Abstract
In the epididymis, prevention of autoimmune responses against spermatozoa and simultaneous protection against pathogens is important for male fertility. We have previously shown that mononuclear phagocytes (MPs) are located either in the epididymal interstitium or in close proximity to the epithelium. In the initial segments (IS), these ‘intraepithelial’ MPs extend slender luminal-reaching projections between epithelial cells. In this study, we performed an in-depth characterisation of MPs isolated from IS, caput–corpus and cauda epididymis of CX3CR1EGFP+/− mice that express EGFP in these cells. Flow cytometry analysis revealed region-specific subsets of MPs that express combinations of markers traditionally described in ‘dendritic cells’ or ‘macrophages’. RNA sequencing identified distinct transcriptomic signatures in MPs from each region and revealed specific genes involved in inflammatory and anti-inflammatory responses, phagosomal activity and antigen processing and presentation. Functional fluorescent in vivo labelling assays showed that higher percentages of CX3CR1+ MPs that captured and processed antigens were detected in the IS compared to other regions. Confocal microscopy showed that in the IS, caput and corpus, circulatory antigens were internalised and processed by interstitial and intraepithelial MPs. However, in the cauda only interstitial MPs internalised and processed antigens, while intraepithelial MPs did not take up antigens, indicating that all antigens have been captured before they reached the epithelial lining. Cauda MPs may thus confer a stronger protection against blood-borne pathogens compared to proximal regions. By identifying immunoregulatory mechanisms in the epididymis, our study may lead to new therapies for male infertility and epididymitis and identify potential targets for immunocontraception.
Keywords: macrophages, dendritic cells, RNA sequencing, immune regulation, male reproductive tract, post-testicular regulation, male fertility
Introduction
Infertility affects 12–15% of couples in the world, and male factors are involved in 40–50% of these cases (http://www.nichd.nih.gov/health/topics/infertility). In about 50% of cases, male infertility is classified as ‘idiopathic’ (Jose-Miller et al., 2007), illustrating our poor knowledge of male reproductive physiology. Therefore, understanding various functions of the male reproductive tract that contribute to the functional viability of spermatozoa is of particular importance to reproductive health. One critical aspect of male reproductive health that has remained understudied so far is the close interaction between the reproductive system and the immune system in the post-testicular environment. The ability of the epididymis, a long convoluted and segmented tube, to prevent the development of autoimmune responses against antigenic spermatozoa, while initiating very efficient immune responses against pathogenic microorganisms and cancer cells, is one of the most intriguing aspects of male reproductive biology.
When sperm leave the testis, they are not fully mature; they acquire motility and potential for capacitation during their transit and storage in the epididymis (Cornwall, 2009; Yanagimachi, 1994). Post-meiotic germ cells start expressing surface and intracellular molecules, not expressed elsewhere in the body, at puberty, long after the establishment of systemic immune tolerance. Therefore, a finely tuned balance between tolerance to antigenic sperm and immune defence is required to maintain epididymal function while protecting sperm against ascending and blood-borne pathogens. The breakdown of the homeostasis between the epididymal mucosa and sperm is potentially involved in a significant number of male infertility cases (Hedger, 2011; Da Silva and Smith, 2015; Da Silva and Barton, 2016; Fijak et al., 2018; Voisin et al., 2018).
The immune system of the epididymis is composed of different cell types including monocytes, macrophages, dendritic cells and B and T lymphocyte cells (Pollanen and Cooper, 1994; Yeung et al., 1994; Flickinger et al., 1997; Serre and Robaire, 2002; Da Silva et al., 2011; Hedger, 2011; Da Silva and Smith, 2015; Pierucci-Alves et al., 2018; Voisin et al., 2018). Currently, macrophages and dendritic cells are widely recognised as major regulators of all immune responses in normally functioning organs. The canonical function of dendritic cells is the activation of T cells to regulate adaptive immune responses, whereas macrophages are professional phagocytic cells that reside in all organs to maintain tissue integrity, clear debris and initiate innate immunity after infection or injury (Gordon and Taylor, 2005; Hume, 2008). Macrophages can also present antigens, but they lack the ability to stimulate T cells, which is a characteristic of dendritic cells, and they usually fail to mobilise to lymphoid tissues in which naive T cells are abundant. However, overlapping molecular profiles reflect similar functions for macrophages and dendritic cells (Geissmann et al., 2010; Hashimoto et al., 2011; Cerovic et al., 2014). In addition, these cells regulate tissue homeostasis in an organ-specific manner. This tremendous plasticity allows for the specialisation of brain-specific (microglia), liver-specific (Kupffer cells) or skin-specific (Langerhans cells) populations of macrophages (Gosselin et al., 2014; Lavin et al., 2014; Amit et al., 2016). Therefore, it is likely that the epididymis shapes subsets of immune cells that are optimised to face the unique immunological challenges that take place in the male excurrent duct.
Using transgenic mice expressing fluorescent surface markers specific for immune cells, such as CX3CR1 (chemokine (C-X3-C motif) receptor 1) and CD11c (integrin alpha X), we previously revealed a dense immune cellular network in the epididymis (Da Silva et al., 2011; Da Silva and Smith, 2015). These markers are expressed by subsets of immune cells called mononuclear phagocytes (MPs) that include monocytes, dendritic cells, macrophages, T cells and natural killer (NK) cells (Jakubzick et al., 2017; Lee et al., 2018). We showed that, in the initial segments (IS) of the epididymis, specialised intraepithelial MPs extend slender projections between epithelial cells towards the lumen. This unique morphometric characteristic was not observed in MPs from the corpus and cauda, where they cover the base of the epithelium. MPs are also located in the interstitium in all epididymal segments (Da Silva et al., 2011). It is known that CX3CR1-expressing immune cells play a major role in either pro-inflammatory or anti-inflammatory responses, depending on the local environmental conditions in different tissues (Lee et al., 2018). In the current study, we investigated how MPs may contribute to the establishment and maintenance of the environment in which sperm mature and are stored. We identified clearly distinct transcriptomic signatures in MPs from proximal, middle and distal epididymal regions and revealed their segment-specific functional characteristics in the protection of spermatozoa against circulatory pathogens and other harmful antigens.
Materials and Methods
Animals
Adult (12 weeks) C57BL/CBAF1 wild-type male mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA). The mice referred to as ‘CX3CR1-EGFP’ (Jung et al., 2000) in this study are exclusively CX3CR1EGFP+/− mice (12–15 weeks old). All procedures were reviewed and approved by the Massachusetts General Hospital (MGH) Subcommittee on Research Animal Care and were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Mice were anesthetised with isoflurane (2%, mixed with oxygen, flow rate of 2 L/min; Baxter, Deerfield, IL). Tail vein injection (i.v.) was performed as previously described (Battistone et al., 2018) with ovalbumin-Alexa 647 (OVA; 4 mg/kg, Life Technologies Corporation, Eugene, OR) or ProSense 680 (Pro; 80 nmol/kg; PerkinElmer, Boston, MA). Mice were euthanised with isoflurane (2%, mixed with oxygen, flow rate of 2 L/min) and dislocated at 1 h following OVA treatment or 24 h following Pro treatment for flow cytometry or microscopy analysis.
