mediated AKT phosphorylation, but that PI3Kα is the isoform responsible for supporting platelet function [88]. PAR4-, thrombin-, CRP- and fucoidan-mediated integrin αIIbβ3 activation and α-granule release, as well as thrombus formation on a collagen-coated surface under flow, were not affected [88]. In contrast, p110α deletion, but not PI3Kα inhibition, resulted in a synergistic enhancement of TPO-mediated priming of platelet function by increasing ERK1/2 phosphorylation and TxA2 formation, suggesting a novel kinase-independent negative regulatory role for PI3Kα in platelet function. Laurent et al. [89] also found more discrete roles for PI3Kα in platelet function, with PI3Kα deficiency and inhibitors reducing ADP secretion at low levels of GPVI activation and impairing platelet adhesion to vWF under shear. PI3Kα, together with PI3Kβ, also contributes to the platelet priming effect of anti-phospholipid antibodies [80]. More importantly, PI3Kα deletion and inhibition resulted in decreased arterial thrombosis without affecting bleeding time, suggesting it has potential as an anti-thrombotic target [89].
The PI3Kδ isoform is expressed at low levels in both mouse and human platelets [68, 90] and plays only a minor role in platelet function [91]. p110δ-deficient platelets, or mouse platelets expressing a catalytically-inactive form of p110δ, showed minor aggregation defects, reduced spreading on fibrinogen and vWF, but normal thrombus formation on collagen under flow [91].
Class II PI3Ks
Class II PI3Ks are currently considered to catalyse phosphorylation of the 3′ position of the inositol ring of PtdIns or PtdIns(4)P in vivo to generate PtdIns(3)P or PtdIns(3,4)P2, respectively [92, 93]. Achieving unambiguous confidence in the relative contributions of PI3Ks to the turnover of specific phosphoinositides in vivo is however highly challenging, due to the complexity of the interconnected phosphoinositide network, and due to technical difficulties in measuring specific lipids. It also appears that the relative production of PtdIns(3)P or PtdIns(3,4)P2 by Class II PI3Ks may depend on the local abundance of their respective substrate at the site of action, such as plasma or endosomal membranes, and on the cell type studied [93, 94].
The Class II PI3K family comprises three isoforms, PI3KC2α (PIK3C2A gene), β (PIK3C2B) and γ (PIK3C2G), which appear to function as catalytic monomers, without regulatory partners (Fig. 1) [93]. Similar to the Class I PI3K p110s, these enzymes possess RBD, C2, helical and catalytic domains, but also possess a poorly-structured N-terminal region, in addition to a C-terminal region containing C2 and PX domains [14, 95]. Understanding of Class II PI3K regulation is in its infancy, but for PI3KC2α, the N-terminal region has been shown to support plasma membrane recruitment of the enzyme via interactions with clathrin [96, 97], while the C2 and PX domains interact with phosphoinositides such as PtdIns(4,5)P2 to release an autoinhibitory mechanism to enable catalytic activity [98–100]. It is likely that multiple further interactions with proteins and lipids can regulate the function of the Class II PI3Ks and, as for the Class I PI3Ks, specificity within such interactions is likely to guide differential usage of the three Class II isoforms [93]. While PI3KC2γ expression appears to be largely restricted to tissues such as liver, breast and prostate, PI3KC2α and PI3KC2β demonstrate more widespread expression [101–103]. In a manner analogous to Class I PI3K-generated PtdIns(3,4,5)P3, the Class II PI3K products PtdIns(3)P and PtdIns(3,4)P2 can regulate cell function via a range of effectors containing 3-phosphoinositide-binding elements such as FYVE, PX and PH domains [104].
Class II PI3Ks predominantly serve to modulate cell function via the regulation of membrane trafficking and dynamics [93], and the lethality of PI3KC2α loss or inactivation in mice demonstrates the critical role of this Class II isoform in embryonic development [105–107]. Indeed, PI3KC2α has been shown to regulate angiogenesis, insulin signalling and glucose transport, sonic hedgehog signalling, primary cilium assembly and clathrin-mediated endocytosis [93, 94, 105, 107–109]. The impact of homozygous loss of PI3KC2α appears to be less severe in humans, potentially reflecting differential usage of Class II PI3Ks between humans and mice, or a differing ability to compensate [110]. Such species difference is an important factor in the consideration of PI3K inhibitor development for human disease. Loss of PI3KC2β or PI3KC2γ expression or activity in mice yields viable animals, albeit with distinct metabolic phenotypes of increased or decreased insulin sensitivity, respectively [101, 111, 112]. Furthermore, heterozygous loss of PI3KC2α activity induces mild, age-dependent obesity, insulin resistance and glucose intolerance, in addition to leptin resistance in male mice, although females display no metabolic phenotype [106]. PI3KC2α’s role in endocytosis includes the synthesis of a local pool of PtdIns(3,4)P2 at late-stage endocytic intermediates to recruit SNX9 to support dynamin-mediated membrane scission for vesicle release from the invaginated membrane, while this class II PI3K also supports the removal of recycling cargo from endosomes via the production of PtdIns(3)P and the activation of RAB11 [97, 107, 113]. Class II PI3KC2β has an important role in mTORC1 regulation on lysosomes and late endosomes [114], in regulation of the potassium channel KCa3.1 in CD4 T-cells and mast cells [115, 116], in mitosis progression [117] and in cell migration [118–120]. Class II PI3K’s can hold non-catalytic scaffolding roles, including a role for PI3KC2α in mitotic spindle stabilization during metaphase [121], with such roles potentially being resistant to inhibition with small molecules targeting the catalytic activity of these enzymes.
Class II PI3Ks in platelet function and thrombosis
Human and mouse platelets express PI3KC2α and β, but not PI3KC2γ [122]. Due to the lethality of PI3KC2α loss in mice, mouse studies have utilised heterozygous knock-in of a kinase-dead mutation (D1268A) at the endogenous locus [123], or inducible shRNA gene targeting [122, 124, 125]. PI3KC2α-deficient mouse platelets show defective thrombus formation, forming accelerated but highly unstable thrombi under haemodynamic shear [122, 123]. Interestingly, although there was a decrease in a ‘housekeeping’ pool of PtdIns(3)P, the platelet phenotype observed with loss of PI3KC2α was not associated with defects in agonist-induced changes in PtdIns3P or PtdIns(3,4)P2. Instead, PI3KC2α-deficient platelets displayed various structural and biophysical changes in their membranes, including an enlarged open canalicular system (OCS), enhanced membrane tethers, an enrichment of barbell proplatelets, a reduction in certain membrane skeleton proteins, decreased filopodia, and defects in α-granules [122, 123]. Megakaryocytes (MKs), large cells residing in the bone marrow involved in platelet production, also showed an abnormal demarcation membrane system, confirming the membrane defects not to be specific to platelets, and defining an important role for PI3KC2α in membrane structure and dynamics [92, 122, 123].
Although an early study suggested a role of PI3KC2β in PtdIns(3,4)P2 generation following integrin αIIbβ3 activation [126], platelets from PI3KC2β-deficient mice showed unaltered basal or agonist-stimulated levels of PtdIns(3)P, PtdIns(3,4)P2 or PtdIns(3,4,5)P3 compared to wild type littermates [122]. Furthermore, PI3KC2β-deficient platelets had normal platelet functional, haemostatic and thrombotic responses [122]. Loss of both PI3KC2α and PI3KC2β had no impact on agonist-stimulated 3-phosphoinositide levels, and confirmed that PI3KC2α’s role in the regulation of platelet open canalicular structure and thrombus stability is non-redundant, although VPS34 expression was increased in this context [125]. Subsequent work using ion beam-scattering electron microscopy and mass spectrometry confirmed that the defect in platelet membrane structure observed for PI3KC2α-deficient platelets is not associated with major changes in membrane lipid composition, but is due to increased OCS dilation, volume, and plasma membrane openings, with a potential impact on membrane tethering during thrombus formation [124]. It is important to note that a lack of selective inhibitors for the Class II PI3Ks has hampered further interrogation of their functional roles in many contexts, including whether the functional significance observed in mice will translate fully to humans. However, Class II PI3K inhibitors are beginning to emerge, and may be of value in thrombosis, cancer and other settings, as discussed below [117, 127–129].
Class III PI3K
The sole Class III PI3K, VPS34 (PIK3C3 gene), is the primordial PI3K conserved across species from yeast to humans, and serves to catalyse the generation of PtdIns(3)P from PtdIns [130–132]. VPS34 is widely expressed across mammalian tissues, and forms two protein complexes, Complex I and Complex II (Fig. 1) [93, 133]. In addition to VPS34, both complexes contain VPS15 (PIK3R4) and Beclin 1, although Complex I also contains ATG14, whereas Complex II contains UVRAG (Fig. 2) [134]. Complex I may also incorporate the regulatory proteins NRBF2 or AMBRA, while Complex II can contain Rubicon, although it appears likely that several VPS34-containing complexes of varying composition may exist in cells [93]. The helical and kinase domains of VPS34 are flexible and regulate its catalytic activity by adopting closed or open conformations, as the C-terminal helix blocks the ATP-binding site until the association of the helix with the membrane removes this inhibition [135]. The helical and kinase domains of VPS34 are positioned on one side of a V-shaped assembly that both Complex I and II appear to form, and interact with VPS15 [14, 93]. Beclin 1, and ATG14 or UVRAG, are positioned on the other arm of the V assembly, which also mediates membrane association (Fig. 2) [14, 93]. ATG14 preferentially associates with highly curved membranes to facilitate the association of Complex I with the growing autophagic isolation membrane, while Complex II associates with relatively flat endosomal membranes potentially due to flexibility between the two arms of its V shape allowing an extended conformation [14, 136, 137]. Class III PI3K is highly regulated by post-translational modifications, including acetylation, ubiquitination, SUMOylation, and phosphorylation by enzymes such as AMPK and mTORC1, which can serve to modulate its catalytic activity and protein–protein interactions [14, 134].
Complex I’s activation and recruitment to the isolation membrane leads to PtdIns(3)P generation to support autophagosome formation and elongation, while Complex II supports endosome maturation and fusion of autophagosomes with late endosomes/lysosomes [93, 134]. As discussed earlier, Class II PI3Ks can also generate PtdIns(3)P, as can lipid phosphatases, and so Class III PI3K is not the sole source of this phosphoinositide in cells, and indeed its contribution appears to vary between cell types [93].
Various strategies have been utilised to assess Class III’s physiological role in differing tissues, including a kinase-dead mouse knock-in approach, knock-out approaches and, more recently, the use of inhibitors. Global homozygous loss of VPS34 catalytic activity or expression leads to embryonic lethality in mice between E6.5 and E8.5 [133, 138]. A corresponding impact on the expression of VPS34 interactors such as VPS15 in these models makes protein-specific phenotype interpretation more challenging, but the comparable embryonic lethality of knock-out and kinase-dead knock-in mice supports a role for this enzyme in early embryonic development, with defects in cell proliferation and mTOR signalling [133, 138]. Tissue-specific targeting has allowed further insight into the physiological roles of VPS34, with loss of VPS34 leading to cardiomyopathy and cardiomegaly in the heart [139, 140], hepatomegaly and hepatic steatosis in the liver [139], rod cell degeneration in the retina [141], neurodegeneration in the nervous system [142–144], and defective T cell survival and homeostasis [145, 146]. Many of these phenotypes were associated with defects in cellular autophagy or endocytic trafficking.
Class III PI3K in platelet function and thrombosis
Both mouse and human platelets express the Class III PI3K protein, VPS34. Two detailed studies assessing mice with targeting of VPS34 in their megakaryocytes and platelets have revealed the functional importance of this enzyme in this cell lineage [147, 148]. The core phenotypes of the distinct VPS34-deficient mouse lines were comparable in that loss of Class III PI3K had no effect on haemostasis, but resulted in defective in vivo and in vitro thrombosis. Both studies reported smaller thrombi under arterial flow conditions, suggesting defects in thrombus growth and stability. This implies that VPS34 might hold value as an antithrombotic target, as discussed later, which has been supported by the use of the VPS34 inhibitors 3-MA, VPS34-IN1 and SAR405 in human platelets [147, 148].
However, the two VPS34-deficient mouse lines did differ in the characterisation of various specific MK and platelet features, potentially due to differences in experimental conditions or gene-targeting approaches and their relative penetration. While Liu et al. [147] reported a normal platelet count and morphology, Valet et al. [148] observed microthrombocytopenia, with a reduction in both platelet count and volume, and multiple phenotypic alterations in VPS34 deficient-megakaryocytes. The latter includes fewer but larger α-granules in MKs in native bone marrow, and increased release of platelets outside of the sinusoids directly into the bone marrow compartment. This ectopic platelet release is likely to underlie the thrombocytopenia in these mice [148]. In addition, VPS34-deficient MKs showed reduced transferrin and fibrinogen endocytosis, and decreased number and increased size of fibrinogen-containing and clathrin-coated vesicles, suggesting a defect in clathrin-mediated endocytosis [148]. RAB11 labelling also suggested impaired endosomal recycling, with VPS34-deficient MKs therefore demonstrating a general trafficking defect [148]. VPS34-deficient MKs showed a 30–40% reduction in PtdIns(3)P which, although confirming Class III PI3K to be a significant source of this phosphoinositide in this cell type, confirms the importance of other enzymatic routes of PtdIns(3)P synthesis in MKs [148].
Valet et al. [148] also revealed VPS34-deficient platelets to show multiple specific defects, many of which correspond to their observations in VSP34-deficient MKs. Although VPS34-deficient platelets showed reduced numbers of α and dense granules, their granule release was faster and exacerbated in response to acute platelet stimulation both in platelet suspensions and in vitro flow studies. VPS34-platelets were however less efficient in recruiting wild type platelets to allow further thrombus growth [148]. As for MKs, VPS34 is also likely to be involved in clathrin-mediated endocytosis in platelets, as platelet Mpl endocytosis and fibrinogen internalisation were both defective [148]. Indeed, the Pf4-Cre-Pik3c3 mice showed elevated serum thrombopoietin (TPO), correlating with the reduced platelet count, and defective platelet Mpl endocytosis [148]. The contribution of VPS34 to total PtdIns(3)P levels in platelets was modest with a 10% decrease in PtdIns(3)P in VPS34 deficient platelets under resting conditions. Although the agonist induced pool of PtdIns(3)P was more markedly affected, the reduction was still partial, supporting the role of other enzymes in PtdIns(3)P generation in platelets [148]. Platelet shape change, filopodia formation, integrin activation, aggregation, ROS production and Thromboxane A2 production responses to a range of agonists were normal in VPS34-deficient murine platelets and in human platelets treated with a VPS34 inhibitor [148].
While the overall phenotype of VPS34-deficient mice was similar in the study by Liu et al. [147] a range of differences in platelet characteristics and responses were observed. In contrast to Valet et al. the number of platelet α and dense granules was normal in VPS34-deficient platelets. However, α and dense granule secretion, integrin αIIbβ3 activation and platelet aggregation were defective in response to collagen and thrombin, in particular at lower agonist concentrations, while downstream phosphorylation of SYK and PLCγ2 (but not other pathways) was affected [147]. Furthermore, clot retraction of VPS34-deficient platelets was delayed, despite platelet spreading on fibrinogen and integrin β3 and SRC phosphorylation being normal, suggesting a defect in later, but not early, integrin outside-in signalling [147]. Interestingly, PtdIns(3)P levels were comparable between wild type and VPS34-deficient platelets, although VPS34-deficient platelets had a significantly lower response to thrombin or convulxin stimulation [147]. The partial effect of VPS34 deficiency on PtdIns(3)P levels is in agreement with Valet et al. [148] and studies investigating platelet PI3KC2α [123], and confirms the involvement of multiple enzymes in platelet PtdIns(3)P synthesis.
Interestingly, Liu et al.’s [147] findings also revealed that VSP34 supports NADH/NADPH oxidase (NOX) activity and subsequent generation of reactive oxygen species (ROS) to impact on platelet activation. VPS34-deficient platelets had reduced agonist-induced translocation of the NOX subunits p40phox and p47phox to the plasma membrane, p40phox phosphorylation and ROS generation [147]. VPS34 deficiency furthermore impaired mTORC1 and 2 activation, as judged by substrate phosphorylation, although this did not appear to influence platelet function. Similarly, although loss of VPS34 affected basal autophagic flux in resting platelets, with increased LC3-II in VPS34-deficient platelets, VPS34 did not hold an important role in autophagic flux associated with platelet activation, and the effects of autophagy inhibition did not match the phenotype of VPS34 loss [147]. Therefore, while loss of VPS34 function appears to drive defects in many tissue types due to an impact on autophagy, the phenotype of VPS34-deficient platelets does not appear to be solely driven by loss of this cellular process, despite potential importance for autophagy in platelets and the suggestion in other studies that its disruption has consequences for haemostasis and thrombosis [149, 150].
