Abstract
Phenolic composition and nutritional attributes of diaphragma juglandis fructus (Djf) and walnut shells (Ws) were investigated. Phenolic acids, hydroxybenzoic acid, isoflavone, and flavone were identified in the free phenolic fractions (FPFs) of both Djf and Ws. Bound phenolic fractions were less than FPFs both in content and diversity. The soluble dietary fiber contents of Djf and Ws were 25.56 g/100 g and 9.5 g/100 g, respectively. The contents of unsaturated fatty acids (1912.28 mg/kg and 9137.56 mg/kg, respectively) were significantly higher than that of saturated fatty acid both in Djf and Ws. The content of essential amino acids in Djf (9.67 mg/g) was significantly higher than that in Ws. More than eight types of monosaccharides were detected in Djf and Ws. The monosaccharide content of Djf (314.16 mg/g) was significantly higher than that of Ws (60.97 mg/g). Trehalose was the predominant component both in Djf (71.2%) and Ws (78.6%).
Electronic supplementary material
The online version of this article (10.1007/s10068-019-00655-z) contains supplementary material, which is available to authorized users.
Keywords: Diaphragma juglandis fructus, Walnut shells, Nutritional analysis, Phenolic acid fraction
Introduction
The walnut (Juglans regia L.) is a high economic value crop in the genus of walnuts with a long history of cultivation. Consumption of walnut kernels offers great benefits for humans such as the inhibition of arteriosclerosis, hypercholesterolaemia, and cardiovascular disease (Tsoukas et al., 2015), as well as the reduction of both oxidative stress and inflammation (Berryman et al., 2013). Thus, walnut kernels have been processed into many types of food via frying, sugar pickling, drying, and smashing. However, the parts of the walnut of diaphragma juglandis fructus (Djf) and walnut shells (Ws) are usually discarded as waste during walnut processing in food factories, because they mainly consist of undigestible substances, such as fiber and lignin. In the history of Chinese medicine, both Djf and Ws were used as effective materials to deal with kidney deficiency, spermatorrhea, enuresis, gonorrhea, hematuria, bring down, diarrhea, and insomnia (Jing et al., 2014). This indicates promising potential for the presence of functional food components in Djf and Ws. Wang et al. (2017) isolated 14 compounds from Djf via preparative high-performance liquid chromatography (pre-HPLC) and high-speed counter-current chromatography (HSCCC). The chemical structure of 7 compounds were identified by nuclear magnetic resonance (NMR) and electrospray ionization mass spectrometry (ESI–MS), including gallic acid, dihydrophaseic acid, blumenol B, ethyl gallate, (4S)-4-hydroxy-1-tetralone, (+)-dehydrovomifoliol, and (6R, 9R)-9-hydroxymegastigman-4-en-3-one. Meng et al. (2017) evaluated both the antioxidant and antibacterial activities of polysaccharides from Djf. The obtained results showed that Djf had remarkable hemolysis inhibitory activity. Pretreatment with Djf markedly weakened the oxidative damage induced by H2O2 in hepatic L02 cells via strengthening the cell viability. Djf also showed clear in vivo and in vitro hypoglycemic activities. Meng et al. (2018) further reported that Djf effectively suppressed the proliferation of HepG2 and BGC-82 cell lines. In addition, Djf significantly enhanced phagocytosis, stimulate the production of NO, tumor necrosis factor-α (TNF-α), and interleukins (IL-6 and IL-1β), and promote their corresponding mRNA expression levels in a dose-dependent manner. At present, only a number of phenolic acids and polysaccharides were reported in the analysis of Djf. However, the chemical composition of Ws has not been described.
Nutrient elements such as: unsaturated fatty acid, and phenolic compounds were the basic information for the development of functional food. Thus, the potential of Djf and Ws for functional food application still needs to be comprehensively understood. However, the nutritional composition and two groups of phenolic compounds in Djf and Ws have not been reported to date. Therefore, this study comparatively investigated the nutritional attributes of Djf and Ws, and identified monosaccharide and phenolic compounds in both Djf and Ws.
Materials and methods
Materials and chemicals
Ws and Djf were collected from an indigenous breed of walnut tree in Hezhang, Guizhou Province, China. The samples were firstly dried under 35 °C in an oven and then crushed to a powder with a grinder (800Y, Boou co., LTD, Yongkang, Zhejiang, China). The crushed powders were sieved through a 100-mesh screen, and stored at 25 °C in a dry environment until analysis. As mobile phase for phenol substance analysis, formic acid and acetonitrile (HPLC grade) were chosen, which were purchased from Kermel (Tianjin, China). Neutral protease (100 U/mg), amyloglucosidase (100,000 U/mL), and α-amylase (10,000 U/mL) were purchased from Yuanye biology (Shanghai, China). 99% Morpholine taurine (MES) and 1-phenyl-3-methyl-5-pyrazolone (PMP) were purchased from Macklin (Shanghai, China). Dihydroquercetin, gallate, mannose, rhamnose, ribose, glucuronic acid, mycose, galacturonic acid, xylose, galactose, and arabinose (≥ 98%) were commercially purchased from Beijing Solarbio (Beijing, China). All other chemicals in this study were of analytical grade.
Proximate composition analysis
Ash, moisture, crude protein, and crude lipid were analyzed by the methods of AOAC (1995). Insoluble dietary fiber (IDF), soluble dietary fiber (SDF), and total dietary fiber (TDF) contents were analyzed by the method of Asp et al. (1983). Reducing and total sugars were analyzed by the 3,5-dinitrosalicylic acid (DNS) method (Saurav et al., 2012). The analyses of total phenolic compounds in the samples were conducted, following the folin–ciocalteu method used before (Conde-Hernandez and Guerrero-Beltran, 2014).
Mineral elements and metals analysis
Eleven elements include Na, K, Fe, Mg, Ca, Zn, Cu, Mn, Se, Hg, and As were analyzed with AOAC methods (1995) by using an inductively coupled plasma-mass spectrometer (ICP-MS) (Plasma Quant MS, Analytik Jena AG, Jena, Germany).
