Introduction
Anaplastic large-cell lymphoma (ALCL), a type of peripheral T-cell lymphoma, is a rare form of non-Hodgkin lymphoma, comprising 1% to 2% of non-Hodgkin lymphoma in adults in the United States.1 There are 2 major types of ALCL: systemic ALCL (S-ALCL) and primary cutaneous ALCL. S-ALCL is the more aggressive form, with high rates of metastasis and a poor prognosis. S-ALCL is divided into 2 subgroups based on the expression of anaplastic lymphoma kinase (ALK): ALK- and ALK+. The ALK- subtype is more likely to relapse than the ALK+ subtype, despite having a good initial response to standard chemotherapy.2 Approximately 10% to 20% of patients with S-ALCL may experience skin metastasis, with a higher prevalence in the ALK- subgroup.3 We present a case with rapid onset and rapid resolution of skin metastases in a patient with ALK- S-ALCL.
Case report
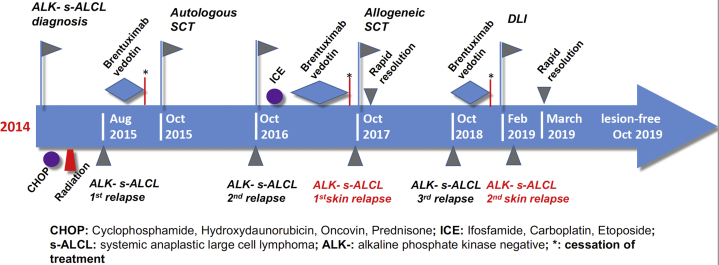
A 47-year-old white man with a past medical history of ALCL, hypertension, congestive heart failure, renal insufficiency, and hepatitis C infection presented with multiple light pink annular papules and nodules with central ulceration on his face and neck in February 2019 (Fig 1). ALK- S-ALCL was originally diagnosed in 2014. He was treated with cyclophosphamide, doxorubicin, vincristine, and prednisone for 6 cycles followed by radiation therapy, and he achieved complete remission for 1 year. In June 2015, he presented with left-sided inguinal lymphadenopathy, which was positive for a relapse of ALK- S-ALCL. He subsequently received 4 cycles of brentuximab vedotin followed by an autologous stem cell transplant (SCT) in October 2015. One year after SCT, he experienced relapse in the right inguinal lymph nodes and underwent right-sided groin lymphadenectomy with adjuvant chemotherapy with ifosfamide, carboplatin, and etoposide therapy for 3 cycles and brentuximab vedotin for 7 months.
Fig 1.
Gross appearance of skin lesions: multiple light pink annular papules and nodules with central ulceration on his face and neck. Photo was taken on February 14, 2019.
The patient was scheduled to undergo a second SCT, this time an allogeneic bone marrow transplant, in October 2017, and his chemotherapy regimen was stopped 3 weeks before the procedure. It was during this period when he first presented with cutaneous metastases. The skin lesions rapidly resolved 10 days after the SCT. In October 2018, he experienced another relapse in the left cervical lymph nodes, and brentuximab vedotin was resumed, with complete remission in 2 months.
Brentuximab vedotin infusion was discontinued in early January 2019 because of peripheral neuropathy. Instead, the patient received a donor lymphocyte infusion (DLI) to boost graft-versus-tumor effect from his prior SCT. About 10 days after DLI, he developed recurrence of skin lesions with a low-grade fever, fatigue, nausea, vomiting, and right-sided cervical lymphadenopathy.
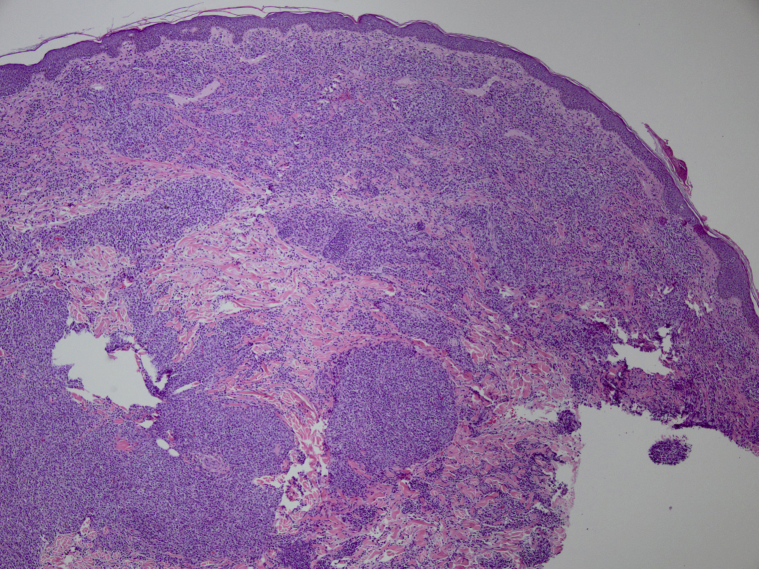
Biopsy samples taken of these lesions showed a patchy to nodular dermal infiltrate of variable and atypical mononuclear cells. Immunohistochemistry showed positive staining of CD3 and CD30 but negative staining for CD20, epithelial membrane antigen, and ALK (Fig 2). The skin lesions rapidly disappeared 2 weeks later, without the need for additional treatment. A follow-up visit in mid-March 2019 indicated no cervical lymphadenopathy. As of October 2019, the patient was not receiving any chemotherapy or brentuximab vedotin treatment (Fig 3).