Isolation of EGFP+ MPs from CX3CR1 EGFP+/− mice, RNA extraction and RNA-sequencing
Isolation of EGFP+ MPs from the epididymis of CX3CR1EGFP+/− mice was performed as we previously described (Da Silva et al., 2011). Briefly, IS, caput/corpus and cauda tissues were incubated for 30 min at 37 C with gentle shaking in dissociation medium (RPMI 1640 with 0.5 mg/mL collagenase type I and 0.5 mg/mL collagenase type II). After enzymatic digestion, cells were passed through a 70-μm nylon mesh strainer, washed in PBS with 2% fetal bovine serum and 2 mM EDTA. Double fluorescence-activated cell sorting (FACS) was performed at the HSCI-CRM Flow Cytometry Core (Boston, MA). The RNA of EGFP+ MPs, from the three anatomical regions (IS, caput/corpus and cauda) was isolated using a PicoRNA KIT (Thermo Fisher Scientific) (Ruan et al., 2014). Each sample was obtained using four epididymides from two mice. Genomic DNA contamination was removed by digestion with the RNase-free DNase set (Qiagen, Hilden, Germany). RNA quality and quantity were assessed using a Bioanalyzer (Agilent RNA 6000 Pico Kit, Agilent Technologies, Santa Clara, CA, USA). RNA-seq libraries were prepared using the Clontech SMARTER Kit v4, followed by sequencing on an Illumina HiSeq 2500 instrument (MGH Next Generation Core Facility, Boston, MA). Transcriptome mapping was performed with STAR (Dobin et al., 2013) using the Ensembl annotation of mm9 reference genome. Read counts for individual genes were produced using HTSeq (Anders et al., 2015). Differential expression analysis was performed using the edgeR package (Robinson et al., 2010) after normalising read counts and including only those genes with CPM (counts per million) >1 for one or more samples. The standard PCA method from edgeR software was used to make PCA plots (differentially expressed genes; false positive rate (FDR): 0.05). Multiplot Studio software was used to obtain the volcano plots (fold change (FC) versus P value) and the differential gene expression. Differentially expressed genes were defined based on the criteria of >2-fold change in expression value, CV < 1.2 and P < 0.05. The web-based software Morpheus was employed to plot heat maps. The RNA-seq dataset from MPs will be deposited in Gene Expression Omnibus (GEO) under accession number GSE136442.
C with gentle shaking in dissociation medium (RPMI 1640 with 0.5 mg/mL collagenase type I and 0.5 mg/mL collagenase type II). After enzymatic digestion, cells were passed through a 70-μm nylon mesh strainer, washed in PBS with 2% fetal bovine serum and 2 mM EDTA. Double fluorescence-activated cell sorting (FACS) was performed at the HSCI-CRM Flow Cytometry Core (Boston, MA). The RNA of EGFP+ MPs, from the three anatomical regions (IS, caput/corpus and cauda) was isolated using a PicoRNA KIT (Thermo Fisher Scientific) (Ruan et al., 2014). Each sample was obtained using four epididymides from two mice. Genomic DNA contamination was removed by digestion with the RNase-free DNase set (Qiagen, Hilden, Germany). RNA quality and quantity were assessed using a Bioanalyzer (Agilent RNA 6000 Pico Kit, Agilent Technologies, Santa Clara, CA, USA). RNA-seq libraries were prepared using the Clontech SMARTER Kit v4, followed by sequencing on an Illumina HiSeq 2500 instrument (MGH Next Generation Core Facility, Boston, MA). Transcriptome mapping was performed with STAR (Dobin et al., 2013) using the Ensembl annotation of mm9 reference genome. Read counts for individual genes were produced using HTSeq (Anders et al., 2015). Differential expression analysis was performed using the edgeR package (Robinson et al., 2010) after normalising read counts and including only those genes with CPM (counts per million) >1 for one or more samples. The standard PCA method from edgeR software was used to make PCA plots (differentially expressed genes; false positive rate (FDR): 0.05). Multiplot Studio software was used to obtain the volcano plots (fold change (FC) versus P value) and the differential gene expression. Differentially expressed genes were defined based on the criteria of >2-fold change in expression value, CV < 1.2 and P < 0.05. The web-based software Morpheus was employed to plot heat maps. The RNA-seq dataset from MPs will be deposited in Gene Expression Omnibus (GEO) under accession number GSE136442.
Confocal microscopy
Tissue fixation was performed by immersion in 4% PFA (paraformaldehyde) for 4 h at room temperature as previously described (Battistone et al., 2019a). Slides were mounted with SlowFade Diamond Antifade Mounting medium (Thermo Fisher Scientific, Waltham, MA) containing the DNA marker DAPI and were examined using a Zeiss LSM 800 Airyscan confocal microscope (Oberkochen, Germany) and a Nikon A1R confocal inverted microscope (Nikon Instruments, Melville, NY). For ProSense 680, to avoid the inherent background fluorescence in the far red excitation in the epididymis, tissue sections were treated with TrueBlack Lipofuscin Autofluorescence Quencher (Biotium, Fremont, CA) for 5 s at a 1:40× dilution.
Immunofluorescence was performed as previously described (Shum et al., 2014; Battistone et al., 2019a). The primary antibody used was a rabbit polyclonal antibody against F4/80 (1:50, clone BM8; eBioscience). The secondary antibody used was a donkey cy3-conjugated anti-rabbit IgG (1400, Jackson ImmunoResearch Laboratories). All antibodies were diluted in DAKO medium (Dako, Carpinteria, CA).
Negative and positive controls
For confocal microscopy images, epididymis from WT mice was imaged to determine negative signals for EGFP, OVA (Alexa 647) and ProSense 680. Epididymis from WT mice injected with OVA was analysed to determine negative signals for EGFP and positive signals for Alexa 647. Epididymis from CX3CR1EGFP+/− transgenic mice was analysed to determine positive signals for EGFP and negative signals for Alexa 647 and ProSense 680. Epididymis from WT mice injected with Pro was analysed to determine negative signals for EGFP and positive signals for Alexa 700. For F4/80 staining, we used the secondary antibody alone (donkey cy3-conjugated anti-rabbit IgG) as a negative control. No staining was detected.
Flow cytometry analysis
Epididymal single-cell suspensions were generated as described above. The quantification of the number of CX3CR1+OVA+ and CX3CR1+ Pro+ cells with respect to the total number of epididymal live cells analysed and, within the CX3CR1+ cell population, were analysed using flow cytometry in IS, caput/corpus and cauda. Cell suspensions were incubated with a cocktail of anti-mouse antibodies (1/250) against PE/Cy7 F4/80 (clone BM8), BV711 CD45 or BV650 CD45 (clone 30-F11), APC/Cy7 CD11b (clone M1/711), BV421 CD11c (clone N418), BV786 CD103 (clone M290), BV605 CD206 (clone C068C2) and PE CD64 (clone X54-5/7.1). Antibodies were purchased from BD Biosciences (San Jose, CA, USA) or BioLegend (San Diego, CA, USA).
