Lung cancer screening is receiving increasing attention worldwide, both in the medical community and the public. There is now definitive evidence that low-dose CT (LDCT) screening can reduce lung cancer mortality, derived from multiple randomized trials including the US National Lung Screening Trial (NLST),1 the Multicentric Italian Lung Detection (MILD) trial,2 and preliminary results from the Dutch-Belgian NELSON trial.3
However, any screening program is associated with both benefits and harms, and accurately communicating these to patients and the public is a complex challenge. In the United States, despite a national recommendation, uptake of lung screening among eligible people is low. In the United Kingdom and throughout Europe, there has been debate in the research community about whether, when, and how screening should be implemented.4,5 Most recently, England’s National Health Service announced a planned rollout of LDCT screening at 10 sites. This prompted disagreement from commentators concerned about the harms of screening and ultimately to a discussion on television (BBC Newsnight).
Given the complexity of the debate, it is difficult for primary care providers to understand and explain the benefits and harms of screening to their patients. Multiple graphical tools have been developed and published to aid this conversation, each based on the NLST, while other bespoke graphics have been used in pilot studies. However, some of the published graphics can be misleading, and all represent outcomes based on the NLST protocol, which is now nearly 20 years old. Current protocols in the US (Lung-RADS v1.06) and the UK (British Thoracic Society7) differ from the NLST protocol in important ways. In particular, they categorize small pulmonary nodules as negative screens, which substantially reduces the number of false-positives and the subsequent need for additional scans and invasive procedures.
We engaged an international group of lung screening experts with the goal of assembling and providing accurate and balanced information on the benefits and harms of NLST-like LDCT screening. To reflect contemporary practice, we analyzed individual-level data from the NLST to represent outcomes that would have been observed if Lung-RADS had been used to manage LDCT findings. The NLST LDCT arm comprised 26,722 participants who were offered three annual screens, then followed for approximately 4 additional years. The Lung-RADS classification of NLST screens was done retrospectively and has been described previously.8 We defined Lung-RADS categories 1 and 2, which recommend return to annual screening, as normal (negative). Categories 3 (6-month follow-up), 4A (3-month follow-up), and 4B/4X (immediate follow-up) comprised an abnormal result.
We classified each NLST LDCT-arm participant into mutually exclusive groups: (1) all normal results by Lung-RADS and no lung cancer diagnosed, (2) one or more abnormal results by Lung-RADS and no lung cancer diagnosed, and (3) lung cancer cases, including those diagnosed after screening ended (through December 31, 2009). Among the group with an abnormal result but no lung cancer, we further identified participants who experienced the following harms as defined in the NLST: (a) an invasive procedure, (b) a major complication from an invasive procedure, and (c) death within 60 days of an invasive procedure from any cause (including those unrelated to the procedure).
Among participants with lung cancer, some represent overdiagnosis and some represent prevented lung cancer deaths, but we cannot know which particular participants fall into these groups. To estimate overdiagnosis, we applied the percentage of screen-detected lung cancer cases that were overdiagnosed in NLST (18.5%, relative to chest X-ray during the trial period)9 to the Lung-RADS screen-detected cases. To estimate lives saved, we took the difference in lung cancer deaths per 1000 between the NLST LDCT and chest X-ray arms, then reduced it by the relative reduction in sensitivity from Lung-RADS (13.3% lower vs. the NLST protocol for screen-detected cancers).8 The 13.3% reduction in sensitivity was consistent by lung cancer stage (data not shown), supporting this approximation.
We found that if Lung-RADS had been used in the NLST, 779 people per 1000 would have had all normal screen results by Lung-RADS and no lung cancer diagnosis. Another 180 would have had one or more abnormal results (“false-positives”) requiring a follow-up low-dose CT at 3 or 6 months, but no lung cancer diagnosis. Among these 180, 13 would require an invasive procedure to rule out lung cancer at some point during the trial, 0.4 (1 in 2500 screened) would have a major complication from an invasive procedure, and 0.2 (1 in 5000 screened) would die within 60 days of an invasive procedure from any cause. Finally, 41 per 1000 would be diagnosed with lung cancer, among whom approximately 4 cases represent overdiagnosis and 3 represent lung cancer deaths prevented because of screening.
