Abstract
Stroke is a leading cause of serious long-term physical disability due to insufficient neurorepair mechanisms. In general, physical activity is an important modifiable risk factor, particularly for stroke and cardiovascular diseases. Physical exercise has shown to be neuroprotective in both animal experiments and clinical settings. Exercise can be considered a mild stressor and follows the prototypical preconditioning stimulus. It has beneficial effects on brain health and cognitive function. Preconditioning exercise, which is prophylactic exercise prior to ischemia, can protect the brain from subsequent serious injury through promotion of angiogenesis, mediation of inflammatory responses, inhibition of glutamate over-activation, protection of the blood-brain barrier, and inhibition of apoptosis. Preconditioning exercise appears to induce brain ischemic tolerance and it has been shown to exert beneficial effects. It is clinically safe and feasible and represents an exciting new paradigm in endogenous neuroprotection for patients with acute stroke. In this review, we describe the neuroprotective potential of preconditioning exercise and clinical applications in patients with acute ischemic stroke.
Keywords: preconditioning exercise, rehabilitation, stroke, neuroprotection
Stroke is a leading cause of serious long-term physical disability. Stroke survivors worldwide experience disability and severe morbidity due to motor and cognitive deficits. Since 1995, intravenous thrombolytic treatment with recombinant tissue plasminogen activator (rt-PA) has been a recommended medical therapy for acute ischemic stroke1). Unfortunately, due to difficulties in differentiating etiology and a limited therapeutic time window, rt-PA is available to only 3%-5% of the patients with stroke2,3). Although rehabilitation interventions improve the outcome, full recovery is often not achieved4).
Exercise is one of the several behavioral interventions that influence neurotrophins, neuroplasticity, and cognition5). In addition, regular exercise promotes general health and reduces the risk of hypokinetic disease-associated sedentary lifestyle6). Furthermore, regular exercise ameliorates abnormal arterial blood pressure, improves glucose and lipid metabolic disorders, reduces obesity and improves endothelial function7). Exercise enhances neurobiological processes that promote brain health in aging and disease, thus resulting in systemic beneficial effects, including the promotion of brain function6,8). It has strong effects on the immune system and alters the production of cytokines such as interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α)8). Moreover, exercise may protect the brain against degenerative events by increasing the expression of brain-derived neurotrophic factor (BDNF) in the brain and insulin-like growth factor-1 (IGF-1) in the blood9,10). A recent study has demonstrated that exercise-induced hormone irisin contributes to the neuroprotective effect of physical exercise against cerebral ischemia11). Exercise, even of moderate intensity, has systemic effects on the body, including the central nervous system8).
Animal studies have demonstrated the beneficial effects of exercise on cerebral ischemia, including amelioration of blood-brain barrier (BBB) dysfunction, maintenance of neurovascular integrity, promotion of enhanced cell survival, and fewer neurological deficits5,7,12). There is growing literature substantiating the benefits of exercise intervention on induced brain injury in animal stroke models13-16). It is now well-established that physical exercise can exert neuroprotection and neuroplasticity both in animal experiments and clinical settings7). This neuroprotective potential of prophylactic exercise as preconditioning has garnered great interest in stroke rehabilitation medicine. In this review, we consider the endogenous neuroprotective potential of preconditioning exercise, its role prior to ischemia, molecular mechanisms, and clinical application.
Exercise Prior to Ischemic Stroke (Preconditioning Exercise)
Preconditioning is defined as the exposure of a system or an organ to a conditioning stimulus to induce tolerance or resistance to a subsequent injury17). Moreover, preconditioning is an endogenous strategy that triggers cells and organisms to initiate the expression of intrinsic protective factors, thus helping them acquire tolerance and self-defense against subsequent damage1). Multiple preconditioning stimuli, such as hypoxia, ischemia, oxidative stress, anoxia, and oxidative phosphorylation inhibitors, have been studied18). However, such prophylactic treatments may be harmful to patients; therefore, more safer and feasible treatments have been sought recently7). Exercise, which can be considered a mild stressor, is a prototypical preconditioning stimulus and has beneficial effects on brain health and cognitive function5,19).
