Abstract
Carbon monoxide (CO) is the leading cause of poisoning deaths in many countries, including Japan. Annually, CO poisoning claims about 2000–5000 lives in Japan, which is over half of the total number of poisoning deaths. This paper discusses the physicochemical properties of CO and the toxicological evaluation of CO poisoning.
Keywords: Carbon monoxide, Carboxyhemoglobin, Pathophysiology, Toxicity, Measurement
1. Introduction
Numerous poisons, from natural toxins [1,2] to synthetic chemicals existing in our environment, can produce a wide variety of deleterious effects in living organisms. This review article discusses carbon monoxide (CO) poisoning from a clinical point of view.
CO – sometimes termed a “silent killer” [3,4] is a colorless, odorless, and non-irritable gas. As the specific gravity of CO is 0.97, it is slightly lighter than air. This gas is mainly produced by incomplete combustion of organic compounds [5,6]. Vehicle exhaust, smoke from fires and improperly maintained heating systems are included as common sources.
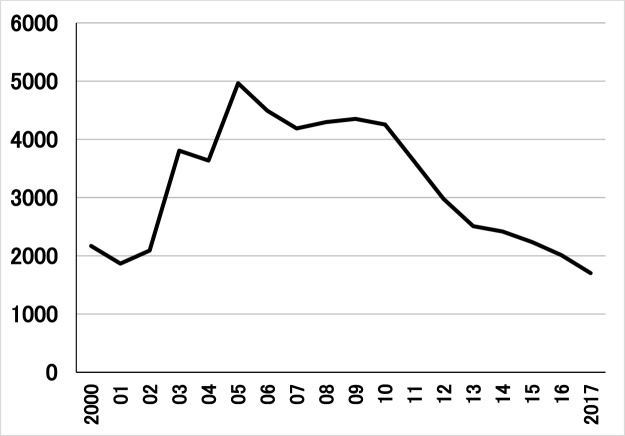
The annual number of CO poisoning death in Japan is about 2000–5000 (Fig. 1), and it is a major cause of poisoning deaths. This is mirrored in several other countries [3,[7], [8], [9], [10], [11], [12], [13]]. The worldwide incidence of CO poisoning has remained stable during the last 25 years [14]. However, deaths from CO poisoning doubled in 2003 compared to that for 2001 in Japan. This may be attributable to an increase of suicides by means of CO inhalation [15]. Information from suicide-related websites may facilitate suicide by CO [16,17].
Fig. 1.
Statistics on annual numbers of death by CO poisoning in Japan (2000–2015).
Forensic toxicologists deal with cases of fatal CO poisoning and are required to evaluate its toxicity in daily practice. This article describes the chemical properties and toxicological characteristics of CO.
2. Sources of CO
CO is formed by incomplete combustion of organic compounds. The main sources of CO encountered in poisoning cases are house fires (the maximum concentration of CO in air is around 5 % in the immediate vicinity of a house fire) [18], incomplete combustion of fuels (e.g., charcoal, briquette, fuel gas, petroleum) using a burner, heating or cooking equipment with insufficient ventilation or improper maintenance, exhaust gas from vehicles using internal combustion engines (the CO concentration in exhaust gas is less than a few percent) [19], and industrial accidents (such as those occurring at iron foundries or chemical plants).
Exhaust from a diesel engine contains approximately 0.01–0.06 % CO, and inhalation does not cause fatal CO poisoning [20]. Concentrations of CO in exhaust from gasoline engine have also decreased in recent years with the introduction of three-way catalytic converters [19].
CO is contained in mainstream smoke from cigarettes (3–4 %) [21], and blood carboxyhemoglobin (CO-Hb) saturation is increased approximately 10–15 % in heavy smokers [[22], [23], [24]].
Poisoning is occurred by inhalation of a relatively high concentration of CO gas. This is not always accidental: it is also used deliberately as a means of suicide. Charcoal-burning suicide has been increasing since the late 1990’s in East-Asian countries such as Hong Kong, Taiwan and Japan [10,15,25,26]. Approximately 80 % of cases under the category of “Other gases and vapors (ICD-10, X67)” in the Vital Statistics of Japan for 2007 were estimated to involve charcoal-burning suicide [15]. Cases of homicide by CO poisoning have also been reported [27].
3. Toxicokinetics of CO
CO is a gas at normal room temperature, and is inhaled from the lungs into the blood stream. Since the affinity of CO for hemoglobin (Hb) is 230- to 270-times greater than that of oxygen, CO-Hb is formed in erythrocytes [6,28,29]. The formation of CO-Hb in blood depends on a wide variety of factors, including the concentration of inspired CO, duration of CO exposure, pulmonary ventilation, exercise and health status [6,28]. A small amount of CO is produced by heme-protein degradation in vivo [28]. CO remains almost completely unoxidized following inhalation, with less than 0.1 % of inhaled CO being converted to carbon dioxide [30]. The rest is eventually discharged from the body.
CO shows a high affinity not only for hemoglobin, but also for other heme-proteins such as myoglobin and cytochrome c oxidase. CO also binds to myoglobin in myocardium and skeletal muscle [6,28,31]. As up to 15 % of the total CO in the body is taken up by tissues [32], CO can diffuse from organs into the blood as CO-Hb saturation in blood decreases [33].
The following formula has been used to estimate CO-Hb saturation in blood:
| CO-Hb (%) = a × CO in the inspired air (%) × time (min), |
where a is a constant with values of 3 at rest, 5 under light activity, 8 under light work, and 11 under heavy work [34], or
| CO-Hb (%) ≈ 0.33 × RMV × CO in the inspired air (%) × time (min), |
where RMV is respiratory minute volume, with standard values of 8.5 at rest, 25 under light exercise, and 50 under heavy exercise [35].
CO-Hb saturation in healthy, non-smoking subjects is less than 2 % [23,24]. It increases to 4–6 % in cases of hemolytic anemia, and can increase to nearly 10 %, depending on the state of the disease [23]. Methylene chloride, a solvent used as a paint or varnish remover, is metabolized to CO [36,37]. Severe CO poisoning with CO-Hb saturation up to 50 %, has been reported following methylene chloride exposure [38].