PI3Ks as clinical targets for thrombosis
PI3K inhibitors have been in development for many years, driven by the therapeutic potential of targeting these enzymes in cancer, inflammatory and immune conditions. First generation compounds such as Wortmannin and LY294002 were limited by pan-PI3K inhibition and off-target action against other cellular kinases but have proven to be valuable tools for characterising PI3K signalling, while subsequent PI3K inhibitors with isoform-selectivity and/or improved pharmacology have received more serious consideration in the clinic in recent years [151–153]. To date, the focus of efforts to clinically target PI3Ks in thrombosis has been Class I PI3Kβ. This is because the Class I PI3Ks have received considerably more attention than Class II or III in this area so far, and because, as discussed above, PI3Kβ is the predominant functional Class I PI3K in platelets. Indeed, platelet PI3Kβ was the target of one of the earliest isoform-selective PI3K inhibitors, TGX-221 [70, 154]. The highly homologous nature of the ATP binding pocket of the Class I PI3Ks makes achieving isoform-selective inhibitors a major challenge, but the observation of two clusters of non-conserved residues at its periphery, and a hard-won understanding of the intricate details of the conformational flexibility and interactions of the binding pocket, have aided the development of inhibitors with impressive selectivity [155]. The use of TGX-221 defined a role for platelet PI3Kβ in initiating and sustaining αIIbβ3 adhesive contacts, most notably under conditions of shear stress, thus proposing PI3Kβ as a new antithrombotic target (Fig. 3) [70]. This was subsequently supported and extended by a wide body of work using TGX-221 and gene-targeted mice, defining roles for PI3Kβ downstream of various platelet receptors to support thrombus formation in vivo, and confirming that PI3Kβ inhibition provides protection from arterial thrombosis, with limited effect on normal haemostasis [48, 69, 71, 75].
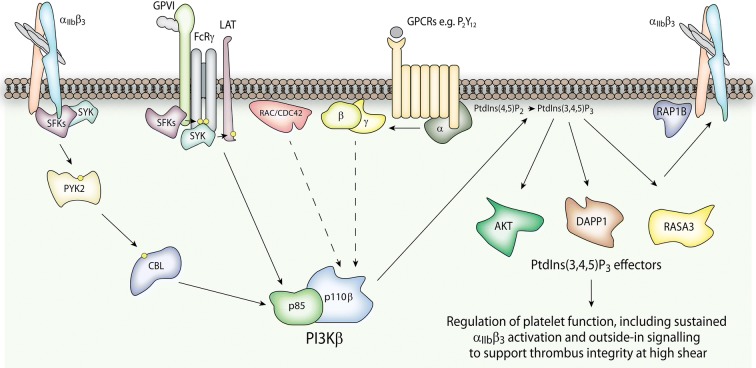
Fig. 3.
The role of PI3Kβ in platelets. Class I PI3Kβ is considered a potential antithrombotic target due to its functional role downstream of various platelet receptors, and has received the most intense clinical interest of the PI3Ks so far in this setting. In particular, it plays a key role in sustaining integrin αIIbβ3 signalling to support stable thrombus formation under high shear, acting via the regulation of various cellular effectors which are responsive to its catalytic product, PtdIns(3,4,5)P3. PI3Kβ can receive activatory input via multiple interactions, including direct interaction of p85 with CBL downstream of αIIbβ3, association of the SH2 domains of p85 with phosphorylated tyrosines on receptors and adaptors downstream of Glycoprotein VI (GPVI), and interactions of p110β with RAC/CDC42 and the Gβγ subunits of activated heterotrimeric G proteins downstream of GPCRs such as P2Y12
Based on this, development of further small molecules targeting PI3Kβ led to the derivation of AZD6482, which is an active enantiomer of a racemic mixture first known as KN-309, an improved structural analogue of TGX-221 [156]. AZD6482 has nanomolar potency against PI3Kβ and is highly selective for this enzyme against a panel of protein kinases, with lowest selectivity against the other Class I PI3Ks, and the related DNA-PK [72]. AZD6482 inhibits agonist- and shear-induced human platelet aggregation, and displayed a concentration-dependent anti-thrombotic effect in vivo in a modified Folt’s model in dogs, with no detectable increase in bleeding time or blood loss even at concentrations considerably higher than required for antithrombotic effect [72]. A 3 h infusion in healthy human male Caucasian subjects confirmed a maximal inhibition of platelet aggregation at 1uM in ex vivo assays, with limited effect on cutaneous bleeding time [72]. AZD6482 demonstrated a mean effective elimination half-life of ~ 20 min for the highest dose groups, and a rapid normalization of platelet function post end of infusion [72]. This was attributed to high clearance and a relatively small distribution volume, with the study authors proposing that AZD6482 may provide value as a parenteral antiplatelet agent in acute stroke where rapid onset of action and low bleeding risk is desirable, and in cardiothoracic surgery. Since extracorporeal circulation can lead to platelet dysfunction, and TGX-221 has been shown to be of value in this setting [157, 158], PI3Kβ inhibitors may be of use in cardiopulmonary bypass surgery, and avoid the bleeding risk associated with integrin αIIbβ3 inhibitors [156]. While AZD6482, like other PI3K inhibitors, has shown some impact on glucose homeostasis, the study authors concluded that this would not be of clinical importance during short-term infusion as an antiplatelet agent [72], although on the basis that inhibition of PI3Kα may underlie any major effect on insulin signalling, PI3Kβ inhibitors with an improved selectivity ratio against PI3Kα are being actively sought [76, 159]. However, even with highly selective agents, it remains unclear whether long-term PI3Kβ inhibition beyond acute usage would be a viable therapeutic approach given PI3Kβ’s roles in multiple aspects of physiology, and considering mice with loss of PI3Kβ activity develop mild insulin resistance with age [160]. Despite this, PI3Kβ inhibitors are under development in cancer and may be of value in contexts of PTEN loss and genomic aberrations of the PI3Kβ locus [161, 162] and studies to date suggest these agents may have an acceptable safety profile, although this remains a major challenge for the development of Class I PI3K inhibitors in cancer (discussed later).
Since anti-platelet therapy is often administered as a combination of drugs, with Aspirin prescribed alongside a P2Y12 antagonist in settings where single therapy is insufficient, Nylander et al. [163] extended on their initial validation of AZD6482 with a study administering this drug as part of combination therapy. AZD6482 was assessed alongside the P2Y12 antagonists ticagrelor or clopidogrel, or alongside aspirin, in studies using dogs and healthy humans. Assessment of ex vivo antiplatelet effect using a conscious dog model confirmed the attractive profile of PI3Kβ inhibition in demonstrating anti-platelet efficacy with limited effect on bleeding time, while with healthy male Caucasian human subjects a combination of PI3Kβ inhibition plus COX inhibition with aspirin provided less bleeding potential, but more potential for greater overall anti-platelet effects, than P2Y12 inhibition plus aspirin [163]. This study therefore suggested that PI3Kβ inhibition could be of value not only as a monotherapy, but in combination with Aspirin, suggesting further clinical investigation of this enzyme as an anti-thrombotic target should take place.
Despite this promise for PI3Kβ as an anti-thrombotic target, its clinical potential may be hampered by the observation that its inhibition may lead to increased risk of embolism of thrombotic material [75, 164]. Laurent et al. [75] demonstrated a key role for PI3Kβ, via a AKT-GSK3 axis, in thrombus stability and recruitment of new platelets to the growing thrombus at high shear rate, observed using mice with selective loss of PI3Kβ in the megakaryocyte lineage and human platelets treated with AZD6482. The thrombus instability with loss of PI3Kβ activity at high shear rate was associated with the formation of platelet emboli from large thrombi, suggesting the potential for secondary ischaemic events, with the growing thrombus itself likely contributing to the elevation of local shear in the blood vessel [75, 164]. Further studies are needed to determine whether this property would rule out PI3Kβ inhibitors for clinical use, particularly since P2Y12 inhibition may cause a similar effect which has not limited its use clinically [156], and tailoring of PI3Kβ inhibitor dosage may mean a suitable level of inhibition can be found [164].
As an understanding of the role of Class II and Class III PI3Ks in platelet function begins to develop, therapeutic targeting of these enzymes in thrombosis becomes a new consideration. As for Class I PI3Kβ, available evidence suggests selective inhibition of Class II PI3KC2α might offer a new antithrombotic approach, and indeed early data from the Hamilton group at Monash University suggests PI3KC2α inhibitors may prove to be potent anti-thrombotics with a promising safety profile, with their lead compound MIPS-19416 producing an antithrombotic effect that may be largely independent of canonical platelet activation, producing similar observations to studies with PI3KC2α-deficient mice (ISTH Academy. Moon M. July 10, 2019; 274013; OC 78.3). Class II PI3K inhibition has also been proposed as a therapeutic approach in cancer and diabetes [165, 166]. With most of our current understanding of Class II PI3K function in organismal physiology coming from mouse gene targeting, the ongoing development [127–129, 165] of selective Class II PI3K inhibitors as tools will allow an improved understanding of the intricate roles and regulation of these enzymes in humans, and provide a better perspective of whether they may be useful and viable therapeutic targets in human disease. Similarly, given the defect in thrombosis observed with loss of VPS34 in platelets, without an impact on haemostasis, pharmacological inhibition of Class III PI3K has been proposed as a novel anti-thrombotic approach [147, 148], and may also be of benefit for cancer and diabetes [133, 167, 168]. This suggests exciting new clinical opportunities for PI3K inhibition in thrombosis although, as for Class I PI3K inhibitors, whether drugs targeting Class II or Class III PI3Ks could have an acceptable safety and efficacy profile given the importance of these enzymes in normal development and physiology in such a broad range of tissues, and the complexity of PI3K signalling, remains unclear. More generally, major barriers to the development of novel antithrombotic drugs include the challenge of establishing good preclinical models that can adequately mimic the human disease setting, and the relatively high bar of deriving novel therapeutic strategies and candidates that are a sufficient improvement on existing drugs to warrant interest from the pharmaceutical industry, prescribers and patients.
PI3Ks in other aspects of cardiovascular disease
Beyond platelet function, PI3Ks are implicated in various other aspects of cardiovascular physiology and disease, including atherosclerosis, hypertension, angiogenesis, heart disease and myocardial infarction. Coronary heart disease is commonly associated with atherosclerosis, whereby thrombosis can lead to acute myocardial infarction and sudden cardiac death [169]. PI3Kγ appears to play a role in the pathogenesis of atherosclerosis, and pharmacological inhibition of this Class I PI3K isoform with AS605240 was shown to significantly reduce early atherosclerotic lesions in Apolipoprotein E (ApoE)-null mice, and attenuate advanced atherosclerotic lesions in LDL receptor-deficient mice [170]. PI3Kγ levels are elevated in mouse and human atherosclerotic lesions and the function of this Class I isoform in the haematopoietic lineage supports atherosclerotic progression via roles in macrophage and T cell infiltration, and plaque stabilization [170]. Furthermore, Chang et al. [171] confirmed the role of PI3Kγ in macrophage activation in response to oxidized low-density lipoprotein, inflammatory chemokines and angiotensin II, and also demonstrated that p110γ deficiency leads to a significant reduction in the size of atherosclerotic plaques in ApoE-deficient mice. In addition, GM-CSF-differentiated mouse macrophages become foam cells by PI3Kγ-dependent fluid-phase pinocytosis of LDL [172]. PI3Kγ may therefore represent a therapeutic target in atherosclerosis, as may other PI3K isoforms involved in various cell types implicated in this condition [104], and the relatively restricted expression of PI3Kγ in particular could limit unwanted effects in other tissues [173].
Hypertension is a strong risk factor for cardiovascular disease, and since PI3Ks have roles in the regulation of vascular tone they may represent therapeutic targets in this context. Mice lacking PI3Kγ are protected from angiotensin II-induced hypertension, due to a role for this Class I PI3K in smooth muscle contraction via signalling pathways involving RAC-driven ROS production, and AKT-driven extracellular calcium entry via L-type calcium channels [174]. As such, loss of PI3Kγ function in vivo decreases peripheral vascular resistance via a vasorelaxing effect mediated by loss of pressure-induced AKT phosphorylation and impaired plasma membrane trafficking of the α1C L-type calcium channel in smooth muscle [175]. In support of this, a single nucleotide polymorphism (SNP) in a region flanking the p110γ (PIK3CG) gene locus in humans was shown to influence pulse pressure and mean arterial pressure, and potential risk of cardiovascular events including hypertension, coronary heart disease and stroke risk scores [176]. PI3Kγ’s role in both hypertension and (the often associated) inflammation means inhibitors of this isoform might be attractive therapeutic candidates via both anti-hypertensive and anti-inflammatory properties [173, 176]. Other Class I PI3K isoforms may also hold roles in hypertension, with p110δ expression being upregulated in aortas of hypertensive rats [177], while Class II PI3KC2α appears to regulate vascular smooth muscle contraction and play a role in spontaneous hypertension in rats via a Ca2+-PI3KC2α-RHO axis [178–180].
As discussed earlier, both Class I PI3Kα and Class II PI3KC2α support angiogenesis through roles in endothelial cell signalling and function, including transduction of VEGFR signalling and coupling to RHOA to facilitate cell migration [40, 105]. The Class I PI3Kβ and γ isoforms also support endothelial cell migration in response to GPCR agonists such as S1P, and PI3Kγ plays a role in endothelial progenitor cells to support neovascularisation and reperfusion after ischaemia [152, 181, 182]. Modulation of PI3K function may therefore offer therapeutic opportunities to regulate vascular and tissue regeneration after ischaemic damage in cardiovascular disease states. Interestingly, it has been observed that low doses of PI3K inhibitors can improve vascular function [183–185], which might be of value in cardiovascular disease. The studies making these observations were focussed on the impact of PI3K inhibitors on the tumour vasculature in cancer, and suggest that the vascular effects of these drugs can be used to improve drug delivery to tumours while also facilitating immune cell recruitment [183–186]. In contrast, in line with the angiogenesis phenotypes of mice with PI3K deficiency, the potential value of PI3K inhibitors used at higher doses in cancer is via their inhibition or eradication of the tumour vasculature [186]. These effects may be via direct action of the inhibitors against PI3K in endothelial cells, or indirectly via action on other tumour cells [186], myeloid cells [182], and even platelets [187]. Thus, consideration of the intersection between the importance of PI3Ks and their inhibition in cardiovascular (patho)physiology and in cancer may be of value.
In addition to the vasculature, platelets and immune cells, PI3Ks’ various roles in cardiac tissue suggest inhibitors might be of direct value in heart disease. Class I PI3Kα is important for both cardiac development and adult cardiac physiology, controlling cardiomyocyte cell size [188], physiological cardiac growth [189], and thus overall heart size [190]. Furthermore, PI3Kα activity protects the heart against myocardial infarction-induced heart failure [191], can improve the function of a failing heart, is important for exercise-induced cardioprotection [192], and can protect against heart disease in response to dilated cardiomyopathy and acute pressure overload [193, 194]. PI3Kα also negatively regulates gelsolin-activity to suppress gelsolin’s actin-severing activity that contributes to cardiac remodelling in heart failure [195]. PI3Kα’s role in mediating insulin signalling to L-type calcium channels to regulate calcium currents in cardiac myocytes is a key mechanism underpinning the importance of this Class I PI3K isoform in the heart, while both PI3Kα and PI3Kβ support cardiac structure and organisation via regulation of junctophilin-2 localisation and T-tubule organisation [152, 196, 197]. Inhibition of PI3Kα would therefore appear to be detrimental to cardiac development and function and may also have acute detrimental effects such as atrial fibrillation [198]. In contrast, inhibition of PI3Kγ may be cardioprotective and thus a more promising approach for heart failure [152]. Indeed, while PI3Kγ holds a key role in normal cardiac physiology as a scaffolding protein for protein kinase A and phosphodiesterases [199–201], the importance of its catalytic activity is more apparent in the disease setting. p110γ expression is upregulated in congestive heart failure, and its pharmacological inhibition improves contractility in failing hearts by preventing a reduction in β-adrenergic receptor density, with mice expressing a kinase dead form of p110γ showing protection from ventricular modelling and failure caused by pressure overload [152, 199, 201]. In addition to a role for PI3Kγ in regulating β-adrenergic receptors and contractility in cardiomyocytes, this Class I isoform likely also functions in leukocytes to regulate inflammatory signalling in heart failure [152, 202]. In agreement with this, the benefits observed with administration of PI3Kγ inhibitors in animal models of heart disease and failure appear to be dependent on both cardiac contractility and immune cell infiltration [152]. In addition, Class III PI3K’s role in the transition of cardiac hypertrophy to heart failure might suggest inhibition of VPS34 to be of clinical value in this context [203], but demonstration that VPS34 prevents hypertrophic cardiomyopathy by regulating myofibril proteostasis, and that its loss leads to cardiomegaly and decreased contractility, suggests this is unlikely to be a beneficial therapeutic approach [139, 140]. Class II PI3KC2α is also required for cardiac looping during embryonic development [107].