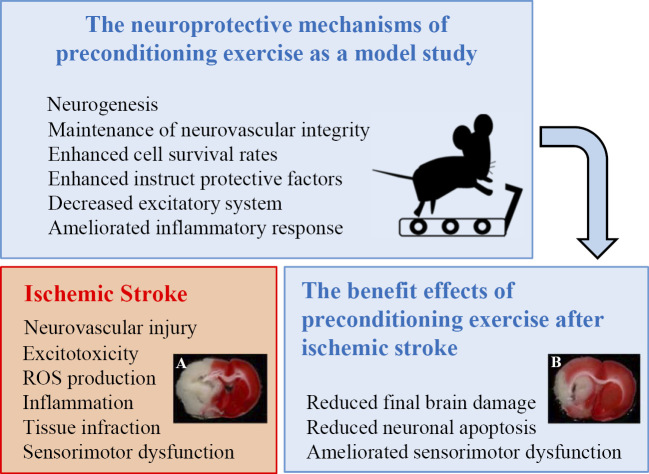
Previous animal experiments have demonstrated the beneficial effect of preconditioning exercise on stroke-induced brain injury7,19). Preconditioning exercise induces a stimuli similar to ischemia in the brain before injury, which induces tolerance or resistance to the subsequent injury such as brain ischemic tolerance. Several studies have demonstrated that preconditioning exercise provides significant neuroprotection against acute stroke through the promotion of angiogenesis, mediation of inflammatory responses, and inhibition of neuronal apoptosis20-23). Therefore, various aspects are possibly involved in the neuroprotective mechanisms of preconditioning exercise in the central nervous system (Figure 1).
Figure 1.
The neuroprotective mechanisms of preconditioning exercise in the brain. Ischemic stroke induces neurovascular injury through mechanisms such as excitotoxicity, reactive oxygen species (ROS) production, and inflammation. Preconditioning exercise, which is prophylactic exercise prior to stroke, has been shown to exert beneficial effects after stroke. In the 2,3,5-triphenyltetrazorlium chloride (TTC) study, the tissue infarction in rats with preconditioning exercise (right, white area) was decreased compared with the brain of rats without preconditioning (left).
Preconditioning Exercise in Animal Models
Most animal studies have used a model of middle cerebral artery occlusion16,24) and traumatic brain injury25). Methods of exercise preconditioning prior to ischemia have included forced treadmill running16,26) and voluntary wheel running27), both of which have shown a neuroprotective effect. Although preconditioning exercise by forced treadmill running can have beneficial effects, the stress induced by the enforcement to run is disadvantageous, thus outweighing the beneficial effects28). Previous studies have demonstrated that at least 2 or 3 weeks of exercise prior to ischemia is necessary to obtain a neuroprotective effect29,30). Forced treadmill running demands that the subjects exercise at a frequency of 30 min to 1 hour for 5-7 days per week26). Several studies have shown that moderate- or high-intensity running is effective in generating a neuroprotective effect compared with that generated with low-intensity exercise. Recently, our laboratory investigated the neuroprotective effects of different frequencies of preconditioning exercise on neuronal apoptosis using the expression of B-cell lymphoma 2 (Bcl-2) family members, such as anti-apoptotic protein Bcl-2, pro-apoptotic Bcl-2-associated X protein (Bax), and caspase-3, which is activated in the apoptotic cell. Our study demonstrated that high-intensity preconditioning exercise for three times or more per week can exert neuroprotective effects through the downregulation of the Bax/Bcl-2 ratio and caspase-3 activation after stroke31). This suggests that a frequency of at least three times per week of preconditioning exercise is necessary to obtain neuroprotective effects. Therefore, exercise as a neuroprotective preconditioning paradigm may depend on the frequency at a given exercise intensity. Therefore, exercise intensity and frequency prior to ischemia may be important contributing factors for stroke outcomes.
Potential Mechanisms of Preconditioning Exercise on Neuroprotection
Animal studies have indicated beneficial effects of preconditioning exercise on cerebral ischemia, including neurogenesis, maintenance of BBB and neurovascular integrity, enhanced cell survival rates and instruct protective factors, decreased excitatory system activation, and ameliorated inflammatory response16,26,32-35) (Figure 1).
Preconditioning Exercise and Neurogenesis
Neurogenesis affords tolerance to brain ischemia, which potentially explains the connection between exercise and neurogenesis36,37). Previous studies have indicated that exercise promotes hippocampal neurogenesis and improves short-term memory and spatial and temporal function through neurogenesis and newly formed neuronal circuitry38-40). Exercise also increases the proliferation of neuronal stem cells around the damaged area following traumatic brain injury41). Taken together, these studies suggest that exercise-induced neurogenesis is a possible mechanism explaining the reduced brain damage and improved functional recovery after stroke in preconditioned patients.