Fatty acid analysis
Samples (100 mg) were extracted with 2 mL of 2% sodium hydroxide-methanol solution and 100 μL of methyl 19 alkanate was added as interior label. This was followed by transmethylation with 3 mL 14% boron trifluoride methanol solution. The obtained supernatant was taken after 1 mL n-hexane was added to the centrifuge tube and shaken for 2 min before extraction. The volume was filled to 1 mL with n-hexane. Then the mixture was passed through a 0.22 μm membrane. The extracted lipid was subjected to fatty acid composition analysis with a GC/MS instrument (Trace1310 ISQ, ThermoFisher, Waltham, MA, USA) with a TG-5MS (30 m × 0.25 mm × 0.25 μm) chromatographic column (Li et al., 2012).
Amino acid analysis
Samples were hydrolyzed by 6 M hydrochloric acid solution at 110 °C for 22–24 h. Then, samples were deacidized and drained in vacuum and fully dissolved with 0.02 mol/L HCl solution, placed at room temperature for 1 h with 1.0 M triethylamine-acetonitrile solution and 0.1 M phenyl isothiocyanate acetonitrile solution, extracted by n-hexane, and filtered with a 0.22 μm nylon membrane. The extracted lipid was then subjected to amino acid composition analysis with a HPLC instrument (1260, Agilent, Palo Alto, CA, USA) with a C18 (4.6 × 250 mm × 5 μm) (Shiseido, Tokyo, Japan) chromatographic column (Anne et al., 2018).
HPLC analysis of monosaccharide compounds
Monosaccharide compounds including mannose, rhamnose, ribose, glucuronic acid, mycose, galacturonic acid, xylose, galactose, and arabinose were determined by using a HPLC instrument (1290, Agilent) equipped with a diode array detector. The sample processing was identical to the method of Schadel et al. (2010) and the derivatization procedure was conducted with 1-phenyl-3-methyl-5-pyrazolone (PMP).
The derivatives were separated on a Thermo BDS C18 column (250 × 4.6 mm i.d., 5 µm). The mobile phase consisted of A and B eluents. Eluent A is a mixture of 15% acetonitrile with 0.05 M phosphate buffer solution (KH2PO4-NaOH, pH 7.1). Eluent B is a mixture of 40% acetonitrile with 0.05 M phosphate buffer solution (KH2PO4–NaOH, pH 7.1). The gradient conditions were as follows: 0 min, 100% A; 0–10 min, 100–90% A; 10–40 min, 90–70% A; 40–45 min, 70–100% A. The eluate was monitored at 250 nm, the flow rate was 1.0 mL min−1, the injection volume was 10 μL, and the column was maintained at 30 °C. Through plotting the ratio of the peak areas of sugar to the internal standard against the sugar concentration, the linearity of each calibration curve could be confirmed. The concentrations of unknown samples were calculated via standard curves.
Free and bound phenolic compound preparation
Extraction of free phenolic compounds was performed as follows (Okarter et al., 2010): the sample (1 g) and 50 mL of 80% chilled acetone were simultaneously added into a 150 mL conical flask, and sealed immediately. Then, the conical flask was fixed to a shaking table at 150 rpm for 20 min for phenolic compound extraction. After shaking, the solution was centrifuged at 2500 g for 10 min. Supernatants were collected and concentrated to less than 10 mL by evaporation at 45 °C. Then, acetonitrile was added to the concentrate, filling it to 25 mL. The mixture was then filtered it with a 0.22 μm nylon membrane filter and stored at − 20 °C.
Extraction of bound phenolic compounds was performed as follows: the residue after extraction of free phenolic compound and 40 mL of 1.2 N HCl-methanol were simultaneously added to a 150 mL conical flask, which was immediately sealed. Then, the conical flask was fixed to a shaking table at 150 rpm and 35 °C for 24 h. After shaking, the solution was filtered through a filter paper and the filtrate was evaporated at 45 °C until complete dryness. 25 mL acetonitrile was added to the tube to redissolve the bound phenolic compounds. Prior to HPLC analysis, the solution was filtered with a 0.22 μm nylon membrane filter and stored at − 20 °C.
HPLC analysis of phenolic compounds
The instrument conditions were identical to the method of monosaccharide compounds. The HPLC conditions were set as follows: column temperature of 40 °C, sample injection volume of 10 μL, flow rate of 0.8 mL/min, mobile phase A (0.2% formic acid), and mobile phase B (100% acetonitrile). The gradient profile was performed as follows: 0 min, 10% B; 0–10 min, 10–15% B; 10–15 min, 15–20% B; 15–25 min, 30% B. Phenolic compounds were monitored at 300 nm. Retention times of internal standards were gallate, 3.32 min and dihydroquercetin, 15.91 min.
HPLC–DAD/ESI–MS analysis of phenolic compound extracts
Phenolic compound extracts were identified by an UHPLC (Accela 1250, Thermo Fisher, Waltham, MA, USA) equipped with a diode array detector, and coupled to an TSQ quantum ultra-triple-quadrupole mass spectrometer. The HPLC was performed as described above. A T-type phase separator was used for the effluent of the HPLC column before flowing into the mass spectrometer (MS) (split ratio = 1:3). The electrospray source of the MS was performed at negative mode. The MS parameters were as follows: vaporizer temperature of 500 °C, drying gas (N2) flow rate of 12.0 L/min, capillary temperature of 350 °C, and spray voltage of 2500 V. The m/z of mass spectra results were collected from 100 to 1200. Data were analyzed via LC–MS Xcalibur workstation software (Version 2.6, Thermo Fisher Scientific).
Statistical analysis
All assays were performed in triplicate. Statistical analyses were carried out using the SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). One-way analysis of variance (one-way ANOVA) was applied to assess the statistically significant differences of data. Significant differences among various treatments were set at p < 0.05 according to Duncan’s multiple range tests.
