Abstract
Fusarium wilt caused by Fusarium oxysporum f. sp. fragariae is one of the most serious indigenous soil-borne fungal disease of strawberry. In this study, we have identified and investigated two sets of bacterial samples: Bacillus licheniformis (X-1) and Bacillus methylotrophicus (Z-1). Both of them were isolated from the rhizosphere soil of healthy strawberries which showed a strong inhibitory effect on Fusarium wilt caused by Fusarium oxysporum f. sp. fragariae. Bioorganic fertilizer developed by our team exhibiting a strong inhibition ability against the pathogen in comparison with the chemical and organic fertilizers. It allowed 80% disease free strawberry production together with improved physical and biochemical indexes in the pot experiments. The enzyme activity analysis of SOD, PPO, POD, and CAT in the bioorganic fertilizer (BOF) group showed significant increase with values; 48.8%, 68.7%, 85.9%, and 41.1% than that of the control group, respectively. The results of bacterial diversity showed that Bacillus in group BOF was almost three times as large as in the healthy soil control group (CK). Besides, the results of microbial diversity showed that Fusarium and Fusicolla of BOF was nearly five times less than that in CK and chemical fertilizer groups, where the Bacillus content reached to three times as much of the CK. Moreover, the enzymes activity and the content of beneficial microorganisms in the rhizosphere increased significantly. In this study, the bioorganic fertilizer developed by the isolated strains had significant effects on the control of strawberry Fusarium wilt disease. Our results demonstrate that BOF is a promising approach to control this disease in strawberry production.
Electronic supplementary material
The online version of this article (10.1007/s13205-020-2060-6) contains supplementary material, which is available to authorized users.
Keywords: Bioorganic fertilizer, Fusarium wilt, Diseases incidence, Strawberry
Introduction
Strawberries (Fragaria × ananassa Duch.) are one of the most economical and popular fruits in the world (Bombarely et al. 2010). However, when planted in the same field for years, the production of strawberries is highly susceptible to the disease of Fusarium wilt which is caused by Fusarium oxysporum f. sp. fragariae (Fof) (Fang et al. 2012). The problem is seriously threatening the commercial strawberry production worldwide, including Western Australia, Korea, China, Spain, the United States, and Serbia (Koike and Gordon 2015), where the development of the agricultural economy and agricultural tourism had already been affected.
Resistant strawberry cultivars are considered to be the first and preferred means of managing Fusarium wilt (Koike and Gordon 2015). Nevertheless, the long-term sustainability is still a problem due to the evolutionary arms race. Current applications of pre-plant chemical treatment of the soil can significantly reduce soil inoculum levels if efficacious materials are used with appropriate application methods (Koike and Gordon 2015). Non-chemical pre-plant treatments such as steam, solarization, and anaerobic soil disinfestation show potential in reducing soil inoculum levels but all have limitations. The use of bioorganic fertilizers (BOF) is emerging as a choice of feasible and environmentally safe disease controlling method in recent years (Ge et al. 2016; Ling et al. 2010). With the discovery of novel natural pathogen antagonists (Bacillus subtilis, et al.) for strawberries, biocontrol has become increasingly popular (Singhalage et al. 2019). It is considered that the applications of BOF as probiotic inoculations is an excellent alternative to commonly used disease controlling methods. Previous studies have proved that BOF can provide nutrients for microorganisms for colonization of antagonistic bacteria in the rhizosphere (Wu et al. 2016). Besides, a few experiments have been reported that BOF could largely control Fusarium wilt disease in peppers (Capsicum annuum L.) (Wu et al. 2015), melon (Cucumis melo L.) (Zhao et al. 2011), and bananas (Musa spp.) (Zhang et al. 2011).
The purpose of this study is to obtain BOF mainly composed of local probiotics against strawberry Fusarium wilt, to increase strawberry yield and reduce environmental pollution. The aim of the study is (1) to isolate and identify beneficial bacteria from the healthy rhizosphere soil of strawberry from local strawberry farms, (2) to develop BOF with one or two beneficial bacteria as inoculant and evaluate the control, efficiency, and fertility on the growth of strawberry, (3) to investigate the preliminary mechanism of these probiotics against the disease.
Materials and methods
Isolation and identification of antagonistic microbes
The pathogen, Fusarium oxysporum f. sp. fragariae (Fof), which was isolated from diseased strawberry plant roots, was kindly provided for the use in this study by Prof. Wenchao Zhen (College of Plant Protection, Hebei Agricultural University). The Fof strains were cultured in potato dextrose agar (PDA) medium. For the isolation of antagonistic bacteria, rhizosphere soil was sampled from healthy strawberry surviving in a strawberry production base in Huajia village, Dalian city, China. After 10 times of 10-fold serial dilutions, each dilute of the soil suspension was spread onto plates containing nutrient broth agar (NA) medium. The plates were incubated at 30 °C for 48 h. The colonies were purified and screened for their antagonists to Fof using a dual culture method described by Zhao et al. (2011). Briefly, a 6-mm plug from the leading edge of a 7-day-old culture of Fof plated by placing onto the center of a new PDA plate. The isolated bacteria were inoculated adjacent to the plug developed on the PDA plate, and further incubated at 28 °C for 3–5 days. The diameter of the inhibition zones and the antifungal index of mycelial growth of Fof were measured following the methods of Wang et al. (2009). The radial growth of mycelia (cm) was measured in two perpendicular directions on each cultured plate after 48 h of incubation, and the diameter (0.5 cm) of the inoculation plug was subtracted. The values were averaged. The antifungal index in percentage was determined in comparison with growth on non-amended medium, and calculated by the following formula:
where Da is the diameter of the growth zone in the test plate, and Db is the diameter of growth zone in the control plate.
Furthermore, the morphological, biochemical, and physiological properties of strains were determined according to the procedures outlined in Bergey’s Manual of Determinative Bacteriology.
Genomic DNA of bacterial strains was extracted using a Genomic DNA Extraction Kit according to the manufacturer’s instruction (Sangon, Shanghai, China). The 16S rRNA gene of mitochondria was obtained by PCR using primers B 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and the U 1492R (5′-TACGGCTACCTTGTTACGACTT-3′) (Sagar et al. 2014). The PCR products were sent to Shanghai Sangon Biological Engineering Technology & Services Co., Ltd. for sequencing. The sequences were analyzed using NCBI Blast server.
SEM analysis of antagonistic bacterial strains
Antagonistic strains were used for inoculation as above. At the 5th day of growth, mycelial plugs of Fof (6 mm) were cut out and fixed in 2% glutaraldehyde for 4 h at room temperature and rinsed three times (15 min/each) in phosphate buffer (0.1 M). Samples were dehydrated in ethanol series dilution series of 30%, 50%, 70%, 80%, 90%, and 100%, each for 10 min. Each sample was then critical point dried with liquid CO2, and coated with gold/palladium. Specimen micrographs were obtained using S-4800 FESEM (Hitachi, Japan) scanning electron microscope (SEM) at the Dalian Jiaotong University.
Bioorganic fertilizer preparation
The organic fertilizer (OF) consisted of a compost of swine manure and corn straw (2–4 cm). Corn stalk was obtained from a straw processing factory (Dalian, China). Swine manure was provided by Wan Xinyuan pig breeding Co., Ltd., which contained Carbon: Nitrogen (C/N) ratio of 14:1 and 69% humidity. The composting experiments were conducted in the plastic boxes with 2.5 cm foam insulation layer outside with the dimensions of 86 × 65 × 62 cm (length × width × height). The swine manure was mixed well with corn straw, and then, the mixture was adjusted to having C/N ratio of 25:1 and 65% humidity. The 150 kg of samples were transferred into each container of three replicates. The composting piles were kept throughout four stages including initial phase, mesophilic phase, thermophilic phase, and maturation phase, in 35 days. The end-product of compost contained 45.6% organic matter, 3.2% N, 2.8% P2O5, and 1.6% K2O.