Preconditioning Exercise and BBB Integrity
The hallmark of stroke injury is endothelial dysfunction leading to BBB leakage and edema. Exercise preconditioning improves different structural and functional components of the BBB. For example, pre-ischemic exercise improves BBB function and reduces cerebral edema42,43). Additionally, pre-ischemic exercise enhances the integrity of the basal lamina after ischemic stroke by inhibiting the overexpression of matrix metalloproteinase-9 (MMP-9)42). Treadmill pre-training also ameliorates brain edema by downregulating aquaporin-4 in a rat ischemic stroke model44). Finally, pre-ischemic exercise alleviates BBB dysfunction through the extracellular signal regulated kinases (ERK1/2) pathway20). In summary, preconditioning exercise may help maintain the integrity of the BBB after stroke through several mechanisms.
Preconditioning Exercise and Neurovascular Integrity
Vascular remodeling is important in improving the outcome of stroke. Preconditioning exercise activates astrocytes and improves angiogenesis in the penumbra areas following brain ischemia16). This association of astroglial proliferation and angiogenesis with preconditioning exercise suggests that both astrocytes and endothelial cells participate in the formation of new blood vessels in the brain. Preconditioning exercise protects the brain from ischemia through improved cerebral blood flow and regulation of endothelin 1 (ET-1) in a rat model of ischemia34). Also, physical exercise increases levels of insulin-like growth factor 1 (IGF-1) and vascular endothelial growth factor (VEGF) in the serum or brain9,25,45). IGF-1 and VEGF both play a vital role in cerebral vasculature angiogenesis. Additionlly, preconditioning exercise elevates midkine (MK) levels after ischemic stroke16), which is a heparin-binding growth factor which is neurotropic and promotes angiogenesis46). Taken together, these studies show that preconditioning exercise may improve cerebral blood flow and regulate angiogenesis of cerebral vasculature following experimentally induced ischemic stroke.
Preconditioning Exercise and Cell Survival Activity
Preconditioning exercise enhances cell survival rates in the penumbral region surrounding ischemia in a model of rat stroke16). The ischemic core consists of tissue necrosis, while the penumbra region surrounding the core shows signs of apoptosis. Thus, the penumbra region can provide neuroprotection after ischemia. Preconditioning exercise can reduce apoptotic activity in the penumbral region by enhancing the expression of neurotrophic factors such as MK and BDNF16). These factors promote neurite outgrowth and enhance neuronal activity47,48), thereby providing a neuroprotective and regenerative role in cerebral ischemia. Heat shock protein (HSP-70) and ERK-mediated signal pathways have been shown to be involved in ischemia-induced apoptosis7). Preconditioning exercise diminishes neuronal injury by upregulating HSP-70 and ERK1/2 in a rat model of stroke30). Moreover, exercise-induced neuroprotection is mediated by reduced MMP-9 expression in a rat stroke model49,50). Bcl-2 plays a pivotal role in the control of cell death and is upregulated by ischemic tolerance51). Our previous work demonstrated that preconditioning exercise exerts neuroprotective effects through the downregulation of the Bax/Bcl-2 ratio and caspase-3 activation after stroke31). Bcl-2 and Bax are anti- and pro-apoptotic proteins, respectively, while caspase-3 is activated in the apoptotic cell.
Preconditioning Exercise and Intrinsic Protective Factors
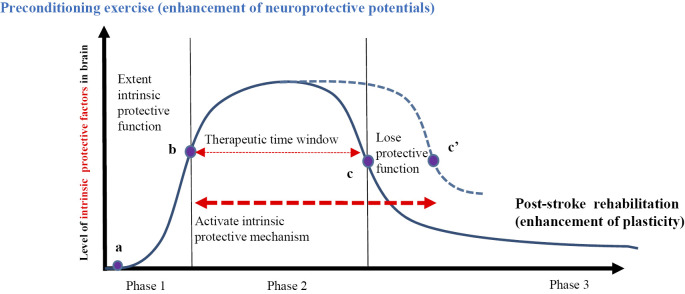
A number of intracellular intrinsic factors are present and can be activated to protect cells from injury, including cerebral ischemia1). Hypoxia-induced factor-1α (HIF-1α) is one of the most important transcriptional factors implicated in the hypoxic or ischemic brain and plays a key role in neuroprotection against ischemic brain injury1,52). In addition, 14-3-3γ is an isotype of the 14-3-3 protein family (β, η, γ, δ, τ, ζ, and ε) and is most abundantly found in the brain1,53). The γ member of the 14-3-3 family exerts pleiotropic effects during various physiological processes, such as cell proliferation and anti-apoptosis54,55). Our previous study demonstrated that preconditioning exercise activates HIF-1α and 14-3-3γ in neurons and astrocytes and subsequently induced neuron- and astrocyte-mediated brain tolerance35). The upregulated 14-3-3γ induced by preconditioning exercise reduces ischemic neuronal cell death through the 14-3-3γ/p-β-catenin Ser37/Bax/caspase 3 anti-apoptotic pathway after ischemic stroke in rats35). Understanding the mode of induction and mechanism of protection for these intrinsic protective factors would be beneficial for administering preconditioning exercise to extend the therapeutic time window, leading to better management of patients with stroke (Figure 2).