Fig 2.
Histologic analysis of biopsy samples confirms the diagnosis of skin metastasis of ALK- systemic anaplastic large-cell lymphoma. ALK, Anaplastic lymphoma kinase.
Fig 3.
The timeline of the patient's medical history (2014-2019). ALK, Anaplastic lymphoma kinase; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; DLI, donor lymphocyte infusion; ICE, ifosfamide, carboplatin, etoposide; S-ALCL, systemic anaplastic large-cell lymphoma; SCT, stem cell transplant.
Discussion
The ALK- subtype of S-ALCL is a rare form of non-Hodgkin lymphoma that is not well studied. Several reports addressed cutaneous metastases in patients with ALK- S-ALCL.4, 5, 6, 7, 8 These lesions appear to be heterogeneous, ranging from erythematous, ulcerated papules and plaques on the head, neck, and trunk to violaceous nodules and tumors in the perineal region. The timeline of cutaneous metastasis is typically between 3 months and 4 years after initial diagnosis of S-ALCL. Our patient was treated according to the National Comprehensive Cancer Network guideline,9 whereby he received the first-line therapy with 6 cycles of cyclophosphamide, doxorubicin, vincristine, and prednisone/radiation therapy and the second-line therapy with brentuximab vedotin, autologous SCT, and allogeneic SCT sequentially (Table I). To our knowledge, no prior studies have clarified the typical timeline for resolution of cutaneous metastasis after treatment.
Table I.
National Comprehensive Cancer Network guideline for management of anaplastic T-cell lymphoma
| Treatment | Treatment options | Test results | Treatment options |
|---|---|---|---|
| First-line treatment |
|
No signs of cancer |
|
| Cancer looks smaller, the same, or larger |
|
||
| Second-line treatment with SCT |
|
No signs of cancer or the cancer looks smaller |
|
| Cancer looks the same or larger |
|
||
| Second-line treatment without SCT |
|
||
CHOEP, Cyclophosphamide, doxorubicin, vincristine, etoposide, and prednisone; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; DHAP, dexamethasone, high-dose Ara-cytarabine, and platinol; EPOCH, etoposide, prednisolone, oncovin, cyclophosphamide, and hydroxydaunorubicin; ESHAP, etoposide, solu-medrol-methylprednisolone, high-dose (A)ra-C, and platinol; GDP, gemcitabine, dexamethasone, and cisplatin; GemOx, gemcitabine , oxaliplatin; HyperCVAD, cyclophosphamide, vincristine, adriamycin, and dexamethasone; ICE, ifosfamide, carboplatin, etoposide; R-MTX-Ara-C, rituximab, methotrexate-cytarabine; SCT, stem cell transplant.
Our report presents an unusual case of ALK- S-ALCL with cutaneous metastases that rapidly appeared and disappeared. It is unlikely that the chemotherapy or immunotherapy played a role in the rapid resolution of the cutaneous metastases because his first episode occurred when he was not receiving chemotherapy and disappeared quickly before resuming treatment. The second episode of skin manifestations appeared and disappeared within a period of a few weeks when he was not receiving any chemotherapy or immunotherapy. It is more likely that the resolution was due to the SCT, DLI, or the nature of the patient's disease itself. Although rapid resolution followed allogeneic SCT, the patient developed recurrence within a year, implying that SCT was not curative. However, since the second resolution of skin lesions after DLI, the patient has been in complete remission.
Generally, DLI is useful in the treatment of recurrent hematologic malignancies in patients receiving allogeneic SCT because it enhances the effect of immune cells in grafts and promotes tumor tissue death. Typically, the time course for DLI to show clinical benefits when other hematologic malignancies are being treated varies from a few months to a year; however, the inclusion of S-ALCL cases was not specified in prior studies.10,11 A recent case series including 2 ALK- patients with recurrent ALCL to the skin reported that, when treated with DLI, 1 patient had complete response, and the other had partial response.12 In addition, another patient with ALCL with relapse to the skin 10 months after chemotherapy and 7 months after allogeneic SCT was treated with DLI and palliative chemotherapy, which led to complete remission more than 3 years later.13 Of note, both of these reports used DLI after the onset of skin lesions. In our case, DLI was used to manage the systemic disease before the development of skin relapse. At the first glance of our timeline, the onset of skin lesions soon after DLI seems to almost exclude DLI from preventing or treating skin relapse. However, the fact that DLI typically takes at least a month to show effect is a possible explanation for the resolution.