For functional in vivo assays, WT epididymis was analysed to determine negative events for EGFP, OVA (Alexa 647) and ProSense 680; epididymis from WT mice injected with OVA was analysed to determine negative events for EGFP and positive events for OVA; epididymis from EGFP transgenic mice was analysed to determine positive events in EGFP and negative events for OVA and ProSense 680; and epididymis from WT mice injected with Pro was analysed to determine negative events for EGFP and positives events for ProSense 680. For the immune phenotype analysis, negative and FMO (fluorescence-minus-one) controls were performed to determine the gate strategies. In the negative control, epididymal cells were not incubated with any antibodies and each FMO control includes all antibodies involved in the experiment, except one (Hulspas et al., 2009).
Statistical analysis
The numerical data were analysed using GraphPad Prism (Version 8; GraphPad Software, La Jolla, CA, USA). Data were analyzed using Student’s t-test or one-way ANOVA followed by a Tukey’s post hoc test. Data were expressed as the mean ± SEM. A value of P < 0.05 was considered significant. For RNA-seq, each sample represents four epididymides from two mice. For flow cytometry analysis, each sample represents one epididymis, and at least four different samples were used for each condition.
Results
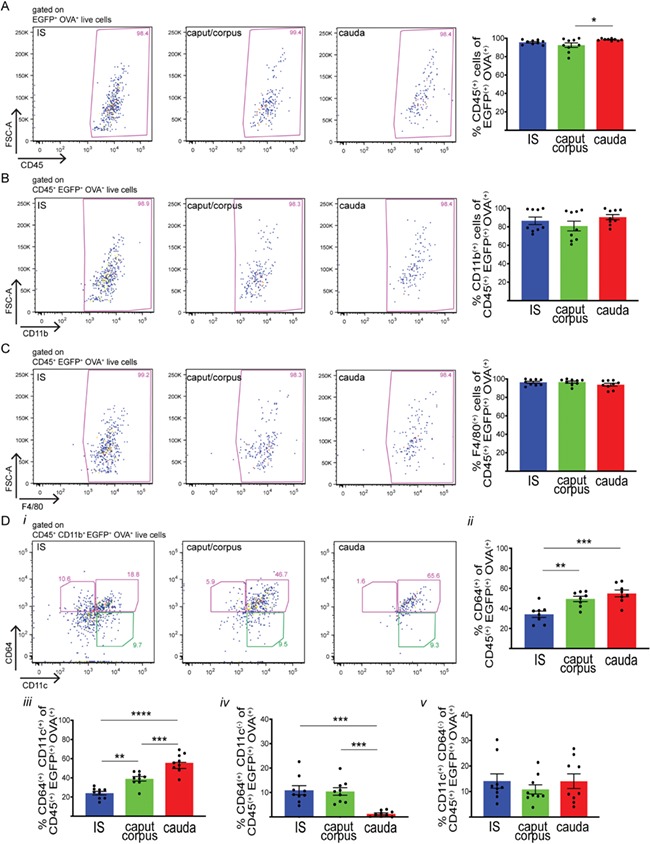
First, we analyzed the immune phenotype of CX3CR1+ (EGFP+) cells, from the proximal, middle and distal regions of the epididymis of CX3CR1EGFP+/− transgenic mice that specifically express EGFP in these cells. The proximal epididymis included only the IS, the middle portion consisting of the caput and the corpus and the distal region representing the cauda. This segmentation was performed in order to reveal phenotypic signatures that may be associated with the unique morphological characteristics of MPs located in the IS, which send intraepithelial projections that reach out between epithelial cells towards the luminal compartment (Da Silva and Barton, 2016; Da Silva et al., 2011). Previous reports showed that intraepithelial MPs located in the more distal epididymal regions do not have luminal-reaching projections. In addition, MPs in the cauda have fewer and less developed peritubular projections compared to IS and caput regions (Da Silva et al., 2011). For these reasons, MPs from the IS and cauda epididymal regions were analysed separately. The caput and corpus MPs were studied together in order to analyse a sufficiently high number of cells. Flow cytometry analysis showed the presence of CX3CR1+ cells in all three epididymal regions examined (Fig. 1A). However, antibodies against different surface markers typically used for the characterisation of MPs revealed distinct populations of CX3CR1+ cells along the epididymis. The majority of CX3CR1+ cells are CD45+ CD11b+ (Fig. 1B and C). Interestingly, in the IS, most of the CX3CR1+ cells are F4/80+ (Fig. 1D), a surface marker broadly expressed by macrophages and a subset of dendritic cells (Geissmann et al., 2010). The percentage of CX3CR1+ F4/80+ cells decreases in the caput/corpus and cauda (Fig. 1D). The MPs that are positive for the high-affinity IgG gamma chain FcγRI surface receptor (CD64) are considered ‘macrophages’, and ‘dendritic’ cells are usually defined as CD11c+ CD64− (Tamoutounour et al., 2012; McGovern et al., 2016). Figure 1Ei shows representative plots of expression of CD64 and CD11c in CX3CR1+ cells in each epididymal region. In all regions, most CX3CR1+ cells show high expression of the ‘macrophage’ marker CD64+, but their percentage decreases slightly from the IS to the cauda (Fig. 1Eii). The majority of these cells are also CD11c+, and their percentages remain constant in each epididymal region examined (Fig. 1Eiii). A small percentage of CX3CR1+ cells are CD64+CD11c−, and their percentage decreases from the IS to the cauda, where they are present in very low numbers (Fig. 1Eiv). Finally, a small percentage of CX3CR1+ cells with the ‘dendritic cell’ signature CD11c+CD64− are found in the IS and their percentage increases in the caput/corpus and cauda (Fig. 1Ev).
Figure 1.