Results and discussion
Proximate chemical composition
The proximate chemical composition of both Djf and Ws are shown in Table 1. The main component of both Djf and Ws was dietary fiber, consisting of less soluble dietary fiber and much insoluble dietary fiber. Djf showed a significantly higher content of SDF than Ws (p < 0.05), which was similar to gracilaria (23.8 g/100 g dw) (Sanz-Pintos et al., 2017), and higher than oat bran (8.9 g/100 g dw) (Zhang et al., 2011) and soybean residue (2.5 g/100 g dw) (Jing and Chi, 2013). The consumption of SDF was associated with reductions of LDL-cholesterol in plasma, attenuating glycemic and insulin response, and reducing the risk of chronic disease (Tungland and Meyer, 2002). Thus, the higher amount of SDF in Djf suggests it as a good food material to enhance human health. The content of crude protein, crude ash, and total sugar in Djf and Ws did not show significant differences. Except for the content of crude lipid, Djf showed significantly higher content of reducing sugar and total phenolic compounds (both free and bound phenolic compounds) than that of Ws (p < 0.05).
Table 1.
Proximate chemical composition (g/100 g dw, except moisture g/100 g ww), minerals and metals (mg/kg dw) content of diaphragma juglandis fructus and walnut shell
| Component | Diaphragma juglandis fructus | Walnut shell |
|---|---|---|
| Moisture | 14.75 ± 0.31a | 10.69 ± 0.71 |
| Crude ash | 2.68 ± 0.08 | 2.51 ± 0.06 |
| Crude protein | 2.02 ± 0.11 | 1.97 ± 0.07 |
| Crude lipid | 2.49 ± 0.36 | 4.13 ± 0.55a |
| Reducing sugar | 0.084 ± 0.001a | 0.044 ± 0.001 |
| Total sugar | 0.134 ± 0.002 | 0.154 ± 0.001 |
| Soluble dietary fiber | 25.56 ± 0.92a | 9.50 ± 0.26 |
| Insoluble dietary fiber | 48.21 ± 0.56 | 57.74 ± 0.71a |
| Total dietary fiber | 73.66 ± 0.73a | 67.28 ± 0.57 |
| Free phenolic compounds | 3.22 ± 0.02a | 1.63 ± 0.01 |
| Bound phenolic compounds | 1.74 ± 0.01a | 1.08 ± 0.01 |
| Total phenolic compounds | 4.84 ± 0.01a | 2.82 ± 0.01 |
| K | 8362.87 ± 21.90 | 8479.53 ± 30.33 |
| Na | 479.17 ± 6.29 | 774.10 ± 14.37a |
| Ca | 3526.37 ± 15.71a | 2378.23 ± 15.06 |
| Mg | 636.80 ± 12.94a | 536.97 ± 10.13 |
| Fe | 36.93 ± 0.90 | 54.33 ± 0.78a |
| Cu | 3.78 ± 0.16 | 4.12 ± 0.17 |
| Zn | 4.93 ± 0.11 | 6.74 ± 0.17a |
| Mn | 14.70 ± 0.10 | 25.47 ± 0.69a |
| Se | 0.049 ± 0.002 | ND |
| Hg/Pb | ND | ND |
aA value that is significantly greater (p < 0.05) than its paired value is denoted with an “a”. Paired results with no letter have no significant difference
Mineral elements and metals
The results of mineral element and metal analyses of both Djf and Ws are shown in Table 1. The content of K in Djf and Ws was highest, reaching more than 8360 mg/kg. Furthermore, both were rich in alkali metal Na, which plays an important role in maintaining the electrolyte balance of the human body. Ws contained more heavy metals (Fe, Mn, and Zn) than Djf (p < 0.05). In contrast, Djf was rich in Ca and Mg (p < 0.05). The contents of these elements were lower than those reported for tea and herbal tea (Martín-Domingoa et al., 2017), but showed a rich variety, indicating potential use as raw materials for the development of alternative tea products. Djf and Ws had higher levels of Fe than Turkish walnuts (Kalkisim et al., 2014) and other nuts (Cristopher and Rosario, 2018) that have been reported, indicating that it could be a potential source of Fe in the human body. Djf contained low levels of Se, which were not detected in Ws. Due to the high elemental content in Djf and Ws, this can be used as a potential raw material source for energy drinks or other food development.
Fatty acids
The fatty acids of Djf and Ws are shown in Table 2. Twenty-six fatty acids were identified in Djf and Ws, with thirteen saturated fatty acids and thirteen unsaturated fatty acids. The saturated and unsaturated fatty acids in Ws were significantly higher than those in Djf (p < 0.05). Palmitic acid had the highest content of saturated fatty acids, which is commonly used as natural emulsifier in food and cosmetics. It was reported that palmitic acid could reduce the content of cholesterol in serum and played a preventive and therapeutic role in atherosclerosis and other thrombotic diseases (Wu et al., 2017). The unsaturated fatty acids in Djf and Ws accounted for 63.5% and 78.8% respectively. Linoleic acid and oleic acid were dominant; linoleic acid belongs to the ω-6 series, which was recognized as an essential nutrient with good oxidation stability that reduces the content of low density lipoprotein cholesterol in human blood, while maintaining the level of high density lipoprotein cholesterol, which was known as “safety fatty acids” (Jiang and Jiang, 2018). Cholesterol must be combined with linoleic acid for normal operation and metabolism in the body. In contrast, cholesterol can be combined with saturated fatty acids if linoleic acid was missing, thus causing metabolic disorders, gradually leading to atherosclerosis with the combo deposited on the vascular wall, and ultimately leading to cardiovascular and cerebrovascular diseases (Ermelinda et al., 2018). The efficacy of Djf in protecting the cardiovascular system and other aspects was likely related to the rich content of linoleic acid (Liu et al., 2015). Djf and Ws also contained small amounts of polyunsaturated fatty acids such as eicosapentaenoic acid (EPA), arachidonic acid (AA), and nervonic acid, which has been used as healthy food ingredient in fortified food.
Table 2.