Figure 2.
Schema of the time course of acute stroke. In the ischemic brain, neuroprotective factors increase during the early phase from a to b (phase 1). The level of protective factor expression peaks from b to c, the therapeutic time window (phase 2). Preconditioning exercise increases intrinsic protective factors in the penumbra region and may extend the therapeutic time window by enhancing the expression of intrinsic protective factors (from c to c'). During phase 3, the level of protective factor expression reduces. In this phase, post-stroke rehabilitation is important to enhance brain plasticity and functional recovery for stroke survivors.
Preconditioning Exercise and Excitatory System
The excessive release of excitatory neurotransmitter glutamate after stroke leads to neuronal excitotoxicity and subsequent brain damage13). Preconditioning exercise reduces the overexpression of glutamate and its receptors, metabotropic glutamate receptor 5 (mGluR5) and N-methyl-D-aspartate receptor subunit type 2B (NR2B) in rat ischemic stroke models56). Another study showed that preconditioning exercise prior to ischemic reperfusion increased the antioxidant ability and decreased the oxidative damage in the rat brain22). Apoptosis and oxidative damage play critical roles following ischemic reperfusion injury. We have shown that preconditioning exercise after ischemic reperfusion reduces peroxynitrite-induced neurotoxicity in the brain16), which suggests that preconditioning exercise decreases oxidative damage to the brain following ischemic reperfusion injury.
Preconditioning Exercise and Inflammatory Response
Brain ischemia induces a serious inflammatory response. Various inflammatory mediators such as TNF-α or IL-6 are released by ischemic brain cells; these mediators can exacerbate the deleterious effects of ischemic brain injury57). Preconditioning inhibits inflammatory injury by reducing the expression of inflammatory mediators as well as reducing the accumulation of leukocytes during reperfusion, thus leading to reduced brain damage13). Preconditioning exercise exerts neuroprotective effects through the regulation of the toll-like receptor (TLR) 4/nuclear factor-κB (NF-κB) signaling pathway and reduced inflammatory mediators (TNF-α or IL-1β) in the peripheral serum during ischemic reperfusion injury24). In summary, preconditioning exercise might ameliorate inflammatory responses by regulating inflammatory cascades in ischemic stroke.
Preconditioning Exercise and Clinical Application
Physical activity is one of the lifestyle factors that are associated with a reduced risk of stroke. Meta-analyses of the physical activity and stroke risk relationship have demonstrated that leisure-time physical activity reduces the risk of total, ischemic, and hemorrhagic stroke58). While many studies have investigated the association between physical activity and risk of stroke, few clinical studies have explored whether prestroke physical activity is associated with better functional outcome of stroke or stroke severity.
Retrospective studies have shown that patients with stroke who reported regular exercising before stroke onset have milder strokes and better functional outcomes after stroke59-63). In a retrospective clinical study of 673 patients with stroke, a high or moderate level of physical activity was associated with a high Barthel Index (BI) score at enrollment and 3-months follow-up as part of the Ischemic Stroke Genetics Study61). Another retrospective clinical study of 265 patients with stroke, which represents a subset of patients with first-time stroke enrolled in the ExStroke Pilot Trial, showed that physical activity prior to stroke was associated with a milder stroke and better long-term outcomes60). In a cross-sectional study of 362 patients with acute ischemic stroke admitted to the Stroke Unit of Lille University Hospital, less severe stroke was associated with the duration of weekly exercise prior to stroke using the National Institutes of Health Stroke Scale, modified Rankin scale (nRS), and BI, thus suggesting that physical activity is a simple way to decrease cerebral ischemia severity63). Taken together, these clinical studies suggest that prestroke physical activity decreases the severity of stroke and improves the subsequent functional outcome.