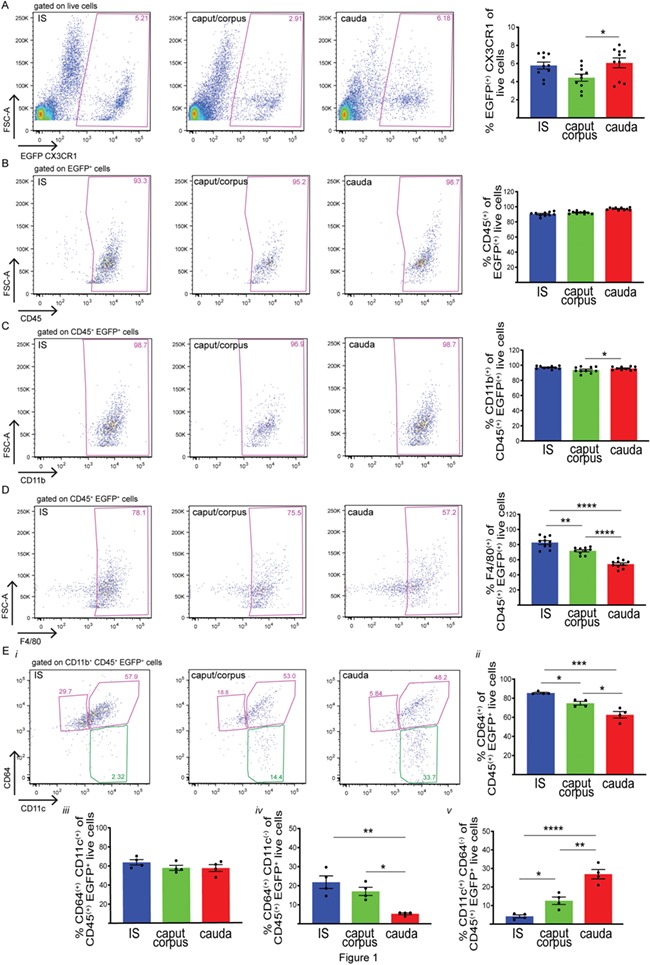
Heterogeneous MP populations from different epididymal segments. (A) Flow cytometry analysis of CX3CR1+ (EGFP+) cells in the initial segments (IS), caput/corpus and cauda epididymis of CX3CR1EGFP+/− transgenic mice that specifically express EGFP in MPs. (B, C, D, E) Flow cytometry analysis of different immune markers, CD45 (B), CD11b (C), F4/80 (D), CD11c and CD64 (E), in IS, caput/corpus and cauda epididymis of CX3CR1EGFP+/− transgenic mice. In all three regions examined, most of the CX3CR1+ cells are CD45+CD11b+F4/80+. (Ei) Representative flow cytometry plots for CD64 and CD11c in IS, caput/corpus and cauda epididymis. (Eii) Percentages (%) of CD64+ cells within the CD45+ CX3CR1+ live cell population (sum of both pink boxes in i). (Eiii) Percentages (%) of CD11c+CD64+ cells within the CD45+CX3CR1+ live cell population (top right pink box in i). (Eiv) Percentages (%) of CD64+CD11c− cells within the CD45+CX3CR1+ live cell population (top left pink box in i). (Ev) Percentages (%) of CD11c+CD64− cells within the CD45+CX3CR1+ live cell population (green box in i). Results are expressed as mean ± SEM of experiments performed with samples from 10 (A–D) or 4 (E) mice. *P < 0.05, **P < 0.001, ****P < 0.0001.
In all epididymal regions, some CX3CR1+ cells are positive for the tolerogenic marker CD103 (Coombes et al., 2007; Coombes and Powrie, 2008; Matteoli et al., 2010) or the mannose receptor CD206 (Supplementary Fig. S1) (Gordon et al., 2014; Martinez and Gordon, 2014). These results indicate that epididymal CX3CR1+ cells represent a mixed population of immune cells that include macrophages and dendritic cells. These cells will be collectively referred to as MPs for the remainder of this article.
We further characterised the transcriptomic profiles of CX3CR1+ MPs that were isolated by fluorescence-activated double-cell sorting from the IS, caput/corpus and cauda epididymal regions of CX3CR1EGFP+/− transgenic mice. For each region, independent samples corresponding to both epididymides from two different animals were included in the analysis. The complete transcriptome dataset is listed in Supplementary Table SI. Principal component analysis (PCA) 2D mapping of RNA-seq data demonstrated that MPs from different epididymal regions were clearly separated from each other based on the global transcriptome expression profiles with IS MP clustering further apart from the others (Fig. 2A). In line with this, MPs isolated from the IS have the highest number of distinct genes compared to MPs from the caput/corpus and cauda, as illustrated in the volcano plots comparing the gene expression profiles of IS MPs (blue), caput/corpus MPs (green) and cauda MPs (red), which showed fewer genes differentially expressed between caput/corpus and cauda MPs, compared to genes of IS versus cauda and IS versus caput/corpus. Interestingly, we also detected groups of genes exclusively expressed in MPs in each epididymal region, shown in light colours in Fig. 2B–D. (The full list is provided in Supplementary Table SII.)
Figure 2.
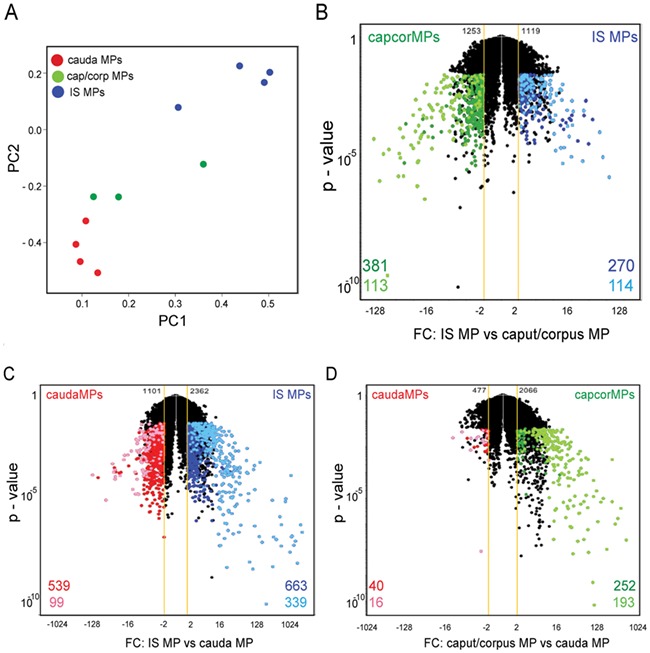
Distinct gene expression profiles of MPs along the epididymal segments. (A) PCA (principal component analysis) plot showing global expression profiles from MPs isolated by double cell sorting from the IS, caput/corpus and cauda epididymal regions of CX3CR1EGFP+/− transgenic mice. Each sample of RNA was obtained from a pool of four epididymides. PC1 (principal component 1) and PC2 (principal component 2). (B, C, D) Volcano plots (fold change (FC) versus P value) of MPs from IS (blue) vs. caput/corpus (capcor; green) (B), from IS (blue) vs. cauda (red) (C) and from caput/corpus (green) vs. cauda MPs (red) (D). Genes exclusively expressed in MPs in each region are shown in light colours and upregulated segment-specific genes are shown in dark colours. FC: fold change. Yellow line shows ±2 FC.
Next, we asked whether MPs have a functional specialisation depending on their location within the epididymis. To that end, we studied pathway enrichment using the Enrichr analysis tool. Genes related to phagosomal activity and antigen processing and presentation were highly expressed in MPs from all regions, in agreement with their capacity to coordinate immune responses. Interestingly, specific sets of genes in each of these pathways were differentially expressed depending on the location of the MPs, revealing that a high number of these transcripts are upregulated in cauda MPs (Fig. 3A). In addition, several genes related to inflammatory and anti-inflammatory signalling pathways were observed in MPs from all regions, and specific gene subsets were differentially expressed depending on their location within the organ (Fig. 3B). For example, pro-inflammatory TNF receptors (Tnfrsf1a, Tnfrsf21, Tnfrsf13b) were predominant in caput/corpus and cauda MPs, and Tnfrsf11a was predominant in IS MPs, while chemokines, such as Ccl6, Cxcl13, Ccl9, Ccl22 and Ccl17, were enriched in cauda MPs, and Ccl12, CxCl2, Cxcl10, Cxcl14, CxCl1 and Cxcl16 were enriched in IS MPs. IS MPs were also enriched with receptors associated with anti-inflammatory responses (e.g. Il10ra, Il10rb, Il21r, Il4ra, Tgfbr1, Tgfbr2), and MPs from the other regions were enriched in transcripts involved in immune tolerance, such as Ido1 and Tgfb1 (caput/corpus) and Lgals1 (cauda).