A pile of the organic fertilizer was inoculated with 108 cfu/mL of the microbial agents (X-1:Z-1 = 1:1) by the ratio of 10% (v/w). The welled-mixture was collected into a plastic box (95 × 65 × 60 cm) for the secondary fermentation at 30 °C for 2 weeks.
Samples of composting material obtained from 5 different areas in the piles (four corners and center of the pile at the depth of about 15 cm). Samples were collected on days of 0, 4, 10, 15, 22, 30, and 35. Approximately 500 g material was collected of each sampling and then was mixed adequately for the following analyses. The pH was determined according to Meier et al. (2017). The electrical conductivity (EC) was measured using a detecting instrument (DDS-307, Shanghai, China) from fresh samples suspended in sterile water at 1:10 (w/w). The measurements were conducted following 20 min oscillation and filtration. Moisture content was measured after drying the compost samples at 105 °C for 24 h. Total organic carbon (TOC) and total nitrogen (TN) were determined by potassium dichromate and sulfuric acid detection method and Kjeldahl digestion method (Lu 2000), and then the C/N was calculated. Cucumber seeds were used for the germination index (GI) measurement. Twenty cucumber seeds were distributed on filter paper (Hangzhou Whatman-Xinhua Filter Paper Co., Ltd.) in Petri dishes (10 cm in diameter) and moistened with 5 mL of the compost extract. Three replicate dishes for each sample of different groups were incubated at 25 °C for 48 h (Jiang et al. 2018). The calculation equation was as:
Pot experiment design
Strawberry cv. Fragaria × ananassa Duch. ‘Benihoppe’ is a high-quality cultivar with large fruit and high yield. The seedlings at 5-leaf stage (0–35-day growth) were purchased from Beijing ShuYou Agricultural Development Co., Ltd., China. Each of the seedlings was cultivated in pots (16 × 16 × 12 cm) containing 500 g of soil. The soil was purchased from Flower Market of Dalian Xijiao Gardens, China, having the following properties: pH 6.5, organic matter 368 g/kg, available N 79.0 mg/kg, available P 129 mg/kg, and available K 40.0 mg/kg having no pathogenic bacteria (healthy soil). Experimental soil was prepared by mixing the healthy soil with Fof suspension (1 × 107 cfu/mL) with a ratio of 100 mL/kg at a final concentration of 109 cfu/kg soil. In all the treatments, 2% (w/w) following fertilizers were added; chemical fertilizer (NPK compound fertilizer), organic fertilizer, and bioorganic fertilizer as three replicates each having five pots. Soils used in this test were mixed with the fertilizers to ensure a uniform distribution prior to preparing the pots.
Disease incidence measurement
The experiment of disease incidence index was conducted in a greenhouse (23 ± 2 °C, 60% Relative Humidity, RH, 10–12 h day-light). The experimental design is presented in Supplementary Table 1. The disease incidence and disease severity of strawberry in each treatment group were observed and recorded daily after transplanting strawberries into the individual pots for 45 days. The disease incidence was calculated as according to the manuscript in Yuan et al. (2016) (Supplementary materials).
Physiochemical analysis of plants
Strawberry plants of different treatment groups are (1) control group (CK), plant-soil (healthy soil) having no inoculations; (2) chemical fertilizer group (CF), plant-soil supplemented with 2% chemical fertilizer (w/w); (3) organic fertilizer (OF), plant-soil supplemented with 2% organic fertilizer; and (4) bioorganic fertilizer (BOF), plant-soil supplemented with 2% bioorganic fertilizer. Each treatment had three blocks of five pots. The plants were pulled out from the pots, shaken off rhizosphere soil gently and washed with clean water. The dry weight, height, and root length were measured. The dry weight measurements were conducted after keeping the plants in the oven under blast dryer at 105 °C for 30 min and kept at 75 °C until obtaining constant weight measurements. The measurements were repeated three times each time, the average values were determined as the growth index of each plant. The pigment in the chloroplast of Chinese Pakchoi leaves was determined after ethanol extractions spectrophotometrically. The soluble sugar and soluble protein values were obtained by anthrone colorimetry and Coomassie Brilliant Blue G-250 staining. Root activity was determined by trichlorophenyl tetrachlorozole chloride method.
Defense enzyme activity assay
The three infected strawberry plants were randomly selected from each treatment group. The same part of the plant and the same growth of strawberry leaves were collected for enzyme activity determination. All leaves were freshly cut, weighed and homogenized in a pre-chilled mortar and pestle using liquid nitrogen after 30 days of growth. The homogenized tissue solution was immediately assessed to determine the activities of superoxide dismutase (SOD), catalase (CAT), peroxidase (POD), and polyphenol oxidase (PPO) using the Enzyme Kit (Nanjing Jiancheng Bioengineering Institute, China) according to the manufacturer’s procedure.
Diversity analysis of rhizosphere microbial community
The microbial community was studied from the samples of rhizosphere soil of strawberry from all the trial groups of DI experiment at the end of 45 days for the purpose of evaluating the effects of bioorganic fertilizer on the enrichment of microbial diversity in the strawberry rhizosphere, including bacteria and fungi. Total DNA of soil samples was extracted using a soil genomic DNA extraction kit (Solarbio, Beijing, China). DNA was quantified using the Qubit2.0 DNA Kit according to the manufacturer’s instructions. The diversity of the rhizosphere fungal community was analyzed using high-throughput sequencing (Illumina MiSeq 2500, BioMarker Technologies Co., Ltd., Beijing, China). The sequence reads were initially processed with Prinseq (PRINSEQ-lite 0.19.5) to remove low-quality data and improve the syncretic rates of the subsequent sequence. Double-ended sequences were fused through FLASH v1.2.7. Sequences with similarities greater than or equal to 0.97 were grouped into operational taxonomic units (OTUs) using UCLUST (version 1.2.22). ITS sequencing data were classified using the Unit algorithm. The relative abundance of OTUs was compared through samples to investigate the effects of different levels of rhizosphere microbial community.
PCR detection of antibiotic biosynthesis genes
Genomic DNA was extracted of the strains (X-1 and Z-1) using a DNA extraction kit (TIANGEN Biotech Co., Ltd). The PCR primer sets, reaction content, and cycling conditions for the PCR amplifications are presented in Supplementary Table 2. The PCR products were sequenced by Shanghai Sangon Biological Engineering Technology & Services Co., Ltd., and compared with existing sequences in the GenBank database using NCBI Blast server.
Statistical analyses
Statistical analyses were performed using GraphPad Prism 7.0 for windows. All values were expressed as their mean ± standard deviations of three repetitions, and levels of significance were evaluated using one-way ANOVA with Duncan’s multiple range tests. Differences were considered significant at the level of p < 0.05.