CO-Hb saturation in blood is easily decreased with oxygen administration [39]. The elimination half-life of CO during respiration depends on various factors, such as the concentration of inspired CO, duration of CO exposure, presence of oxygenation following rescue the oxygen concentration administered, and RMV [40,41]. This results in a half-life for a resting adult of about 4−5 h under room air ventilation at sea level, 80 min breathing 100 % oxygen at normobaric pressure, and 23 min breathing oxygen at 3 atmospheres absolute (ATA) [42,43].
4. Toxicity and pathophysiology of CO poisoning
Tissue hypoxia is the main toxic effect of acute CO poisoning, which is due the formation of CO-Hb. It causes decreases the oxygen transport capacity, resulting in insufficient oxygenation at the tissue level [6,28,29,44]. When CO binds to a hemoglobin subunit, other binding sites show increased affinity for the oxygen molecule. Hence, CO shifts the oxygen-hemoglobin dissociation curve to the left, inhibiting oxygen dissociation in the low-oxygen region, and potentiating tissue hypoxia [39,41,42,[45], [46], [47]].
Due to the much greater affinity of CO, as compared to oxygen, for hemoglobin, the bond between CO and hemoglobin is strong. However, this bond can be broken is reversible, CO is only displaced by oxygen slowly [6,28,29,47].
CO also binds to myoglobin in myocardium and skeletal muscle, causing dysfunctional tissue oxygen transport. In myocardium, this results in cardiac dysfunction [47]. It also has direct effects by inhibiting the activity of enzymes such as cytochrome c oxidase. CO poisoning may thus also be implicated in impairment of cardiac and neurological functions.
Apoptosis is a key factor in the pathogenesis of heart failure [48,49]. CO poisoning leads to apoptosis in myocardial cells. Neurotoxicity following CO exposure involves apoptosis and intracellular oxidative stress, and erythropoietin, resveratrol and hyperbaric oxygen all reduce dysfunction of the myocardium and brain by suppressing apoptosis or through other pathways [[48], [49], [50], [51]].
Tissue hypoxia due to CO potentiates vascular permeability and causes increased accumulation of interstitial fluid with decreased circulating blood volume (hemoconcentration) affecting multiple organs. This includes brain edema with neurological symptoms and disorders of consciousness; pulmonary edema with respiratory failure; decreased myocardial contractility, arrhythmias and heart failure; and renal failure [46,47].
5. Symptoms and management of CO poisoning
The CO-Hb concentration in healthy, non-smoking subjects is less than 2 %, and less than 15 % in smokers. At CO-Hb levels below 10 %, no notable symptoms are observed. Neurological symptoms such as nausea, headache and dizziness are observed with CO-Hb levels over 10 %. Increases in respiratory and heart rates, syncope, motor paralysis and confusion are observed with CO-Hb level of 30–50 %. CO-Hb levels exceeding 50 % are considered life-threatening, and values in this range are central to the diagnosis of CO-poisoning [6,28,29,47].
Signs and symptoms such as headache, dizziness, fatigue and nausea are nonspecific [6,47]. Since behavioral disorders such as agitation, confusion and hallucination are sometimes observed, differential diagnoses such as psychosis, brain metastasis of tumor, stroke and coagulation disorders are required in clinical practice [[52], [53], [54], [55]].
Motor function is depressed prior to impairment of consciousness in cases of CO poisoning [56]. This means that a victim may notice CO poisoning and try to improve the room ventilation, but may not be able to move at all.
The symptoms shown in Table 1 reportedly reflect the CO-Hb level. However, clinical symptoms of acute CO poisoning and their severity do not always correlate with concentrations of CO-Hb on admission [22,41,57,58]. This discrepancy may be due to two reasons. First, the CO-Hb saturation in blood is affected by various factors such as the concentration of inspired CO and the exposure time, oxygenation following rescue and the oxygen concentration applied [6,28], and the time elapsed between termination of CO exposure and blood sampling [57,58]. Second, as CO has a high affinity for heme proteins, CO that has diffused into tissues may not readily dissociate from them. As a result, considerable amounts of CO may be left in the body, even after CO-Hb saturation has been decreased by oxygenation [31]. So, while Table 1 describe the symptoms related to increasing levels of CO-Hb following CO inhalation [41], we must also consider medical interventions and pre-hospital procedures such as oxygen administration when evaluating the toxicity of CO.
Table 1.
Levels of carboxyhemoglobin (CO-Hb) saturation (%) and symptoms.
| CO-Hb (%) | clinical symptom |
|---|---|
| < 1 | normal range (due to endogenous production) |
| < 10 | smoker’s blood (no symptom) |
| 10–20 | headache, fatigue, ear ringing |
| 20–30 | headache, weakness, nausea, vomitting |
| 30–40 | severe headache, dizziness, nausea, vomitting |
| 40–50 | syncope, confusion, increased respiration and heart rate |
| 50k60 | coma, convulsions, depressed respiration |
| 60–70 | coma, convulsions, cardiopulmonary depression, often fatal |
| 70 < | respiratory failure, death |
The first step of patient management is immediate evacuation from the contaminated environment. Control of the airway, intravenous access and cardiac monitoring are required for the management in the hospital. Administration of 100 % oxygen via facemask or endotracheal tube is required for the elimination of CO from blood as an initial treatment. Hyperbaric oxygen therapy can also be considered, where available, to accelerates the elimination of CO and reverse effects on inflammation and mitochondrial dysfunction induced by CO poisoning [6,47,59].
6. Autopsy findings
The cherry-red coloration of the skin is most characteristic appearance of the body surface is in CO poisoning cases. This is usually observed with CO-Hb concentrations exceeding 30 % [60]. Autopsy reveals blood, organs and muscles with similar cherry-red coloring, by the CO-Hb and carboxymyoglobin formation. Pulmonary edema and generalized organ congestion are also observed [60].