Figure 3.
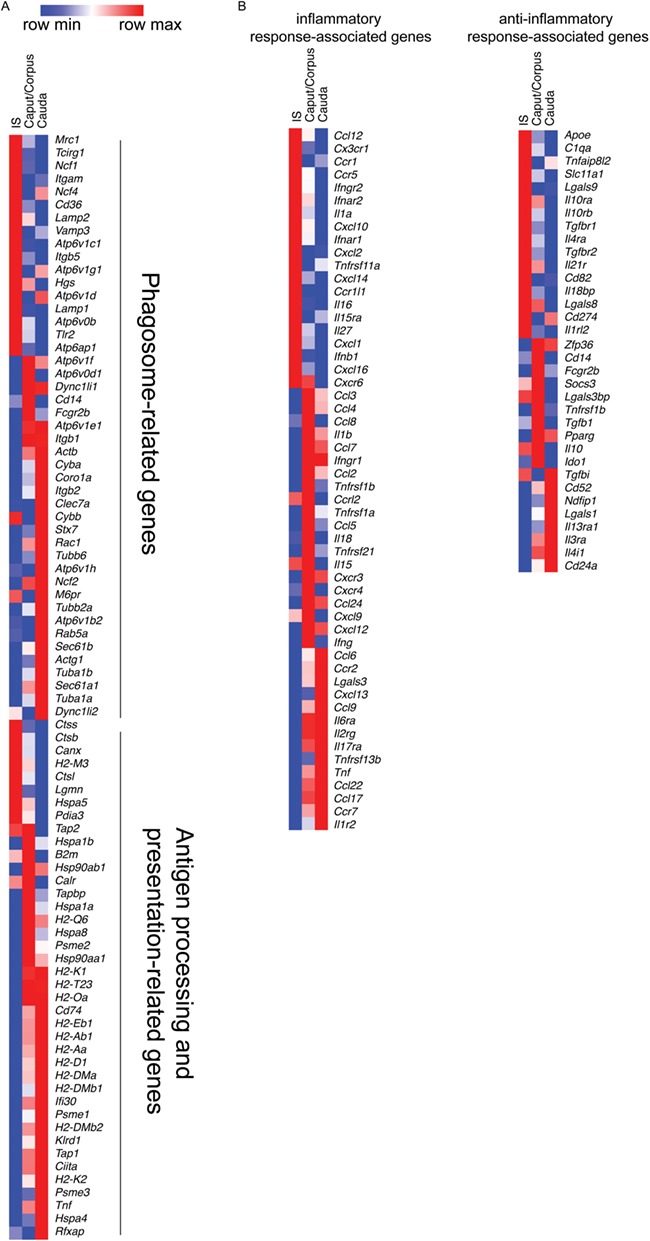
Location-dependent functional specialisation of MPs. (A) Heat map of identified transcripts in MPs related to phagosome (top), as well as antigen processing and presentation in MPs (bottom) from the three epididymal segments examined. (B) Heat map of most representative inflammatory response-associated genes (left) and anti-inflammatory response-associated genes (right) in the MP transcriptome from the three epididymal regions examined. Reads Per Kilobase Million (RPKM) values are listed in Supplementary Table SIII. Heatmaps represent row-normalized gene expression of the indicated genes using a color gradient scale ranging from higher (red) to lower (blue) relative levels.
Moreover, components of the cell adhesion molecule and leukocyte transendothelial migration pathways (i.e. Cldn10, Mag, Vcam1, Cadm1, Itga6, Cldn2, Itga9, Comp, Itgb5, Tln2 and Vcam1) were highly enriched in IS MPs in agreement with their typical morphological phenotypes that include elongation of thin projections between epithelial cells toward the lumen (see Supplementary Table SI for a complete list of transcripts). In cauda MPs, components of NF-kappa signalling (Gadd45b, Tnfaip3, Traf1, Tnfrsf13c, Card11, Malt1), and B lymphocyte signalling (Il4, Grap2, Fyn, Cblb, Card11, Malt1, Pak4) pathways were enriched compared to IS MPs.
To study the ability of CX3CR1+ MPs to take up circulatory antigens, we injected WT and CX3CR1EGFP+/− mice with fluorescent ovalbumin-Alexa 647 (OVA) intravenously. One hour after injection, the epididymides were processed for flow cytometry analysis to characterise the types of MPs that had captured circulatory OVA. WT mice were used as controls to determine the threshold for CX3CR1 green fluorescence positivity (Fig. 4A, top panels). When CX3CR1EGFP+/− mice were injected with OVA (Fig. 4A, lower panels), the percentage of CX3CR1+ MPs that internalised OVA was significantly higher in the IS compared to the other two regions, both considering the number of CX3CR1+OVA+ cells with respect to the total number of epididymal live cells analysed (Fig. 4B), or within CX3CR1+ cell population (Fig. 4C). Most of these double-positive cells (CX3CR1+ and OVA+) are CD45+, CD11b+ and F4/80+ (Fig. 5A–C). Subsets of MPs that had internalised OVA were further defined by their CD64 and CD11c expression (Fig. 5Di). CX3CR1+OVA+CD64+ ‘macrophage’ populations increased from IS to cauda (Fig. 5Dii). Within this population, CX3CR1+OVA+CD64+CD11c+ cells also significantly increased from IS to cauda (Fig. 5Diii). In addition, a small proportion of the CX3CR1+OVA+CD64+ population lacked expression of CD11c and was even less abundant in cauda compared to the other regions (Fig. 5Div). In all regions, CX3CR1+OVA+ MPs that exhibited the ‘dendritic cell’ phenotype CD11c+CD64− constituted a minor fraction (Fig. 5Dv). In addition, we detected some CX3CR1+OVA+ MPs that were CD103+ or CD206+ in all regions, with the highest percentage observed in the caput/corpus region (Supplementary Fig. SII).
Figure 4.
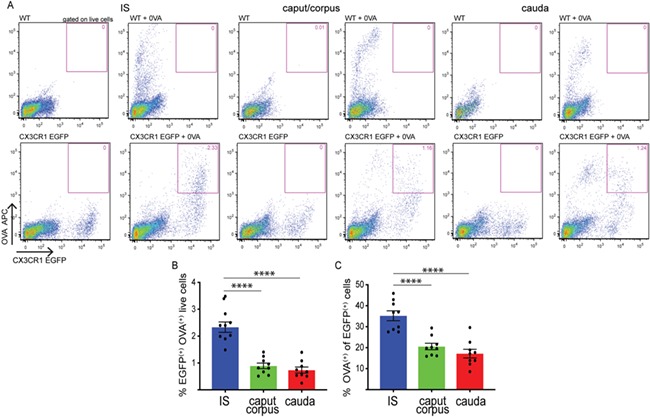
Capture of circulatory antigens by epididymal MPs. (A) Circulatory antigens (Alexa 647-labelled OVA) were administered by tail vein injection (4 mg/kg) into WT (control) or CX3CR1EGFP+/− mice. Gating parameters for CX3CR1+ cells were determined using WT mice (upper panels, pink box). Flow cytometry showed subsets of CX3CR1+ cells that internalised fluorescent OVA in IS, caput/corpus and cauda epididymis (lower panels, pink box). (B, C) Quantification of CX3CR1+ cells with OVA in IS (blue), caput/corpus (green) and cauda (red), showing the number of CX3CR1+OVA+ cells with respect to the total number of epididymal live cells analysed (B), and within the CX3CR1+ cell population (C) from CX3CR1EGFP+/− mice. A higher percentage of CX3CR1+OVA+ cells was detected in the IS. Results are expressed as mean ± SEM of experiments performed with nine samples. ****P < 0.0001.