Results
Antagonistic bacteria
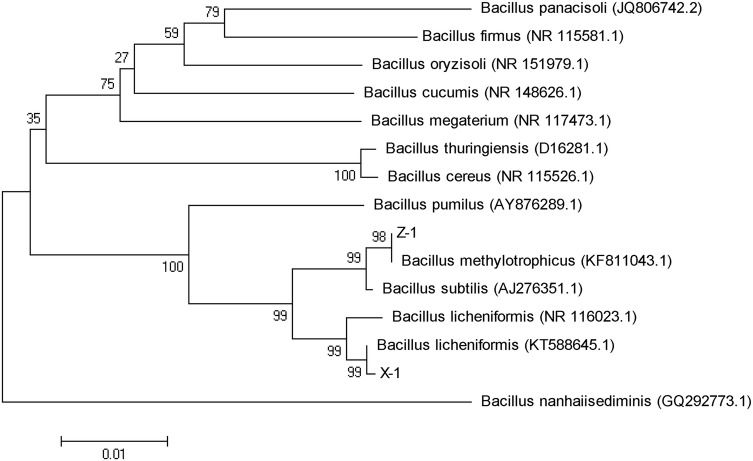
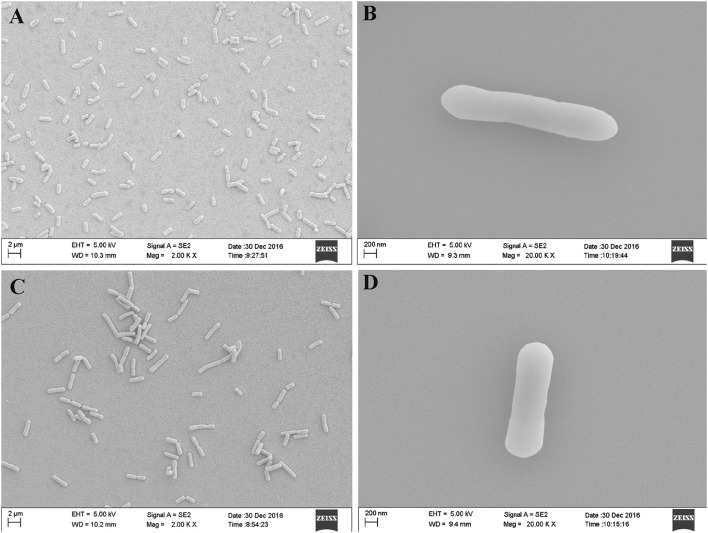
Eighteen bacterial isolates were obtained from the healthy strawberry rhizosphere soil. Two of them (X-1 and Z-1) showed strong inhibitory effects against the pathogen, with inhibition rates of 75.7% and 70.2%, respectively (Fig. 1, Table 1). Analyses of 16S rDNA sequences (Fig. 2) showed that X-1 (GenBank accession number: KT588645.1) had 99% similarity to Bacillus licheniformis, Z-1 (GenBank accession number: KT588645.1) had 98% similarity to Bacillus methylotrophicus (GenBank accession number: KC790303.1). These two strains were preliminary identified as Bacillus licheniformis and Bacillus methylotrophicus. Besides, scanning electron microscope micrographs of X-1 and Z-1 (Fig. 3) had typical rod-shaped morphology with a width and length of 0.8–1.0 × 2.2–3.0 μm. Further analysis of physiological and biochemical characteristics of two strains are presented in Table 2. Both of them belong to Gram-positive bacteria having an ability to utilize glycols such as glucose, mannitol, and xylose. They also showed the ability to produce catalase and hydrolyze starch. The reaction of V–P, nitrate reduction, and citrate tests were positive. X-1 strain showed no ability to liquefy gelatin and produce hydrogen sulfide, while Z-1 strain presented negative methyl red test. As a result, these two strains were confirmed as Bacillus licheniformis and Bacillus methylotrophicus.
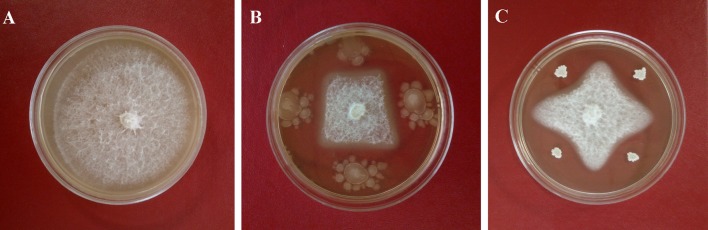
Fig. 1.
Inhibitory effects of antagonistic strains on the pathogen Fof after culturing for 7 days in vitro. a Fof as a control; b Fof co-culture with X-1; c Fof co-culture with Z-1
Table 1.
Inhibition diameter of X-1 and Z-1 against pathogen Fusarium oxysporum f. sp. fragariae in vitro
| Antagonistic bacteria | Inhibition rate | Inhibition diameter/mm |
|---|---|---|
| X-1 | 75.7% ± 0.004 | 31.8 ± 0.16 |
| Z-1 | 70.2% ± 0.010 | 29.5 ± 0.41 |
The data in the table are the mean of three replicates as the mean ± standard deviations with p < 0.05 according to Duncan’s multiple range test
Fig. 2.
Neighbor-joining tree based on 16S rDNA gene sequences of strains X-1 and Z-1, and related species of the genus Bacillus with 1000 bootstraps
Fig. 3.
The morphological characteristics of X-1 and Z-1 by SEM. a X-1 (× 2000); b X-1 (× 20,000); c Z-1 (× 2000); d Z-1 (× 20,000)
Table 2.
Physiological and biochemical characteristics of strain X-1 and Z-1
| Characteristics test | Results | Characteristics test | Results | ||
|---|---|---|---|---|---|
| X-1 | Z-1 | X-1 | Z-1 | ||
| Gram stain | + | + | Catalase test | + | + |
| Methyl red | + | − | Glucose | + | + |
| Hydrogen sulfate | − | + | Mannitol | + | + |
| Nitrate reduction | + | + | Esculin hydrolysis | + | + |
| Starch hydrolysis | + | + | Salicin | − | − |
| Citrate solution | + | + | Cellobiose | + | − |
| V—P test | + | + | Xylose | + | + |
| Glutinhydrolyzation | − | + | Lactose | − | + |
“+, −” stand for positive or negative of the reaction results
Properties of composts
The physical and chemical parameters of composts in OF and BOF groups are presented in Table 3. The values of pH, EC, moisture, TOC, TN, the ratio of C/N, and GI were not significantly different in OF and BOF. The ratio of C/N in both of the trials decreased from 25 at the beginning of composting to 12 at the final stage of composting. The GI value of the resulting composts was 86.5% in OF and 87.2% in BOF, respectively. The two antagonistic strains of X-1 and Z-1in the BOF reached to 1.7 × 109 cfu/(g dry weight) and 1.8 × 109 cfu/(g dry weight), respectively.
Table 3.
Selected chemical and physical properties of composts
| Properties | Compost (OF) | Compost (BOF) |
|---|---|---|
| pH | 7.95 ± 0.5 | 8.02 ± 0.4 |
| EC (μS cm−1) | 4.08 ± 0.4 | 4.02 ± 0.5 |
| Moisture (%) | 26.5 ± 0.3 | 25.2 ± 0.5 |
| TOC (%) | 28.7 ± 0.5 | 26.2 ± 0.5 |
| TN (%) | 2.4 ± 0.3 | 2.2 ± 0.5 |
| C/N | 12 ± 0.1 | 11.9 ± 0.1 |
| GI (%) | 86.5 ± 0.5 | 87.2 ± 0.5 |
The data in the table are the mean of three replicates as the mean ± standard deviations with p < 0.05 according to Duncan’s multiple range test. EC, electric conductivity. TOC, total organic carbon. TN, total nitrogen. GI, germination index
Disease incidence
Compared with other groups (Table 4), the BOF had the lowest value in disease incidence and disease index, 13.33% and 23.33%, respectively. The highest value was observed in control efficacy as 75.39%. Statistical analysis showed that there were significant differences between group BOF and other experimental groups in all three indicators (p < 0.05). The disease incidence and disease incidence index of group BOF were decreased by about 80% and 70%, respectively, in comparison with the control groups.