However, a large prospective cohort study enrolling healthy men without a history of stroke at baseline, showed little evidence that prestroke physical activity influences functional outcome after stroke64). In another prospective observational multicenter study conducted in French and Japanese patients with stroke treated with intravenous recombinant tissue plasminogen activator (rt-PA), prestroke physical activity had little or no influence on outcome 3 months after treatment for cerebral ischemia with rt-PA65). A prospective clinical study monitored 18,117 adults without a history of stroke for 12 years and reported that physical inactivity before stroke was associated with a higher risk of being dependent both before and after a stroke event66). These studies show that the beneficial effects of physical activity prior to stroke are controversial in clinical settings. A possible explanation for this discrepancy in study findings might be related to the different populations and design of studies. In addition, the molecular mechanism underling preconditioning exercise in stroke has not yet been explored in detail. In a review of exercise studies in elderly individuals, peripheral markers such as BDNF, IGF-1, and VEGF were potentially useful indicators of neuroplasticity67). However, additional longitudinal studies are required to examine the beneficial effects of physical activity prior to stroke.
As mentioned before, preconditioning using a preceding sublethal ischemic insult is an attractive strategy for protecting neurons by inducing ischemic tolerance in the brain. Physical exercise may be a promising preconditioning method to induce brain ischemic tolerance after stroke; however, some elderly patients are unable or unwilling to exercise after stroke. In such patients, remote ischemic conditioning (RIC) can be potentially effective in inducing neuroprotection in patients with a neuronal disorder. RIC triggers endogenous protective pathways in distant organs such as the heart and brain, thereby inducing neuroprotection. RIC involves the repetitive inflation and deflation of a blood pressure cuff on the limb; it has been shown to improve cerebral circulation in patients with symptomatic intracranial arterial stenosis and is a safe and effective therapy for elderly patients68). In addition, RIC is potentially effective in patients with cerebral small-vessel disease in slowing cognitive decline and reducing white matter hyperintensities69). It is a feasible therapeutic approach with good compliance for targeted population. The experimental evidence suggests that RIC and physical exercise share common mechanisms70); therefore, preconditioning exercise can be considered a form of remote conditioning, and conversely, RIC can be viewed as an equivalent to preconditioning exercise in patients unable or unwilling to exercise.
Conclusion
In conclusion, preconditioning exercise appears to induce brain ischemia tolerance and has been shown to exert beneficial effects in both preclinical and clinical studies. Elucidating the mechanisms of preconditioning exercise-induced neuroprotection after stroke will help in the development of new treatment strategies for patients with stroke. Clinically, preconditioning exercise and remote preconditioning exercise are safe and feasible, thus representing an exciting new paradigm in endogenous neuroprotection for patients with acute and chronic stroke.
Conflict of Interest
The author declares no conflicts of interest.
Acknowledgments
This work was supported by a grant from JSPS KAKENHI (grant No. JP17K01459 to Harutoshi Sakakima).
References
- 1. Dong Y, Zhao R, et al.: 14-3-3gamma and neuroglobin are new intrinsic protective factors for cerebral ischemia. Mol Neurobiol. 2010; 41: 218-231. [DOI] [PubMed] [Google Scholar]
- 2. Fonarow GC, Smith EE, et al.: Timeliness of tissue-type plasminogen activator therapy in acute ischemic stroke: patient characteristics, hospital factors, and outcomes associated with door-to-needle times within 60 minutes. Circulation. 2011; 123: 750-758. [DOI] [PubMed] [Google Scholar]
- 3. Kikuchi K, Tancharoen S, et al.: The efficacy of edaravone (radicut), a free radical scavenger, for cardiovascular disease. Int J Mol Sci. 2013; 14: 13909-13930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Langhorne P, Bernhardt J, et al.: Stroke rehabilitation. Lancet. 2011; 377: 1693-1702. [DOI] [PubMed] [Google Scholar]
- 5. Ploughman M, Austin MW, et al.: The effects of poststroke aerobic exercise on neuroplasticity: a systematic review of animal and clinical studies. Transl Stroke Res. 2015; 6: 13-28. [DOI] [PubMed] [Google Scholar]