Figure 5.
Segment-specific MP populations that take up OVA. Flow cytometry analysis of different immune markers (CD45 (A), CD11b (B), F4/80 (C), CD64 and CD11c (D)) in CX3CR1+ MPs in the IS (blue), caput/corpus (green) and cauda (red) epididymis of CX3CR1EGFP+/− mice injected with OVA. In all three regions, most of the CX3CR1+OVA+ cells are CD45+CD11b+F4/80+. (Di) Representative flow cytometry plots for CD64 and CD11c within the CD45+CD11b+CX3CR1+OVA+ cell population in IS, caput/corpus and cauda epididymis from mice injected with OVA. (Dii) Percentages (%) of CD64+ cells within the CD45+CD11b+CX3CR1+OVA+ live cell population (pink boxes in i). (Diii) Percentages (%) of CD11c+CD64+ cells within the CD45+CD11b+CX3CR1+OVA+ live cell population (top right pink box in i). (Div) Percentages (%) of CD64+CD11c− cells within the CD45+CD11b+CX3CR1+OVA+ live cell population (top left pink box in i). (Dv) Percentages (%) of CD11c+CD64− within the CD45+CD11b+CX3CR1+OVA+ live cell population (green box in i). Results are expressed as mean ± SEM of experiments performed with samples from nine mice. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Confocal microscopy imaging showed that in all epididymal regions, some CX3CR1+ cells located in the interstitium had internalised OVA (Fig. 6). Higher numbers of CX3CR1+ MPs that internalised OVA were detected in the IS compared to the other epididymal segments, in agreement with our flow cytometry analysis (Fig. 6A–F). In this region, several OVA+ MPs were in close proximity with the epithelium and their intraepithelial projections also contained OVA (Fig. 6D–F, arrows). In the caput, some intraepithelial MPs internalised OVA (Fig. 6G–L). A few CX3CR1+ MPs with slender luminal reaching projection were observed, but these cells did not internalise OVA. In the corpus (Fig. 6M–R), the majority of cells that internalised OVA were located in the interstitium, but a few were also in close proximity to the basal side of the epithelium (Fig. 6P–R, arrows). In the cauda, the cells that internalised OVA (red) were located exclusively in the interstitium and were not in close proximity to the epithelium (Fig. 6S–X). In all regions, some CX3CR1+ cells did not take up OVA, in agreement with our flow cytometry analysis. In the corpus and cauda epididymis, the majority of interstitial cells that internalised OVA (red) were CX3CR1-negative. Immunofluorescence microscopy showed that the majority of these cells are F4/80+ (Supplementary Fig. SIII). These results indicate that CX3CR1+ cells show distinct antigen-capture activities and immune phenotypes depending on their localisation within the epididymis.
Figure 6.
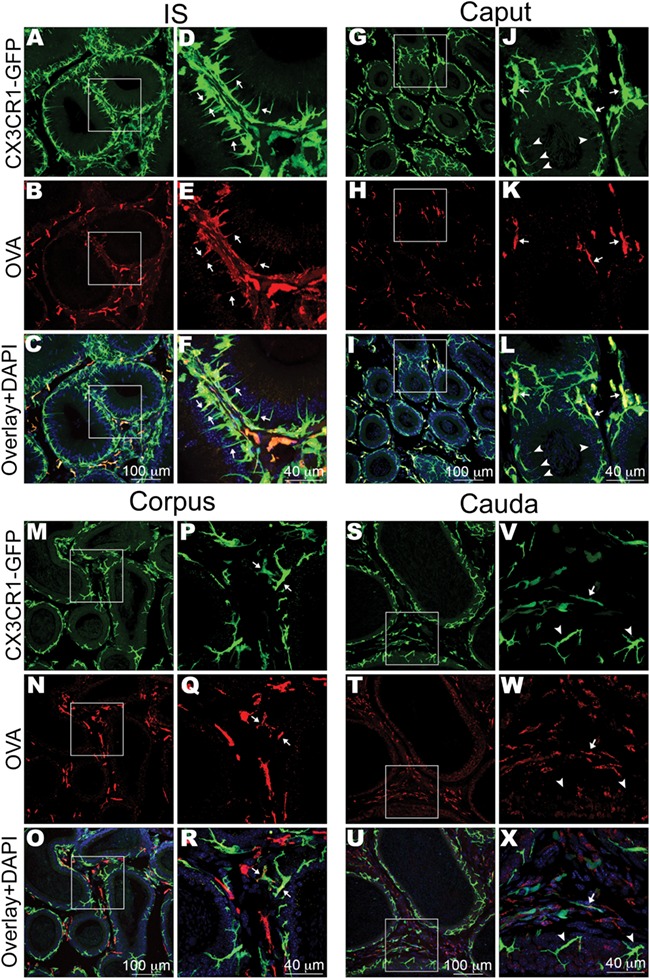
Localisation of different MP populations that take up OVA. Confocal microscopy images showing CX3CR1+ MPs (green) and OVA+ (red) cells in IS (initial segments), caput, corpus and cauda. (A–C) In IS, interstitial and intraepithelial MPs internalised OVA. Most cells that internalised OVA were CX3CR1+. Panels (D–F) show higher magnifications of the regions shown in the boxes in (A–C). Several CX3CR1+OVA+ MPs were in close proximity with the epithelium, and their intraepithelial projections also contained OVA (arrows). (G–I) In the caput, the majority of CX3CR1+OVA+ MPs were located in the interstitium, but a few were in close proximity with the epithelium (arrows). Panels (J–L) show higher magnification of the regions delineated by the white boxes in the left panels. Arrows points to CX3CR1+OVA+MPs that are in close proximity to the epithelium. In this region, a few CX3CR1+ MPs have small and slender intraepithelial projections (arrowheads), but these cells did not internalise OVA. (M–O) In the corpus, several cells that had internalised OVA were located in the interstitium and were negative for CX3CR1 (red alone in O and R). Panels (P–R) show higher magnifications of the regions delineated by the boxes in (M–O). A few CX3CR1+ MPs had internalised OVA and were in close proximity to the basal side of the epithelium (arrows). In this region, MPs do not exhibit intraepithelial luminal reaching projections. (S–U) In the cauda, interstitial MPs had internalised OVA, but no OVA signal was detected in intraepithelial MPs. Panels (V–X) show higher magnifications of the regions delineated by the boxes in (S–U). MPs that are in close proximity to the epithelium were negative for OVA (arrowheads). In the interstitium, a mixed population of OVA+ cells that were either positive for CX3CR1 (arrows) or negative for CX3CR1 (red in U and X) were detected. In the merged panels, nuclei are labelled with DAPI (blue). This figure shows representative images from different epididymal regions, at least four epididymides from four mice were analysed by confocal microscopy.