Necrosis of the globus pallidum is observed in cases of CO poisoning that occur over a prolonged period [61,62]. The underlying mechanisms are thought to involve hypoxic brain damage, as well as apoptosis [[61], [62], [63]].
7. Measurement of CO
Toxicological evaluation of CO poisoning is based on autopsy findings and CO-Hb saturation in blood. As most autopsy findings are non-specific for CO poisoning- other than the cherry-red color changes in the skin, organs and blood, the basic point of evaluation in forensic practice is CO-Hb saturation.
As a test for CO, spectrophotometric methods, gas chromatography, detection tubes and oximetry are employed [64,65], and various methods have been reported. The spectrophotometric method is the most widely used. The presence of CO-Hb can be determined by changes in the absorption spectrum [[66], [67], [68], [69]]. This is simple procedure.
CO is liberated from Hb and introduced in gas chromatography. Various methods have been reported for liberation of CO from blood [[70], [71], [72], [73], [74], [75], [76]]. The released CO is detectable by various detectors, including a thermal conductivity detector [[70], [71], [72],74,75], barrier discharge ionization detector [76], and flame ionization detector with the catalytic reduction of CO to methane (methanizer) [73]. Gas chromatography using a semiconductor detector (sensor gas chromatography) has been applied in forensic practice [77]. This system has some advantages such as portability and easy handling. As gas chromatography measures CO directly, the hemoglobin content also has to be measured for each sample, to enable calculation of the percentage CO-Hb.
Alternatively, a method using detector tube can be applied [78]. This system consists of an aspirating pump, tube for separation and tube for detection. The tube for CO separation is packed with ferricyanide coated silica gel particles [78]. The tube for detection of CO is packed with potassium palladium sulfite coated silica gel particles [79,80]. CO is released from blood following sample injection (200 μl) into the CO-separator tube, and the CO-detector tube detects the released CO gas, followed by aspiration by the pump. The detector tube can be easily used at the scene or in on-site testing.
An oximeter is widely used at the clinical laboratory [81], and also in daily forensic practice [[82], [83], [84], [85], [86], [87], [88], [89], [90]]. The instrument we use (AVOX 4000; International Technidyne Corp, Edison, NJ, USA) applies multiple wavelengths for the determination of various hemoglobin species, including CO-Hb, and requires small amounts of blood for measurement. This system allows easy handling of samples and has some advantages for on-site testing.
For details of measurement conditions and equipment, refer to the individual references [[64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90]].
CO-Hb is relatively stable at 4 °C for up to 24 months [91,92], and without refrigeration for up to 4 weeks [93].
8. Evaluation of CO toxicity
The fatal concentration for CO poisoning is a CO-Hb saturation over 50–60 % [65,94]. As mentioned above: since the CO-Hb saturation in blood is affected by multiple factors, medical interventions such as oxygen administration or cardiopulmonary resuscitation must be considered when evaluating CO toxicity. The measured value of CO-Hb at the time of death is generally found to be higher for younger casualties than for the elderly [[94], [95], [96]]. Elderly individuals may die at lower concentrations [6] - with a level is around 25 %, sometimes measured, with no other cause of death found. This may reflect the fact that younger individuals tend to have fewer comorbidities and are better able to tolerate the tissue hypoxia.
The brain is an organ with a very high oxygen demand, and so is especially sensitive to the effects of tissue hypoxia that results from acute CO poisoning. The heart is an organ with a high oxygen demand and is thus often affected, similar to the brain. Patients with cardiovascular disease experience reduced thresholds for angina, arrhythmias and myocardial infarction. These conditions have been observed even with CO-Hb of 5–10 % [6], with sudden death from severe arteriosclerotic heart disease reported at CO-Hb of 20–30 % [6,60]. During the last few years, several in vivo or in vitro experiments have examined various compounds such as magnesium sulfate, insulin, hesperidin, resveratrol, granulocyte colony stimulating factor and erythropoietin that can potentially combat early complications and late consequences of CO poisoning in the brain and heart [[48], [49], [50],[97], [98], [99], [100], [101], [102], [103]].
In cases of automobile exhaust gas inhalation, the inhalation of nitrogen oxide leads to the production of methemoglobin (MetHb), and this need to be considered in addition to CO-Hb. High concentrations of MetHb have been reported in some cases, although methemoglobinemia is uncommon [[104], [105], [106]].
Additional consideration in cases of fire are other toxic gases (such as cyanide and phosgene) and oxygen deficiency that results from the consumption of oxygen in combustion. As cyanide is detoxicated by binding to MetHb, attention must be paid to the concentration of MetHb in the victim’s blood when evaluating toxicity [107] - and so CO-Hb, cyanide and MetHb should be measured in cases of suspected fire victims [107].
Postmortem formation of CO due to putrefaction has been reported in a sample obtained from a case with a long postmortem interval. This was attributed to the degradation of heme-proteins such as hemoglobin and myoglobin. Postmortem formation of CO has also been reported in conditions and samples such as immersion in water for long periods. Values of CO-Hb over 10 % in pleural effusion are sometimes observed in cases of drowning without CO inhalation [[108], [109], [110], [111], [112]]. Since no indicators have been identified for postmortem CO formation, we should not use body cavity fluids for measurement of CO in cases involving severe putrefaction.
9. Conclusion
We have discussed various issues related to CO described in previous reports. These data may be valuable for interpreting CO poisoning and may provide valuable information for forensic diagnosis. Recently, CO has been recognized as not only a toxic substance, but also a signaling gas, and research into therapeutic applications is been underway [113,114]. Further study of potential applications in daily practice are required.