- 6. White LJ and Castellano V: Exercise and brain health--implications for multiple sclerosis: Part 1--neuronal growth factors. Sports Med. 2008; 38: 91-100. [DOI] [PubMed] [Google Scholar]
- 7. Zhang F, Wu Y, et al.: Exercise preconditioning and brain ischemic tolerance. Neuroscience. 2011; 177: 170-176. [DOI] [PubMed] [Google Scholar]
- 8. Radak Z, Suzuki K, et al.: Physical exercise, reactive oxygen species and neuroprotection. Free Radic Biol Med. 2016; 98: 187-196. [DOI] [PubMed] [Google Scholar]
- 9. Carro E, Nuñez A, et al.: Circulating insulin-like growth factor I mediates effects of exercise on the brain. J Neurosci. 2000; 20: 2926-2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cotman CW and Berchtold NC: Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002; 25: 295-301. [DOI] [PubMed] [Google Scholar]
- 11. Li DJ, Li YH, et al.: The novel exercise-induced hormone irisin protects against neuronal injury via activation of the Akt and ERK1/2 signaling pathways and contributes to the neuroprotection of physical exercise in cerebral ischemia. Metabolism. 2017; 68: 31-42. [DOI] [PubMed] [Google Scholar]
- 12. Wang X, Zhang M, et al.: Physical exercise training and neurovascular unit in ischemic stroke. Neuroscience. 2014; 271: 99-107. [DOI] [PubMed] [Google Scholar]
- 13. Ding YH, Young CN, et al.: Exercise preconditioning ameliorates inflammatory injury in ischemic rats during reperfusion. Acta Neuropathol. 2005; 109: 237-246. [DOI] [PubMed] [Google Scholar]
- 14. Matsuda F, Sakakima H, et al.: The effects of early exercise on brain damage and recovery after focal cerebral infarction in rats. Acta Physiol (Oxf). 2011; 201: 275-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sakakima H, Khan M, et al.: Stimulation of functional recovery via the mechanisms of neurorepair by S-nitrosoglutathione and motor exercise in a rat model of transient cerebral ischemia and reperfusion. Restor Neurol Neurosci. 2012; 30: 383-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Otsuka S, Sakakima H, et al.: The neuroprotective effects of preconditioning exercise on brain damage and neurotrophic factors after focal brain ischemia in rats. Behav Brain Res. 2016; 303: 9-18. [DOI] [PubMed] [Google Scholar]
- 17. Baillieul S, Chacaroun S, et al.: Hypoxic conditioning and the central nervous system: A new therapeutic opportunity for brain and spinal cord injuries? Exp Biol Med (Maywood). 2017; 242: 1198-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dirnagl U, Becker K, et al.: Preconditioning and tolerance against cerebral ischaemia: from experimental strategies to clinical use. Lancet Neurol. 2009; 8: 398-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Islam MR, Young MF, et al.: Neuroprotective potential of exercise preconditioning in stroke. Cond Med. 2017; 1: 27-34. [PMC free article] [PubMed] [Google Scholar]
- 20. Guo M, Lin V, et al.: Preischemic induction of TNF-alpha by physical exercise reduces blood-brain barrier dysfunction in stroke. J Cereb Blood Flow Metab. 2008; 28: 1422-1430. [DOI] [PubMed] [Google Scholar]
- 21. Dornbos D 3rd, Zwagerman N, et al.: Preischemic exercise reduces brain damage by ameliorating metabolic disorder in ischemia/reperfusion injury. J Neurosci Res. 2013; 91: 818-827. [DOI] [PubMed] [Google Scholar]
- 22. Feng R, Zhang M, et al.: Pre-ischemic exercise alleviates oxidative damage following ischemic stroke in rats. Exp Ther Med. 2014; 8: 1325-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aboutaleb N, Shamsaei N, et al.: Pre-ischemic exercise reduces apoptosis in hippocampal CA3 cells after cerebral ischemia by modulation of the Bax/Bcl-2 proteins ratio and prevention of caspase-3 activation. J Physiol Sci. 2015; 65: 435-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhu L, Ye T, et al.: Exercise Preconditioning regulates the toll-like receptor 4/nuclear factor-κB signaling pathway and reduces cerebral ischemia/reperfusion inflammatory injury: A study in rats. J Stroke Cerebrovasc Dis. 2016; 25: 2770-2779. [DOI] [PubMed] [Google Scholar]
- 25. Taylor JM, Montgomery MH, et al.: Exercise preconditioning improves traumatic brain injury outcomes. Brain Res. 2015; 1622: 414-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ding YH, Li J, et al.: Exercise preconditioning upregulates cerebral integrins and enhances cerebrovascular integrity in ischemic rats. Acta Neuropathol. 2006; 112: 74-84. [DOI] [PubMed] [Google Scholar]