Fatty acid content of diaphragma juglandis fructus and walnut shell (mg/kg dw)
| Component | Diaphragma juglandis fructus | Walnut shell |
|---|---|---|
| Octanoic acid (C8:0) | 1.37 ± 0.05a | 0.85 ± 0.04 |
| Decylic acid (C10:0) | 1.86 ± 0.02a | 0.53 ± 0.02 |
| Lauric acid (C12:0) | 5.33 ± 0.10a | 2.32 ± 0.03 |
| Myristic acid (C14:0) | 11.62 ± 0.20a | 7.42 ± 0.18 |
| Pentadecanoic acid (C15:0) | 22.24 ± 0.26 | 22.1 ± 0.19 |
| Palmitic acid (C16:0) | 700.55 ± 8.80 | 1778.28 ± 36.38a |
| Margaric acid (C17:0) | 28.95 ± 0.16 | 30.48 ± 0.42 |
| Stearic acid (C18:0) | 143.96 ± 4.02 | 483.74 ± 20.70a |
| Arachidic acid (C20:0) | 18.98 ± 0.27 | 22.71 ± 0.05a |
| Heneicosanoic acid (C21:0) | 18.66 ± 0.06 | 16.02 ± 0.17 |
| Behenic acid (C22:0) | 50.79 ± 0.31a | 36.53 ± 0.04 |
| Tricosanoic acid (C23:0) | 44.91 ± 0.28a | 27.72 ± 0.07 |
| Lignoceric acid (C24:0) | 49.89 ± 0.24a | 31.16 ± 0.19 |
| Total saturated | 1099.10 ± 12.80 | 2459.86 ± 16.69a |
| Palmitoleic acid (C16:1) | 17.45 ± 0.24 | 47.71 ± 0.77a |
| cis-10-heptadecenoic acid (C17:1) | 7.51 ± 0.14 | 10.17 ± 0.11a |
| Oleic acid (C18:1N9C) | 549.92 ± 18.98 | 2001.72 ± 26.12a |
| Linoleic acid (C18:2N6C) | 1314.06 ± 10.71 | 7041.75 ± 56.54a |
| γ-linolenic acid (C18:3N6) | 5.39 ± 0.09 | 5.49 ± 0.18 |
| cis-11-eicosenoic acid (C20:1) | 5.47 ± 0.16 | 15.57 ± 0.42a |
| cis-11,14-eicosadienoic acid (C20:2) | 3.90 ± 0.15 | 5.33 ± 0.12a |
| cis-8,11,14-epoxyeicosatrienoic acids (C20:3N6) | 0.77 ± 0.05 | 0.90 ± 0.06 |
| Arachidonic acid (C20:4N6) | 1.06 ± 0.05 | 1.29 ± 0.04 |
| cis-5,8,11,14,17-timnodonic acid (C20:5N3) | 2.20 ± 0.08 | 4.63 ± 0.06a |
| Erucic acid (C22:1N9) | 1.82 ± 0.06 | 1.91 ± 0.07 |
| cis-13,16-docosadienoic acid (C22:2) | 1.36 ± 0.09a | 0.52 ± 0.04 |
| Nervonic acid (C24:1) | 1.36 ± 0.11a | 0.57 ± 0.03 |
| Total unsaturated | 1912.28 ± 30.46 | 9137.56 ± 28.84a |
aA value that is significantly greater (p < 0.05) than its paired value is denoted with an “a”. Paired results with no letter have no significant difference
Amino acids
The amino acid compositions of both Djf and Ws are shown in Table 3. Six essential amino acids (EAA) and 11 non-essential amino acids (NEAA) were detected in both Djf and Ws, which was lower than that in walnut kernel (Mao et al., 2014). EAA and NEAA contents in Djf were 9.67 mg/g and 19.85 mg/g, respectively, which were significantly higher than those in Ws (p < 0.05). The highest concentrations of EAA in Djf and Ws were lysine (2.41 mg/g dw) and leucine (1.53 mg/g dw), respectively. Lysine can improve the body’s absorption and utilization of food protein, thus promoting growth and enhance immunity and other functions. Leucine also regulates protein synthesis and catabolism of the body, controls blood glucose level, provides energy, and increases the production of growth hormone to burn visceral fat (FAO/WHO, 1991). The highest content of NEAA in Djf and Ws was glutamic acid, with contents of 3.86 mg/g and 3.05 mg/g, respectively. Glutamic acid is an important excitatory neurotransmitter, participates in protein and sugar metabolism in the brain, promotes oxidation process, can be combined with ammonia in the body into non-toxic glutamine, which reduces blood ammonia levels, and repairs wounds (Zhang et al., 2018). EAA accounted for 32.76% and 22.65% of total amino acid (TAA) in Djf and Ws, respectively, and EAA/NEAA were 48.79% and 36.33%, respectively. The EAA content in Djf was significantly higher than that in Ws. The ideal protein standard for the human body proposed by FAO/WHO was about 40% of EAA/TAA and 60% of EAA/NEAA (FAO/WHO, 1991). From this point of view, the protein in Djf was more balanced, with a certain protein supplement function.
Table 3.
Amino acid content of diaphragma juglandis fructus and walnut shell (mg/g dw)
| Amino acids | Diaphragma juglandis fructus | Walnut shell |
|---|---|---|
| Lysine (Lys) | 2.41 ± 0.02a | 0.13 ± 0.01 |
| Phenylalanine (Phe) | 1.23 ± 0.02a | 0.90 ± 0.03 |
| Threonine (Thr) | 1.04 ± 0.03 | 0.85 ± 0.03 |
| Isoleucine (Ile) | 1.25 ± 0.04a | 0.87 ± 0.02 |
| Leucine (Leu) | 2.09 ± 0.07a | 1.53 ± 0.04 |
| Valine (Val) | 1.66 ± 0.04a | 1.14 ± 0.02 |
| Total essential amino acids | 9.67 ± 0.10a | 5.42 ± 0.06 |
| Aspartic acid (Asp) | 0.59 ± 0.03 | 0.56 ± 0.03 |
| Serine (Ser) | 1.61 ± 0.03a | 1.24 ± 0.03 |
| Glutamate (Glu) | 3.86 ± 0.02a | 3.05 ± 0.04 |
| Glycine (Gly) | 2.10 ± 0.05a | 1.33 ± 0.04 |
| Alanine (Ala) | 1.56 ± 0.02a | 1.12 ± 0.05 |
| Cystine (Cys) | 2.15 ± 0.04 | 2.07 ± 0.04 |
| Tyrosine (Tyr) | 0.88 ± 0.04a | 0.49 ± 0.01 |
| Proline (Pro) | 2.40 ± 0.02a | 1.30 ± 0.03 |
| Arginine (Arg) | 1.13 ± 0.03 | 1.55 ± 0.04a |
| Histidine (His) | 2.61 ± 0.04a | 1.07 ± 0.02 |
| Methionine(Met) | 0.98 ± 0.04 | 1.13 ± 0.05 |
| Total nonessential anmino acids | 19.85 ± 0.11a | 14.92 ± 0.13 |
| Total amino acids | 29.52 ± 0.15a | 20.34 ± 0.11 |
aA value that is significantly greater (p < 0.05) than its paired value is denoted with an “a”. Paired results with no letter have no significant difference
The scores of EAA to the reference protein of FAO/WHO (1991) are shown in supplementary source 1. The obtained amino acid score indicates the ratio of the amino acid in 1 g of the sample protein to that in 1 g of the standard protein. Threonine and lysine were the lowest in the Djf and Ws, respectively, which is the first limiting amino acid. The closer the amino acid score is to 1, the closer the amino acid composition of the food protein is to the FAO/WHO recommended protein amino acids. Most amino acid scores of Djf and Ws were closer to l, indicating that their essential amino acid composition was reasonable. The amino acid score of Djf was higher than that of Ws except for methionine + cystine, indicating that the amino acid nutrition of the Djf was more balanced.