Declaration of Competing Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Acknowledgements
This work was supported by JSPS KAKENHI Grant-in-aid for Scientific Research (C) Grant Number JP18K10127.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.toxrep.2020.01.005.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Tavassoli M., Afshari A., Arsene A.L., Mégarbane B., Dumanov J., Bastos Paoliello M.M., Tsatsakis A., Carvalho F., Hashemzaei M., Karimi G., Rezaee R. Toxicological profile of Amanita virosa – a narrative review. Toxicol. Rep. 2019;6:143–150. doi: 10.1016/j.toxrep.2019.01.002. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Govorushko S., Rezaee R., Dumanov J., Tsatsakis A. Poisoning associated with the use of mushrooms: a review of the global pattern and main characteristics. Food Chem. Toxicol. 2019;128:267–279. doi: 10.1016/j.fct.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 3.Can G., Sayili U., Sayman Ö.A., Kuyumcu Ö.F., Yilmaz D., Esen E., Yurtseven E., Erginöz E. Mapping of carbon monoxide related death risk in Turkey: a ten-year analysis based on news agency records. BMC Public Health. 2019;19:9. doi: 10.1186/s12889-018-6342-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byard R.W. Carbon monoxide-the silent killer. Forensic Sci. Med. Pathol. 2019;15:1–2. doi: 10.1007/s12024-018-0040-5. [DOI] [PubMed] [Google Scholar]
- 5.Baselt R.C. 11th ed. Biochemical Publications; Seal Beach: 2017. Disposition of Toxic Drugs and Chemicals in Man. [Google Scholar]
- 6.Elenhorn M.J. 2nd ed. Williams &Wilkins, Baltimore; 1997. Medical Toxicology Diagnosis and Treatment of Human Poisoning. [Google Scholar]
- 7.Ministry of Health, Labour and Walfare, Vital Statistics of Japan, 1998–2015.
- 8.National Research Institute of Police Science, Annual case report of drug and toxic poisoning in Japan., No. 44 -No. 61. 2000–2019.
- 9.Tsunoda N. Drug and toxic poisoning in recent Japan. J. Health Sci. 1999;45:356–366. [Google Scholar]
- 10.Ito T., Nakamura Y. Death from carbon monoxide poisoning in Japan between 1968-2007 through data from vital statistics. J. Jpn. Soc. Emerg. Med. 2010;13:275–282. [Google Scholar]
- 11.Centers for Disease Control and Prevention (CDC) Carbon monoxide --related deaths--United States, 1999-2004. MMWR. 2007;56:1309–1312. [PubMed] [Google Scholar]
- 12.Anderson A.R., Centers for Disease Control and Prevention (CDC) Top five chemicals resulting in injuries from acute chemical incidents – Hazardous substances emergency events surveillance, nine states, 1999-2008. MMWR suppl. 2015;64:39–46. [PubMed] [Google Scholar]
- 13.Sircar K., Clower J., Shin M.K., Bailey C., King M., Yip F. Carbon monoxide poisoning deaths in the United States, 1999 to 2012. Am. J. Emerg. Med. 2015;33:1140–1145. doi: 10.1016/j.ajem.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mattiuzzi C., Lippi G. Worldwide epidemiology of carbon monoxide poisoning. Hum. Exp. Toxicol. 2019 doi: 10.1177/0960327119891214. [DOI] [PubMed] [Google Scholar]
- 15.Yoshioka E., Hanley S.J.B., Kawanishi Y., Saijo Y. Epidemic of charcoal burning suicide in Japan. Br. J. Psychiatry. 2014;204:274–282. doi: 10.1192/bjp.bp.113.135392. [DOI] [PubMed] [Google Scholar]
- 16.Hitosugi M. Suicide due to carbon monoxide poisoning – trends and preventive measures. Rinsho Byori. Suppl. 2008;141:40–44. [PubMed] [Google Scholar]
- 17.Ozawa-de Silva C. Too lonely to die alone: internet suicide pacts and existential suffering in Japan. Cult. Med. Psychiatry. 2008;32:516–551. doi: 10.1007/s11013-008-9108-0. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki Y., Takeda M., Inamura T., Tanaka Y. Analyzed gases of burning products collected at fire scenes. Rep. Fire Sci. Lab. 1989;26:45–52. [Google Scholar]
- 19.Kawaraya T., Garivait H., Laowagul W., Sukasem P., Tabucanon M.S., Sakata M., Okuno T. Exhaust gases from new gasoline vehicles in Thailand. Seikatsu. Eisei. 1997;41:93–96. [Google Scholar]
- 20.Yamanouchi H., Honma N., Shigeno R. A fatal case due to the exhaust of diesel engine. Res. Pract. Forens. Med. 1985;28:115–118. [Google Scholar]
- 21.Kano M. Pharmacology of the smoking. Diagn. Treat. 2009;97:1327–1331. [Google Scholar]
- 22.Raub J.A., Mathieu-Norf M., Hampson N.B., Thom S.R. Carbon monoxide poisoning - a public perspective. Toxicology. 2000;145:1–14. doi: 10.1016/s0300-483x(99)00217-6. [DOI] [PubMed] [Google Scholar]
- 23.Owens E.O. Endogenous carbon monoxide production in disease. Clin. Biochem. 2010;43:1183–1188. doi: 10.1016/j.clinbiochem.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 24.Prockop L.D., Chichkova R.I. Carbon monoxide intoxication: an updated review. J. Neurol. Sci. 2007;262:122–130. doi: 10.1016/j.jns.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 25.Liu K.Y., Beautrais A., Caine E., Chan K., Chao A., Conwell Y., Law C., Lee D., Li P., Yip P. Charcoal burning suicides in Hong Kong and urban Taiwan: an illustration of the impact of a novel suicide method on overall regional rates. J. Epidemiol. Community Health. 2007;61:248–253. doi: 10.1136/jech.2006.048553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yi-Han C., Chia-Yueh H., Qijin C., Shu-sen C., Paul Y. The evolution of the characteristics of charcoal-burning suicide in Hong Kong, 2002-2013. J. Affect. Disord. 2019;257:390–395. doi: 10.1016/j.jad.2019.07.041. [DOI] [PubMed] [Google Scholar]