Table 4.
Effect of different treatments on control of strawberry Fusarium wilt
| Treatments | Disease incidence (%) | Disease index (%) | Control efficacy (%) |
|---|---|---|---|
| CK | 93.33 ± 0.09a | 94.67 ± 1.89a | – |
| CF | 86.67±0.09a | 88.00 ± 3.27a | 4.19 ± 5.87c |
| OF | 80.00 ± 0.16a | 73.00 ± 3.56b | 22.83 ± 4.59b |
| BOF | 13.33 ± 0.19b | 23.33 ± 4.71c | 75.39 ± 4.71a |
“–”, represents no value. The data in the table are the mean of three replicates as the mean ± standard deviations with p < 0.05 according to Duncan’s multiple range test
Growth indexes of strawberry
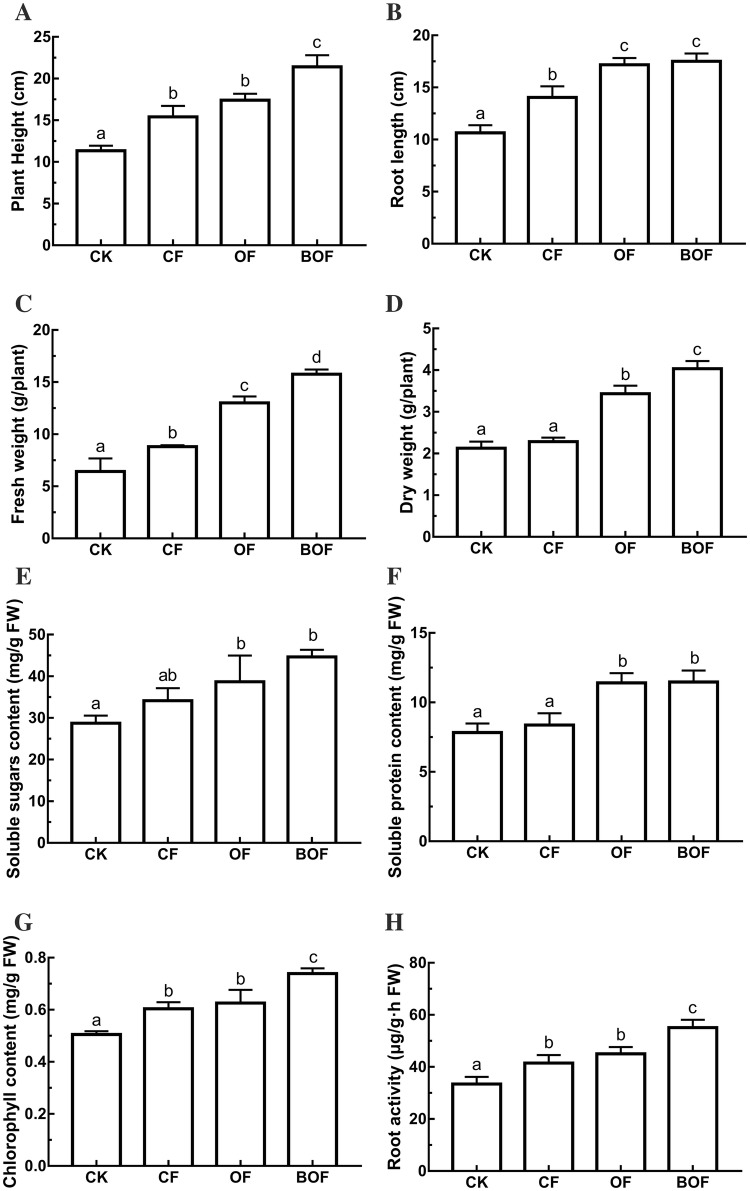
Eight basic growth parameters of strawberry are presented in Fig. 4. Compared with other experimental groups, three of the four sensory indexes (Fig. 4a, c, d) in group BOF showed significant differences in plant height (21.4 cm), fresh weight (15.8 g), and dry weight (4.0 g). The indexes of soluble sugar content (Fig. 4e) and soluble protein content (Fig. 4f) showed no significant differences between OF and BOF groups; however, they were significantly higher than that of the control group. Compared with the other two groups (group CF and OF), the chlorophyll content (Fig. 4g) and root activity (Fig. 4h) of group BOF were significantly increased. The average value of the chlorophyll content and root activity of BOF was detected as 0.739 mg/g FW and 55.13 μg/g·h−1 FW, respectively. The chlorophyll content of group BOF was 22.6% higher than that of group CF and 18.1% higher than that of OF group. The root activity of BOF was 32.9% higher than that of group CF and 22.3% higher than that of group OF, respectively.
Fig. 4.
The physiological and biochemical growth indexes of strawberry. a Plant height (cm); b root length (cm); c fresh weight (FW) (g); d dry weight (g); e soluble sugars content (mg/g FW); f soluble protein content (mg/g FW); g Chlorophyll content (mg/g FW); h root activity (μg/g·h FW). Bars with different letters indicate significant differences according to Duncan’s multiple range tests at p < 0.05 level
Enzymes
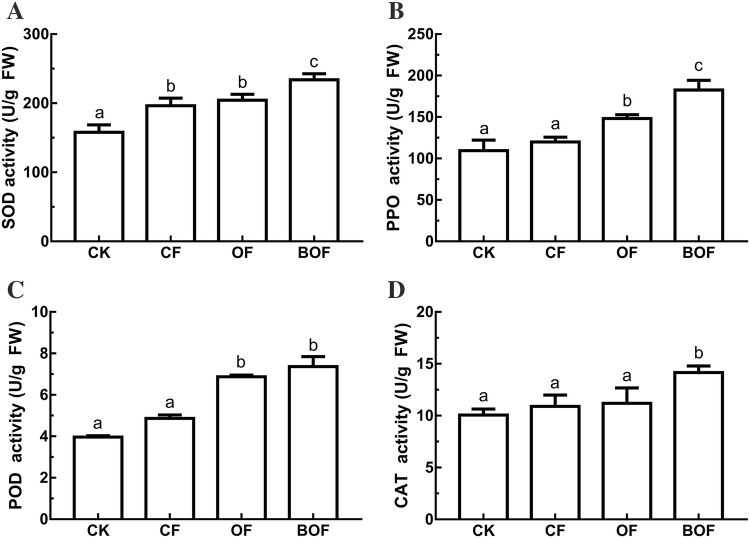
In the soil-planting trials containing Fof, the defensive enzyme activities of strawberry were found higher in BOF than that of the other groups (Fig. 5). The comparison between groups BOF and OF revealed that the activities of SOD, PPO, and CAT in leaves of strawberry seedlings treated with BOF were significantly higher than those treated with OF, which were 14.6%, 23.4%, and 28.1%, respectively. Although not significant, the difference observed in the enzyme activity of POD in BOF was 7.3% higher. Further analysis of enzymes activity between groups of BOF and CK, of the enzymes, SOD, PPO, POD, and CAT, wherein BOF group the activities were higher 48.8%, 68.7%, 85.9%, and 41.1%, respectively.
Fig. 5.