To study the antigen processing activity of MPs, WT and CX3CR1EGFP+/− mice were injected (i.v.) with ProSense 680 (Pro), which fluoresces upon processing by intracellular proteases (Fig. 7A). Flow cytometry analysis showed that more CX3CR1+ MPs were positive for Pro in the IS than in the more distal regions 24 h after injection (Fig. 7B and C). Similar to OVA positive cells, the CX3CR1+Pro+cells were CD45+CD11b+F4/80+ in all epididymal regions (Fig. 8A–C), but they also showed specific phenotypic signatures depending on their location within the epididymis. As such, CX3CR1+Pro+CD64+ ‘macrophages’ were predominant in all regions (Fig. 8ii). The percentage of CX3CR1+Pro+CD64+CD11c+ cells significantly increased from IS to cauda (Fig. 8Diii). A small percentage of the CX3CR1+Pro+CD64+ cells did not express CD11c, with the lowest value seen in the cauda (Fig. 8Div). Finally, the percentage of cells with ‘dendritic cell-like’ phenotype (CD11c+CD64−) was similar in the IS, caput/corpus and cauda (Fig. 8D). In all regions, some CX3CR1+Pro+ cells were CD103+ and CD206+, and the caput/corpus region contained the highest percentage of these populations (Supplementary Fig. SIV).
Figure 7.
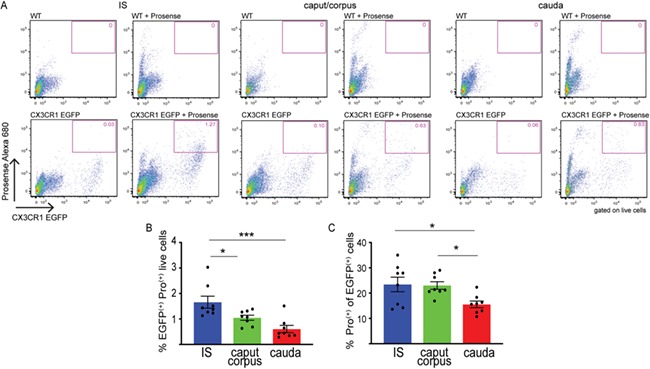
Processing of circulatory antigens by epididymal MPs. (A) Circulatory antigens (ProSense 680, Pro) were administered by tail vein injection (80 nmol/kg) into WT (control) or CX3CR1EGFP+/− mice. Pro fluoresces upon processing inside cells. Gating parameters for CX3CR1+ cells were determined using WT mice (upper panels, pink boxes). Flow cytometry showed subsets of CX3CR1+Pro+ cells in IS, caput/corpus and cauda epididymis (lower panels, pink boxes). (B, C) Quantification of CX3CR1+Pro+ cells in IS (blue), caput/corpus (green) and cauda (red), showing the number of CX3CR1+Pro+ cells with respect to the total number of epididymal live cells analysed (B) and within the CX3CR1+ cell population (C). Results are expressed as mean ± SEM of experiments performed with samples from nine mice. *P < 0.05, ***P < 0.001.
Figure 8.
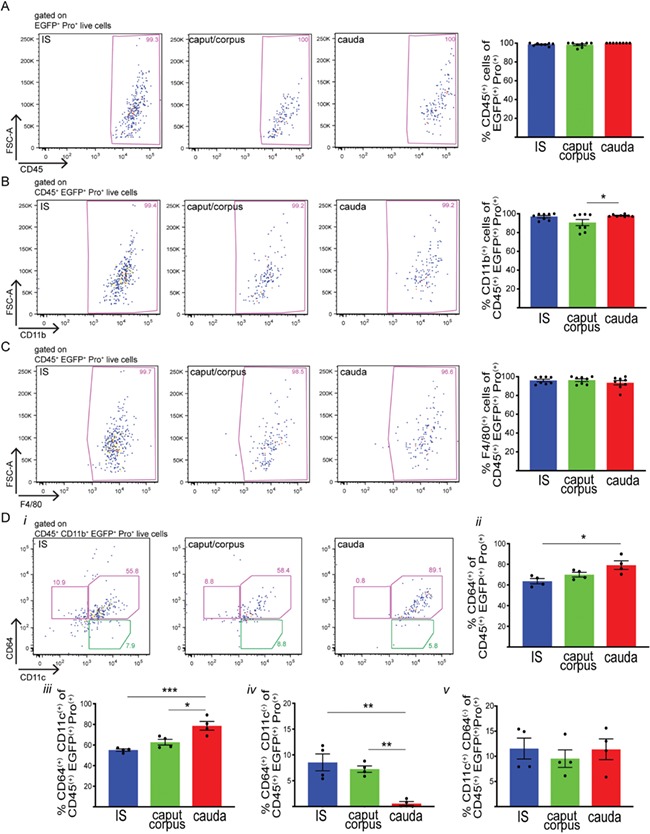
Segment-specific processing of circulatory antigens by epididymal MPs. Flow cytometry analysis of different immune markers (CD45 (A), CD11b (B), F4/80 (C), CD64 and CD11c (D)) in CX3CR1+ MPs in the IS (blue), caput/corpus (green) and cauda (red) epididymis of CX3CR1EGFP+/− mice injected with Pro. In all three regions, most of the CX3CR1+Pro+ cells are CD45+CD11b+F4/80+. (Di) Representative flow cytometry plots for CD64 and CD11c in IS, caput/corpus and cauda epididymis from mice injected with Pro. (Dii) Percentages (%) of CD64+ within CD45+CD11b+EGFP+Pro+ live cells (both pink boxes in i). (Diii) Percentages (%) of CD11c+CD64+ within CD45+CD11b+CX3CR1+Pro+ live cells (right pink box in i). (Div) Percentages (%) of CD64+CD11c− within CD45+CD11b+CX3CR1+Pro+ live cells (left pink box in I). (Dv) Percentages (%) of CD11c+CD64− within CD45+CD11b+CX3CR1+Pro+ live cells (green box in i). Results are expressed as mean ± SEM of experiments performed with samples from 8 (A–C) or four mice (D). *P < 0.05, ***P < 0.001, ****P < 0.0001.
Confocal microscopy showed that in the IS, caput and corpus (Fig. 9A–R), CX3CR1+ MPs that were in close proximity to the basolateral side of the epithelium (arrows in panels D-F J-L, and P-R) and interstitial CX3CR1+ MPs had processed Pro. In the cauda (Fig. 9S–X), only interstitial MPs had processed Pro, and CX3CR1+ MPs that were adjacent to epithelial cells were negative for Pro. In the IS, almost all Pro+ cells were CX3CR1+ in the interstitium and epithelium. In contrast, a significant population of Pro+ cells located in the interstitium of the corpus and cauda were negative for CX3CR1. These results indicate that CX3CR1+ MPs show distinct antigen-processing activity and immune phenotype depending on their localisation in the epididymis and that a population of cells that can process antigens exists in the distal regions.
Figure 9.