- 27.Marufuji K., Ohnuma T., Ohmi M., Niwase T. A homicide case camouflaged as an accidental CO poisoning. Res. Pract. Forens. Med. 1975;18:59–70. [Google Scholar]
- 28.World Health Organization . 2nd ed. World Health Organization; Geneva: 1999. Environmental Health Criteria 213, Carbon Monoxide. [Google Scholar]
- 29.Powers R.H., Dean D.E. Forensic Toxicology Mechanisms and Pathology. CRC Press; Boca Raton: 2016. Pulmonary toxicology; pp. 147–164. [Google Scholar]
- 30.Tobias C.A., Lawrence J.H., Roughton F.J.W., Root W.S., Gregersen M.I. The elimination of carbon monoxide from the human body with reference to the possible conversion of CO to CO2. Am. J. Physiol. 1945;145:253–263. doi: 10.1152/ajplegacy.1945.145.2.253. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto I., Inokuchi S. Carbon monoxide. In: Japanese Society for Clinical Toxicology, editor. Standard Clinical Practice Guide for Acute Poisoning. Jiho, Tokyo. 2008. pp. 179–186. [Google Scholar]
- 32.Coburn R.F. The carbon monoxide body stores. Ann. N.Y. Acad. Sci. 1970;174:11–22. doi: 10.1111/j.1749-6632.1970.tb49768.x. [DOI] [PubMed] [Google Scholar]
- 33.Roughton F.J.W., Root W.S. The fate of CO in the body during recovery from mild carbon monoxide poisoning in man. Am. J. Physiol. 1945;145:239–252. doi: 10.1152/ajplegacy.1945.145.2.239. [DOI] [PubMed] [Google Scholar]
- 34.Forbes W.H., Sargent F., Roughton F.J.W. The rate of carbon monoxide uptake by normal men. Am. J. Physiol. 1945;143:594–608. [Google Scholar]
- 35.Quintiere J.G. Principles of fire behavior. Delmar, New York. 1997 [Google Scholar]
- 36.Ratney R.S., Wegman D.H., Elkins H.B. In vivo conversion of methylene chloride to carbon monoxide. Arch. Environ. Health. 1974;28:223–226. doi: 10.1080/00039896.1974.10666472. [DOI] [PubMed] [Google Scholar]
- 37.Carlsson A., Hultengren M. Exposure to methylene chloride. Ⅲ. Metabolism of 14C-labelled methylene chloride in rat. Scand. J. Work Environ. Health. 1975;1:104–108. doi: 10.5271/sjweh.2856. [DOI] [PubMed] [Google Scholar]
- 38.Fagin J., Bradley J., Williams D. Carbon monoxide poisoning secondary to inhaling methylene chloride. Br. Med. J. 1980;281:1461. doi: 10.1136/bmj.281.6253.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taki K. Hyperbaric oxygen therapy (HBOT) for CO poisoning – survey of acute CO poisoning in Japan. J. Jpn. Assoc. Clin. Hyperbaric Oxygen Div. 2009;6:7–12. [Google Scholar]
- 40.Shimazu T. Half-life of blood carboxyhemoglobin. Chest. 2001;119:661–662. doi: 10.1378/chest.119.2.661. [DOI] [PubMed] [Google Scholar]
- 41.Shimazu T. Pathophysiology, myths and mysteries of acute carbon monoxide poisoning. Chudoku Kenkyu. 2006;19:23–33. [PubMed] [Google Scholar]
- 42.Naito H. 2nd ed. Nankodo Co., Ltd; Tokyo: 2001. Poisoning of Industrial Products, Gases Pesticides, Drugs and Natural Toxins -cases, Pathogenesis and Its Treatment- [Google Scholar]
- 43.Weaver L.K., Howe S., Hopkins R., Chan K.J. Carboxyhemoglobin half-life in carbon monoxide-poisoned patients treated with 100% oxygen at atmospheric pressure. Chest. 2000;117:801–808. doi: 10.1378/chest.117.3.801. [DOI] [PubMed] [Google Scholar]
- 44.Ghosh A., Banerjee S., Mitra A., Muralidharan M., Roy B., Banerjee R., Mandal A.K., Chatterjee I.B. Interaction of p-benzoquinone with hemoglobin in smoker’s blood causes alteration of structure and loss of oxygen binding capacity. Toxicol. Rep. 2016;3:295–305. doi: 10.1016/j.toxrep.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Douglas C.G., Haldane J.S., Haldane J.B.S. The laws of combination of haemoglobin with carbon monoxide and oxygen. J. Physiol. 1912;44:275–304. doi: 10.1113/jphysiol.1912.sp001517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Okada Y. Carbon monoxide poisoning. Jpn. J. Acute Med. 1979;3:1114–1122. [Google Scholar]
- 47.Tomaszewski C. Carbon monoxide. In: Nelson L.S., editor. Goldfrank’s Toxicologic Emergencies. 9th ed. McGraw-Hill; New York, Chicago: 2011. pp. 1658–1670. [Google Scholar]