Effects of different treatments on defense enzymes activity (U/g fresh weight) in strawberry seedling leaves; SOD, PPO, POD, and CAT in a–d, respectively. Bars with different letters indicate significant differences according to Duncan’s multiple range tests at p < 0.05 level
Diversity of bacterial and fungal populations
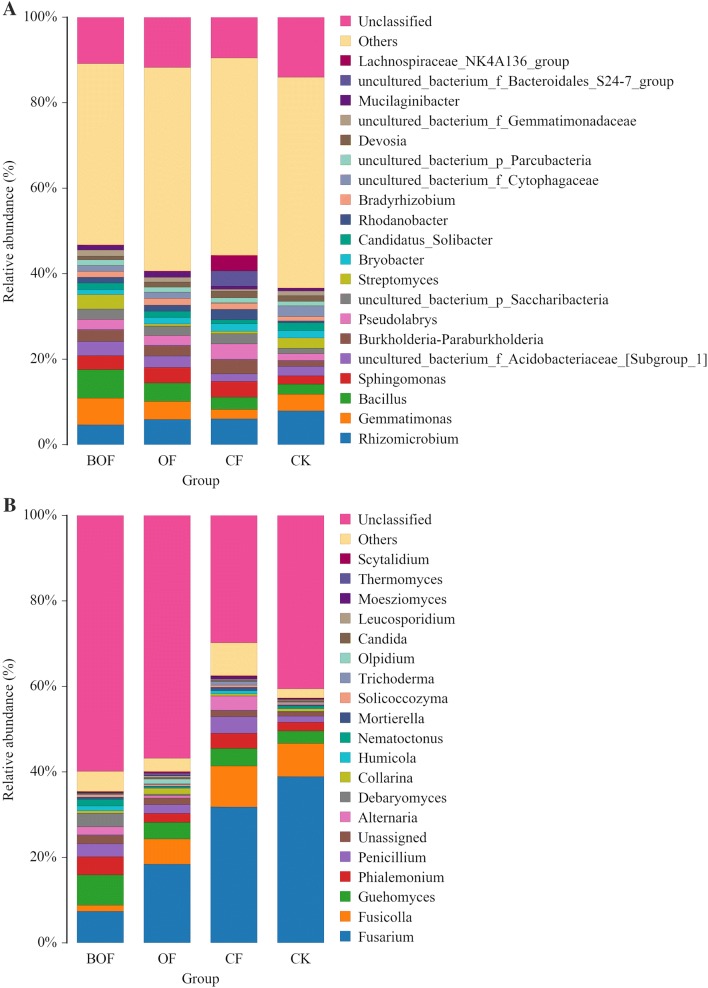
The result of the microbial diversity of rhizosphere in the soil of strawberry after 45 days of transplanting is presented in Table 5. Group BOF had the highest value among the four indexes (OTU, ACE, Shannon, and Chao1) in bacterial community diversity. However, there were no significant differences between group BOF, OF and CK in parameters Shannon and Chao 1. The results of fungal diversity analysis showed that there was a significant difference only when the parameters of Shannon were compared, that is, there were significant differences between group CK and other three experimental groups, while there was no significant difference between the other three parameters, OTU, ACE, and Chao1. Figure 6 presents the changes in their relative abundance of the genus level of the top 20 bacterial and fungal classified genera. Bacillus reached 6.63% in group BOF and 2.32% in group CK, and group BOF was almost three times as large as group CK (Fig. 6a). Besides, the amount of Bacillus in group BOF increased by 55% compared to group OF. In Fig. 6b, two fungi, Fusarium and Fusicolla, accounted for 8.82% in group BOF, 24.31% in group OF, 41.43% in group CF, and 46.67% in group CK, respectively. Compared with group CF and group CK, two kinds of fungal content (Fusarium and Fusicolla) in group BOF decreased nearly five times.
Table 5.
Diversity indexes of bacterial and fungal communities of experiment soil of strawberry in the different treatments
| Treatment | Bacterial | Fungal | ||||||
|---|---|---|---|---|---|---|---|---|
| OTU | ACE | Shannon | Chao1 | OTU | ACE | Shannon | Chao1 | |
| CK | 1877.3 ± 46.8b | 2299.7 ± 81.7b | 6.20 ± 0.04b | 2340.5 ± 62.5b | 477.7 ± 6.7a | 556.6 ± 17.5a | 2.63 ± 0.22a | 553.9 ± 17.2a |
| CF | 1517.3 ± 97.4a | 1849.2 ± 126.2a | 5.60 ± 0.06a | 1884.4 ± 148.5a | 610.0 ± 81.7a | 642.9 ± 10.6a | 3.42 ± 0.62b | 646.0 ± 70.1a |
| OF | 2129.0 ± 47.7c | 2518.2 ± 26.9bc | 6.28 ± 0.05b | 2549.1 ± 26.9b | 615.3 ± 72.9a | 643.9 ± 64.5a | 3.92 ± 0.14b | 648.6 ± 17.6a |
| BOF | 2390.3 ± 37.6d | 2535.3 ± 97.1c | 6.27 ± 0.11b | 2557.1 ± 95.0b | 602.7 ± 16.0a | 632.8 ± 78.5a | 3.41 ± 0.47b | 639.6 ± 75.1a |
The data in the table are the mean of three replicates as the mean ± standard deviations with p < 0.05 according to Duncan’s multiple range test. CK, control group in experiment soil without any inoculation. CF, chemical fertilizer group in in experiment soil with chemical fertilizer 2% (w/w). OF, organic fertilizer group in in experiment soil with organic fertilizer 2% (w/w). BOF, bioorganic fertilizer group in in experiment soil with bioorganic fertilizer 2% (w/w)
Fig. 6.
Bacterial community structure and comparison of bacteria groups at genus level. Others and unclassified: others contain the genus representing < top 20 of the total reads; unclassified means all of the unclassified reads. (a Bacterial community structure at the genus level; b comparison of bacteria groups at genus level)
Ultrastructure and gene
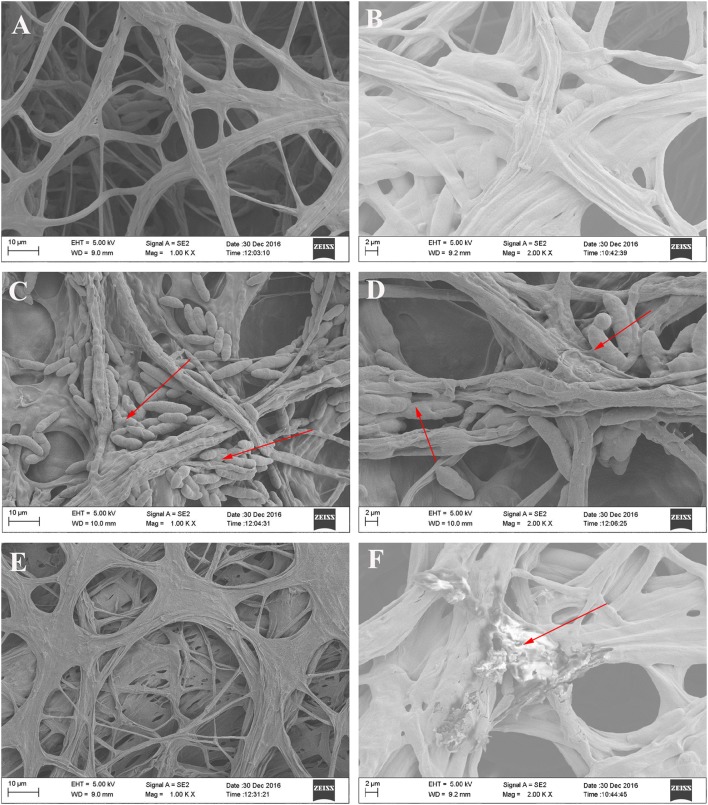
Normal hyphae were regular in shape and their surfaces were smooth (Fig. 7a, b). Noticeable morphological changes were found in the hyphae of Fof in the presence of the antagonistic bacteria X-1 and Z-1. It was observed that strain X-1 adhered on hyphae, evoking hyphae shriveling and deformity (Fig. 7c, d). Disruption of hyphae in the presence of strain Z-1 was also present, where hyphal swellings (Fig. 7e), collapse, protoplasm extrusion, as well as micropores were visible (Fig. 7f).