Identification of monosaccharide compounds
The HPLC chromatograms of monosaccharide compounds in Djf and Ws are shown in supplementary source 2. The samples were compared to standard monosaccharide samples. The monosaccharide components were qualitatively and quantitatively analyzed via retention time and peak area, respectively. The content of monosaccharide compounds in Djf and Ws are shown in Table 4. Nine types of monosaccharide compounds were detected in Djf and eight in Ws. Significantly more monosaccharide compounds were found in Djf than in Ws (p < 0.05). The trehalose content in Djf (223.76 mg/g) was significantly higher than that in Ws, and was close to that reported for Cordyceps militaris (247.1 mg/g) (Reis et al., 2013). Trehalose can be used as a stabilizer for blood products, vaccines, and other biologically active substances in medicine, and can also effectively protect the epidermal cell membrane structure and keep skin healthy (Sciarretta et al., 2018). The contents of xylose, mannose, arabinose, and galactose in Djf were significantly higher than in Ws (p < 0.05), which was consistent with the report of Meng et al. (2017) in Djf. These results indicate that hemicellulose is abundant in Djf and Ws, which providing an important alternate renewable energy resource. The determination of monosaccharide components in Djf and Ws provides a theoretical basis for future research and development.
Table 4.
Retention times and indentification of monosaccharide compounds extracted from diaphragma juglandis fructus and walnut shell (mg/g dw)
| Peak | Retention time (min) | Identity | Diaphragma juglandis fructus | Walnut shell |
|---|---|---|---|---|
| 1 | 11.873 | Mannose | 11.45 ± 0.52a | 1.94 ± 0.04 |
| 2 | 14.009 | Rhamnose | 8.99 ± 0.41a | 0.97 ± 0.06 |
| 3 | 14.487 | Ribose | 2.99 ± 0.27a | 1.98 ± 0.06 |
| 4 | 15.331 | Glucuronic acid | 3.09 ± 0.09 | ND |
| 5 | 16.763 | Trehalose | 223.76 ± 10.01a | 47.91 ± 3.75 |
| 6 | 19.326 | Galacturonic acid | 8.18 ± 0.05a | 0.95 ± 0.01 |
| 7 | 20.787 | Xylose | 44.79 ± 2.08a | 3.77 ± 0.26 |
| 8 | 23.669 | Galactose | 2.77 ± 0.02a | 0.58 ± 0.10 |
| 9 | 28.478 | Arabinose | 8.11 ± 1.57a | 2.87 ± 0.54 |
| Total content | 314.16 ± 10.75a | 60.97 ± 3.86 |
aA value that is significantly greater (p < 0.05) than its paired value is denoted with an “a”. Paired results with no letter have no significant difference
Identification of phenolic compounds
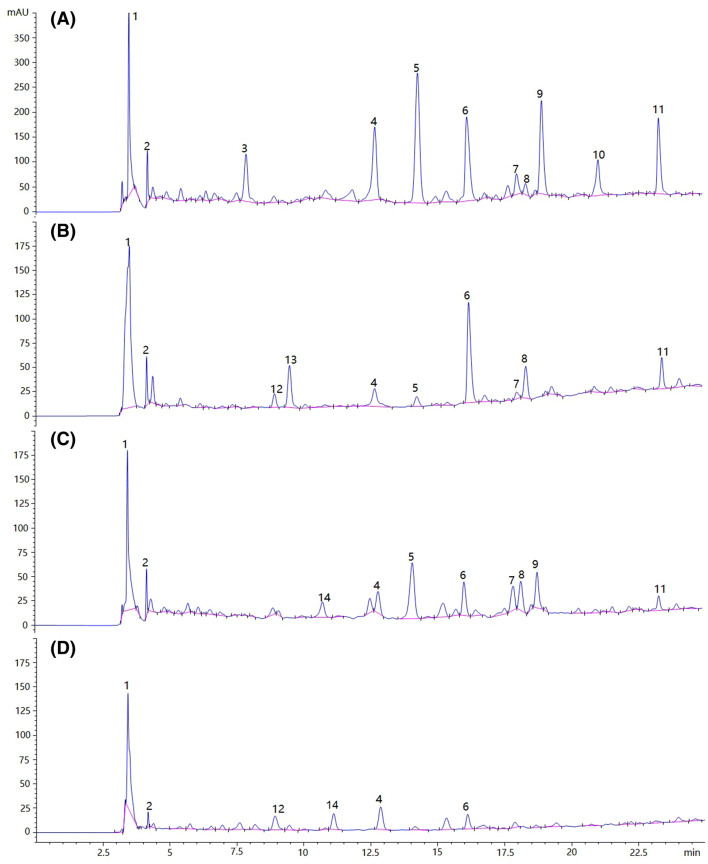
The chromatograms of two groups of phenolic compounds in Djf and Ws are shown in Fig. 1A–D. All peaks of phenolic compounds were efficiently separated and appeared in a range of 2.5–25 min. Most phenolic compounds showed maximum UV absorbance at 280–330 nm (Liang et al., 2013). The detection wavelength was set to 300 nm by scanning the whole wavelength and comparing the peak under different wavelength conditions. Eleven and 10 peaks were identified as phenolic compounds (Fig. 1A) in both the free phenolic compounds (FPC) and bound phenolic compounds (BPC) extraction of Djf, respectively. All identified phenolic compounds (peak 1–13) are listed in Table 5. Phenolic compounds in FPC and BPC showed a similar result with eight types of phenolic compounds that existed in both Djf and Ws. However, a small difference was also found in the results (compound 3, 9, and 10 only existed in the FPC extraction of Djf, while compounds 12 and 13 only existed in the BPC extraction of Ws). Ten and six peaks were identified as phenolic compounds (Fig. 1B) in the FPC and BPC extraction of Ws, respectively. In contrast to the results of Djf, only five phenolic compounds were found in the FPC and BPC extraction of Ws. The new phenolic compounds (peak 14) are listed in Table 4. Compounds 7, 8, 9, and 11 were only found in the FPC extraction of Ws, while compound 14 was only found in the BPC extraction of Ws. Hilbig et al. (2018) reported 29 phenolic compounds in pecan nut shells, 24 of which had not been reported in the literature for this raw material before. The difference in the number of species may depend on the sample. Vu et al. (2018) identified 16 phenolic compounds in black walnut kernels and Milena et al. (2017) found 15 phenolic compounds in hazelnut shell. Therefore, the phenolic compounds in Djf and Ws are an innovative source of phenolic compounds and offer potential use in pharmaceutical and food industries.