- 48.Rezaee M.A., Mohammadpour A.H., Imenshahidi M., Mahmoudi M., Sankian M., Tsarouhas K., Tsakalof A., Tsatsakis A.M., Moallem S.A. Protective effect of erythropoietin on myocardial apoptosis in rats exposed to carbon monoxide. Life Sci. 2016;148:118–124. doi: 10.1016/j.lfs.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 49.Hashemzaei M., Barani A.K., Iranshahi M., Rezaee R., Tsarouhas K., Tsatsakis A.M., Wilks M.F., Tabrizian K. Effects of resveratrol on carbon monoxide-induced cardiotoxicity in rats. Environ. Toxicol. Pharmacol. 2016;46:110–115. doi: 10.1016/j.etap.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 50.Moallem S.A., Mohamadpour A.H., Abnous K., Sankian M., Sadeghnia H.R., Tsatsakis A., Shahsavand S. Erythropoietin in the treatment of carbon monoxide neurotoxicity in rat. Food Chem. Toxicol. 2015;86:56–64. doi: 10.1016/j.fct.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 51.Jurič D.M., Šuput D., Brvar M. Hyperbaric oxygen preserves neurotrophic activity of carbon monoxide-exposed astrocytes. Toxicol. Lett. 2016;253:1–6. doi: 10.1016/j.toxlet.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 52.Wojciechowski V.V., Calina D., Tsarouhas K., Pivnik A.V., Sergievich A.A., Kodintsev V.V., Filatova E.A., Ozcagli E., Docea A.O., Arsene A.L., Gofita E., Tsitsimpikou C., Tsatsakis A.M., Golokhvast K.S. A guide to acquired vitamin K coagulophathy diagnosis and treatment: the Russian perspective. Daru. 2017;25:10. doi: 10.1186/s40199-017-0175-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nassbaum L., Hogea L.M., Călina D., Andreescu N., Grădinaru R., Ştefănescu R., Puiu M. Modern treatment approaches in psychoses pharmacogenetic, neuroimagistic and clinical implications. Farmacia. 2017;65:75–81. [Google Scholar]
- 54.Buga A.-M., Docea A.O., Albu C., Malin R.D., Branisteanu D.E., Ianosi G., Ianosi S.L., Iordache A., Calina D. Molecular and cellular stratagem of brain metastases associated with melanoma. Oncol. Lett. 2019;17:4170–4175. doi: 10.3892/ol.2019.9933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tsatsakis A.M., Docea A.O., Calina D., Tsarouhas K., Zamfira L.-M., Mitrut R., Sharifi-Rad J., Kovatsi L., Siokas V., Dardiotis E., Drakoulis N., Lazopoulos G., Tsitsimpikou C., Mitsias P., Neagu M. A mechanistic and pathophysiological approach for stroke associated with drugs of abuse. J. Clin. Med. 2019;8:1295. doi: 10.3390/jcm8091295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suzuki O. Forensic toxicology. In: Katsumata Y., Suzuki O., editors. NEW Legal Medicine and Medical Law. Nankodo Co., Ltd; Tokyo: 2008. pp. 131–163. [Google Scholar]
- 57.Sokal J.A., Kralkowska E. The relationship between exposure duration, carboxyhemoglobin, blood glucose, pyruvate and lactate and the severity of intoxication in 39 cases of acute carbon monoxide poisoning in man. Arch. Toxicol. 1985;57:196–199. doi: 10.1007/BF00290887. [DOI] [PubMed] [Google Scholar]
- 58.Hampson N.B., Hauff N.M. Carboxyhemoglobin levels in carbon monoxide poisoning: do they correlate with the clinical picture? Am. J. Emerg. Med. 2008;26:665–669. doi: 10.1016/j.ajem.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 59.Rose J.J., Wang L., Xu Q., McTiernan C.F., Shiva S., Tejero J., Glandwin M.T. Carbon monoxide poisoning: pathogenesis, management, and future directions of therapy. Am. J. Respir. Crit. Care Med. 2017;195:596–606. doi: 10.1164/rccm.201606-1275CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saukko P., Knight B. 4th ed. CRC Press; Boca Raton, FL: 2016. Knight’s Forensic Pathology; pp. 589–594. [Google Scholar]
- 61.Okada M., Okuda B., Okae S. Bilateral necrosis of the pallidum in a case of carbon monoxide poisoning. Neurol. Med. 1982;17:304–305. [Google Scholar]
- 62.Sawada Y., Sakamoto T., Sadamitsu D., Yokota J., Ohashi N., Yoshioka T., Onishi S. CT and pathological findings of globus pallidus after severe carbon monoxide poisoning. Jpn. J. Acute Med. 1983;7:1721–1724. [Google Scholar]
- 63.Uemura K., Harada K., Sadamitsu D., Tsuruta R., Takahashi M., Aki T., Yasuhara M., Maekawa T., Yoshida K.-i. Apoptotic and necrotic brain lesions in a fatal case of carbon monoxide poisoning. Forensic Sci. Int. 2001;116:213–219. doi: 10.1016/s0379-0738(00)00375-3. [DOI] [PubMed] [Google Scholar]
- 64.Akane A., Fukui Y. A review on the development of carboxyhemoglobin analysis. Res. Pract. Forens. Med. 1985;28:185–190. [Google Scholar]
- 65.Sato K. Carbon monoxide. In: Suzuki O., Watanabe K., editors. Drugs and Poisons in Humans, -a Handbook of Practical Analysis. Springer-Verlag; Berlin Heidelberg: 2005. pp. 91–99. [Google Scholar]
- 66.Akiya S., Tanimura A. Microdetermination of carbon monoxide-hemoglobin. Yakugaku Zasshi. 1952;72:453–454. [Google Scholar]
- 67.Kozuka H., Niwase T., Taniguchi T. Spectrophotometric determination of carboxyhemoglobin. J. Hygiene Chem. 1969;15:342–345. [Google Scholar]
- 68.Katusmata Y., Aoki M., Sato K., Suzuki O., Oya M., Yada S. A simple spectrophotometry for determination of carboxyhemoglobin in blood. J. Forensic Sci. 1982;27:928–934. [PubMed] [Google Scholar]
- 69.Ishizu H., Ameno S., Yamamoto Y., Okamura Y., Shogano N. A simple spectrophotometric method for CO-Hb determination in blood and its application in legal medicine. Acta. Crim. Japon. 1982;48:187–196. [Google Scholar]