Fig. 7.
SEM micrographs of antagonistic bacteria interacting with hyphae of Fof on PDA medium. a, c, e Magnification: 1000×. b, d, f Magnification: 2000×. a, b Fof hyphae alone; c, d Fof hyphae treated with strain X-1 (red arrows show Fof hyphae surrounded by X-1); e, f Fof hyphae treated with strain Z-1 (red arrows indicate Fof hyphae damaged by Z-1)
The results of PCR detection of lipopeptide antibiotic-related synthetic genes are presented in Supplementary Fig. 1. Only the gene bamB related to Bacillomycin synthesis was amplified in X-1. Five synthetic genes related to lipopeptide antibiotics were detected in Z-1, ituA, ituB, ituD, bamB, and sur. Gene ituA, ituB, and ituD were synthesis genes of Iturin, gene bamB was of Bacillomycin, gene sur was of Surfactin. Blast homology analysis was performed on the detected sequences of lipopeptide antibiotic synthesis genes (Supplemental Table 2). The homologous similarity between the gene bamB of X-1 and the related gene sequence of Bacillus amyloliquefaciens (KY111359.1) strain was 99%. The homologous similarity between the related gene of Z-1 and Bacillus was: ituA of Z-1 and the related gene sequence of B. subtilis MH25 (EU263005.1) strain was 99%; ituB of Z-1 and that of B. subtilis (AB050629.1) was 98%; ituD of Z-1 and that of B. amyloliquefaciens PPCB004 (FJ815155.1) was 99%; bamB of Z-1 and that of B. methylotrophicus CBMB205 (CP0119 37.1) was 99%; sur of Z-1 and that of B. subtilis SQR 9 (JN084036.1) was 97%, respectively.
Discussion
Bacteria
Among various bacterial antagonists reported, Bacillus spp. like B. amyloliquefaciens, B. licheniformis and B. methylotrophicus have been used for effective control of the diseases by producing a variety of biologically active compounds with a broad spectrum of activities toward phytopathogens and that can induce host systemic resistance (Nakkeeran et al. 2019; Ongena and Jacques 2008). Bacillus licheniformis and methylotrophicus are the one of the most popular bacteria in controlling disease either in plant or soil borne. In this study, we screened antimicrobial microorganisms with highly targeted and regional characteristics in terms of the local situation, especially in Northeast China. Two strains, X-1 (Bacillus licheniformis) and Z-1 (Bacillus methylotrophicus), showed the strongest antagonistic in all separated strains which could control Fusarium wilt. The mechanism of antagonism is devoted to the biosynthesis of secondary metabolites that could prevent or control diseases and promote the growth of microorganisms (Diep and Nes 2002). In another previous study (Compant et al. 2005), the mechanisms of antagonistic bacteria (biocontrol agents) against soil-borne pathogens has been proposed: (1) antagonism, which is a direct mechanism, and (2) the induction of plant systemic resistance against the pathogen, which is an indirect mechanism. It was indicated that Bacillus spp. could induce the systemic resistance of plants to suppress various diseases in diversity of hosts.
Growth of strawberry
Based on the results of strawberry pot experiments, it could be concluded that: the bioorganic fertilizer developed by inoculating with two strains of bacteria (X-1 and Z-1) had strong antimicrobial activity and excellent growth-promoting ability. These improvements were attributed to the application of bioorganic fertilizers containing two isolated antagonistic strains (X-1 and Z-1). The results of this study were consistent with those reported previously (Singhalage et al. 2019). This may be related to the nutrition provided by bioorganic fertilizer to strawberry rhizosphere microorganisms which could promote the biosynthesis of secondary metabolites. The previous study has proved that the antagonistic microbes could survive and form populations in the treated soils (Wu et al. 2016). Besides, the beneficial bacteria have two effects, antifungal and fungicidal. One may be due to the antagonistic bacteria competing for nutrients with inhibited growth of Fof. The other one is that the bacteria secrete some hydrolytic enzymes, such as chitinase and glycanase which can degrade hypha, cell walls, and/or damage the normal structure of Fof hyphae (Di Francesco et al. 2016; Chan and Tian 2005).
In general, the addition of bioorganic fertilizer has positive effects on the photosynthetic pigment content in treated leaves compared with that in the control leaves. The increase of pigment content led to an increase of fresh and dry weight, which was attributed to the fact that pigment was the key factor of energy conversion. The yield of strawberry is directly related to the photosynthetic capacity of the plant. Many studies have been carried out on the influence of bioorganic fertilizer for the plant growth and photosynthetic rate (Doni et al. 2018). The chlorophyll content indicated a better and healthy root system that functions properly leading to empower the plants for better performance. Furthermore, the results of root activity analysis also prove the validity of this conclusion. Root activity of group BOF was significantly higher than that of the other experimental groups. The microorganisms of strawberry rhizosphere to trigger the synthesis of hormones and metabolites that had a significant role in promoting the growth indexes of strawberry. It has also been proved in the studies of root activity of other plants (Guler et al. 2016).
Preliminary mechanism
When plants suffer from stressful conditions such as injury, pathogen infection, or extreme temperatures, activities in defense enzymes increase (Kloepper et al. 2004; García-Limones et al. 2002; Jogaiah et al. 2013). The enzyme activities of SOD, PPO, POD, and CAT increased in group BOF which may be due to the reason that the antagonistic bacteria X-1 (Bacillus licheniformis) and Z-1 (Bacillus methylotrophicus) could be the dominant bacteria in the experiment soil of strawberry rhizosphere. Enhancing system resistance of strawberry stimulates the activity of defense enzymes equally to protect seedlings from pathogenic infections. These results were consistent with previous findings, including Gnanaprakash et al. (2013), Liu et al. (2016), Wu et al. (2015) and Pieterse et al. (2014). Although the activity of defense enzymes was conducted in soils containing pathogenic bacteria, and the activities were improved. A similar study (Ansari and Mahmood 2017) reported that the activity of defense enzymes was increased under the condition of soil culture without pathogenic bacteria. In another study with B. licheniformis was shown that bacteria could produce different types of extracellular proteases which can degrade heterologously produced extracellular proteins. Bacillus methylotrophicus can also produce many lipopeptides isoforms belonging to different families (surfactin, iturin and fengycin) allowing the use in various fields of biotechnological applications (Jemil et al. 2017).
The results of high-throughput sequencing of ITS rDNA genes to assess the biocontrol efficacy of Fusarium wilt revealed that the application of bioorganic fertilizer could increase the abundance of beneficial bacterial groups and decrease the abundance of Fusarium effectively. The previous studies had shown that the application of bioorganic fertilizer could decrease in the abundance of Fusarium effectively and suppress Fusarium wilt disease on bananas (Shen et al. 2015) and watermelons (Li et al. 2019). Some microorganisms in strawberry rhizosphere that were beneficial to strawberry growth could grow efficiently by applying bioorganic fertilizer. In addition to promoting the growth of strawberry, these beneficial microorganisms could also compete with some indigenous microorganisms (Xiong et al. 2017). One of the family in Bacillus is Gemmatimonas which has the function of nitrogen fixation providing sufficient nitrogen source for plant growth. They are the strains with some probiotic properties (Lodemann et al. 2008). Fusarium and Fusicolla are two plant pathogenic fungi that can cause soil-borne diseases of plants; however, they all can be suppressed by the family of Bacillus (Falardeau et al. 2013; Rebib et al. 2012).