The value of PI3K inhibition for myocardial infarction (MI) remains unclear. The Class I PI3Kδ/γ inhibitor, TG100-115, entered phase I and II clinical trials for acute MI, with observation that it provided cardioprotection in animal models by reducing infarct development and preserving myocardial function even when administered up to 3 h after myocardial infusion [204, 205]. However, selective PI3Kγ inhibition with AS605240 led to an increased infarct size with defective reparative neovascularisation and impaired recovery of left ventricular function in a mouse model of MI, which was supported by the use of PI3Kγ-deficient mice [206]. Furthermore, Haubner et al. [207] reported a protective role for PI3Kγ in myocardial ischaemia–reperfusion injury in mice, mediated via a kinase-independent mechanism. Similarly, PI3Kα has a cardioprotective role in ischaemia–reperfusion injury to limit myocardial infarct size via inhibition of mitochondrial permeability transition pore opening, thus suggesting that promoting, rather than inhibiting, PI3Kα would be preferable in this setting [208]. PI3Kβ also has cell-specific effects in the ischaemic heart, with PI3Kβ activity being protective against myocardial ischaemic injury in cardiac myocytes, while loss of PI3Kβ activity in endothelial cells leads to cardioprotective effects [209].
The cardiovascular system as an unintended target of PI3K inhibitors
In addition to the consideration of PI3Ks as therapeutic targets for cardiovascular disease, it is clearly also important to consider the significance of their inhibition in cardiovascular tissues as an unintended consequence of PI3K inhibitor administration in other settings, such as oncology. Indeed, at present the predominant focus of PI3K inhibitor development is cancer, with small molecules targeting all Class I PI3K isoforms (either individually or non-selectively) currently in clinical trials, and Class II and Class III PI3K inhibitors being considered for future promise in this setting. From the discussion above, it is clear that PI3K inhibition has the potential to lead to unwanted cardiovascular events, including arrhythmia and cardiac remodelling [198, 210–215]. In some scenarios, the benefits may outweigh the risks, and cardiotoxicity may not apply to all PI3K inhibitors or dosing regimes, but careful cardiovascular monitoring of patients on PI3K-targeted therapy should be in place, particularly for compromised individuals. The authors refer the readers to an excellent discussion by McLean et al. [210] covering this topic, which includes suggestions for strategies to optimize the benefit:risk ratio.
Conclusions and future perspective
There is now considerable evidence of roles for the PI3K isoforms in various aspects of cardiovascular physiology and disease. This implies these enzymes might prove to be valuable therapeutic targets in contexts such as thrombosis, atherosclerosis and heart failure. In particular, their key roles in platelets suggest Class I, II and III PI3Ks might all be valid anti-thrombotic targets, with the aim of inhibiting thrombosis with limited effect on normal haemostasis. However, it is important to reflect on lessons learned so far in the pursuit of Class I PI3K inhibitors, particularly in the field of cancer, where issues with toxicity, lack of efficacy, and a still limited appreciation of the complexity, redundancy and cell type-specificity of cell signalling has led to much disappointment. It seems clear that in any given cardiovascular context, multiple PI3K isoforms are operating, across multiple cell types, often with both positive and negative roles. Furthermore, given emerging evidence of their important organismal roles, it appears likely that attempts to therapeutically target the Class II and Class III PI3Ks may face similar challenges to those met for Class I PI3K, particularly for the broadly expressed isoforms where managing unwanted inhibition in off-target tissues could be a major challenge.
Nevertheless, the often critical importance of the PI3Ks in various aspects of physiology and disease means they continue to be attractive targets for the development of drugs, and progress continues to be made with innovative new strategies. Indeed, for cancer, while the concept of PI3K inhibition as a monotherapy directly targeting solid tumours has faced challenges [186, 216, 217], value may still come through the use of specific dosing regimes (e.g. higher, but intermittent, dosing) [218], through the combination of PI3K inhibitors with other drugs (e.g. alpelisib/piqray with fulvestrant) [219], through the development of p110α mutant-selective agents (e.g. taselisib) [220], via dietary and pharmacological approaches [221], through understanding the effect of PI3K inhibition on cells beyond the cancer cells themselves (e.g. idelalisib) [186, 217], and through new drug delivery approaches [222]. Time will tell whether these approaches permit PI3K inhibition to be a viable long term therapeutic strategy in cancer, and whether this progress will benefit other conditions such as thrombosis, immune and inflammatory states, and PI3K-related syndromes (e.g. PIK3CA-related overgrowth spectrum (PROS) [223]) with an acceptable safety profile. Platelets and leukocytes may hold more potential for targeting with PI3K inhibitors in comparison to other cell types in more complex, heterogeneous, less accessible, solid tissues or tumours, and the greatest value of PI3K inhibitors may prove to be in acute clinical settings where a therapeutic window may be easier to find. It may be that some inhibitors originally developed for oncology prove to be of more value in other settings. If targeting the PI3K isoforms themselves continues to prove challenging, considering other elements in the PI3K pathway may yet be of value. For example, inhibition of phosphoinositide effectors downstream of the PI3Ks, if druggable, may offer greater functional selectivity and/or target cell selectivity than their upstream masters, to provide drugs with reduced unwanted effects on other PI3K-regulated pathways and tissues. Ultimately, as ever, safer and more efficacious drugs will come from a more detailed understanding of the function and regulation of the individual PI3K isoforms, and their interplay with each other and other cellular effectors across multiple cell types in complex in vivo settings of human health and disease.
Acknowledgements
We apologise to all authors whose excellent work we were unable to cite due to space limitations.
Abbreviations
- PI3K
phosphoinositide 3-kinase
- PtdIns(3,4,5)P3
phosphatidylinositol (3,4,5)-trisphosphate
- PtdIns(3,4)P2
phosphatidylinositol (3,4)-bisphosphate
- PtdIns(4,5)P2
phosphatidylinositol (4,5)-bisphosphate
- PtdIns(3)P
phosphatidylinositol (3)-monophosphate
- GEF
guanine nucleotide exchange factor
- GAP
GTPase accelerating protein
- SH2
Src homology 2 domain
- RBD
Ras-binding domain
- GPCR
G protein-coupled receptor
Authors’ contributions
The authors contributed equally to this article. Both authors read and approved the final manuscript.
Funding
The authors gratefully acknowledge funding from the British Heart Foundation (Grant Nos. PG/12/79/29884, PG/13/11/30016, PG/14/3/30565).
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tom N. Durrant, Email: tom.durrant@chem.ox.ac.uk
Ingeborg Hers, Email: i.hers@bris.ac.uk.
References
- 1.Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, Ahmed M, Aksut B, Alam T, Alam K, Alla F, Alvis-Guzman N, Amrock S, Ansari H, Arnlov J, Asayesh H, Atey TM, Avila-Burgos L, Awasthi A, Banerjee A, Barac A, Barnighausen T, Barregard L, Bedi N, Belay Ketema E, Bennett D, Berhe G, Bhutta Z, Bitew S, Carapetis J, Carrero JJ, Malta DC, Castaneda-Orjuela CA, Castillo-Rivas J, Catala-Lopez F, Choi JY, Christensen H, Cirillo M, Cooper L, Jr, Criqui M, Cundiff D, Damasceno A, Dandona L, Dandona R, Davletov K, Dharmaratne S, Dorairaj P, Dubey M, Ehrenkranz R, El Sayed Zaki M, Faraon EJA, Esteghamati A, Farid T, Farvid M, Feigin V, Ding EL, Fowkes G, Gebrehiwot T, Gillum R, Gold A, Gona P, Gupta R, Habtewold TD, Hafezi-Nejad N, Hailu T, Hailu GB, Hankey G, Hassen HY, Abate KH, Havmoeller R, Hay SI, Horino M, Hotez PJ, Jacobsen K, James S, Javanbakht M, Jeemon P, John D, Jonas J, Kalkonde Y, Karimkhani C, Kasaeian A, Khader Y, Khan A, Khang YH, Khera S, Khoja AT, Khubchandani J, Kim D, Kolte D, Kosen S, Krohn KJ, Kumar GA, Kwan GF, Lal DK, Larsson A, Linn S, Lopez A, Lotufo PA, El Razek HMA, Malekzadeh R, Mazidi M, Meier T, Meles KG, Mensah G, Meretoja A, Mezgebe H, Miller T, Mirrakhimov E, Mohammed S, Moran AE, Musa KI, Narula J, Neal B, Ngalesoni F, Nguyen G, Obermeyer CM, Owolabi M, Patton G, Pedro J, Qato D, Qorbani M, Rahimi K, Rai RK, Rawaf S, Ribeiro A, Safiri S, Salomon JA, Santos I, Santric Milicevic M, Sartorius B, Schutte A, Sepanlou S, Shaikh MA, Shin MJ, Shishehbor M, Shore H, Silva DAS, Sobngwi E, Stranges S, Swaminathan S, Tabares-Seisdedos R, Tadele Atnafu N, Tesfay F, Thakur JS, Thrift A, Topor-Madry R, Truelsen T, Tyrovolas S, Ukwaja KN, Uthman O, Vasankari T, Vlassov V, Vollset SE, Wakayo T, Watkins D, Weintraub R, Werdecker A, Westerman R, Wiysonge CS, Wolfe C, Workicho A, Xu G, Yano Y, Yip P, Yonemoto N, Younis M, Yu C, Vos T, Naghavi M, Murray C. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017;70(1):1–25. doi: 10.1016/j.jacc.2017.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laurent PA, Payrastre B, Gratacap MP. Class I PI3Ks in arterial thrombosis. Aging. 2019;11(5):1321–1322. doi: 10.18632/aging.101841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stegner D, Haining EJ, Nieswandt B. Targeting glycoprotein VI and the immunoreceptor tyrosine-based activation motif signaling pathway. Arterioscler Thromb Vasc Biol. 2014;34(8):1615–1620. doi: 10.1161/ATVBAHA.114.303408. [DOI] [PubMed] [Google Scholar]
- 4.Hunter RW, Hers I. Insulin/IGF-1 hybrid receptor expression on human platelets: consequences for the effect of insulin on platelet function. J Thromb Haemost. 2009;7(12):2123–2130. doi: 10.1111/j.1538-7836.2009.03637.x. [DOI] [PubMed] [Google Scholar]
- 5.Koenig-Oberhuber V, Filipovic M. New antiplatelet drugs and new oral anticoagulants. Br J Anaesth. 2016;117(Suppl 2):ii74–ii84. doi: 10.1093/bja/aew214. [DOI] [PubMed] [Google Scholar]
- 6.Fuster V, Sweeny JM. Aspirin: a historical and contemporary therapeutic overview. Circulation. 2011;123(7):768–778. doi: 10.1161/CIRCULATIONAHA.110.963843. [DOI] [PubMed] [Google Scholar]
- 7.Aungraheeta R, Conibear A, Butler M, Kelly E, Nylander S, Mumford A, Mundell SJ. Inverse agonism at the P2Y12 receptor and ENT1 transporter blockade contribute to platelet inhibition by ticagrelor. Blood. 2016;128(23):2717–2728. doi: 10.1182/blood-2016-03-707844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Giezen JJ, Nilsson L, Berntsson P, Wissing BM, Giordanetto F, Tomlinson W, Greasley PJ. Ticagrelor binds to human P2Y(12) independently from ADP but antagonizes ADP-induced receptor signaling and platelet aggregation. J Thromb Haemost. 2009;7(9):1556–1565. doi: 10.1111/j.1538-7836.2009.03527.x. [DOI] [PubMed] [Google Scholar]
- 9.Taylor L, Vasudevan SR, Jones CI, Gibbins JM, Churchill GC, Campbell RD, Coxon CH. Discovery of novel GPVI receptor antagonists by structure-based repurposing. PLoS ONE. 2014;9(6):e101209. doi: 10.1371/journal.pone.0101209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller MM, Banville J, Friends TJ, Gagnon M, Hangeland JJ, Lavallee JF, Martel A, O’Grady H, Remillard R, Ruediger E, Tremblay F, Posy SL, Allegretto NJ, Guarino VR, Harden DG, Harper TW, Hartl K, Josephs J, Malmstrom S, Watson C, Yang Y, Zhang G, Wong P, Yang J, Bouvier M, Seiffert DA, Wexler RR, Lawrence RM, Priestley ES, Marinier A. Discovery of potent protease-activated receptor 4 antagonists with in vivo antithrombotic efficacy. J Med Chem. 2019 doi: 10.1021/acs.jmedchem.9b00186. [DOI] [PubMed] [Google Scholar]
- 11.Michelson AD. Antiplatelet therapies for the treatment of cardiovascular disease. Nat Rev Drug Discov. 2010;9(2):154–169. doi: 10.1038/nrd2957. [DOI] [PubMed] [Google Scholar]
- 12.Vanhaesebroeck B, Stephens L, Hawkins P. PI3K signalling: the path to discovery and understanding. Nat Rev Mol Cell Biol. 2012;13(3):195–203. doi: 10.1038/nrm3290. [DOI] [PubMed] [Google Scholar]
- 13.Durrant TN, Hutchinson JL, Heesom KJ, Anderson KE, Stephens LR, Hawkins PT, Marshall AJ, Moore SF, Hers I. In-depth PtdIns(3,4,5)P3 signalosome analysis identifies DAPP1 as a negative regulator of GPVI-driven platelet function. Blood Adv. 2017;1(14):918–932. doi: 10.1182/bloodadvances.2017005173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burke JE. Structural basis for regulation of phosphoinositide kinases and their involvement in human disease. Mol Cell. 2018;71(5):653–673. doi: 10.1016/j.molcel.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Tsolakos N, Durrant TN, Chessa T, Suire SM, Oxley D, Kulkarni S, Downward J, Perisic O, Williams RL, Stephens L, Hawkins PT. Quantitation of class IA PI3Ks in mice reveals p110-free-p85s and isoform-selective subunit associations and recruitment to receptors. Proc Natl Acad Sci USA. 2018;115(48):12176–12181. doi: 10.1073/pnas.1803446115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burke JE, Williams RL. Synergy in activating class I PI3Ks. Trends Biochem Sci. 2015;40(2):88–100. doi: 10.1016/j.tibs.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Burke JE, Williams RL. Dynamic steps in receptor tyrosine kinase mediated activation of class IA phosphoinositide 3-kinases (PI3K) captured by H/D exchange (HDX-MS) Adv Biol Regul. 2013;53(1):97–110. doi: 10.1016/j.jbior.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vadas O, Dbouk HA, Shymanets A, Perisic O, Burke JE, Abi Saab WF, Khalil BD, Harteneck C, Bresnick AR, Nurnberg B, Backer JM, Williams RL. Molecular determinants of PI3Kgamma-mediated activation downstream of G-protein-coupled receptors (GPCRs) Proc Natl Acad Sci USA. 2013;110(47):18862–18867. doi: 10.1073/pnas.1304801110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walser R, Burke JE, Gogvadze E, Bohnacker T, Zhang X, Hess D, Kuenzi P, Leitges M, Hirsch E, Williams RL, Laffargue M, Wymann MP. PKCbeta phosphorylates PI3Kgamma to activate it and release it from GPCR control. PLoS Biol. 2013;11(6):e1001587. doi: 10.1371/journal.pbio.1001587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang MZ, Jang H, Nussinov R. The mechanism of PI3K activation at the atomic level. Chem Sci. 2019;10(12):3671–3680. doi: 10.1039/C8SC04498H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bohnacker T, Marone R, Collmann E, Calvez R, Hirsch E, Wymann MP. PI3Kgamma adaptor subunits define coupling to degranulation and cell motility by distinct PtdIns(3,4,5)P3 pools in mast cells. Sci Signal. 2009;2(74):ra27. doi: 10.1126/scisignal.2000259. [DOI] [PubMed] [Google Scholar]