Fig. 1.
High-performance liquid chromatograms of (A) free phenolic compounds of diaphragma juglandis fructus; (B) bound phenolic compounds of diaphragma juglandis fructus; (C) free phenolic compounds of walnut shell; and d bound phenolic compounds of walnut shell. The numbers of the peaks in this figure coincide with the compound numbers in Table 5
Table 5.
Retention Times, Mass Spectrometic Data, and Indentification of Phenolic Compounds Extracted from Djf and Ws
| Peak | Retention time (min) | Identity | [M-H]− m/z | Refs. |
|---|---|---|---|---|
| 1 | 3.417 | Gallic acid | 169.12 | Yang et al. (2012) |
| 2 | 4.172 | Phthalic acid | 164.82 | Jing et al. (2015) |
| 3 | 7.846 | Catechin | 289.11 | Jing et al. (2015) |
| 4 | 12.862 | Vanillin | 151.27 | Yang et al. (2012) |
| 5 | 14.107 | Ethyl gallate | 197.14 | Jing et al. (2015) |
| 6 | 16.113 | Dihydroquercetin | 303.05 | Jing et al. (2015) |
| 7/8 | 17.873/18.162 | Kaempferol | 285.09 | Olabiyi et al. (2018) |
| 9 | 18.769 | Taxifolin-3-O-α-l-arabinofuranoside | 435.22 | Wang et al. (2017) |
| 10 | 20.995 | Quercetin-3-rhamnoside | 447.19 | Wang et al. (2017) |
| 11 | 23.314 | Quercetin-3-O-(4″-O-acetyl)-α-l-rhamnopyranoside | 489.14 | Wang et al. (2017) |
| 12 | 8.914 | Blumenol B | 225.21 | Wang et al. (2017) |
| 13 | 9.476 | Propyl gallate | 211.34 | Emira et al. (2012) |
| 14 | 11.107 | Vanillic acid | 167.08 | Emira et al. (2012) |
The LC–MS data of phenolic compounds are shown in Table 5. Compound 1 (gallic acid) and compound 6 (dihydroquercetin) were identified by contrasting their retention times to the corresponding standards. At the same time, the quasi-molecular ion ([M–H]− m/z 169.12) of compound 1 was consistent with the findings of Yang et al. (2012). The [M–H]− quasi-molecular ion of compound 6 at m/z 301.04/303.05 were similar to quercetin and dihydroquercetin. However, the retention time of quercetin was not consistent with the peaks that exhibited an [M–H]− ion at m/z 301.04. Therefore, compound 6 could not be identified as quercetin. Compound 6 was tentatively considered as dihydroquercetin based on previous findings (Jing et al., 2015).
Compounds 2 and 3 were observed at 4.172 and 7.846 min, and showed an [M–H]− ion at m/z 164.82 and 289.11, respectively. These were tentatively considered as phthalic acid and catechin based on previous findings (Jing et al., 2015). Compounds 4 and 5 were observed at 12.862 and 14.107 min, showing an [M–H]− ion at m/z 151.27 and 197.14, respectively. These were tentatively considered as vanillin and ethyl gallate (Jing et al., 2015).
Compounds 7 and 8 were observed at 17.873 and 18.162 min respectively, but they all showed an [M–H]− ion at m/z 285.09. These were tentatively considered as kaempferol (Olabiyi et al., 2018). Compounds 9, 10, and 11 observed at 18.769, 20.995, and 23.314 min, showed [M–H]− ions at m/z 435.22, 447.19, and 489.14, and were tentatively considered as taxifolin-3-O-α-l-arabinofuranoside, quercetin-3-rhamnoside, and quercetin-3-O-(4″-O-acetyl)-α-l-rhamnopyranoside, respectively (Wang et al., 2017).
Compounds 12 and 13 were bound phenolic compounds in the samples, which were observed at 8.914 and 9.476 min, showing an [M–H]− ion at m/z 225.21 and 211.34, and were tentatively considered as blumenol B and propyl gallate, respectively (Wang et al., 2017). Compound 14 was only detected in Ws, at 11.107 min, showing an [M–H]− ion at m/z 167.08, and was tentatively considered as vanillic acid (Emira et al., 2012).
In summary, the main phenolic compounds in Djf and Ws existed in their free state, with an abundant species. Both Djf and Ws could be utilized for the development of health beneficial functional foods, e.g., as phenolic antioxidants, for regulating the gut eco-environment, weight loss, and as a Fe supplement. It is also interesting to develop a beverage incorporated with micronized Djf and Ws. Further research will address the effect of micronization technology on the chemical composition of Djf and Ws.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This study was supported by “national natural science foundation of China (31760463); construction project of innovative talents base of Guizhou provence ([2016] 22)”. The authors would like to express sincere gratitude to the Food Processing Institute of the Guizhou Academy of Agricultural Sciences, Potato Engineering Research Center of Guizhou Province, Agricultural Product Processing Institute of the Guizhou Academy of Agricultural Sciences for the financial support of this research. In particular, the authors would like to extend their gratitude to the Guizhou Institute of Walunt, for providing the walunt material.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Qiang Hu, Email: huqiang@foods.ac.cn.