The presence of antibiotic biosynthesis genes could also attribute to the biocontrol of plant pathogens in several Bacillus strains (González-Sánchez et al. 2010). In the present study, five antibiotic biosynthesis genes (ituA, ituB, ituD, bamB, and sur) were detected in strain Z-1. The gene, bamB, involved in Bacillomycin biosynthesis, was detected in the strain X-1 based on PCR assays. These antibiotic biosynthesis genes were linked to synthesize Iturin, Bacillomycin, Fengycin, and Surfactin. Therefore, it was possible to synthesize lipopeptide antibiotics in antagonistic bacteria X-1 and Z-1 for the suppression of Fof growth. Bioorganic fertilizer could suppress the diseases for the reason that the antagonistic lipopeptide as surfactant inducing morphological changes on the hyphae (Tendulkar et al. 2007). In several studies, iturin-like and/or fengycin-like lipopeptides were responsible for inhibitory activity against the growth of F. oxysporum f. sp. lycopersici, Aspergillus phoenicis, and Bipolaris sorokiniana (Benitez et al. 2010). In our study too, the bioorganic fertilizer (X-1 and Z-1) producing lipopeptides may be the main reason controlling the Fusarium wilt. The combination of these surfactants are reasoned for controlling the diseases (Alvarez et al. 2012) with a suggested mechanism by pore formation (Inès and Dhouha 2015). Further basic research is needed to understand the mechanisms in details for the mode of action of these beneficial microorganism in fighting against pathogenic organisms such as Fusarium oxysporum f. sp. fragariae.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This research was financially supported by “13th Five-Year” National Key R&D Program of China (Grant No. 2016YFD0501406), the China Postdoctoral Science Foundation (Grant No. 2017M613260) and Science and Technology Tackling and Achievement Conversion Project of Corp (Grant No. 2016AD025).
Authors contribution
YPX designed the study. TZ conducted the experiments. YC and TZ wrote the manuscript. XYL supervised experiments at all stages. MSA revised the manuscript. LLW and SYL assisted in data analysis and molecular analysis. All authors read and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
The authors have no financial disclosures or conflicts of interest.
References
- Alvarez F, Castro M, Príncipe A, Borioli G, Fischer S, Mori G, Jofre E. The plant-associated Bacillus amyloliquefaciens strains MEP218 and ARP23 capable of producing the cyclic lipopeptides iturin or surfactin and fengycin are effective in biocontrol of sclerotinia stem rot disease. J Appl Microbiol. 2012;112(1):159–174. doi: 10.1111/j.1365-2672.2011.05182.x. [DOI] [PubMed] [Google Scholar]
- Ansari RA, Mahmood I. Optimization of organic and bio-organic fertilizers on soil properties and growth of pigeon pea. Sci Hortic. 2017;226:1–9. [Google Scholar]
- Benitez LB, Velho RV, Lisboa MP, da Costa Medina LF, Brandelli A. Isolation and characterization of antifungal peptides produced by Bacillus amyloliquefaciens LBM5006. J Microbiol. 2010;48(6):791–797. doi: 10.1007/s12275-010-0164-0. [DOI] [PubMed] [Google Scholar]
- Bombarely A, Merchante C, Csukasi F, Cruz-Rus E, Caballero JL, Medina-Escobar N, Blanco-Portales R, Botella MA, Muñoz-Blanco J, Sánchez-Sevilla JF. Generation and analysis of ESTs from strawberry (Fragaria xananassa) fruits and evaluation of their utility in genetic and molecular studies. BMC Genom. 2010;11(1):503. doi: 10.1186/1471-2164-11-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan Z, Tian S. Interaction of antagonistic yeasts against postharvest pathogens of apple fruit and possible mode of action. Postharvest Biol Technol. 2005;36(2):215–223. [Google Scholar]
- Compant S, Duffy B, Nowak J, Clément C, Barka EA. Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microbiol. 2005;71(9):4951–4959. doi: 10.1128/AEM.71.9.4951-4959.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Francesco A, Martini C, Mari M. Biological control of postharvest diseases by microbial antagonists: How many mechanisms of action? Eur J Plant Pathol. 2016;145(4):711–717. [Google Scholar]
- Diep DB, Nes IF. Ribosomally synthesized antibacterial peptides in Gram positive bacteria. Curr Drug Targets. 2002;3(2):107–122. doi: 10.2174/1389450024605409. [DOI] [PubMed] [Google Scholar]
- Doni F, Zain CRCM, Isahak A, Fathurrahman F, Anhar A, Mohamad WNAW, Yusoff WMW, Uphoff N. A simple, efficient, and farmer-friendly Trichoderma-based biofertilizer evaluated with the SRI Rice Management System. Org Agric. 2018;8(3):207–223. [Google Scholar]
- Falardeau J, Wise C, Novitsky L, Avis T. Ecological and mechanistic insights into the direct and indirect antimicrobial properties of Bacillus subtilis lipopeptides on plant pathogens. J Chem Ecol. 2013;39(7):869–878. doi: 10.1007/s10886-013-0319-7. [DOI] [PubMed] [Google Scholar]
- Fang X, You MP, Barbetti MJ. Reduced severity and impact of Fusarium wilt on strawberry by manipulation of soil pH, soil organic amendments and crop rotation. Eur J Plant Pathol. 2012;134(3):619–629. [Google Scholar]
- García-Limones C, Hervás A, Navas-Cortés JA, Jiménez-Díaz RM, Tena M. Induction of an antioxidant enzyme system and other oxidative stress markers associated with compatible and incompatible interactions between chickpea (Cicer arietinum L.) and Fusarium oxysporum f. sp. ciceris. Physiol Mol Plant Pathol. 2002;61(6):325–337. [Google Scholar]
- Ge B, Liu B, Nwet TT, Zhao W, Shi L, Zhang K. Bacillus methylotrophicus strain NKG-1, isolated from Changbai Mountain, China, has potential applications as a biofertilizer or biocontrol agent. PLoS ONE. 2016;11(11):e0166079. doi: 10.1371/journal.pone.0166079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnanaprakash PH, Jogaiah S, Sreedhara AP, Prashanth GN, Kini RK, Shetty SH. Association between accumulation of allene oxide synthase activity and development of resistance against downy mildew disease of pearl millet. Mol Biol Rep. 2013;40(12):6821–6829. doi: 10.1007/s11033-013-2799-5. [DOI] [PubMed] [Google Scholar]
- González-Sánchez M, Pérez-Jiménez R, Pliego C, Ramos C, De Vicente A, Cazorla F. Biocontrol bacteria selected by a direct plant protection strategy against avocado white root rot show antagonism as a prevalent trait. J Appl Microbiol. 2010;109(1):65–78. doi: 10.1111/j.1365-2672.2009.04628.x. [DOI] [PubMed] [Google Scholar]
- Guler NS, Pehlivan N, Karaoglu SA, Guzel S, Bozdeveci A. Trichoderma atroviride ID20G inoculation ameliorates drought stress-induced damages by improving antioxidant defence in maize seedlings. Acta Physiol Plant. 2016;38(6):132. doi: 10.1007/s11738-016-2153-3. [DOI] [Google Scholar]