- 27. Kalogeraki E, Pielecka-Fortuna J, et al.: Physical exercise preserves adult visual plasticity in mice and restores it after a stroke in the somatosensory cortex. Front Aging Neurosci. 2016; 8: 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Svensson M, Rosvall P, et al.: Forced treadmill exercise can induce stress and increase neuronal damage in a mouse model of global cerebral ischemia. Neurobiol Stress. 2016; 5: 8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang RY, Yang YR, et al.: Protective effects of treadmill training on infarction in rats. Brain Res. 2001; 922: 140-143. [DOI] [PubMed] [Google Scholar]
- 30. Liebelt B, Papapetrou P, et al.: Exercise preconditioning reduces neuronal apoptosis in stroke by up-regulating heat shock protein-70 (heat shock protein-72) and extracellular-signal-regulated-kinase 1/2. Neuroscience. 2010; 166: 1091-1100. [DOI] [PubMed] [Google Scholar]
- 31. Terashi T, Otsuka S, et al.: Neuroprotective effects of different frequency preconditioning exercise on neuronal apoptosis after focal brain ischemia in rats. Neurol Res. 2019; 41: 510-518. [DOI] [PubMed] [Google Scholar]
- 32. Ding Y, Li J, et al.: Exercise pre-conditioning reduces brain damage in ischemic rats that may be associated with regional angiogenesis and cellular overexpression of neurotrophin. Neuroscience. 2004; 124: 583-591. [DOI] [PubMed] [Google Scholar]
- 33. Zhang F, Wu Y, et al.: Pre-ischemic treadmill training induces tolerance to brain ischemia: involvement of glutamate and ERK1/2. Molecules. 2010; 15: 5246-5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang Q, Zhang L, et al.: The effects of exercise preconditioning on cerebral blood flow change and endothelin-1 expression after cerebral ischemia in rats. J Stroke Cerebrovasc Dis. 2014; 23: 1696-1702. [DOI] [PubMed] [Google Scholar]
- 35. Otsuka S, Sakakima H, et al.: Preconditioning exercise reduces brain damage and neuronal apoptosis through enhanced endogenous 14-3-3γ after focal brain ischemia in rats. Brain Struct Funct. 2019; 224: 727-738. [DOI] [PubMed] [Google Scholar]
- 36. Rhodes JS, van Praag H, et al.: Exercise increases hippocampal neurogenesis to high levels but does not improve spatial learning in mice bred for increased voluntary wheel running. Behav Neurosci. 2003; 117: 1006-1016. [DOI] [PubMed] [Google Scholar]
- 37. Maysami S, Lan JQ, et al.: Proliferating progenitor cells: a required cellular element for induction of ischemic tolerance in the brain. J Cereb Blood Flow Metab. 2008; 28: 1104-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brown J, Cooper-Kuhn CM, et al.: Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur J Neurosci. 2003; 17: 2042-2046. [DOI] [PubMed] [Google Scholar]
- 39. Bednarczyk MR, Aumont A, et al.: Prolonged voluntary wheel-running stimulates neural precursors in the hippocampus and forebrain of adult CD1 mice. Hippocampus. 2009; 19: 913-927. [DOI] [PubMed] [Google Scholar]
- 40. Sah N, Peterson BD, et al.: Running reorganizes the circuitry of one-week-old adult-born hippocampal neurons. Sci Rep. 2017; 7: 10903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Itoh T, Imano M, et al.: Exercise increases neural stem cell proliferation surrounding the area of damage following rat traumatic brain injury. J Neural Transm (Vienna). 2011; 118: 193-202. [DOI] [PubMed] [Google Scholar]
- 42. Guo M, Cox B, et al.: Pre-ischemic exercise reduces matrix metalloproteinase-9 expression and ameliorates blood-brain barrier dysfunction in stroke. Neuroscience. 2008; 151: 340-351. [DOI] [PubMed] [Google Scholar]
- 43. Shamsaei N, Erfani S, et al.: Neuroprotective Effects of exercise on brain edema and neurological Movement disorders following the cerebral ischemia and reperfusion in rats. Basic Clin Neurosci. 2017; 8: 77-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. He Z, Wang X, et al.: Treadmill pre-training ameliorates brain edema in ischemic stroke via down-regulation of aquaporin-4: an MRI study in rats. PLoS One. 2014; 9: e84602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cotman CW, Berchtold NC, et al.: Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007; 30: 464-472. [DOI] [PubMed] [Google Scholar]
- 46. Muramatsu T: Midkine and pleiotrophin: two related proteins involved in development, survival, inflammation and tumorigenesis. J Biochem. 2002; 132: 359-371. [DOI] [PubMed] [Google Scholar]