- 22.Deladeriere A, Gambardella L, Pan D, Anderson KE, Hawkins PT, Stephens LR. The regulatory subunits of PI3Kgamma control distinct neutrophil responses. Sci Signal. 2015;8(360):ra8. doi: 10.1126/scisignal.2005564. [DOI] [PubMed] [Google Scholar]
- 23.Cheung LWT, Walkiewicz KW, Besong TMD, Guo HF, Hawke DH, Arold ST, Mills GB. Regulation of the PI3K pathway through a p85 alpha monomer-homodimer equilibrium. Elife. 2015;4:e06866. doi: 10.7554/eLife.06866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thorpe LM, Spangle JM, Ohlson CE, Cheng H, Roberts TM, Cantley LC, Zhao JJ. PI3K-p110alpha mediates the oncogenic activity induced by loss of the novel tumor suppressor PI3K-p85alpha. Proc Natl Acad Sci USA. 2017;114(27):7095–7100. doi: 10.1073/pnas.1704706114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suire S, Coadwell J, Ferguson GJ, Davidson K, Hawkins P, Stephens L. p84, a new Gbetagamma-activated regulatory subunit of the type IB phosphoinositide 3-kinase p110gamma. Curr Biol. 2005;15(6):566–570. doi: 10.1016/j.cub.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 26.Stephens LR, Eguinoa A, ErdjumentBromage H, Lui M, Cooke F, Coadwell J, Smrcka AS, Thelen M, Cadwallader K, Tempst P, Hawkins PT. The G beta gamma sensitivity of a PI3K is dependent upon a tightly associated adaptor, p101. Cell. 1997;89(1):105–114. doi: 10.1016/S0092-8674(00)80187-7. [DOI] [PubMed] [Google Scholar]
- 27.Stoyanov B, Volinia S, Hanck T, Rubio I, Loubtchenkov M, Malek D, Stoyanova S, Vanhaesebroeck B, Dhand R, Nurnberg B, et al. Cloning and characterization of a G protein-activated human phosphoinositide-3 kinase. Science. 1995;269(5224):690–693. doi: 10.1126/science.7624799. [DOI] [PubMed] [Google Scholar]
- 28.Dbouk HA, Vadas O, Shymanets A, Burke JE, Salamon RS, Khalil BD, Barrett MO, Waldo GL, Surve C, Hsueh C, Perisic O, Harteneck C, Shepherd PR, Harden TK, Smrcka AV, Taussig R, Bresnick AR, Nurnberg B, Williams RL, Backer JM. G protein-coupled receptor-mediated activation of p110beta by Gbetagamma is required for cellular transformation and invasiveness. Sci Signal. 2012;5(253):ra89. doi: 10.1126/scisignal.2003264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurosu H, Maehama T, Okada T, Yamamoto T, Hoshino S, Fukui Y, Ui M, Hazeki O, Katada T. Heterodimeric phosphoinositide 3-kinase consisting of p85 and p110beta is synergistically activated by the betagamma subunits of G proteins and phosphotyrosyl peptide. J Biol Chem. 1997;272(39):24252–24256. doi: 10.1074/jbc.272.39.24252. [DOI] [PubMed] [Google Scholar]
- 30.Fritsch R, de Krijger I, Fritsch K, George R, Reason B, Kumar MS, Diefenbacher M, Stamp G, Downward J. RAS and RHO families of GTPases directly regulate distinct phosphoinositide 3-kinase isoforms. Cell. 2013;153(5):1050–1063. doi: 10.1016/j.cell.2013.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salamon RS, Dbouk HA, Collado D, Lopiccolo J, Bresnick AR, Backer JM. Identification of the Rab5 binding site in p110beta: assays for PI3Kbeta binding to Rab5. Methods Mol Biol. 2015;1298:271–281. doi: 10.1007/978-1-4939-2569-8_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodriguezviciana P, Warne PH, Dhand R, Vanhaesebroeck B, Gout I, Fry MJ, Waterfield MD, Downward J. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature. 1994;370(6490):527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- 33.Pacold ME, Suire S, Perisic O, Lara-Gonzalez S, Davis CT, Walker EH, Hawkins PT, Stephens L, Eccleston JF, Williams RL. Crystal structure and functional analysis of Ras binding to its effector phosphoinositide 3-kinase gamma. Cell. 2000;103(6):931–943. doi: 10.1016/S0092-8674(00)00196-3. [DOI] [PubMed] [Google Scholar]
- 34.Vanhaesebroeck B, Welham MJ, Kotani K, Stein R, Warne PH, Zvelebil MJ, Higashi K, Volinia S, Downward J, Waterfield MD. P110delta, a novel phosphoinositide 3-kinase in leukocytes. Proc Natl Acad Sci USA. 1997;94(9):4330–4335. doi: 10.1073/pnas.94.9.4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Houslay DM, Anderson KE, Chessa T, Kulkarni S, Fritsch R, Downward J, Backer JM, Stephens LR, Hawkins PT. Coincident signals from GPCRs and receptor tyrosine kinases are uniquely transduced by PI3K beta in myeloid cells. Sci Signal. 2016;9(441):ra82. doi: 10.1126/scisignal.aae0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bresnick AR, Backer JM. PI3Kbeta-A versatile transducer for GPCR, RTK, and small GTPase signaling. Endocrinology. 2019;160(3):536–555. doi: 10.1210/en.2018-00843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goggs R, Poole AW. Platelet signaling-a primer. J Vet Emerg Crit Care. 2012;22(1):5–29. doi: 10.1111/j.1476-4431.2011.00704.x. [DOI] [PubMed] [Google Scholar]
- 38.Bi L, Okabe I, Bernard DJ, Wynshaw-Boris A, Nussbaum RL. Proliferative defect and embryonic lethality in mice homozygous for a deletion in the p110alpha subunit of phosphoinositide 3-kinase. J Biol Chem. 1999;274(16):10963–10968. doi: 10.1074/jbc.274.16.10963. [DOI] [PubMed] [Google Scholar]
- 39.Lelievre E, Bourbon PM, Duan LJ, Nussbaum RL, Fong GH. Deficiency in the p110alpha subunit of PI3K results in diminished Tie2 expression and Tie2(−/−)-like vascular defects in mice. Blood. 2005;105(10):3935–3938. doi: 10.1182/blood-2004-10-3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Graupera M, Guillermet-Guibert J, Foukas LC, Phng LK, Cain RJ, Salpekar A, Pearce W, Meek S, Millan J, Cutillas PR, Smith AJ, Ridley AJ, Ruhrberg C, Gerhardt H, Vanhaesebroeck B. Angiogenesis selectively requires the p110alpha isoform of PI3K to control endothelial cell migration. Nature. 2008;453(7195):662–666. doi: 10.1038/nature06892. [DOI] [PubMed] [Google Scholar]
- 41.Zhao JJ, Cheng HL, Jia SD, Wang L, Gjoerup OV, Mikami A, Roberts TM. The p110 alpha isoform of PI3K is essential for proper growth factor signaling and oncogenic transformation. Proc Natl Acad Sci USA. 2006;103(44):16296–16300. doi: 10.1073/pnas.0607899103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Foukas LC, Claret M, Pearce W, Okkenhaug K, Meek S, Peskett E, Sancho S, Smith AJ, Withers DJ, Vanhaesebroeck B. Critical role for the p110alpha phosphoinositide-3-OH kinase in growth and metabolic regulation. Nature. 2006;441(7091):366–370. doi: 10.1038/nature04694. [DOI] [PubMed] [Google Scholar]
- 43.Knight ZA, Gonzalez B, Feldman ME, Zunder ER, Goldenberg DD, Williams O, Loewith R, Stokoe D, Balla A, Toth B, Balla T, Weiss WA, Williams RL, Shokat KM. A pharmacological map of the PI3-K family defines a role for p110alpha in insulin signaling. Cell. 2006;125(4):733–747. doi: 10.1016/j.cell.2006.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ciraolo E, Morello F, Hobbs RM, Wolf F, Marone R, Iezzi M, Lu X, Mengozzi G, Altruda F, Sorba G, Guan K, Pandolfi PP, Wymann MP, Hirsch E. Essential role of the p110beta subunit of phosphoinositide 3-OH kinase in male fertility. Mol Biol Cell. 2010;21(5):704–711. doi: 10.1091/mbc.e09-08-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guillermet-Guibert J, Smith LB, Halet G, Whitehead MA, Pearce W, Rebourcet D, Leon K, Crepieux P, Nock G, Stromstedt M, Enerback M, Chelala C, Graupera M, Carroll J, Cosulich S, Saunders PTK, Huhtaniemi I, Vanhaesebroeck B. Novel role for p110 beta PI 3-kinase in male fertility through regulation of androgen receptor activity in sertoli cells. Plos Genet. 2015;11(7):e1005304. doi: 10.1371/journal.pgen.1005304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dou ZX, Chattopadhyay M, Pan JA, Guerriero JL, Jiang YP, Ballou LM, Yue ZY, Lin RZ, Zong WX. The class IA phosphatidylinositol 3-kinase p110-beta subunit is a positive regulator of autophagy. J Cell Biol. 2010;191(4):827–843. doi: 10.1083/jcb.201006056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kulkarni S, Sitaru C, Jakus Z, Anderson KE, Damoulakis G, Davidson K, Hirose M, Juss J, Oxley D, Chessa TA, Ramadani F, Guillou H, Segonds-Pichon A, Fritsch A, Jarvis GE, Okkenhaug K, Ludwig R, Zillikens D, Mocsai A, Vanhaesebroeck B, Stephens LR, Hawkins PT. PI3Kbeta plays a critical role in neutrophil activation by immune complexes. Sci Signal. 2011;4(168):ra23. doi: 10.1126/scisignal.2001617. [DOI] [PubMed] [Google Scholar]
- 48.Gratacap MP, Guillermet-Guibert J, Martin V, Chicanne G, Tronchere H, Gaits-Iacovoni F, Payrastre B. Regulation and roles of PI3Kbeta, a major actor in platelet signaling and functions. Adv Enzyme Regul. 2011;51(1):106–116. doi: 10.1016/j.advenzreg.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 49.Okkenhaug K, Bilancio A, Farjot G, Priddle H, Sancho S, Peskett E, Pearce W, Meek SE, Salpekar A, Waterfield MD, Smith AJ, Vanhaesebroeck B. Impaired B and T cell antigen receptor signaling in p110delta PI 3-kinase mutant mice. Science. 2002;297(5583):1031–1034. doi: 10.1126/science.1073560. [DOI] [PubMed] [Google Scholar]
- 50.Sasaki T, Irie-Sasaki J, Jones RG, Oliveira-dos-Santos AJ, Stanford WL, Bolon B, Wakeham A, Itie A, Bouchard D, Kozieradzki I, Joza N, Mak TW, Ohashi PS, Suzuki A, Penninger JM. Function of PI3Kgamma in thymocyte development, T cell activation, and neutrophil migration. Science. 2000;287(5455):1040–1046. doi: 10.1126/science.287.5455.1040. [DOI] [PubMed] [Google Scholar]
- 51.Hirsch E, Katanaev VL, Garlanda C, Azzolino O, Pirola L, Silengo L, Sozzani S, Mantovani A, Altruda F, Wymann MP. Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science. 2000;287(5455):1049–1053. doi: 10.1126/science.287.5455.1049. [DOI] [PubMed] [Google Scholar]
- 52.Ferguson GJ, Milne L, Kulkarni S, Sasaki T, Walker S, Andrews S, Crabbe T, Finan P, Jones G, Jackson S, Camps M, Rommel C, Wymann M, Hirsch E, Hawkins P, Stephens L. PI(3)Kgamma has an important context-dependent role in neutrophil chemokinesis. Nat Cell Biol. 2007;9(1):86–91. doi: 10.1038/ncb1517. [DOI] [PubMed] [Google Scholar]
- 53.Suire S, Condliffe AM, Ferguson GJ, Ellson CD, Guillou H, Davidson K, Welch H, Coadwell J, Turner M, Chilvers ER, Hawkins PT, Stephens L. Gbetagammas and the Ras binding domain of p110gamma are both important regulators of PI(3)Kgamma signalling in neutrophils. Nat Cell Biol. 2006;8(11):1303–1309. doi: 10.1038/ncb1494. [DOI] [PubMed] [Google Scholar]
- 54.Leslie NR, Dixon MJ, Schenning M, Gray A, Batty IH. Distinct inactivation of PI3K signalling by PTEN and 5-phosphatases. Adv Biol Regul. 2012;52(1):205–213. doi: 10.1016/j.advenzreg.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 55.Cote JF, Motoyama AB, Bush JA, Vuori K. A novel and evolutionarily conserved PtdIns(3,4,5)P-3-binding domain is necessary for DOCK180 signalling. Nat Cell Biol. 2005;7(8):797-U63. doi: 10.1038/ncb1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chandra M, Chin YKY, Mas C, Feathers JR, Paul B, Datta S, Chen KE, Jia XY, Yang Z, Norwood SJ, Mohanty B, Bugarcic A, Teasdale RD, Henne WM, Mobli M, Collins BM. Classification of the human phox homology (PX) domains based on their phosphoinositide binding specificities. Nat Commun. 2019;10:1528. doi: 10.1038/s41467-019-09355-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lemmon MA. Membrane recognition by phospholipid-binding domains. Nat Rev Mol Cell Biol. 2008;9(2):99–111. doi: 10.1038/nrm2328. [DOI] [PubMed] [Google Scholar]
- 58.Laurent PA, Severin S, Gratacap MP, Payrastre B. Class I PI 3-kinases signaling in platelet activation and thrombosis: PDK1/Akt/GSK3 axis and impact of PTEN and SHIP1. Adv Biol Regul. 2014;54:162–174. doi: 10.1016/j.jbior.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 59.Battram AM, Durrant TN, Agbani EO, Heesom KJ, Paul DS, Piatt R, Poole AW, Cullen PJ, Bergmeier W, Moore SF, Hers I. The phosphatidylinositol 3,4,5-trisphosphate (PI(3,4,5)P3) binder Rasa3 Regulates phosphoinositide 3-kinase (PI3K)-dependent integrin alphaIIbbeta3 outside-in signaling. J Biol Chem. 2017;292(5):1691–1704. doi: 10.1074/jbc.M116.746867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stefanini L, Paul DS, Robledo RF, Chan ER, Getz TM, Campbell RA, Kechele DO, Casari C, Piatt R, Caron KM, Mackman N, Weyrich AS, Parrott MC, Boulaftali Y, Adams MD, Peters LL, Bergmeier W. RASA3 is a critical inhibitor of RAP1-dependent platelet activation. J Clin Invest. 2015;125(4):1419–1432. doi: 10.1172/JCI77993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Patel A, Kostyak J, Dangelmaier C, Badolia R, Bhavanasi D, Aslan JE, Merali S, Kim S, Eble JA, Goldfinger L, Kunapuli S. ELMO1 deficiency enhances platelet function. Blood Adv. 2019;3(4):575–587. doi: 10.1182/bloodadvances.2018016444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goggs R, Williams CM, Mellor H, Poole AW. Platelet Rho GTPases-a focus on novel players, roles and relationships. Biochem J. 2015;466(3):431–442. doi: 10.1042/BJ20141404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hawkins PT, Stephens LR. Emerging evidence of signalling roles for PI(3,4)P2 in Class I and II PI3K-regulated pathways. Biochem Soc Trans. 2016;44(1):307–314. doi: 10.1042/BST20150248. [DOI] [PubMed] [Google Scholar]