- Inès M, Dhouha G. Lipopeptide surfactants: production, recovery and pore forming capacity. Peptides. 2015;71:100–112. doi: 10.1016/j.peptides.2015.07.006. [DOI] [PubMed] [Google Scholar]
- Jemil N, Manresa A, Rabanal F, Ayed HB, Hmidet N, Nasri M. Structural characterization and identification of cyclic lipopeptides produced by Bacillus methylotrophicus DCS1 strain. J Chromatogr B. 2017;1060:374–386. doi: 10.1016/j.jchromb.2017.06.013. [DOI] [PubMed] [Google Scholar]
- Jiang J, Huang H, Huang Y, Liu X, Liu D. Relationship between maturity and microbial communities during pig manure composting by phospholipid fatty acid (PLFA) and correlation analysis. J Environ Manag. 2018;206:532–539. doi: 10.1016/j.jenvman.2017.10.067. [DOI] [PubMed] [Google Scholar]
- Jogaiah S, Abdelrahman M, Tran L-SP, Shin-ichi I. Characterization of rhizosphere fungi that mediate resistance in tomato against bacterial wilt disease. J Exp Bot. 2013;64(12):3829–3842. doi: 10.1093/jxb/ert212. [DOI] [PubMed] [Google Scholar]
- Kloepper JW, Ryu C-M, Zhang S. Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology. 2004;94(11):1259–1266. doi: 10.1094/PHYTO.2004.94.11.1259. [DOI] [PubMed] [Google Scholar]
- Koike ST, Gordon TR. Management of Fusarium wilt of strawberry. Crop Prot. 2015;73:67–72. [Google Scholar]
- Li H, Yuan G, Zhu C, Zhao T, Zhang R, Wang X, Yang J, Ma J, Zhang Y, Zhang X. Soil fumigation with ammonium bicarbonate or metam sodium under high temperature alleviates continuous cropping-induced Fusarium wilt in watermelon. Sci Hortic. 2019;246:979–986. [Google Scholar]
- Ling N, Xue C, Huang Q, Yang X, Xu Y, Shen Q. Development of a mode of application of bioorganic fertilizer for improving the biocontrol efficacy to Fusarium wilt. Biocontrol. 2010;55(5):673–683. [Google Scholar]
- Liu K, Garrett C, Fadamiro H, Kloepper JW. Induction of systemic resistance in Chinese cabbage against black rot by plant growth-promoting rhizobacteria. Biol Control. 2016;99:8–13. [Google Scholar]
- Lodemann U, Lorenz BM, Weyrauch KD, Martens H. Effects of Bacillus cereus var. toyoi as probiotic feed supplement on intestinal transport and barrier function in piglets. Arch Anim Nutr. 2008;62(2):87–106. doi: 10.1080/17450390801912068. [DOI] [PubMed] [Google Scholar]
- Lu R. Methods of soil and agricultural chemistry analysis. Beijing: Chinese Agricultural Science and Technology Press; 2000. pp. 1–627. [Google Scholar]
- Meier S, Curaqueo G, Khan N, Bolan N, Rilling J, Vidal C, Fernández N, Acuña J, González M-E, Cornejo P. Effects of biochar on copper immobilization and soil microbial communities in a metal-contaminated soil. J Soils Sediments. 2017;17(5):1237–1250. [Google Scholar]
- Nakkeeran S, Vinodkumar S, Renukadevi P, Rajamanickam S, Jogaiah S. Bioactive molecules from Bacillus spp.: an effective tool for plant stress management. In: Jogaiah S, Abdelrahman M, editors. Bioactive molecules in plant defense. Berlin: Springer; 2019. pp. 1–23. [Google Scholar]
- Ongena M, Jacques P. Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol. 2008;16(3):115–125. doi: 10.1016/j.tim.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Pieterse CM, Zamioudis C, Berendsen RL, Weller DM, Van Wees SC, Bakker PA. Induced systemic resistance by beneficial microbes. Annu Rev Phytopathol. 2014;52:347–375. doi: 10.1146/annurev-phyto-082712-102340. [DOI] [PubMed] [Google Scholar]
- Rebib H, Hedi A, Rousset M, Boudabous A, Limam F, Sadfi-Zouaoui N. Biological control of Fusarium foot rot of wheat using fengycin-producing Bacillus subtilis isolated from salty soil. Afr J Biotechnol. 2012;11(34):8464–8475. [Google Scholar]
- Sagar K, Singh SP, Goutam KK, Konwar BK. Assessment of five soil DNA extraction methods and a rapid laboratory-developed method for quality soil DNA extraction for 16S rDNA-based amplification and library construction. J Microbiol Methods. 2014;97:68–73. doi: 10.1016/j.mimet.2013.11.008. [DOI] [PubMed] [Google Scholar]
- Shen Z, Ruan Y, Chao X, Zhang J, Li R, Shen Q. Rhizosphere microbial community manipulated by 2 years of consecutive biofertilizer application associated with banana Fusarium wilt disease suppression. Biol Fertil Soils. 2015;51(5):553–562. [Google Scholar]
- Singhalage I, Seneviratne G, Madawala H, Wijepala P. Profitability of strawberry (Fragaria ananassa) production with biofilmed biofertilizer application. Sci Hortic. 2019;243:411–413. [Google Scholar]
- Tendulkar S, Saikumari Y, Patel V, Raghotama S, Munshi T, Balaram P, Chattoo B. Isolation, purification and characterization of an antifungal molecule produced by Bacillus licheniformis BC98, and its effect on phytopathogen Magnaporthe grisea. J Appl Microbiol. 2007;103(6):2331–2339. doi: 10.1111/j.1365-2672.2007.03501.x. [DOI] [PubMed] [Google Scholar]
- Wang H, Wen K, Zhao X, Wang X, Li A, Hong H. The inhibitory activity of endophytic Bacillus sp. strain CHM1 against plant pathogenic fungi and its plant growth-promoting effect. Crop Prot. 2009;28(8):634–639. [Google Scholar]
- Wu Y, Zhao C, Farmer J, Sun J. Effects of bio-organic fertilizer on pepper growth and Fusarium wilt biocontrol. Sci Hortic. 2015;193:114–120. [Google Scholar]
- Wu K, Fang Z, Wang L, Yuan S, Guo R, Shen B, Shen Q. Biological potential of bioorganic fertilizer fortified with bacterial antagonist for the control of tomato bacterial wilt and the promotion of crop yields. J Microbiol Biotechnol. 2016;26(10):1755–1764. doi: 10.4014/jmb.1604.04021. [DOI] [PubMed] [Google Scholar]
- Xiong W, Guo S, Jousset A, Zhao Q, Wu H, Li R, Kowalchuk GA, Shen Q. Bio-fertilizer application induces soil suppressiveness against Fusarium wilt disease by reshaping the soil microbiome. Soil Biol Biochem. 2017;114:238–247. [Google Scholar]
- Yuan L, Qi A, Cheng Y, Sagen G, Qu Y, Liu B. Fecal microbiota of three bactrian camels (Camelus ferus and Camelus bactrianus) in China by high throughout sequencing of the V3-V4 region of the 16S rRNA gene. J Arid Land. 2016;9(1):153–159. [Google Scholar]
- Zhang N, Wu K, He X, Li S, Zhang Z, Shen B, Yang X, Zhang R, Huang Q, Shen Q. A new bioorganic fertilizer can effectively control banana wilt by strong colonization with Bacillus subtilis N11. Plant Soil. 2011;344(1–2):87–97. [Google Scholar]
- Zhao Q, Dong C, Yang X, Mei X, Ran W, Shen Q, Xu Y. Biocontrol of Fusarium wilt disease for Cucumis melo melon using bio-organic fertilizer. Appl Soil Ecol. 2011;47(1):67–75. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.