We compiled these results into an infographic (Figure), along with a full-page version with explanatory text (https://www.iarc.fr/infographics/benefits-and-harms-of-lung-cancer-screening/). We did not attempt to quantify invasive procedures, complications, and deaths among people with lung cancer, but instead emphasize harms occurring in people with benign disease. For those with lung cancer, the full-page version states that most or all would require invasive procedures and treatments.
Figure:
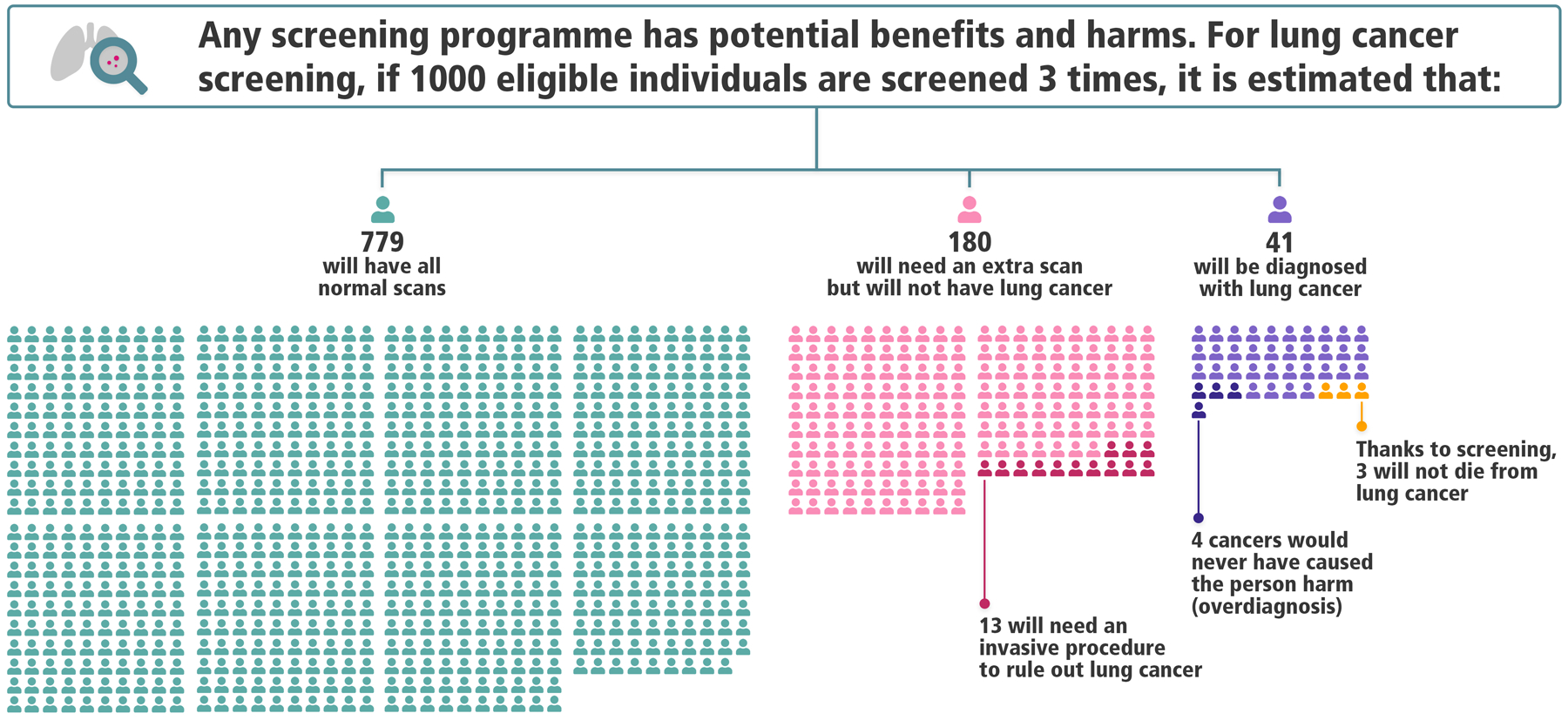
Infographic depicting estimated outcomes in the US National Lung Screening Trial under the Lung-RADS nodule management protocol
Reproduced with permission from the International Agency for Research on Cancer; full-page infographic available at https://www.iarc.fr/infographics/benefits-and-harms-of-lung-cancer-screening/
Our new infographic represents a contemporary interpretation of the findings of NLST using a modern protocol. However, our data will underestimate the lung cancer mortality benefit from continual LDCT screening, both because the NLST had only 3 annual screens and because the control arm used chest X-ray screening (which may slightly reduce lung cancer mortality). Preliminary results from the NELSON trial, which offered 4 screens over 5.5 years with a no-screening control arm, show a substantially larger relative benefit than the NLST.3 Further, benefits and harms of screening vary based on underlying lung cancer risk, even among eligible individuals.10 With these caveats, we hope that our infographic will facilitate improved communication about lung screening to providers, patients, and the public. It will be updated as protocols change and additional lung screening data become available.
Funding
This work was supported by the INTEGRAL project (NCI U19 CA203654).
We thank Dr. Paul Pinsky and Dr. Emily Hurley for their input on this work, and Morena Sarzo and Nicholas O’Connor for their assistance with graphic design. The authors alone are responsible for the views expressed in this article and they do not necessarily represent the views, decisions, or policies of the institutions with which they are affiliated.