- 70.Miyauchi C., Sakaki K. An improved method for gas chromatographic quantification of carboxyhemoglobin in blood. Nihon Hoigaku Zasshi. 1974;28:59–64. [Google Scholar]
- 71.Hishida S., Mizoi Y. Studies on the quantitative determination of carbon monoxide in human blood by gas chromatography. Nihon Hoigaku Zasshi. 1976;30:319–326. [Google Scholar]
- 72.Sakata M., Haga M. Determination of carbon monoxide in blood by head space analysis. J. Toxicol. Sci. 1980;5:35–43. doi: 10.2131/jts.5.35. [DOI] [PubMed] [Google Scholar]
- 73.Guillot J.G., Weber J.P., Savoie J.Y. Quantitative determination of carbon monoxide in blood by head-space gas chromatography. J. Anal. Toxicol. 1981;5:264–266. doi: 10.1093/jat/5.6.264. [DOI] [PubMed] [Google Scholar]
- 74.Seto Y., Kataoka M., Tsuge K. Stability of blood carbon monoxide and hemoglobins during heating. Forensic Sci. Int. 2001;121:144–150. doi: 10.1016/s0379-0738(01)00465-0. [DOI] [PubMed] [Google Scholar]
- 75.Lewis R.J., Johnson R.D., Canfield D.V. An accurate method for the determination of carboxyhemoglobin in postmortem blood using GC-TCD. J. Anal. Toxicol. 2004;28:59–62. doi: 10.1093/jat/28.1.59. [DOI] [PubMed] [Google Scholar]
- 76.Ohmori T., Otsuka M., Seto Y. Measurement of carbon monoxide in blood sample using gas-chromatography with barrier discharge ionization detection. Rep. Natl. Res. Inst. Police Sci. 2017;66:59–64. [Google Scholar]
- 77.Tanaka N., Kinoshita H., Takakura A., Jamal M., Kumihashi M., Uchiyama Y., Tsutsui K., Ameno K. Application of sensor gas chromatography for the determination of carbon monoxide in forensic medicine. Curr. Environ. Med. Sci. 2014;7:9–11. [Google Scholar]
- 78.Ishizawa F., Misawa S. A handy and simple apparatus for the quantitative determination of COHb in blood. Acta. Crim. Japon. 1986;52:26–32. [PubMed] [Google Scholar]
- 79.4th ed. Komyo rikagaku kogyo; Tokyo: 2003. Komyo Rikagaku Kogyo. Gas Detector Tube System Handbook. [Google Scholar]
- 80.Pharmaceutical Society of Japan . Kanehara shuppan; Tokyo: 1990. Standard Methods of Analysis for Hygienic Chemists -with Commentary- (1990) [Google Scholar]
- 81.Mahoney J.J., Vreman H.J., Stevenson D.K., Van Kessel A.L. Measurement of carboxyhemoglobin and total hemoglobin by five specialized spectrophotometers (CO-oximeters) in comparison with reference methods. Clin. Chem. 1993;39:1693–1700. [PubMed] [Google Scholar]
- 82.Okada M., Okada T., Ide K. Utilization of a CO-oximeter in the medico-legal field. Nihon Houigaku Zasshi. 1985;39:318–325. [PubMed] [Google Scholar]
- 83.Higuchi T., Noguchi K., Maeda H. An evaluation of analyzed data of hemoglobin derivatives by CO-oximeter in medico-legal autopsy. Nihon Houigaku Zasshi. 1992;46:416–418. [PubMed] [Google Scholar]
- 84.Maeda H., Fukita K., Oritani S., Nagai K., Zhu B.L. Evaluation of post-mortem oxymetry in fire victims. Forensic Sci. Int. 1996;81:201–209. doi: 10.1016/s0379-0738(96)01952-4. [DOI] [PubMed] [Google Scholar]
- 85.Shimizu K., Mizukami H., Fukushima T., Sasaki M., Shiono H. Use of a CO-oximeter for forensic diagnosis of hypothermia. Nihon Houigaku Zasshi. 1998;52:196–201. [PubMed] [Google Scholar]
- 86.Brehmer C., Iten P.X. Rapid determination of carboxyhemoglobin in blood by Oximeter. Forensic Sci. Int. 2003;133:179–181. doi: 10.1016/s0379-0738(03)00066-5. [DOI] [PubMed] [Google Scholar]
- 87.Watanabe N. Medico-legal application of hemoglobin analysis using CO-oximeter. Hokkaido Igaku Zasshi. 2003;78:557–566. [PubMed] [Google Scholar]
- 88.Olson K.N., Hillyer M.A., Kloss J.S., Geiselhart R.J., Apple F.S. Accident or arson: is Co-oximetry reliable for carboxyhemoglobin measurement postmortem? Clin. Chem. 2010;56:515–520. doi: 10.1373/clinchem.2009.131334. [DOI] [PubMed] [Google Scholar]
- 89.Tanaka N., Ameno K., Jamal M., Ohkubo E., Kumihashi M., Kinoshita H. Application of oximeter AVOX 4000 for the determination of CO-Hb in the forensic practice. Res. Pract. Forens. Med. 2010;53:39–43. [Google Scholar]
- 90.Fujihara J., Kinoshita H., Tanaka N., Yasuda T., Takeshita H. Accuracy and usefulness of the AVOXimeter 4000 as routine analysis of carboxyhemoglobin. J. Forensic Sci. 2013;58:1047–1049. doi: 10.1111/1556-4029.12144. [DOI] [PubMed] [Google Scholar]
- 91.Kunsman G.W., Presses C.L., Prodriguez P. Carbon monoxide stability in stored postmortem blood samples. J. Anal. Toxicol. 2000;24:572–578. doi: 10.1093/jat/24.7.572. [DOI] [PubMed] [Google Scholar]
- 92.Tanaka N., Ameno K., Jamal M., Kumihashi M., Miyatake N., Kinoshita H. Effects of sampling methods and storage on the value of oxyhemoglobin ratio and carboxyhemoglobin ratio. Res. Pract. Forens. Med. 2012;55:51–55. [Google Scholar]
- 93.Hampson N.B. Stability of carboxyhemoglobin in stored and mailed blood samples. Am. J. Emerg. Med. 2008;26:191–195. doi: 10.1016/j.ajem.2007.04.028. [DOI] [PubMed] [Google Scholar]
- 94.Mitsui T., Ito M. Concentration of carboxyhemoglobin in blood to death. Eisei Kagaku. 1990;36:158–161. [Google Scholar]