Jia Liu, Email: mcgrady456@163.com.
Jun Li, Email: lijunsjs2015@163.com.
Hui Liu, Email: wtl505@126.com.
Nan Dong, Email: 460288583@qq.com.
Yang-yang Geng, Email: yygengfood@sina.cn.
Yang Lu, Email: 499528997@qq.com.
Yan Wang, Email: 529046517@qq.com.
References
- Anne L, Martin K, Oliver L. Improved HPLC-method for estimation and correction of amino acid losses during hydrolysis of unknown samples. Anal. Biochem. 2018;543:140–145. doi: 10.1016/j.ab.2017.12.009. [DOI] [PubMed] [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International. 16th ed. Method 996. AOAC International, Gaithersburg, MD, USA (1995)
- Asp NG, Johansson CG, Hallmer H, Siljestrom M. Rapid enzymatic assay of insoluble and soluble dietary fiber. J. Agric. Food Chem. 1983;31:476–482. doi: 10.1021/jf00117a003. [DOI] [PubMed] [Google Scholar]
- Berryman CE, Grieger JA, West SG, Chen CYO, Blumberg JB, Rothblat GH, Sankaranarayanan S, Kris-Etherton PM. Acute consumption of walnuts and walnut components differentially affect postprandial lipemia endothelial function, oxidative stress, and cholesterol efflux in humans with mild hypercholesterolemia. J. Nutr. 2013;143:788–794. doi: 10.3945/jn.112.170993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde-Hernandez LA, Guerrero-Beltran JA. Total phenolics and antioxidant activity of Piper auritum and Porophyllum ruderale. Food Chem. 2014;142:455–460. doi: 10.1016/j.foodchem.2013.07.078. [DOI] [PubMed] [Google Scholar]
- Cristopher GM, Rosario SS. Philippine Pili (Canarium ovatum. Engl.) varieties as source of essential minerals and trace elements in human nutrition. J. Food Compos. Anal. 2018;69:53–61. doi: 10.1016/j.jfca.2018.02.008. [DOI] [Google Scholar]
- Emira N, Mejdi S, Najla T, Riadh K, Gaith H, Lamjed B, Amina B. Antioxidant activities and reversed phase-high performance liquid chromatography (RP-HPLC) identification of polyphenols in the ethyl acetate extract of Tunisian Juglans regia L. treated barks. J. Med. Plants Res. 2012;6:1468–1475. [Google Scholar]
- Ermelinda P, Francesca B, Isabella P, Loredana P, Maeve K, Giovanni F. Bioactive fatty acids of three commercial scallop species. Int. J. Food Prop. 2018;21:519–532. doi: 10.1080/10942912.2018.1425703. [DOI] [Google Scholar]
- FAO/WHO . Protein Quality Evaluation. Rome: Food and Agriculture Organization of the United Nations; 1991. p. 66. [Google Scholar]
- Hilbig J, Alves VR, Müller CMO, Micke GA, Vitali L, Pedrosa RC, Block JM. Ultrasonic-assisted extraction combined with sample preparation and analysis using LC-ESI-MS/MS allowed the identification of 24 new phenolic compounds in pecan nut shell [Carya illinoinensis (Wangenh) C. Koch] extracts. Food Res. Int. 2018;106:549–557. doi: 10.1016/j.foodres.2018.01.010. [DOI] [PubMed] [Google Scholar]
- Jiang XL, Jiang YH. Ultrasonic wave extraction process and fatty acid composition of Fatsia japonica seed oil. J. Chin. Cereals Oil Assoc. 2018;11:31–36. [Google Scholar]
- Jing Y, Chi YJ. Effects of twin-screw extrusion on soluble dietary fibre and physicochemical properties of soybean residue. Food Chem. 2013;138:884–889. doi: 10.1016/j.foodchem.2012.12.003. [DOI] [PubMed] [Google Scholar]
- Jing YC, Zhao HX, Bai H. Research progress of Diaphragma juglandis fructus. J. Pharm. Res. 2014;24:167–169. [Google Scholar]
- Jing YC, Zhao HX, Sun YL, Bai H. Chemical constituents from diaphragma juglandis fructus. Food Drug. 2015;17:87–89. [Google Scholar]
- Kalkisim O, Ozdes D, Onaran A. Assessment of mineral elements and heavy metal cintents of walnut samples (Juglans Regia L.) Adv. Food Sci. 2014;36:24–29. [Google Scholar]
- Li M, Xia JL, Li SH, Huang K, Wang M. Study on fatty acid composition and variation analysis of tung oils in China by GC/MS. Adv. Mater. Res. 2012;554–556:2018–2023. doi: 10.4028/www.scientific.net/AMR.554-556.2018. [DOI] [Google Scholar]
- Liang Q, Cui J, Li H, Liu J, Zhao GH. Florets of sunflower (Helianthus annuus L.): potential new sources of dietary fiber and phenolic acids. J. Agric. Food Chem. 2013;61:3435–3442. doi: 10.1021/jf400569a. [DOI] [PubMed] [Google Scholar]
- Liu SS, Wang ZH, Lu L, Hou WW, Yang HY. Optimization of brewing process for walnut diaphragm tea bags using response surface methodology. Sci. Technol. Food Ind. 2015;21:249–253, 258. [Google Scholar]
- Mao XY, Hua YF, Chen GG. Amino acid composition, molecular weight distribution and gel electrophoresis of walnut (Juglans regia L.) proteins and protein fractionations identification of monosaccharide compounds. Int. J. Mol. Sci. 2014;15:2003–2014. doi: 10.3390/ijms15022003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Domingoa MC, Plaa A, Hernándeza AF, Olmedo P, Navas-Acien A, Lozano-Paniagia D, Gil F. Determination of metalloid, metallic and mineral elements in herbal teas. Risk assessment for the consumers. J. Food Compos. Anal. 2017;60:81–89. doi: 10.1016/j.jfca.2017.03.009. [DOI] [Google Scholar]