Footnotes
Conflicts of Interest
CDB receives consulting fees from Medial EarlySign, LLC and GRAIL, Inc. All other authors report no conflicts of interest.
References
- 1.National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365(5):395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sabia F, Sestini S, Pastorino U, et al. Prolonged lung cancer screening reduced 10-year mortality in the MILD trial: New confirmation of lung cancer screening efficacy. 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The ASCO Post. WCLC 2018: NELSON Study: CT Screening for Early Lung Cancer Reduces Lung Cancer Mortality. 2018. [Google Scholar]
- 4.Oudkerk M, Devaraj A, Vliegenthart R, et al. European position statement on lung cancer screening. Lancet Oncol 2017;18(12):e754–66. [DOI] [PubMed] [Google Scholar]
- 5.Ruano-Ravina A, Pérez-Ríos M, Casàn-Clará P, Provencio-Pulla M. Low-dose CT for lung cancer screening. Lancet Oncol 2018;19(3):e131–2. [DOI] [PubMed] [Google Scholar]
- 6.American College of Radiology. Lung CT Screening Reporting and Data System (Lung-RADS). Accessed 2017 May 18 Available at https://www.acr.org/Quality-Safety/Resources/LungRADS.
- 7.Callister MEJ, Baldwin DR, Akram AR, et al. British Thoracic Society guidelines for the investigation and management of pulmonary nodules: accredited by NICE. Thorax 2015;70(Suppl 2):ii1 LP–ii54. [DOI] [PubMed] [Google Scholar]
- 8.Pinsky PF, Gierada DS, Black W, et al. Performance of Lung-RADS in the National Lung Screening Trial: a retrospective assessment. Ann Intern Med 2015;162(7):485–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patz EF, Pinsky P, Gatsonis C, et al. Overdiagnosis in low-dose computed tomography screening for lung cancer. JAMA Intern Med 2014;174(2):269–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kovalchik SA, Tammemagi M, Berg CD, et al. Targeting of low-dose CT screening according to the risk of lung-cancer death. N Engl J Med 2013;369(3):245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]