To our knowledge, remission of skin metastasis of ALK- S-ALCL in such a short period of time has not been reported. Therefore, our case is valuable in showing that there is a potential for rapid resolution of ALCL cutaneous metastasis. Although the follow-up time has been short and the prognosis is still unknown, DLI may be an option for the management of cutaneous metastasis. However, no conclusions can be reached until further research is conducted.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
References
- 1.Al-Hamadani M., Habermann T.M., Cerhan J.R., Macon W.R., Maurer M.J., Go R.S. Non-Hodgkin lymphoma subtype distribution, geodemographic patterns, and survival in the US: a longitudinal analysis of the National Cancer Data Base from 1998 to 2011. Am J Hematol. 2015;90(9):790–795. doi: 10.1002/ajh.24086. [DOI] [PubMed] [Google Scholar]
- 2.Savage K.J., Harris N.L., Vose J.M. ALK- anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the International Peripheral T-Cell Lymphoma Project. Blood. 2008;111(12):5496–5504. doi: 10.1182/blood-2008-01-134270. [DOI] [PubMed] [Google Scholar]
- 3.Parrilla Castellar E.R., Jaffe E.S., Said J.W. ALK-negative anaplastic large cell lymphoma is a genetically heterogeneous disease with widely disparate clinical outcomes. Blood. 2014;124(9):1473–1480. doi: 10.1182/blood-2014-04-571091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mencia-Gutierrez E., Gutierrez-Diaz E., Salamanca J., Martinez-Gonzalez M.A. Cutaneous presentation on the eyelid of primary, systemic, CD30+, anaplastic lymphoma kinase (ALK)-negative, anaplastic large-cell lymphoma (ALCL) Int J Dermatol. 2006;45(6):766–769. doi: 10.1111/j.1365-4632.2004.02412.x. [DOI] [PubMed] [Google Scholar]
- 5.Hernandez C., Puangsuvan S.N., Peterson A., Robinson J.K. Localized perineal cutaneous nodules: a case of recurrent systemic anaplastic large-cell lymphoma. Clin Exp Dermatol. 2009;34(8):e722–e725. doi: 10.1111/j.1365-2230.2009.03448.x. [DOI] [PubMed] [Google Scholar]
- 6.Metcalf R.A., Bashey S., Wysong A., Kim J., Kim Y.H., Gratzinger D. Intravascular ALK-negative anaplastic large cell lymphoma with localized cutaneous involvement and an indolent clinical course: toward recognition of a distinct clinicopathologic entity. Am J Surg Pathol. 2013;37(4):617–623. doi: 10.1097/PAS.0b013e318280aa9c. [DOI] [PubMed] [Google Scholar]
- 7.Nambudiri V.E., Aboutalebi A., Granter S.R., Saavedra A. Recurrent ALK-negative anaplastic large T-cell lymphoma presenting as necrotizing vasculitis. Am J Dermatopathol. 2013;35(4):512–516. doi: 10.1097/DAD.0b013e31827a0cda. [DOI] [PubMed] [Google Scholar]
- 8.Marschalko M., Eros N., Hollo P. Secondary ALK negative anaplastic large cell lymphoma in a patient with lymphomatoid papulosis of 40 years duration. Am J Dermatopathol. 2010;32(7):708–712. doi: 10.1097/DAD.0b013e3181d46eba. [DOI] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network . National Comprehensive Cancer Network; Plymouth Meeting, PA: 2019. T-Cell Lymphomas. NCCN Guidelines Version 2.2019. [Google Scholar]
- 10.Deol A., Lum L.G. Role of donor lymphocyte infusions in relapsed hematological malignancies after stem cell transplantation revisited. Cancer Treat Rev. 2010;36(7):528–538. doi: 10.1016/j.ctrv.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morris E., Thomson K., Craddock C. Outcomes after alemtuzumab-containing reduced-intensity allogeneic transplantation regimen for relapsed and refractory non-Hodgkin lymphoma. Blood. 2004;104(13):3865–3871. doi: 10.1182/blood-2004-03-1105. [DOI] [PubMed] [Google Scholar]
- 12.Mamez A.C., Levy V., Chevallier P. Effect of immune modulation in relapsed peripheral T-cell lymphomas after post-allogeneic stem cell transplantation: a study by the Societe Francaise de Greffe de Moelle et de Therapie Cellulaire (SFGM-TC) Bone Marrow Transplant. 2016;51(3):358–364. doi: 10.1038/bmt.2015.280. [DOI] [PubMed] [Google Scholar]
- 13.Machaczka M., Nahi H., Karbach H., Klimkowska M., Hagglund H. Successful treatment of recurrent malignancy-associated hemophagocytic lymphohistiocytosis with a modified HLH-94 immunochemotherapy and allogeneic stem cell transplantation. Med Oncol. 2012;29(2):1231–1236. doi: 10.1007/s12032-011-9963-3. [DOI] [PubMed] [Google Scholar]