- 64.Li H, Marshall AJ. Phosphatidylinositol (3,4) bisphosphate-specific phosphatases and effector proteins: a distinct branch of PI3K signaling. Cell Signal. 2015;27(9):1789–1798. doi: 10.1016/j.cellsig.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 65.Dyson JM, Fedele CG, Davies EM, Becanovic J, Mitchell CA. Phosphoinositide phosphatases: just as important as the kinases. Subcell Biochem. 2012;58:215–279. doi: 10.1007/978-94-007-3012-0_7. [DOI] [PubMed] [Google Scholar]
- 66.Malek M, Kielkowska A, Chessa T, Anderson KE, Barneda D, Pir P, Nakanishi H, Eguchi S, Koizumi A, Sasaki J, Juvin V, Kiselev VY, Niewczas I, Gray A, Valayer A, Spensberger D, Imbert M, Felisbino S, Habuchi T, Beinke S, Cosulich S, Le Novere N, Sasaki T, Clark J, Hawkins PT, Stephens LR. PTEN regulates PI(3,4)P2 signaling downstream of class I PI3K. Mol Cell. 2017;68(3):566.e10–580.e10. doi: 10.1016/j.molcel.2017.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hirsch E, Braccini L, Ciraolo E, Morello F, Perino A. Twice upon a time: PI3K’s secret double life exposed. Trends Biochem Sci. 2009;34(5):244–248. doi: 10.1016/j.tibs.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 68.Kim MS, Pinto SM, Getnet D, Nirujogi RS, Manda SS, Chaerkady R, Madugundu AK, Kelkar DS, Isserlin R, Jain S, Thomas JK, Muthusamy B, Leal-Rojas P, Kumar P, Sahasrabuddhe NA, Balakrishnan L, Advani J, George B, Renuse S, Selvan LD, Patil AH, Nanjappa V, Radhakrishnan A, Prasad S, Subbannayya T, Raju R, Kumar M, Sreenivasamurthy SK, Marimuthu A, Sathe GJ, Chavan S, Datta KK, Subbannayya Y, Sahu A, Yelamanchi SD, Jayaram S, Rajagopalan P, Sharma J, Murthy KR, Syed N, Goel R, Khan AA, Ahmad S, Dey G, Mudgal K, Chatterjee A, Huang TC, Zhong J, Wu X, Shaw PG, Freed D, Zahari MS, Mukherjee KK, Shankar S, Mahadevan A, Lam H, Mitchell CJ, Shankar SK, Satishchandra P, Schroeder JT, Sirdeshmukh R, Maitra A, Leach SD, Drake CG, Halushka MK, Prasad TS, Hruban RH, Kerr CL, Bader GD, Iacobuzio-Donahue CA, Gowda H, Pandey A. A draft map of the human proteome. Nature. 2014;509(7502):575–581. doi: 10.1038/nature13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Canobbio I, Stefanini L, Cipolla L, Ciraolo E, Gruppi C, Balduini C, Hirsch E, Torti M. Genetic evidence for a predominant role of PI3Kbeta catalytic activity in ITAM- and integrin-mediated signaling in platelets. Blood. 2009;114(10):2193–2196. doi: 10.1182/blood-2009-03-208074. [DOI] [PubMed] [Google Scholar]
- 70.Jackson SP, Schoenwaelder SM, Goncalves I, Nesbitt WS, Yap CL, Wright CE, Kenche V, Anderson KE, Dopheide SM, Yuan Y, Sturgeon SA, Prabaharan H, Thompson PE, Smith GD, Shepherd PR, Daniele N, Kulkarni S, Abbott B, Saylik D, Jones C, Lu L, Giuliano S, Hughan SC, Angus JA, Robertson AD, Salem HH. PI 3-kinase p110beta: a new target for antithrombotic therapy. Nat Med. 2005;11(5):507–514. doi: 10.1038/nm1232. [DOI] [PubMed] [Google Scholar]
- 71.Martin V, Guillermet-Guibert J, Chicanne G, Cabou C, Jandrot-Perrus M, Plantavid M, Vanhaesebroeck B, Payrastre B, Gratacap MP. Deletion of the p110beta isoform of phosphoinositide 3-kinase in platelets reveals its central role in Akt activation and thrombus formation in vitro and in vivo. Blood. 2010;115(10):2008–2013. doi: 10.1182/blood-2009-04-217224. [DOI] [PubMed] [Google Scholar]
- 72.Nylander S, Kull B, Bjorkman JA, Ulvinge JC, Oakes N, Emanuelsson BM, Andersson M, Skarby T, Inghardt T, Fjellstrom O, Gustafsson D. Human target validation of phosphoinositide 3-kinase (PI3K)beta: effects on platelets and insulin sensitivity, using AZD6482 a novel PI3Kbeta inhibitor. J Thromb Haemost. 2012;10(10):2127–2136. doi: 10.1111/j.1538-7836.2012.04898.x. [DOI] [PubMed] [Google Scholar]
- 73.Cosemans JM, Munnix IC, Wetzker R, Heller R, Jackson SP, Heemskerk JW. Continuous signaling via PI3K isoforms beta and gamma is required for platelet ADP receptor function in dynamic thrombus stabilization. Blood. 2006;108(9):3045–3052. doi: 10.1182/blood-2006-03-006338. [DOI] [PubMed] [Google Scholar]
- 74.Laurent PA, Severin S, Hechler B, Vanhaesebroeck B, Payrastre B, Gratacap MP. Platelet PI3Kbeta and GSK3 regulate thrombus stability at high-shear rate. Blood. 2014 doi: 10.1182/blood-2014-07-588335. [DOI] [PubMed] [Google Scholar]
- 75.Laurent PA, Severin S, Hechler B, Vanhaesebroeck B, Payrastre B, Gratacap MP. Platelet PI3K beta and GSK3 regulate thrombus stability at a high shear rate. Blood. 2015;125(5):881–888. doi: 10.1182/blood-2014-07-588335. [DOI] [PubMed] [Google Scholar]
- 76.Giordanetto F, Wallberg A, Ghosal S, Iliefski T, Cassel J, Yuan ZQ, von Wachenfeldt H, Andersen SM, Inghardt T, Tunek A, Nylander S. Discovery of phosphoinositide 3-kinases (PI3K) p110beta isoform inhibitor 4-[2-hydroxyethyl(1-naphthylmethyl)amino]-6-[(2S)-2-methylmorpholin-4-yl]-1H-pyri midin-2-one, an effective antithrombotic agent without associated bleeding and insulin resistance. Bioorg Med Chem Lett. 2012;22(21):6671–6676. doi: 10.1016/j.bmcl.2012.08.102. [DOI] [PubMed] [Google Scholar]
- 77.Garcia A, Kim S, Bhavaraju K, Schoenwaelder SM, Kunapuli SP. Role of phosphoinositide 3-kinase beta in platelet aggregation and thromboxane A2 generation mediated by Gi signalling pathways. Biochem J. 2010;429(2):369–377. doi: 10.1042/BJ20100166. [DOI] [PubMed] [Google Scholar]
- 78.van der Meijden PE, Schoenwaelder SM, Feijge MA, Cosemans JM, Munnix IC, Wetzker R, Heller R, Jackson SP, Heemskerk JW. Dual P2Y 12 receptor signaling in thrombin-stimulated platelets—involvement of phosphoinositide 3-kinase beta but not gamma isoform in Ca2+ mobilization and procoagulant activity. FEBS J. 2008;275(2):371–385. doi: 10.1111/j.1742-4658.2007.06207.x. [DOI] [PubMed] [Google Scholar]
- 79.Moore SF, Smith NR, Blair TA, Durrant TN, Hers I. Critical roles for the phosphatidylinositide 3-kinase isoforms p110beta and p110gamma in thrombopoietin-mediated priming of platelet function. Sci Rep. 2019;9(1):1468. doi: 10.1038/s41598-018-37012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Terrisse AD, Laurent PA, Garcia C, Gratacap MP, Vanhaesebroeck B, Sie P, Payrastre B. The class I phosphoinositide 3-kinases alpha and beta control antiphospholipid antibodies-induced platelet activation. Thromb Haemost. 2016;115(6):1138–1146. doi: 10.1160/TH15-08-0661. [DOI] [PubMed] [Google Scholar]
- 81.Moore SF, van den Bosch MT, Hunter RW, Sakamoto K, Poole AW, Hers I. Dual regulation of glycogen synthase kinase 3 (GSK3)alpha/beta by protein kinase C (PKC)alpha and Akt promotes thrombin-mediated integrin alphaIIbbeta3 activation and granule secretion in platelets. J Biol Chem. 2013;288(6):3918–3928. doi: 10.1074/jbc.M112.429936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.O’Brien KA, Gartner TK, Hay N, Du X. ADP-stimulated activation of Akt during integrin outside-in signaling promotes platelet spreading by inhibiting glycogen synthase kinase-3beta. Arterioscler Thromb Vasc Biol. 2012;32(9):2232–2240. doi: 10.1161/ATVBAHA.112.254680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hirsch E, Bosco O, Tropel P, Laffargue M, Calvez R, Altruda F, Wymann M, Montrucchio G. Resistance to thromboembolism in PI3Kgamma-deficient mice. FASEB J. 2001;15(11):2019–2021. doi: 10.1096/fj.00-0810fje. [DOI] [PubMed] [Google Scholar]
- 84.Schoenwaelder SM, Ono A, Sturgeon S, Chan SM, Mangin P, Maxwell MJ, Turnbull S, Mulchandani M, Anderson K, Kauffenstein G, Rewcastle GW, Kendall J, Gachet C, Salem HH, Jackson SP. Identification of a unique co-operative phosphoinositide 3-kinase signaling mechanism regulating integrin alpha IIb beta 3 adhesive function in platelets. J Biol Chem. 2007;282(39):28648–28658. doi: 10.1074/jbc.M704358200. [DOI] [PubMed] [Google Scholar]
- 85.Hers I. Insulin-like growth factor-1 potentiates platelet activation via the IRS/PI3Kalpha pathway. Blood. 2007;110(13):4243–4252. doi: 10.1182/blood-2006-10-050633. [DOI] [PubMed] [Google Scholar]
- 86.Kim S, Garcia A, Jackson SP, Kunapuli SP. Insulin-like growth factor-1 regulates platelet activation through PI3-Kalpha isoform. Blood. 2007;110(13):4206–4213. doi: 10.1182/blood-2007-03-080804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gilio K, Munnix IC, Mangin P, Cosemans JM, Feijge MA, van der Meijden PE, Olieslagers S, Chrzanowska-Wodnicka MB, Lillian R, Schoenwaelder S, Koyasu S, Sage SO, Jackson SP, Heemskerk JW. Non-redundant roles of phosphoinositide 3-kinase isoforms alpha and beta in glycoprotein VI-induced platelet signaling and thrombus formation. J Biol Chem. 2009;284(49):33750–33762. doi: 10.1074/jbc.M109.048439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Blair TA, Moore SF, Williams CM, Poole AW, Vanhaesebroeck B, Hers I. Phosphoinositide 3-kinases p110alpha and p110beta have differential roles in insulin-like growth factor-1-mediated Akt phosphorylation and platelet priming. Arterioscler Thromb Vasc Biol. 2014 doi: 10.1161/ATVBAHA.114.303954. [DOI] [PubMed] [Google Scholar]
- 89.Laurent PA, Hechler B, Solinhac R, Ragab A, Cabou C, Anquetil T, Severin S, Denis CV, Mangin PH, Vanhaesebroeck B, Payrastre B, Gratacap MP. Impact of PI3Kalpha (phosphoinositide 3-kinase alpha) inhibition on hemostasis and thrombosis. Arterioscler Thromb Vasc Biol. 2018;38(9):2041–2053. doi: 10.1161/ATVBAHA.118.311410. [DOI] [PubMed] [Google Scholar]
- 90.Zhang J, Vanhaesebroeck B, Rittenhouse SE. Human platelets contain p110delta phosphoinositide 3-kinase. Biochem Biophys Res Commun. 2002;296(1):178–181. doi: 10.1016/S0006-291X(02)00744-1. [DOI] [PubMed] [Google Scholar]
- 91.Senis YA, Atkinson BT, Pearce AC, Wonerow P, Auger JM, Okkenhaug K, Pearce W, Vigorito E, Vanhaesebroeck B, Turner M, Watson SP. Role of the p110delta PI 3-kinase in integrin and ITAM receptor signalling in platelets. Platelets. 2005;16(3–4):191–202. doi: 10.1080/09537100400016711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Valet C, Severin S, Chicanne G, Laurent PA, Gaits-Iacovoni F, Gratacap MP, Payrastre B. The role of class I, II and III PI 3-kinases in platelet production and activation and their implication in thrombosis. Adv Biol Regul. 2016;61:33–41. doi: 10.1016/j.jbior.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 93.Bilanges B, Posor Y, Vanhaesebroeck B. PI3K isoforms in cell signalling and vesicle trafficking. Nat Rev Mol Cell Biol. 2019 doi: 10.1038/s41580-019-0129-z. [DOI] [PubMed] [Google Scholar]
- 94.Campa CC, Franco I, Hirsch E. PI3K-C2alpha: one enzyme for two products coupling vesicle trafficking and signal transduction. FEBS Lett. 2015;589(14):1552–1558. doi: 10.1016/j.febslet.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 95.Liu L, Song X, He D, Komma C, Kita A, Virbasius JV, Huang G, Bellamy HD, Miki K, Czech MP, Zhou GW. Crystal structure of the C2 domain of class II phosphatidylinositide 3-kinase C2alpha. J Biol Chem. 2006;281(7):4254–4260. doi: 10.1074/jbc.M510791200. [DOI] [PubMed] [Google Scholar]
- 96.Gaidarov I, Smith ME, Domin J, Keen JH. The class II phosphoinositide 3-kinase C2alpha is activated by clathrin and regulates clathrin-mediated membrane trafficking. Mol Cell. 2001;7(2):443–449. doi: 10.1016/S1097-2765(01)00191-5. [DOI] [PubMed] [Google Scholar]
- 97.Posor Y, Eichhorn-Gruenig M, Puchkov D, Schoneberg J, Ullrich A, Lampe A, Muller R, Zarbakhsh S, Gulluni F, Hirsch E, Krauss M, Schultz C, Schmoranzer J, Noe F, Haucke V. Spatiotemporal control of endocytosis by phosphatidylinositol-3,4-bisphosphate. Nature. 2013;499(7457):233–237. doi: 10.1038/nature12360. [DOI] [PubMed] [Google Scholar]
- 98.Stahelin RV, Karathanassis D, Bruzik KS, Waterfield MD, Bravo J, Williams RL, Cho W. Structural and membrane binding analysis of the Phox homology domain of phosphoinositide 3-kinase-C2alpha. J Biol Chem. 2006;281(51):39396–39406. doi: 10.1074/jbc.M607079200. [DOI] [PubMed] [Google Scholar]
- 99.Chen KE, Tillu VA, Chandra M, Collins BM. Molecular basis for membrane recruitment by the PX and C2 domains of class II phosphoinositide 3-kinase-C2alpha. Structure. 2018;26(12):1612.e4–1625.e4. doi: 10.1016/j.str.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 100.Wang H, Lo WT, Vujicic Zagar A, Gulluni F, Lehmann M, Scapozza L, Haucke V, Vadas O. Autoregulation of class II alpha PI3K activity by its lipid-binding PX-C2 domain module. Mol Cell. 2018;71(2):343.e4–351.e4. doi: 10.1016/j.molcel.2018.06.042. [DOI] [PubMed] [Google Scholar]
- 101.Braccini L, Ciraolo E, Campa CC, Perino A, Longo DL, Tibolla G, Pregnolato M, Cao Y, Tassone B, Damilano F, Laffargue M, Calautti E, Falasca M, Norata GD, Backer JM, Hirsch E. PI3K-C2gamma is a Rab5 effector selectively controlling endosomal Akt2 activation downstream of insulin signalling. Nat Commun. 2015;6:7400. doi: 10.1038/ncomms8400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rozycka M, Lu YJ, Brown RA, Lau MR, Shipley JM, Fry MJ. cDNA cloning of a third human C2-domain-containing class II phosphoinositide 3-kinase, PI3K-C2gamma, and chromosomal assignment of this gene (PIK3C2G) to 12p12. Genomics. 1998;54(3):569–574. doi: 10.1006/geno.1998.5621. [DOI] [PubMed] [Google Scholar]
- 103.Ho LK, Liu D, Rozycka M, Brown RA, Fry MJ. Identification of four novel human phosphoinositide 3-kinases defines a multi-isoform subfamily. Biochem Biophys Res Commun. 1997;235(1):130–137. doi: 10.1006/bbrc.1997.6747. [DOI] [PubMed] [Google Scholar]