- 47. Yoshida Y, Sakakima H, et al.: Midkine in repair of the injured nervous system. Br J Pharmacol. 2014; 171: 924-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ho VM, Lee JA, et al.: The cell biology of synaptic plasticity. Science. 2011; 334: 623-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhang FY, Chen XC, et al.: Effects of ischemic preconditioning on blood-brain barrier permeability and MMP-9 expression of ischemic brain. Neurol Res. 2006; 28: 21-24. [DOI] [PubMed] [Google Scholar]
- 50. Chaudhry K, Rogers R, et al.: Matrix metalloproteinase-9 (MMP-9) expression and extracellular signal-regulated kinase 1 and 2 (ERK1/2) activation in exercise-reduced neuronal apoptosis after stroke. Neurosci Lett. 2010; 474: 109-114. [DOI] [PubMed] [Google Scholar]
- 51. Meller R, Minami M, et al.: CREB-mediated Bcl-2 protein expression after ischemic preconditioning. J Cereb Blood Flow Metab. 2005; 25: 234-246. [DOI] [PubMed] [Google Scholar]
- 52. Yang J, Liu C, et al.: Hypoxia inducible factor 1α plays a key role in remote ischemic preconditioning against stroke by modulating inflammatory responses in rats. J Am Heart Assoc. 2018; 7: e007589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chen XQ, Chen JG, et al.: 14-3-3gamma is upregulated by in vitro ischemia and binds to protein kinase Raf in primary cultures of astrocytes. Glia. 2003; 42: 315-324. [DOI] [PubMed] [Google Scholar]
- 54. Zhao J, Meyerkord CL, et al.: 14-3-3 proteins as potential therapeutic targets. Semin Cell Dev Biol. 2011; 22: 705-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pang Y, Chai CR, et al.: Ischemia preconditioning protects astrocytes from ischemic injury through 14-3-3γ. J Neurosci Res. 2015; 93: 1507-1518. [DOI] [PubMed] [Google Scholar]
- 56. Zhang F, Jia J, et al.: The effect of treadmill training pre-exercise on glutamate receptor expression in rats after cerebral ischemia. Int J Mol Sci. 2010; 11: 2658-2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mizuma A and Yenari MA: Anti-inflammatory targets for the treatment of reperfusion injury in stroke. Front Neurol. 2017; 8: 467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lee CD, Folsom AR, et al.: Physical activity and stroke risk: a meta-analysis. Stroke. 2003; 34: 2475-2481. [DOI] [PubMed] [Google Scholar]
- 59. Deplanque D, Masse I, et al.: Prior TIA, lipid-lowering drug use, and physical activity decrease ischemic stroke severity. Neurology. 2006; 67: 1403-1410. [DOI] [PubMed] [Google Scholar]
- 60. Krarup LH, Truelsen T, et al.: Prestroke physical activity is associated with severity and long-term outcome from first-ever stroke. Neurology. 2008; 71: 1313-1318. [DOI] [PubMed] [Google Scholar]
- 61. Stroud N, Mazwi TM, et al.: Prestroke physical activity and early functional status after stroke. J Neurol Neurosurg Psychiatry. 2009; 80: 1019-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ricciardi AC, López-Cancio E, et al.: Prestroke physical activity is associated with good functional outcome and arterial recanalization after stroke due to a large vessel occlusion. Cerebrovasc Dis. 2014; 37: 304-311. [DOI] [PubMed] [Google Scholar]
- 63. Deplanque D, Masse I, et al.: Previous leisure-time physical activity dose dependently decreases ischemic stroke severity. Stroke Res Treat. 2012; 2012: 614925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rist PM, Lee IM, et al.: Physical activity and functional outcomes from cerebral vascular events in men. Stroke. 2011; 42: 3352-3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Decourcelle A, Moulin S, et al.: Influence of previous physical activity on the outcome of patients treated by thrombolytic therapy for stroke. J Neurol. 2015; 262: 2513-2519. [DOI] [PubMed] [Google Scholar]
- 66. Rist PM, Capistrant BD, et al.: Physical activity, but not body mass index, predicts less disability before and after stroke. Neurology. 2017; 88: 1718-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Voss MW, Erickson KI, et al.: Neurobiological markers of exercise-related brain plasticity in older adults. Brain Behav Immun. 2013; 28: 90-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Meng R, Ding Y, et al.: Ischemic Conditioning is safe and effective for octo- and nonagenarians in stroke prevention and treatment. Neurotherapeutics. 2015; 12: 667-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wang Y, Meng R, et al.: Remote ischemic conditioning may improve outcomes of patients with cerebral small-vessel disease. Stroke. 2017; 48: 3064-3072. [DOI] [PubMed] [Google Scholar]
- 70. Hess DC and Blauenfeldt RA: Remote ischaemic conditioning-a new paradigm of self-protection in the brain. Nat Rev Neurol. 2015; 11: 698-710. [DOI] [PubMed] [Google Scholar]