Introduction
Tumor necrosis factor (TNF) inhibitor–induced palmoplantar pustulosis (PPP) can present with debilitating, refractory disease that requires changing or stopping anti-TNF agents or adding systemic treatments. Here, we report an update of a previously reported case of severely recalcitrant PPP successfully treated with the Janus kinase (JAK) inhibitor tofacitinib.
Case report
A white woman in her 40s initially presented with vesiculopustules on her palms and soles, clinically diagnosed as PPP, in the setting of adalimumab treatment for Crohn's disease. Despite cessation of adalimumab, the patient's eruption persisted. Throughout the course of her care, she failed to respond to multiple treatment regimens (Table I). Eventually, she had complete clearance after 4 doses of ustekinumab 45 mg subcutaneous injections, with remission achieved for several months, and her case was reported in Archives of Dermatology.1
Table I.
Therapies and treatment response
| Date started | Date ended | Medication | Reason for discontinuation |
|---|---|---|---|
| Before first appointment | June 2008 | Efalizumab | Worsening of symptoms |
| June 2008 | July 2008 | Cyclosporine 400 mg | Esophagitis |
| July 2008 | August 2008 | Intravenous cyclosporine | Issues with midline access, pain |
| August 2008 | August 2008 | Mycophenolic acid | Worsening of symptoms |
| August 2008 | October 2008 | Psoralen plus ultraviolet A with oxsoralen ×8 | Sustained burn and stopped Mild improvement |
| October 2008 | March 2009 | Topical steroid and oral steroid taper | Worsening of symptoms |
| May 2009 | July 2009 | Alefacept | Worsening of symptoms |
| August 2009 | February 2010 | Cyclosporine 200 mg | Creatinine rise |
| October 2009 | February 2010 | Isotretinoin | No improvement |
| February 2010 | November 2010 | Ustekinumab 45 mg ×4 injections | Completely clear with residual response |
| November 2010 | February 2013 | Topical dapsone, cyclosporine | Residual response from ustekinumab |
| February 2013 | June 2013 | Cyclosporine 200 mg | Creatinine rise |
| June 2013 | November 2013 | Ustekinumab 90 mg ×4 injections | No improvement |
| January 2014 | March 2014 | Anakinra | National Institutes of Health trial, limited by adverse effects (severe injection site reaction, headache) |
| April 2014 | July 2014 | Cyclosporine 200 mg | Creatinine rise |
| April 2014 | July 2014 | Methotrexate | Creatinine rise |
| September 2014 | December 2014 | Acitretin | No improvement |
| September 2014 | December 2014 | Cyclosporine 200 mg | Nausea and vomiting |
| January 2015 | November 2017 | Apremilast | No improvement by itself Clear in combination with tocilizumab initially Ultimately relapsed with residual disease |
| May 2015 | November 2017 | Tocilizumab | No improvement by itself Clear in combination with apremilast initially Ultimately relapsed with residual disease |
| November 2017 | November 2017 | Cyclosporine 200 mg | Flare requiring short-term cyclosporine |
| December 2017 | December 2017 | Guselkumab 100 mg ×1 injection | No improvement |
| June 2018 | Present | Tofacitinib | Completely clear |
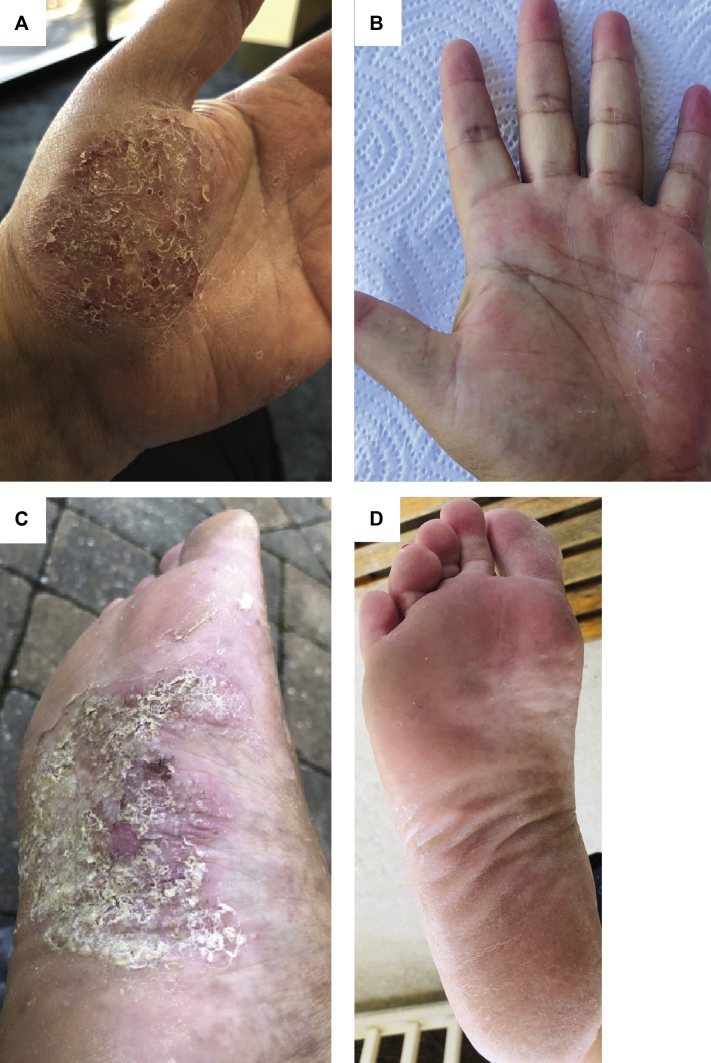