- 104.Hawkins PT, Stephens LR. PI3K signalling in inflammation. Biochim Biophys Acta. 2015;1851(6):882–897. doi: 10.1016/j.bbalip.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 105.Yoshioka K, Yoshida K, Cui H, Wakayama T, Takuwa N, Okamoto Y, Du W, Qi X, Asanuma K, Sugihara K, Aki S, Miyazawa H, Biswas K, Nagakura C, Ueno M, Iseki S, Schwartz RJ, Okamoto H, Sasaki T, Matsui O, Asano M, Adams RH, Takakura N, Takuwa Y. Endothelial PI3K-C2alpha, a class II PI3K, has an essential role in angiogenesis and vascular barrier function. Nat Med. 2012;18(10):1560–1569. doi: 10.1038/nm.2928. [DOI] [PubMed] [Google Scholar]
- 106.Alliouachene S, Bilanges B, Chaussade C, Pearce W, Foukas LC, Scudamore CL, Moniz LS, Vanhaesebroeck B. Inactivation of class II PI3K-C2alpha induces leptin resistance, age-dependent insulin resistance and obesity in male mice. Diabetologia. 2016;59(7):1503–1512. doi: 10.1007/s00125-016-3963-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Franco I, Gulluni F, Campa CC, Costa C, Margaria JP, Ciraolo E, Martini M, Monteyne D, De Luca E, Germena G, Posor Y, Maffucci T, Marengo S, Haucke V, Falasca M, Perez-Morga D, Boletta A, Merlo GR, Hirsch E. PI3K class II alpha controls spatially restricted endosomal PtdIns3P and Rab11 activation to promote primary cilium function. Dev Cell. 2014;28(6):647–658. doi: 10.1016/j.devcel.2014.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dominguez V, Raimondi C, Somanath S, Bugliani M, Loder MK, Edling CE, Divecha N, da Silva-Xavier G, Marselli L, Persaud SJ, Turner MD, Rutter GA, Marchetti P, Falasca M, Maffucci T. Class II phosphoinositide 3-kinase regulates exocytosis of insulin granules in pancreatic beta cells. J Biol Chem. 2011;286(6):4216–4225. doi: 10.1074/jbc.M110.200295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Leibiger B, Moede T, Paschen M, Yunn NO, Lim JH, Ryu SH, Pereira T, Berggren PO, Leibiger IB. PI3K-C2alpha knockdown results in rerouting of insulin signaling and pancreatic beta cell proliferation. Cell Rep. 2015;13(1):15–22. doi: 10.1016/j.celrep.2015.08.058. [DOI] [PubMed] [Google Scholar]
- 110.Tiosano D, Baris HN, Chen A, Hitzert MM, Schueler M, Gulluni F, Wiesener A, Bergua A, Mory A, Copeland B, Gleeson JG, Rump P, van Meer H, Sival DA, Haucke V, Kriwinsky J, Knaup KX, Reis A, Hauer NN, Hirsch E, Roepman R, Pfundt R, Thiel CT, Wiesener MS, Aslanyan MG, Buchner DA. Mutations in PIK3C2A cause syndromic short stature, skeletal abnormalities, and cataracts associated with ciliary dysfunction. PLoS Genet. 2019;15(4):e1008088. doi: 10.1371/journal.pgen.1008088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Harada K, Truong AB, Cai T, Khavari PA. The class II phosphoinositide 3-kinase C2 beta is not essential for epidermal differentiation. Mol Cell Biol. 2005;25(24):11122–11130. doi: 10.1128/MCB.25.24.11122-11130.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Alliouachene S, Bilanges B, Chicanne G, Anderson KE, Pearce W, Ali K, Valet C, Posor Y, Low PC, Chaussade C, Scudamore CL, Salamon RS, Backer JM, Stephens L, Hawkins PT, Payrastre B, Vanhaesebroeck B. Inactivation of the class II PI3K-C2beta potentiates insulin signaling and sensitivity. Cell Rep. 2015;13(9):1881–1894. doi: 10.1016/j.celrep.2015.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Campa CC, Margaria JP, Derle A, Del Giudice M, De Santis MC, Gozzelino L, Copperi F, Bosia C, Hirsch E. Rab11 activity and PtdIns(3)P turnover removes recycling cargo from endosomes. Nat Chem Biol. 2018;14(8):801–810. doi: 10.1038/s41589-018-0086-4. [DOI] [PubMed] [Google Scholar]
- 114.Marat AL, Wallroth A, Lo WT, Muller R, Norata GD, Falasca M, Schultz C, Haucke V. mTORC1 activity repression by late endosomal phosphatidylinositol 3,4-bisphosphate. Science. 2017;356(6341):968–972. doi: 10.1126/science.aaf8310. [DOI] [PubMed] [Google Scholar]
- 115.Srivastava S, Di L, Zhdanova O, Li Z, Vardhana S, Wan Q, Yan Y, Varma R, Backer J, Wulff H, Dustin ML, Skolnik EY. The class II phosphatidylinositol 3 kinase C2beta is required for the activation of the K+ channel KCa3.1 and CD4 T-cells. Mol Biol Cell. 2009;20(17):3783–3791. doi: 10.1091/mbc.e09-05-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Srivastava S, Cai X, Li Z, Sun Y, Skolnik EY. Phosphatidylinositol-3-kinase C2beta and TRIM27 function to positively and negatively regulate IgE receptor activation of mast cells. Mol Cell Biol. 2012;32(15):3132–3139. doi: 10.1128/MCB.00019-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cisse O, Quraishi M, Gulluni F, Guffanti F, Mavrommati I, Suthanthirakumaran M, Oh LCR, Schlatter JN, Sarvananthan A, Broggini M, Hirsch E, Falasca M, Maffucci T. Downregulation of class II phosphoinositide 3-kinase PI3K-C2beta delays cell division and potentiates the effect of docetaxel on cancer cell growth. J Exp Clin Cancer Res. 2019;38(1):472. doi: 10.1186/s13046-019-1472-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Katso RM, Pardo OE, Palamidessi A, Franz CM, Marinov M, De Laurentiis A, Downward J, Scita G, Ridley AJ, Waterfield MD, Arcaro A. Phosphoinositide 3-kinase C2beta regulates cytoskeletal organization and cell migration via Rac-dependent mechanisms. Mol Biol Cell. 2006;17(9):3729–3744. doi: 10.1091/mbc.e05-11-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Domin J, Harper L, Aubyn D, Wheeler M, Florey O, Haskard D, Yuan M, Zicha D. The class II phosphoinositide 3-kinase PI3K-C2 beta regulates cell migration by a PtdIns(3)P dependent mechanism. J Cell Physiol. 2005;205(3):452–462. doi: 10.1002/jcp.20478. [DOI] [PubMed] [Google Scholar]
- 120.Maffucci T, Cooke FT, Foster FM, Traer CJ, Fry MJ, Falasca M. Class II phosphoinositide 3-kinase defines a novel signaling pathway in cell migration. J Cell Biol. 2005;169(5):789–799. doi: 10.1083/jcb.200408005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gulluni F, Martini M, De Santis MC, Campa CC, Ghigo A, Margaria JP, Ciraolo E, Franco I, Ala U, Annaratone L, Disalvatore D, Bertalot G, Viale G, Noatynska A, Compagno M, Sigismund S, Montemurro F, Thelen M, Fan F, Meraldi P, Marchio C, Pece S, Sapino A, Chiarle R, Di Fiore PP, Hirsch E. Mitotic spindle assembly and genomic stability in breast cancer require PI3K-C2alpha scaffolding function. Cancer Cell. 2017;32(4):444.e7–459.e7. doi: 10.1016/j.ccell.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 122.Mountford JK, Petitjean C, Putra HW, McCafferty JA, Setiabakti NM, Lee H, Tonnesen LL, McFadyen JD, Schoenwaelder SM, Eckly A, Gachet C, Ellis S, Voss AK, Dickins RA, Hamilton JR, Jackson SP. The class II PI 3-kinase, PI3KC2alpha, links platelet internal membrane structure to shear-dependent adhesive function. Nat Commun. 2015;6:6535. doi: 10.1038/ncomms7535. [DOI] [PubMed] [Google Scholar]
- 123.Valet C, Chicanne G, Severac C, Chaussade C, Whitehead MA, Cabou C, Gratacap MP, Gaits-Iacovoni F, Vanhaesebroeck B, Payrastre B, Severin S. Essential role of class II PI3K-C2alpha in platelet membrane morphology. Blood. 2015;126(9):1128–1137. doi: 10.1182/blood-2015-03-636670. [DOI] [PubMed] [Google Scholar]
- 124.Selvadurai MV, Brazilek RJ, Moon MJ, Rinckel JY, Eckly A, Gachet C, Meikle PJ, Nandurkar HH, Nesbitt WS, Hamilton JR. The PI 3-kinase PI3KC2alpha regulates mouse platelet membrane structure and function independently of membrane lipid composition. FEBS Lett. 2019;593(1):88–96. doi: 10.1002/1873-3468.13295. [DOI] [PubMed] [Google Scholar]
- 125.Petitjean C, Setiabakti NM, Mountford JK, Arthur JF, Ellis S, Hamilton JR. Combined deficiency of PI3KC2alpha and PI3KC2beta reveals a nonredundant role for PI3KC2alpha in regulating mouse platelet structure and thrombus stability. Platelets. 2016;27(5):402–409. doi: 10.3109/09537104.2016.1145202. [DOI] [PubMed] [Google Scholar]
- 126.Zhang J, Banfic H, Straforini F, Tosi L, Volinia S, Rittenhouse SE. A type II phosphoinositide 3-kinase is stimulated via activated integrin in platelets—a source of phosphatidylinositol 3-phosphate. J Biol Chem. 1998;273(23):14081–14084. doi: 10.1074/jbc.273.23.14081. [DOI] [PubMed] [Google Scholar]
- 127.Mountford SJ, Zheng ZH, Sundaram K, Jennings IG, Hamilton JR, Thompson PE. Class II but not second class-prospects for the development of class II PI3K inhibitors. ACS Med Chem Lett. 2015;6(1):3–6. doi: 10.1021/ml500354e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Boller D, Doepfner KT, De Laurentiis A, Guerreiro AS, Marinov M, Shalaby T, Depledge P, Robson A, Saghir N, Hayakawa M, Kaizawa H, Koizumi T, Ohishi T, Fattet S, Delattre O, Schweri-Olac A, Holand K, Grotzer MA, Frei K, Spertini O, Waterfield MD, Arcaro A. Targeting PI3KC2beta impairs proliferation and survival in acute leukemia, brain tumours and neuroendocrine tumours. Anticancer Res. 2012;32(8):3015–3027. [PubMed] [Google Scholar]
- 129.Freitag A, Prajwal P, Shymanets A, Harteneck C, Nurnberg B, Schachtele C, Kubbutat M, Totzke F, Laufer SA. Development of first lead structures for phosphoinositide 3-kinase-C2gamma inhibitors. J Med Chem. 2015;58(1):212–221. doi: 10.1021/jm5006034. [DOI] [PubMed] [Google Scholar]
- 130.Herman PK, Emr SD. Characterization of VPS34, a gene required for vacuolar protein sorting and vacuole segregation in Saccharomyces cerevisiae. Mol Cell Biol. 1990;10(12):6742–6754. doi: 10.1128/MCB.10.12.6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schu PV, Takegawa K, Fry MJ, Stack JH, Waterfield MD, Emr SD. Phosphatidylinositol 3-kinase encoded by yeast Vps34 gene essential for protein sorting. Science. 1993;260(5104):88–91. doi: 10.1126/science.8385367. [DOI] [PubMed] [Google Scholar]
- 132.Stephens L, Cooke FT, Walters R, Jackson T, Volinia S, Gout I, Waterfield MD, Hawkins PT. Characterization of a phosphatidylinositol-specific phosphoinositide 3-kinase from mammalian cells. Curr Biol. 1994;4(3):203–214. doi: 10.1016/S0960-9822(00)00049-X. [DOI] [PubMed] [Google Scholar]
- 133.Bilanges B, Alliouachene S, Pearce W, Morelli D, Szabadkai G, Chung YL, Chicanne G, Valet C, Hill JM, Voshol PJ, Collinson L, Peddie C, Ali K, Ghazaly E, Rajeeve V, Trichas G, Srinivas S, Chaussade C, Salamon RS, Backer JM, Scudamore CL, Whitehead MA, Keaney EP, Murphy LO, Semple RK, Payrastre B, Tooze SA, Vanhaesebroeck B. Vps34 PI 3-kinase inactivation enhances insulin sensitivity through reprogramming of mitochondrial metabolism. Nat Commun. 2017;8(1):1804. doi: 10.1038/s41467-017-01969-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Backer JM. The intricate regulation and complex functions of the class III phosphoinositide 3-kinase Vps34. Biochem J. 2016;473(15):2251–2271. doi: 10.1042/BCJ20160170. [DOI] [PubMed] [Google Scholar]
- 135.Stjepanovic G, Baskaran S, Lin MG, Hurley JH. Vps34 kinase domain dynamics regulate the autophagic PI 3-kinase complex. Mol Cell. 2017;67(3):528.e3–534.e3. doi: 10.1016/j.molcel.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rostislavleva K, Soler N, Ohashi Y, Zhang L, Pardon E, Burke JE, Masson GR, Johnson C, Steyaert J, Ktistakis NT, Williams RL. Structure and flexibility of the endosomal Vps34 complex reveals the basis of its function on membranes. Science. 2015;350(6257):aac7365. doi: 10.1126/science.aac7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fan W, Nassiri A, Zhong Q. Autophagosome targeting and membrane curvature sensing by Barkor/Atg14(L) Proc Natl Acad Sci USA. 2011;108(19):7769–7774. doi: 10.1073/pnas.1016472108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhou XA, Takatoh J, Wang F. The mammalian class 3 PI3K (PIK3C3) is required for early embryogenesis and cell proliferation. PLoS ONE. 2011;6(1):e16358. doi: 10.1371/journal.pone.0016358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jaber N, Dou Z, Chen JS, Catanzaro J, Jiang YP, Ballou LM, Selinger E, Ouyang X, Lin RZ, Zhang J, Zong WX. Class III PI3K Vps34 plays an essential role in autophagy and in heart and liver function. Proc Natl Acad Sci USA. 2012;109(6):2003–2008. doi: 10.1073/pnas.1112848109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kimura H, Eguchi S, Sasaki J, Kuba K, Nakanishi H, Takasuga S, Yamazaki M, Goto A, Watanabe H, Itoh H, Imai Y, Suzuki A, Mizushima N, Sasaki T. Vps34 regulates myofibril proteostasis to prevent hypertrophic cardiomyopathy. JCI Insight. 2017;2(1):e89462. doi: 10.1172/jci.insight.89462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.He F, Agosto MA, Anastassov IA, Tse DY, Wu SM, Wensel TG. Phosphatidylinositol-3-phosphate is light-regulated and essential for survival in retinal rods. Sci Rep. 2016;6:26978. doi: 10.1038/srep26978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Logan AM, Mammel AE, Robinson DC, Chin AL, Condon AF, Robinson FL. Schwann cell-specific deletion of the endosomal PI 3-kinase Vps34 leads to delayed radial sorting of axons, arrested myelination, and abnormal ErbB2-ErbB3 tyrosine kinase signaling. Glia. 2017;65(9):1452–1470. doi: 10.1002/glia.23173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang L, Budolfson K, Wang F. Pik3c3 deletion in pyramidal neurons results in loss of synapses, extensive gliosis and progressive neurodegeneration. Neuroscience. 2011;172:427–442. doi: 10.1016/j.neuroscience.2010.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhou X, Wang L, Hasegawa H, Amin P, Han BX, Kaneko S, He Y, Wang F. Deletion of PIK3C3/Vps34 in sensory neurons causes rapid neurodegeneration by disrupting the endosomal but not the autophagic pathway. Proc Natl Acad Sci USA. 2010;107(20):9424–9429. doi: 10.1073/pnas.0914725107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.McLeod IX, Zhou X, Li QJ, Wang F, He YW. The class III kinase Vps34 promotes T lymphocyte survival through regulating IL-7Ralpha surface expression. J Immunol. 2011;187(10):5051–5061. doi: 10.4049/jimmunol.1100710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Willinger T, Flavell RA. Canonical autophagy dependent on the class III phosphoinositide-3 kinase Vps34 is required for naive T-cell homeostasis. Proc Natl Acad Sci USA. 2012;109(22):8670–8675. doi: 10.1073/pnas.1205305109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Liu Y, Hu M, Luo D, Yue M, Wang S, Chen X, Zhou Y, Wang Y, Cai Y, Hu X, Ke Y, Yang Z, Hu H. Class III PI3K positively regulates platelet activation and thrombosis via PI(3)P-directed function of NADPH oxidase. Arterioscler Thromb Vasc Biol. 2017;37(11):2075–2086. doi: 10.1161/ATVBAHA.117.309751. [DOI] [PubMed] [Google Scholar]