- 95.Teige B., Lundevall J., Fleischer E E. Carboxyhemoglobin concentrations in fire victims and in cases of fatal carbon monoxide poisoning. Z. Rechtsmed. 1977;80:17–21. doi: 10.1007/BF02332342. [DOI] [PubMed] [Google Scholar]
- 96.Yoshida M., Adachi J., Watabiki T., Tatsuno Y., Ishida N. A study on house fire victims: age, carboxyhemoglobin, hydrogen cyanide and hemolysis. Forensic Sci. Int. 1991;52:13–20. doi: 10.1016/0379-0738(91)90091-v. [DOI] [PubMed] [Google Scholar]
- 97.Bagheri G., Rezaee R., Tsarouhas K., Docea A.O., Shahraki J., Shahriari M., Wilks M.F., Jahantigh H., Tabrizian K., Moghadam A.A., Bagheri S., Spandidos D.A., Tsatsakis A., Hashemzaei M. Magnesium sulfate ameliorates carbon monoxide-induced cerebral injury in male rats. Mol. Med. Rep. 2019;19:1032–1039. doi: 10.3892/mmr.2018.9771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tabrizian K., Shahriari Z., Rezaee R., Jahantigh H., Bagheri G., Tsarouhas K., Docea A.O., Tsatsakis A., Hashemzaei M. Cardioprotective effects of insulin on carbon monoxide-induced toxicity in male rats. Hum. Exp. Toxicol. 2019;38:148–154. doi: 10.1177/0960327118788134. [DOI] [PubMed] [Google Scholar]
- 99.Rezaee R., Sheidary A., Jangjoo S., Ekhtiary S., Bagheri S., Kohkan Z., Dadres M., Oana Docea A., Tsarouhas K., Sarigiannis D.A., Karakitsios S., Tsatsakis A., Kovatsi L., Hashemzaei M. Cardioprotective effects of hesperidin on carbon monoxide poisoned in rats. Drug Chem. Toxicol. 2019;14:1–6. doi: 10.1080/01480545.2019.1650753. [DOI] [PubMed] [Google Scholar]
- 100.Hashemzaei M., Mohammadpour A.H., Imenshahidi M., Rezaee R. Does granulocyte colony stimulating factor have protective effects against carbon monoxide-induced apoptosis? Biologia. 2018;73:1153–1157. [Google Scholar]
- 101.Tabrizian K., Khodayari H., Rezaee R., Jahantigh H., Bagheri G., Tsarouhas K., Hashemzaei M. Magnesium sulfate protects the heart against carbon monoxide-induced cardiotoxicity in rats. Res. Pharm. Sci. 2018;13:65–72. doi: 10.4103/1735-5362.220969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tabrizian K., Shahraki J., Bazzi M., Rezaee R., Jahantigh H., Hashemzaei M. Neuro-protective effects of resveratrol on carbon monoxide-induced toxicity in male rats. Phytother. Res. 2017;31:1310–1315. doi: 10.1002/ptr.5855. [DOI] [PubMed] [Google Scholar]
- 103.Shahsavand S., Mohammadpour A.H., Rezaee R., Behravan E., Sakhtianchi R., Moallem S.A. Effect of erythropoietin on serum brain-derived biomarkers after carbon monoxide poisoning in rats. Iran. J. Basic Med. Sci. 2012;15:752–758. [PMC free article] [PubMed] [Google Scholar]
- 104.Suyama H., Morikawa S., Noma-Tanaka S., Adachi H., Kawano Y., Kaneko K., Ishihara S. Methemoglobinemia induced by automobile exhaust fumes. J. Anesth. 2005;19:333–335. doi: 10.1007/s00540-005-0344-y. [DOI] [PubMed] [Google Scholar]
- 105.Vevelstad M., Morild I. Lethal methemoglobinemia and automobile exhaust inhalation. Forensic Sci. Int. 2009;187:e1–e5. doi: 10.1016/j.forsciint.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 106.Kuo Y.-M., Nussbaum R.L. Prolongation of chemically-induced methemoglobinemia in mice lacking α-synuclein: a novel pharmacologic and toxicologic phenotype. Toxicol. Rep. 2016;3:295–305. doi: 10.1016/j.toxrep.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Moriya F. Poisoning due to carbon monoxide and cyanide gas generated in the occurrence of fire. Chudoku Kenkyu. 2015;28:339–345. [PubMed] [Google Scholar]
- 108.Kojima T., Yashiki M., Une I., Nishiyama Y. Post-mortem formation of carbon monoxide in a drowned body. Nihon Hoigaku Zasshi. 1980;34:163–168. [Google Scholar]
- 109.Kojima T., Nishiyama Y., Yashiki M., Une I. Postmortem formation of carbon monoxide. Forensic Sci. Int. 1982;19:243–248. doi: 10.1016/0379-0738(82)90085-8. [DOI] [PubMed] [Google Scholar]
- 110.Kojima T., Yashiki M., Une I. Experimental study on postmortem formation of carbon monoxide. Forensic Sci. Int. 1983;22:131–135. doi: 10.1016/0379-0738(83)90005-1. [DOI] [PubMed] [Google Scholar]
- 111.Kojima T., Yashiki M., Okamoto I., Noda J., Une I., Miyazaki T., Chikasue F. Postmortem formation of carbon monoxide in blood and body cavity fluids of rats drowned and kept immersed in fresh water. Hiroshima J. Med. Sci. 1984;33:591–594. [PubMed] [Google Scholar]
- 112.Kojima T., Okamoto I., Yashiki M., Miyazaki T., Chikasue F., Degawa K., Oshida S., Sagisaka K. Production of carbon monoxide in cadavers. Forensic Sci. Int. 1986;32:67–77. doi: 10.1016/0379-0738(86)90141-6. [DOI] [PubMed] [Google Scholar]
- 113.Nakao A., Kotani J. Application of therapeutic signaling gas for acute lung injury. J. Jpn. Assoc. Acute Med. 2013;24:59–68. [Google Scholar]
- 114.Hess D.R. Inhalated carbon monoxide: from toxin to therapy. Respir. Care. 2017;62:1333–1342. doi: 10.4187/respcare.05781. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.