Over time, however, the patient experienced worsening of her disease and subsequently failed to improve despite an increased dose of 90 mg ustekinumab. Because of debilitating symptoms, she was intermittently treated with cyclosporine at low doses. She had complete clearance temporarily while taking a combination of apremilast and tocilizumab, but she was unable to be tapered off either medication without a recurrence of symptoms, and she ultimately relapsed with active disease despite combination therapy. She was started on tofacitinib 5 mg tablets twice daily. Since initiation of this medication, her PPP has cleared completely without intermittent flares (Fig 1, A-D). During a follow-up period of 1 year after initiation of tofacitinib, she was able to discontinue all other topical and systemic immunosuppressive agents. Her Crohn's disease was in remission for the duration of her treatment for PPP, without flares of her gastrointestinal disease on any of the medications.
Fig 1.
The left hand (A) before and (B) after tofacitinib initiation and the right foot (C) before and (D) after tofacitinib initiation.
Discussion
Because TNF inhibitor–induced PPP remains a relatively uncommon, understudied phenomenon, its pathophysiology and long-term treatment have not been well established. Here, we present a case of refractory TNF inhibitor–induced PPP that improved with tofacitinib, a JAK inhibitor.
Tofacitinib is an oral JAK inhibitor that inhibits the JAK–signal transducer and activator of transcription pathway, with the greatest effect on JAK1 and JAK3. It decreases the production of a multitude of cytokines, most notably interferon γ, interleukin 6, and interleukin 17A,2 which have been shown to play a role in the pathogenesis of PPP.3 However, because tofacitinib has also been implicated as a trigger for PPP, additional cytokines may be involved.4
Consistent with our current findings, a previous case report has shown the success of tofacitinib for recalcitrant TNF inhibitor–induced PPP in the setting of rheumatoid arthritis treatment.5 We recommend consideration of the use of tofacitinib as a potential long-term management agent for refractory TNF inhibitor–induced PPP. We also hope to encourage further investigation of this agent.
Footnotes
Funding sources: None.
Disclosures: Dr Rosenbach is the deputy editor of JAMA Dermatology. He has received research support from Processa Pharma and consulting fees from Merck, aTyr, and Processa. Ms Wang has no conflicts of interest to declare.
References
- 1.Chu D.H., Van Voorhees A.S., Rosenbach M. Treatment of refractory tumor necrosis factor inhibitor–induced palmoplantar pustulosis: a report of 2 cases. Arch Dermatol. 2011;147(10):1228–1230. doi: 10.1001/archdermatol.2011.275. [DOI] [PubMed] [Google Scholar]
- 2.Ghoreschi K., Gadina M. Jakpot! New small molecules in autoimmune and inflammatory diseases. Exp Dermatol. 2014;23(1):7–11. doi: 10.1111/exd.12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bissonnette R., Nigen S., Langley R.G. Increased expression of IL-17A and limited involvement of IL-23 in patients with palmo-plantar (PP) pustular psoriasis or PP pustulosis; results from a randomised controlled trial. J Eur Acad Dermatol Venereol. 2014;28(10):1298–1305. doi: 10.1111/jdv.12272. [DOI] [PubMed] [Google Scholar]
- 4.Shibata T., Muto J., Hirano Y. Palmoplantar pustulosis–like eruption following tofacitinib therapy for juvenile idiopathic arthritis. JAAD Case Rep. 2019;5(6):518–521. doi: 10.1016/j.jdcr.2019.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koga T., Sato T., Umeda M. Successful treatment of palmoplantar pustulosis with rheumatoid arthritis, with tofacitinib: impact of this JAK inhibitor on T-cell differentiation. Clin Immunol. 2016;173:147. doi: 10.1016/j.clim.2016.10.003. [DOI] [PubMed] [Google Scholar]