- 148.Valet C, Levade M, Chicanne G, Bilanges B, Cabou C, Viaud J, Gratacap MP, Gaits-Iacovoni F, Vanhaesebroeck B, Payrastre B, Severin S. A dual role for the class III PI3K, Vps34, in platelet production and thrombus growth. Blood. 2017;130(18):2032–2042. doi: 10.1182/blood-2017-04-781641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ouseph MM, Huang YJ, Banerjee M, Joshi S, MacDonald L, Zhong Y, Liu HJ, Li XT, Xiang BG, Zhang GY, Komatsu M, Yue ZY, Li ZY, Storrie B, Whiteheart SW, Wang QJ. Autophagy is induced upon platelet activation and is essential for hemostasis and thrombosis. Blood. 2015;126(10):1224–1233. doi: 10.1182/blood-2014-09-598722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Feng W, Chang C, Luo D, Su H, Yu S, Hua W, Chen Z, Hu H, Liu W. Dissection of autophagy in human platelets. Autophagy. 2014;10(4):642–651. doi: 10.4161/auto.27832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Arcaro A, Wymann MP. Wortmannin is a potent phosphatidylinositol 3-kinase inhibitor—the role of phosphatidylinositol 3,4,5-trisphosphate in neutrophil responses. Biochem J. 1993;296:297–301. doi: 10.1042/bj2960297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ghigo A, Morello F, Perino A, Hirsch E. Therapeutic applications of PI3K inhibitors in cardiovascular diseases. Future Med Chem. 2013;5(4):479–492. doi: 10.4155/fmc.13.11. [DOI] [PubMed] [Google Scholar]
- 153.Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269(7):5241–5248. [PubMed] [Google Scholar]
- 154.Vanhaesebroeck B, Whitehead MA, Pineiro R. Molecules in medicine mini-review: isoforms of PI3K in biology and disease. J Mol Med. 2016;94(1):5–11. doi: 10.1007/s00109-015-1352-5. [DOI] [PubMed] [Google Scholar]
- 155.Miller MS, Thompson PE, Gabelli SB. Structural determinants of isoform selectivity in PI3K inhibitors. Biomolecules. 2019;9(3):82. doi: 10.3390/biom9030082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jackson SP, Schoenwaelder SM. Antithrombotic phosphoinositide 3-kinase beta inhibitors in humans: a ‘shear’ delight! J Thromb Haemost. 2012;10(10):2123–2126. doi: 10.1111/j.1538-7836.2012.04912.x. [DOI] [PubMed] [Google Scholar]
- 157.Straub A, Wendel HP, Dietz K, Schiebold D, Peter K, Schoenwaelder SM, Ziemer G. Selective inhibition of the platelet phosphoinositide 3-kinase p110beta as promising new strategy for platelet protection during extracorporeal circulation. Thromb Haemost. 2008;99(3):609–615. doi: 10.1160/TH07-07-0452. [DOI] [PubMed] [Google Scholar]
- 158.Krajewski S, Kurz J, Geisler T, Peter K, Wendel HP, Straub A. Combined blockade of ADP receptors and PI3-kinase p110beta fully prevents platelet and leukocyte activation during hypothermic extracorporeal circulation. PLoS ONE. 2012;7(6):e38455. doi: 10.1371/journal.pone.0038455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zheng Z, Pinson JA, Mountford SJ, Orive S, Schoenwaelder SM, Shackleford D, Powell A, Nelson EM, Hamilton JR, Jackson SP, Jennings IG, Thompson PE. Discovery and antiplatelet activity of a selective PI3Kbeta inhibitor (MIPS-9922) Eur J Med Chem. 2016;122:339–351. doi: 10.1016/j.ejmech.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 160.Ciraolo E, Iezzi M, Marone R, Marengo S, Curcio C, Costa C, Azzolino O, Gonella C, Rubinetto C, Wu HY, Dastru W, Martin EL, Silengo L, Altruda F, Turco E, Lanzetti L, Musiani P, Ruckle T, Rommel C, Backer JM, Forni G, Wymann MP, Hirsch E. Phosphoinositide 3-kinase p110 beta activity: key role in metabolism and mammary gland cancer but not development. Sci Signal. 2008;1(36):ra3. doi: 10.1126/scisignal.1161577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mateo J, Ganji G, Lemech C, Burris HA, Han SW, Swales K, Decordova S, DeYoung MP, Smith DA, Kalyana-Sundaram S, Wu J, Motwani M, Kumar R, Tolson JM, Rha SY, Chung HC, Eder JP, Sharma S, Bang YJ, Infante JR, Yan L, de Bono JS, Arkenau HT. A first-time-in-human study of GSK2636771, a phosphoinositide 3 kinase beta-selective inhibitor, in patients with advanced solid tumors. Clin Cancer Res. 2017;23(19):5981–5992. doi: 10.1158/1078-0432.CCR-17-0725. [DOI] [PubMed] [Google Scholar]
- 162.Tawbi HAH, Peng WY, Milton D, Amaria RN, Glitza IC, Hwu WJ. Phase I/II study of the PI3K beta inhibitor GSK2636771 in combination with pembrolizumab (P) in patients (pts) with PD-1 refractory metastatic melanoma (MM) and PTEN loss. J Clin Oncol. 2018 doi: 10.1200/JCO.2018.36.15_suppl.TPS9596. [DOI] [Google Scholar]
- 163.Nylander S, Wagberg F, Andersson M, Skarby T, Gustafsson D. Exploration of efficacy and bleeding with combined phosphoinositide 3-kinase beta inhibition and aspirin in man. J Thromb Haemost. 2015;13(8):1494–1502. doi: 10.1111/jth.13027. [DOI] [PubMed] [Google Scholar]
- 164.Torti M. PI3K beta inhibition: all that glitters is not gold. Blood. 2015;125(5):750–751. doi: 10.1182/blood-2014-12-612564. [DOI] [PubMed] [Google Scholar]
- 165.Falasca M, Hamilton JR, Selvadurai M, Sundaram K, Adamska A, Thompson PE. Class II phosphoinositide 3-kinases as novel drug targets. J Med Chem. 2017;60(1):47–65. doi: 10.1021/acs.jmedchem.6b00963. [DOI] [PubMed] [Google Scholar]
- 166.Arcaro A, Borgstrom A, Blajecka K. Class II phosphoinositide 3-kinases as potential novel drug targets. Curr Signal Transduct Ther. 2013;8(2):101–112. doi: 10.2174/15743624113086660002. [DOI] [Google Scholar]
- 167.Dyczynski M, Yu Y, Otrocka M, Parpal S, Braga T, Henley AB, Zazzi H, Lerner M, Wennerberg K, Viklund J, Martinsson J, Grander D, De Milito A, Pokrovskaja Tamm K. Targeting autophagy by small molecule inhibitors of vacuolar protein sorting 34 (Vps34) improves the sensitivity of breast cancer cells to sunitinib. Cancer Lett. 2018;435:32–43. doi: 10.1016/j.canlet.2018.07.028. [DOI] [PubMed] [Google Scholar]
- 168.Liu XC, Wang AL, Liang XF, Liu JJ, Zou FM, Chen C, Zhao Z, Deng YX, Wu H, Qi ZP, Wang BL, Wang L, Liu FY, Xu YH, Wang WC, Fernandes SM, Stone RM, Galinsky IA, Brown JR, Loh T, Griffin JD, Zhang SC, Weisberg EL, Zhang X, Liu J, Liu QS. Simultaneous inhibition of Vps34 kinase would enhance PI3K delta inhibitor cytotoxicity in the B-cell malignancies. Oncotarget. 2016;7(33):53515–53525. doi: 10.18632/oncotarget.10650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Otsuka F, Yasuda S, Noguchi T, Ishibashi-Ueda H. Pathology of coronary atherosclerosis and thrombosis. Cardiovasc Diagn Ther. 2016;6(4):396–408. doi: 10.21037/cdt.2016.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Fougerat A, Gayral S, Gourdy P, Schambourg A, Ruckle T, Schwarz MK, Rommel C, Hirsch E, Arnal JF, Salles JP, Perret B, Breton-Douillon M, Wymann MP, Laffargue M. Genetic and pharmacological targeting of phosphoinositide 3-kinase-gamma reduces atherosclerosis and favors plaque stability by modulating inflammatory processes. Circulation. 2008;117(10):1310–1317. doi: 10.1161/CIRCULATIONAHA.107.720466. [DOI] [PubMed] [Google Scholar]
- 171.Chang JD, Sukhova GK, Libby P, Schvartz E, Lichtenstein AH, Field SJ, Kennedy C, Madhavarapu S, Luo J, Wu D, Cantley LC. Deletion of the phosphoinositide 3-kinase p110gamma gene attenuates murine atherosclerosis. Proc Natl Acad Sci USA. 2007;104(19):8077–8082. doi: 10.1073/pnas.0702663104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Anzinger JJ, Chang J, Xu Q, Barthwal MK, Bohnacker T, Wymann MP, Kruth HS. Murine bone marrow-derived macrophages differentiated with GM-CSF become foam cells by PI3Kgamma-dependent fluid-phase pinocytosis of native LDL. J Lipid Res. 2012;53(1):34–42. doi: 10.1194/jlr.M018887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ruckle T, Schwarz MK, Rommel C. PI3K gamma inhibition: towards an ‘aspirin of the 21st century’? Nat Rev Drug Discovery. 2006;5(11):903–918. doi: 10.1038/nrd2145. [DOI] [PubMed] [Google Scholar]
- 174.Vecchione C, Patrucco E, Marino G, Barberis L, Poulet R, Aretini A, Maffei A, Gentile MT, Storto M, Azzolino O, Brancaccio M, Colussi GL, Bettarini U, Altruda F, Silengo L, Tarone G, Wymann MP, Hirsch E, Lembo G. Protection from angiotensin II-mediated vasculotoxic and hypertensive response in mice lacking PI3Kgamma. J Exp Med. 2005;201(8):1217–1228. doi: 10.1084/jem.20040995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Carnevale D, Vecchione C, Mascio G, Esposito G, Cifelli G, Martinello K, Landolfi A, Selvetella G, Grieco P, Damato A, Franco E, Haase H, Maffei A, Ciraolo E, Fucile S, Frati G, Mazzoni O, Hirsch E, Lembo G. PI3Kgamma inhibition reduces blood pressure by a vasorelaxant Akt/L-type calcium channel mechanism. Cardiovasc Res. 2012;93(1):200–209. doi: 10.1093/cvr/cvr288. [DOI] [PubMed] [Google Scholar]
- 176.Wain LV, Verwoert GC, O’Reilly PF, Shi G, Johnson T, Johnson AD, Bochud M, Rice KM, Henneman P, Smith AV, Ehret GB, Amin N, Larson MG, Mooser V, Hadley D, Dorr M, Bis JC, Aspelund T, Esko T, Janssens AC, Zhao JH, Heath S, Laan M, Fu J, Pistis G, Luan J, Arora P, Lucas G, Pirastu N, Pichler I, Jackson AU, Webster RJ, Zhang F, Peden JF, Schmidt H, Tanaka T, Campbell H, Igl W, Milaneschi Y, Hottenga JJ, Vitart V, Chasman DI, Trompet S, Bragg-Gresham JL, Alizadeh BZ, Chambers JC, Guo X, Lehtimaki T, Kuhnel B, Lopez LM, Polasek O, Boban M, Nelson CP, Morrison AC, Pihur V, Ganesh SK, Hofman A, Kundu S, Mattace-Raso FU, Rivadeneira F, Sijbrands EJ, Uitterlinden AG, Hwang SJ, Vasan RS, Wang TJ, Bergmann S, Vollenweider P, Waeber G, Laitinen J, Pouta A, Zitting P, McArdle WL, Kroemer HK, Volker U, Volzke H, Glazer NL, Taylor KD, Harris TB, Alavere H, Haller T, Keis A, Tammesoo ML, Aulchenko Y, Barroso I, Khaw KT, Galan P, Hercberg S, Lathrop M, Eyheramendy S, Org E, Sober S, Lu X, Nolte IM, Penninx BW, Corre T, Masciullo C, Sala C, Groop L, Voight BF, Melander O, O’Donnell CJ, Salomaa V, d’Adamo AP, Fabretto A, Faletra F, Ulivi S, Del Greco F, Facheris M, Collins FS, Bergman RN, Beilby JP, Hung J, Musk AW, Mangino M, Shin SY, Soranzo N, Watkins H, Goel A, Hamsten A, Gider P, Loitfelder M, Zeginigg M, Hernandez D, Najjar SS, Navarro P, Wild SH, Corsi AM, Singleton A, de Geus EJ, Willemsen G, Parker AN, Rose LM, Buckley B, Stott D, Orru M, Uda M, LifeLines Cohort Study. van der Klauw MM, Zhang W, Li X, Scott J, Chen YD, Burke GL, Kahonen M, Viikari J, Doring A, Meitinger T, Davies G, Starr JM, Emilsson V, Plump A, Lindeman JH, Hoen PA, Konig IR, EchoGen C, Felix JF, Clarke R, Hopewell JC, Ongen H, Breteler M, Debette S, Destefano AL, Fornage M, AortaGen Consortium. Mitchell GF, CHARGE Consortium Heart Failure Working Group. Smith NL, KidneyGen consortium. Holm H, Stefansson K, Thorleifsson G, Thorsteinsdottir U, CKDGen Consortium. Cardiogenics Consortium. CardioGram. Samani NJ, Preuss M, Rudan I, Hayward C, Deary IJ, Wichmann HE, Raitakari OT, Palmas W, Kooner JS, Stolk RP, Jukema JW, Wright AF, Boomsma DI, Bandinelli S, Gyllensten UB, Wilson JF, Ferrucci L, Schmidt R, Farrall M, Spector TD, Palmer LJ, Tuomilehto J, Pfeufer A, Gasparini P, Siscovick D, Altshuler D, Loos RJ, Toniolo D, Snieder H, Gieger C, Meneton P, Wareham NJ, Oostra BA, Metspalu A, Launer L, Rettig R, Strachan DP, Beckmann JS, Witteman JC, Erdmann J, van Dijk KW, Boerwinkle E, Boehnke M, Ridker PM, Jarvelin MR, Chakravarti A, Abecasis GR, Gudnason V, Newton-Cheh C, Levy D, Munroe PB, Psaty BM, Caulfield MJ, Rao DC, Tobin MD, Elliott P, van Duijn CM. Genome-wide association study identifies six new loci influencing pulse pressure and mean arterial pressure. Nat Genet. 2011;43(10):1005–1011. doi: 10.1038/ng.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Northcott CA, Poy MN, Najjar SM, Watts SW. Phosphoinositide 3-kinase mediates enhanced spontaneous and agonist-induced contraction in aorta of deoxycorticosterone acetate-salt hypertensive rats. Circ Res. 2002;91(4):360–369. doi: 10.1161/01.RES.0000030861.13850.F1. [DOI] [PubMed] [Google Scholar]
- 178.Seok YM, Azam MA, Okamoto Y, Sato A, Yoshioka K, Maeda M, Kim I, Takuwa Y. Enhanced Ca2+-dependent activation of phosphoinositide 3-kinase class IIalpha isoform-Rho axis in blood vessels of spontaneously hypertensive rats. Hypertension. 2010;56(5):934–941. doi: 10.1161/HYPERTENSIONAHA.110.160853. [DOI] [PubMed] [Google Scholar]
- 179.Yoshioka K, Sugimoto N, Takuwa N, Takuwa Y. Essential role for class II phosphoinositide 3-kinase alpha-isoform in Ca2+-induced, Rho- and Rho kinase-dependent regulation of myosin phosphatase and contraction in isolated vascular smooth muscle cells. Mol Pharmacol. 2007;71(3):912–920. doi: 10.1124/mol.106.032599. [DOI] [PubMed] [Google Scholar]
- 180.Yang S, Wu Q, Huang S, Wang Z, Qi F. Sevoflurane and isoflurane inhibit KCl-induced class II phosphoinositide 3-kinase alpha subunit mediated vasoconstriction in rat aorta. BMC Anesthesiol. 2016;16(1):63. doi: 10.1186/s12871-016-0227-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Heller R, Chang Q, Ehrlich G, Hsieh SN, Schoenwaelder SM, Kuhlencordt PJ, Preissner KT, Hirsch E, Wetzker R. Overlapping and distinct roles for PI3Kbeta and gamma isoforms in S1P-induced migration of human and mouse endothelial cells. Cardiovasc Res. 2008;80(1):96–105. doi: 10.1093/cvr/cvn159. [DOI] [PubMed] [Google Scholar]