- Meng QR, Li YH, Xiao TC, Zhang LF, Xu D. Antioxidant and antibacterial activities of polysaccharides isolatedand purified from Diaphragma juglandis fructus. Int. J. Biol. Macromol. 2017;105:431–437. doi: 10.1016/j.ijbiomac.2017.07.062. [DOI] [PubMed] [Google Scholar]
- Meng QR, Wang YQ, Chen F, Xiao TC, Zhang LF. Polysaccharides from Diaphragma juglandis fructus: extraction optimization, antitumor, and immune-enhancement effects. Int. J. Biol. Macromol. 2018;115:835–845. doi: 10.1016/j.ijbiomac.2018.04.121. [DOI] [PubMed] [Google Scholar]
- Milena M, Antonietta C, Angela M, Carla CSS, Cosimo P, Sonia P. LC-MS profiling highlights hazelnut (Nocciola di Giffoni PGI) shells as a byproduct rich in antioxidant phenolics. Food Res. Int. 2017;101:180–187. doi: 10.1016/j.foodres.2017.08.063. [DOI] [PubMed] [Google Scholar]
- Okarter N, Liu CS, Sorrells ME, Liu RH. Phytochemical content and antioxidant activity of six diverse varieties of whole wheat. Food Chem. 2010;119:249–257. doi: 10.1016/j.foodchem.2009.06.021. [DOI] [Google Scholar]
- Olabiyi AA, Carvalho FB, Bottari NB, Morsch VM, Morel AF, Oboh G, Schetinger MR. Tiger nut and walnut extracts modulate extracellular metabolism of ATP and adenosine through the NOS/cGMP/PKG signalling pathway in kidney slices. Phytomedicine. 2018;43:140–149. doi: 10.1016/j.phymed.2018.04.035. [DOI] [PubMed] [Google Scholar]
- Reis FS, Barros L, Calhelha RC, Cirić A, Griensven LJ, Soković M, Ferreira IC. The methanolic extract of Cordyceps militaris (L.) Link fruiting body shows antioxidant, antibacterial, antifungal and antihuman tumor cell lines properties. Food Chem. Toxicol. 2013;62:91–98. doi: 10.1016/j.fct.2013.08.033. [DOI] [PubMed] [Google Scholar]
- Sanz-Pintos N, Perez-Jimenez J, Buschmann AH, Vergara-Salinas JR, Perez-Correa JR, Saura-Calixto F. Macromolecular antioxidants and dietary fiber in edible seaweeds. J. Food Sci. 2017;82:289–295. doi: 10.1111/1750-3841.13592. [DOI] [PubMed] [Google Scholar]
- Saurav B, Siddhartha D, Chiranjib B. Sonication boost the total reducing sugar (TRS) extraction from sugarcane bagasse after dilute acid hydrolysis. Waste Biomass Valori. 2012;3:81–87. doi: 10.1007/s12649-011-9078-2. [DOI] [Google Scholar]
- Schadel C, Blochl A, Richter A, Hoch G. Quantification and monosaccharide composition of hemicelluloses from different plant functional types. Plant Physiol. Biochem. 2010;48(1):1–8. doi: 10.1016/j.plaphy.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Sciarretta S, Yee D, Nagarajan N, Bianchi F, Saito T, Valenti V, Tong M, Del Re DP, Vecchione C, Schirone L, Forte M, Rubattu S, Shirakabe A, Boppana VS, Volpe M, Frati G, Zhai P, Sadoshima J. Trehalose-induced activation of autophagy improves cardiac remodeling after myocardial infarction. J. Am. Coll. Cardiol. 2018;71:1999–2010. doi: 10.1016/j.jacc.2018.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoukas MA, Ko BJ, Witte TR, Dincer F, Hardman WE, Mantzoros CS. Dietary walnut suppression of colorectal cancer in mice: mediation by miRNApatterns and fatty acid incorporation. J. Nutr. Biochem. 2015;26:776–783. doi: 10.1016/j.jnutbio.2015.02.009. [DOI] [PubMed] [Google Scholar]
- Tungland BC, Meyer D. Nondigestible oligo and polysaccharides (Dietary Fiber): their physiology and role in human health and food. Compr. Rev. Food Sci. Food Saf. 2002;1:90–109. doi: 10.1111/j.1541-4337.2002.tb00009.x. [DOI] [PubMed] [Google Scholar]
- Vu DC, Vo PH, Coggeshall MV, Lin CH. Identification and characterization of phenolic compounds in black walnut kernels. J. Agric. Food Chem. 2018;66:4503–4511. doi: 10.1021/acs.jafc.8b01181. [DOI] [PubMed] [Google Scholar]
- Wang D, Mu Y, Dong HJ, Yan H, Hao C, Wang X, Zhang L. Chemical constituents of the ethyl acetate extract from Diaphragma juglandis fructus and their inhibitory activity on nitric oxide production in vitro. Molecules. 2017;23:72. doi: 10.3390/molecules23010072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LJ, Kobayashi T, Li YY, Xu KQ, Lv YK. Determination and abatement of methanogenic inhibition from oleic and palmitic acids. Int. Biodeter. Biodegr. 2017;123:10–16. doi: 10.1016/j.ibiod.2017.05.021. [DOI] [Google Scholar]
- Yang MZ, Tian XY, Xiao CJ, Han BY, Jiang B. Chemical constituents and bioactivity studies of Diaphragma juglandis fructus. Nat. Prod. Res. Dev. 2012;24:1707–1711. [Google Scholar]
- Zhang M, Bai X, Zhang ZS. Extrusion process improves the functionality of soluble dietary fiber in oat bran. J. Cereal Sci. 2011;54:98–103. doi: 10.1016/j.jcs.2011.04.001. [DOI] [Google Scholar]
- Zhang J, He H, Jie XD, Tian X, Cheng YT, Chen PY, Liu AF. Analysis of amino acid composition and nutritional evaluation of Sichuan white goose. Food Mach. 2018;34:62–67. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.