- 182.Madeddu P, Kraenkel N, Barcelos LS, Siragusa M, Campagnolo P, Oikawa A, Caporali A, Herman A, Azzolino O, Barberis L, Perino A, Damilano F, Emanueli C, Hirsch E. Phosphoinositide 3-kinase gamma gene knockout impairs postischemic neovascularization and endothelial progenitor cell functions. Arterioscler Thromb Vasc Biol. 2008;28(1):68–76. doi: 10.1161/ATVBAHA.107.145573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Qayum N, Muschel RJ, Im JH, Balathasan L, Koch CJ, Patel S, McKenna WG, Bernhard EJ. Tumor vascular changes mediated by inhibition of oncogenic signaling. Cancer Res. 2009;69(15):6347–6354. doi: 10.1158/0008-5472.CAN-09-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Fokas E, Im JH, Hill S, Yameen S, Stratford M, Beech J, Hackl W, Maira SM, Bernhard EJ, McKenna WG, Muschel RJ. Dual inhibition of the PI3K/mTOR pathway increases tumor radiosensitivity by normalizing tumor vasculature. Cancer Res. 2012;72(1):239–248. doi: 10.1158/0008-5472.CAN-11-2263. [DOI] [PubMed] [Google Scholar]
- 185.Qayum N, Im J, Stratford MR, Bernhard EJ, McKenna WG, Muschel RJ. Modulation of the tumor microvasculature by phosphoinositide-3 kinase inhibition increases doxorubicin delivery in vivo. Clin Cancer Res. 2012;18(1):161–169. doi: 10.1158/1078-0432.CCR-11-1413. [DOI] [PubMed] [Google Scholar]
- 186.Okkenhaug K, Graupera M, Vanhaesebroeck B. Targeting PI3K in cancer: impact on tumor cells, their protective stroma, angiogenesis, and immunotherapy. Cancer Discov. 2016;6(10):1090–1105. doi: 10.1158/2159-8290.CD-16-0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wojtukiewicz MZ, Sierko E, Hempel D, Tucker SC, Honn KV. Platelets and cancer angiogenesis nexus. Cancer Metastasis Rev. 2017;36(2):249–262. doi: 10.1007/s10555-017-9673-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Crackower MA, Oudit GY, Kozieradzki I, Sarao R, Sun H, Sasaki T, Hirsch E, Suzuki A, Shioi T, Irie-Sasaki J, Sah R, Cheng HY, Rybin VO, Lembo G, Fratta L, Oliveira-dos-Santos AJ, Benovic JL, Kahn CR, Izumo S, Steinberg SF, Wymann MP, Backx PH, Penninger JM. Regulation of myocardial contractility and cell size by distinct PI3K-PTEN signaling pathways. Cell. 2002;110(6):737–749. doi: 10.1016/S0092-8674(02)00969-8. [DOI] [PubMed] [Google Scholar]
- 189.McMullen JR, Shioi T, Zhang L, Tarnavski O, Sherwood MC, Kang PM, Izumo S. Phosphoinositide 3-kinase(p110alpha) plays a critical role for the induction of physiological, but not pathological, cardiac hypertrophy. Proc Natl Acad Sci USA. 2003;100(21):12355–12360. doi: 10.1073/pnas.1934654100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Shioi T, Kang PM, Douglas PS, Hampe J, Yballe CM, Lawitts J, Cantley LC, Izumo S. The conserved phosphoinositide 3-kinase pathway determines heart size in mice. EMBO J. 2000;19(11):2537–2548. doi: 10.1093/emboj/19.11.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lin RC, Weeks KL, Gao XM, Williams RB, Bernardo BC, Kiriazis H, Matthews VB, Woodcock EA, Bouwman RD, Mollica JP, Speirs HJ, Dawes IW, Daly RJ, Shioi T, Izumo S, Febbraio MA, Du XJ, McMullen JR. PI3K(p110 alpha) protects against myocardial infarction-induced heart failure: identification of PI3K-regulated miRNA and mRNA. Arterioscler Thromb Vasc Biol. 2010;30(4):724–732. doi: 10.1161/ATVBAHA.109.201988. [DOI] [PubMed] [Google Scholar]
- 192.Weeks KL, Gao X, Du XJ, Boey EJ, Matsumoto A, Bernardo BC, Kiriazis H, Cemerlang N, Tan JW, Tham YK, Franke TF, Qian H, Bogoyevitch MA, Woodcock EA, Febbraio MA, Gregorevic P, McMullen JR. Phosphoinositide 3-kinase p110alpha is a master regulator of exercise-induced cardioprotection and PI3K gene therapy rescues cardiac dysfunction. Circ Heart Fail. 2012;5(4):523–534. doi: 10.1161/CIRCHEARTFAILURE.112.966622. [DOI] [PubMed] [Google Scholar]
- 193.McMullen JR, Amirahmadi F, Woodcock EA, Schinke-Braun M, Bouwman RD, Hewitt KA, Mollica JP, Zhang L, Zhang Y, Shioi T, Buerger A, Izumo S, Jay PY, Jennings GL. Protective effects of exercise and phosphoinositide 3-kinase(p110alpha) signaling in dilated and hypertrophic cardiomyopathy. Proc Natl Acad Sci USA. 2007;104(2):612–617. doi: 10.1073/pnas.0606663104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zhabyeyev P, McLean B, Patel VB, Wang W, Ramprasath T, Oudit GY. Dual loss of PI3Kalpha and PI3Kgamma signaling leads to an age-dependent cardiomyopathy. J Mol Cell Cardiol. 2014;77:155–159. doi: 10.1016/j.yjmcc.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 195.Patel VB, Zhabyeyev P, Chen X, Wang F, Paul M, Fan D, McLean BA, Basu R, Zhang P, Shah S, Dawson JF, Pyle WG, Hazra M, Kassiri Z, Hazra S, Vanhaesebroeck B, McCulloch CA, Oudit GY. PI3Kalpha-regulated gelsolin activity is a critical determinant of cardiac cytoskeletal remodeling and heart disease. Nat Commun. 2018;9(1):5390. doi: 10.1038/s41467-018-07812-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wu CY, Jia Z, Wang W, Ballou LM, Jiang YP, Chen B, Mathias RT, Cohen IS, Song LS, Entcheva E, Lin RZ. PI3Ks maintain the structural integrity of T-tubules in cardiac myocytes. PLoS ONE. 2011;6(9):e24404. doi: 10.1371/journal.pone.0024404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ghigo A, Laffargue M, Li M, Hirsch E. PI3K and calcium signaling in cardiovascular disease. Circ Res. 2017;121(3):282–292. doi: 10.1161/CIRCRESAHA.117.310183. [DOI] [PubMed] [Google Scholar]
- 198.Pretorius L, Du XJ, Woodcock EA, Kiriazis H, Lin RC, Marasco S, Medcalf RL, Ming Z, Head GA, Tan JW, Cemerlang N, Sadoshima J, Shioi T, Izumo S, Lukoshkova EV, Dart AM, Jennings GL, McMullen JR. Reduced phosphoinositide 3-kinase (p110alpha) activation increases the susceptibility to atrial fibrillation. Am J Pathol. 2009;175(3):998–1009. doi: 10.2353/ajpath.2009.090126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Patrucco E, Notte A, Barberis L, Selvetella G, Maffei A, Brancaccio M, Marengo S, Russo G, Azzolino O, Rybalkin SD, Silengo L, Altruda F, Wetzker R, Wymann MP, Lembo G, Hirsch E. PI3Kgamma modulates the cardiac response to chronic pressure overload by distinct kinase-dependent and -independent effects. Cell. 2004;118(3):375–387. doi: 10.1016/j.cell.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 200.Ghigo A, Perino A, Damilano F, Leroy J, Nikolaev VO, Richter W, Conti M, Vandecasteele G, Hirsch E, Fischmeister R. PI3Kgamma protects against catecholamine-induced ventricular arrhythmia through PKA-mediated regulation of distinct phosphodiesterases. Cardiovasc Res. 2012;93:S20. doi: 10.1161/CIRCULATIONAHA.112.114074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Perino A, Ghigo A, Ferrero E, More F, Santulli G, Baillie GS, Damilano F, Dunlop AJ, Pawson C, Walser R, Levi R, Altruda F, Silengo L, Langeberg LK, Neubauer G, Heymans S, Lembo G, Wymann MP, Wetzker R, Houslay MD, Iaccarino G, Scott JD, Hirsch E. Integrating cardiac PIP3 and cAMP signaling through a PKA anchoring function of p110 gamma. Mol Cell. 2011;42(1):84–95. doi: 10.1016/j.molcel.2011.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ghigo A, Li M. Phosphoinositide 3-kinase: friend and foe in cardiovascular disease. Front Pharmacol. 2015;6:169. doi: 10.3389/fphar.2015.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Yu P, Zhang Y, Li C, Li Y, Jiang S, Zhang X, Ding Z, Tu F, Wu J, Gao X, Li L. Class III PI3K-mediated prolonged activation of autophagy plays a critical role in the transition of cardiac hypertrophy to heart failure. J Cell Mol Med. 2015;19(7):1710–1719. doi: 10.1111/jcmm.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Doukas J, Wrasidlo W, Noronha G, Dneprovskaia E, Fine R, Weis S, Hood J, Demaria A, Soll R, Cheresh D. Phosphoinositide 3-kinase gamma/delta inhibition limits infarct size after myocardial ischemia/reperfusion injury. Proc Natl Acad Sci USA. 2006;103(52):19866–19871. doi: 10.1073/pnas.0606956103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Eisenreich A, Rauch U. PI3K inhibitors in cardiovascular disease. Cardiovasc Ther. 2011;29(1):29–36. doi: 10.1111/j.1755-5922.2010.00206.x. [DOI] [PubMed] [Google Scholar]
- 206.Siragusa M, Katare R, Meloni M, Damilano F, Hirsch E, Emanueli C, Madeddu P. Involvement of phosphoinositide 3-kinase gamma in angiogenesis and healing of experimental myocardial infarction in mice. Circ Res. 2010;106(4):757–768. doi: 10.1161/CIRCRESAHA.109.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Haubner BJ, Neely GG, Voelkl JGJ, Damilano F, Kuba K, Imai Y, Komnenovic V, Mayr A, Pachinger O, Hirsch E, Penninger JM, Metzler B. PI3K gamma protects from myocardial ischemia and reperfusion injury through a kinase-independent pathway. PLoS ONE. 2010;5(2):e9350. doi: 10.1371/journal.pone.0009350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Rossello X, Riquelme JA, He ZH, Taferner S, Vanhaesebroeck B, Davidson SM, Yellon DM. The role of PI3K alpha isoform in cardioprotection. Basic Res Cardiol. 2017;112(6):66. doi: 10.1007/s00395-017-0657-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Chen X, Zhabyeyev P, Azad AK, Wang W, Minerath RA, DesAulniers J, Grueter CE, Murray AG, Kassiri Z, Vanhaesebroeck B, Oudit GY. Endothelial and cardiomyocyte PI3Kbeta divergently regulate cardiac remodelling in response to ischaemic injury. Cardiovasc Res. 2019;115(8):1343–1356. doi: 10.1093/cvr/cvy298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Mclean BA, Zhabyeyev P, Pituskin E, Paterson I, Haykowsky MJ, Oudit GY. PI3K inhibitors as novel cancer therapies: implications for cardiovascular medicine. J Card Fail. 2013;19(4):268–282. doi: 10.1016/j.cardfail.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 211.Zhabyeyev P, McLean B, Chen XY, Vanhaesebroeck B, Oudit GY. Inhibition of PI3Kinase-alpha is pro-arrhythmic and associated with enhanced late Na4+ current, contractility, and Ca2+ release in murine hearts. J Mol Cell Cardiol. 2019;132:98–109. doi: 10.1016/j.yjmcc.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 212.Ezeani M, Elom S. Necessity to evaluate PI3K/Akt signalling pathway in proarrhythmia. Open Heart. 2017;4(2):e000596. doi: 10.1136/openhrt-2017-000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.McLean BA, Patel VB, Zhabyeyev P, Chen X, Basu R, Wang F, Shah S, Vanhaesebroeck B, Oudit GY. PI3Kalpha pathway inhibition with doxorubicin treatment results in distinct biventricular atrophy and remodeling with right ventricular dysfunction. J Am Heart Assoc. 2019;8(9):e010961. doi: 10.1161/JAHA.118.010961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.McMullen JR, Jay PY. PI3K(p110 alpha) inhibitors as anti-cancer agents—minding the heart. Cell Cycle. 2007;6(8):910–913. doi: 10.4161/cc.6.8.4124. [DOI] [PubMed] [Google Scholar]
- 215.Zhabyeyev P, Chen X, Vanhaesebroeck B, Oudit GY. PI3Kalpha in cardioprotection: cytoskeleton, late Na(+) current, and mechanism of arrhythmias. Channels. 2019;13(1):520–532. doi: 10.1080/19336950.2019.1697127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Rodon J, Dienstmann R, Serra V, Tabernero J. Development of PI3K inhibitors: lessons learned from early clinical trials. Nat Rev Clin Oncol. 2013;10(3):143–153. doi: 10.1038/nrclinonc.2013.10. [DOI] [PubMed] [Google Scholar]
- 217.Fruman DA, Chiu H, Hopkins BD, Bagrodia S, Cantley LC, Abraham RT. The PI3K pathway in human disease. Cell. 2017;170(4):605–635. doi: 10.1016/j.cell.2017.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Juric D, de Bono JS, LoRusso PM, Nemunaitis J, Heath EI, Kwak EL, Macarulla Mercade T, Geuna E, de Miguel-Luken MJ, Patel C, Kuida K, Sankoh S, Westin EH, Zohren F, Shou Y, Tabernero J. A first-in-human, phase I, dose-escalation study of TAK-117, a selective PI3Kalpha isoform inhibitor, in patients with advanced solid malignancies. Clin Cancer Res. 2017;23(17):5015–5023. doi: 10.1158/1078-0432.CCR-16-2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Andre F, Ciruelos E, Rubovszky G, Campone M, Loibl S, Rugo HS, Iwata H, Conte P, Mayer IA, Kaufman B, Yamashita T, Lu YS, Inoue K, Takahashi M, Papai Z, Longin AS, Mills D, Wilke C, Hirawat S, Juric D, Grp S-S. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N Engl J Med. 2019;380(20):1929–1940. doi: 10.1056/NEJMoa1813904. [DOI] [PubMed] [Google Scholar]
- 220.Juric D, Krop I, Ramanathan RK, Wilson TR, Ware JA, Bohorquez SMS, Savage HM, Sampath D, Salphati L, Lin RS, Jin H, Parmar H, Hsu JY, Von Hoff DD, Baselga J. Phase I dose-escalation study of taselisib, an oral PI3K inhibitor, in patients with advanced solid tumors. Cancer Discov. 2017;7(7):704–715. doi: 10.1158/2159-8290.CD-16-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Hopkins BD, Pauli C, Du X, Wang DG, Li X, Wu D, Amadiume SC, Goncalves MD, Hodakoski C, Lundquist MR, Bareja R, Ma Y, Harris EM, Sboner A, Beltran H, Rubin MA, Mukherjee S, Cantley LC. Suppression of insulin feedback enhances the efficacy of PI3K inhibitors. Nature. 2018;560(7719):499–503. doi: 10.1038/s41586-018-0343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Campa CC, Silva RL, Margaria JP, Pirali T, Mattos MS, Kraemer LR, Reis DC, Grosa G, Copperi F, Dalmarco EM, Lima-Junior RCP, Aprile S, Sala V, Dal Bello F, Prado DS, Alves-Filho JC, Medana C, Cassali GD, Tron GC, Teixeira MM, Ciraolo E, Russo RC, Hirsch E. Inhalation of the prodrug PI3K inhibitor CL27c improves lung function in asthma and fibrosis. Nat Commun. 2018;9(1):5232. doi: 10.1038/s41467-018-07698-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Venot Q, Blanc T, Rabia SH, Berteloot L, Ladraa S, Duong JP, Blanc E, Johnson SC, Hoguin C, Boccara O, Sarnacki S, Boddaert N, Pannier S, Martinez F, Magassa S, Yamaguchi J, Knebelmann B, Merville P, Grenier N, Joly D, Cormier-Daire V, Michot C, Bole-Feysot C, Picard A, Soupre V, Lyonnet S, Sadoine J, Slimani L, Chaussain C, Laroche-Raynaud C, Guibaud L, Broissand C, Amiel J, Legendre C, Terzi F, Canaud G. Targeted therapy in patients with PIK3CA-related overgrowth syndrome. Nature. 2018;558(7711):540–546. doi: 10.1038/s41586-018-0217-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.