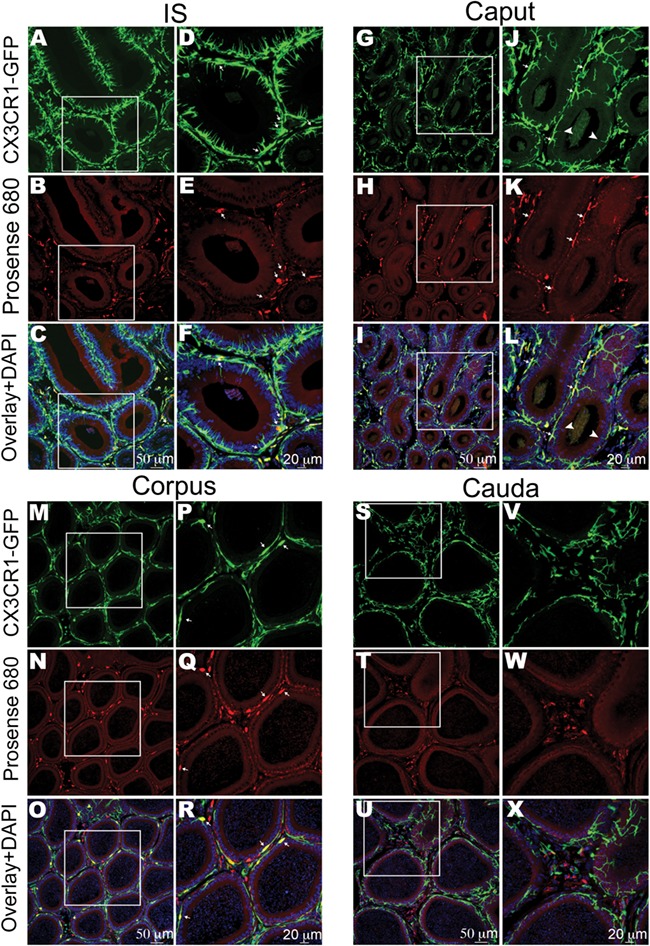
Localisation of heterogeneous MP populations that process circulatory antigens. Confocal microscopy images showing CX3CR1+ MPs (green) and Pro+ (red) cells in IS (initial segments), caput, corpus and cauda. (A–C) In IS, interstitial and intraepithelial CX3CR1+ MPs had processed circulatory antigens (Pro+). (D–F) show higher magnifications of the regions shown in the boxes in (A–C). Several Pro+ CX3CR1+MPs were in close proximity with the epithelium (arrows), but their intraepithelial projections did not contain detectable Pro. (G–I) In the caput, interstitial and intraepithelial MPs had processed antigens. (J–L) show higher magnifications of the regions delineated by the boxes in (G–I). Several MPs that had processed antigens were in close proximity to the basal side of the epithelium (arrows). Arrowheads show slender intraepithelial projections positive for CX3CR1 but negative for Pro. (M–O) In the corpus, interstitial and intraepithelial MPs had processed antigens. Panels (P–R) show higher-magnification images from the boxed regions in panels (M–O). There were some Pro-positive cells in the interstitium that are negative for CX3CR1 (red alone in panels O and R). Arrows indicate Pro+ MPs that are in close proximity to the epithelium. In this region, MPs do not exhibit intraepithelial luminal reaching projections. (S–U) In the cauda, only interstitial MPs processed antigens, and MPs that are in close proximity to the epithelium were negative for Pro. (V–X) show higher magnifications of the regions shown in the boxes in (S–U). In the overlay panels, nuclei are labelled with DAPI (blue). This figure shows representative images from the different epididymal regions; at least four epididymides from four mice were analysed by confocal microscopy.
Discussion
The epididymal mucosal system protects spermatozoa from destruction by the immune system as well as by invading pathogens. We previously characterised the mucosal immune system that resides in the ‘blood–epididymis barrier’ and described an extensive network of distinct MPs that populate different regions of the epididymis and are strategically positioned to actively regulate the interactions between the reproductive and immune systems (Da Silva et al., 2011; Da Silva and Smith, 2015; Da Silva and Barton, 2016). In this study, we show that epididymal MPs exhibit microenvironment-specific phenotypic and functional characteristics depending on their location within the epididymis.
MPs are key players in the immune response of numerous tissues (Banchereau and Steinman, 1998; Steinman et al., 2003; Steinman and Banchereau, 2007; Helft et al., 2010) and were described in other non-lymphoid tissues such as the skin, liver, kidney and intestine (Helft et al., 2010; Gosselin et al., 2014; Lavin et al., 2014; Amit et al., 2016). In the epididymal epithelium, lymphocytes (referred to as ‘halo cells’) and myeloid cells respond to changes initiated by physiopathological conditions, such as aging and infections (Pollanen and Cooper, 1994; Yeung et al., 1994; Flickinger et al., 1997; Serre and Robaire, 2002; Itoh et al., 2005; Da Silva et al., 2011; Da Silva and Smith, 2015; Pierucci-Alves et al., 2018; Voisin et al., 2018). CD64-positive cells are considered to have a ‘macrophage’ phenotype, while ‘dendritic cells’ are defined based on their expression of CD11c and their lack of CD64 expression (Tamoutounour et al., 2012; McGovern et al., 2016). Here we demonstrate that the epididymal tubule and interstitium are populated by several subsets of heterogeneous MPs which express complex combinations of markers traditionally described as ‘dendritic cell’ or ‘macrophage’ markers. Interestingly, in all regions, most of the epididymal MPs express CD64, indicating that these cells exhibit a ‘macrophage’ phenotype. The percentage of CD64+ cells decreases from the proximal to the distal epididymis, in agreement with a previous study showing that macrophages are significantly more abundant in the caput compared to the cauda (Voisin et al., 2018). Moreover, we observed two distinct populations of CD64+ CX3CR1+ cells (CD11c-negative and CD11c-positive), the latter being the most abundant in all regions. In contrast, the percentage of CX3CR1+ MPs with a ‘dendritic cell’ phenotype progressively increases toward the more distal region of the epididymal duct.
Moreover, we reveal region-specific gene signatures in epididymal MPs and show a higher degree of diversity of MPs isolated from the IS compared to MPs from the more distal regions of the epididymis. This is in agreement with previous observations showing distinct morphology of MPs in the IS (Da Silva et al., 2011). In all epididymal regions, subsets of MPs are located either at the base of the epithelium or in the interstitium, while in the IS of the epididymis, some MPs also extend thin luminal-reaching projections between epithelial cells (Da Silva et al., 2011; Da Silva and Smith, 2015). In this regard, we found that IS MPs were highly enriched in transcripts related to cell adhesion molecules, and leukocyte transendothelial and migration pathways. In this study, we found a few MPs located in the caput that extended luminal-reaching projections. These projections were thinner and shorter compared to those observed in the IS, and they were absent in the corpus and cauda, indicating that the ability of MPs to reach out toward the lumen is stronger in the very proximal epididymis and is progressively lost towards the more distal regions.
A fine-tuning balance between sperm tolerance and immune defence is required to maintain the immune privileged function of the epididymis, while protecting sperm against pathogens. As expected from the role of MPs (macrophages and dendritic cells) in other tissues (Geissmann et al., 2010; Hashimoto et al., 2011; Cerovic et al., 2014), epididymal CX3CR1+ MPs express several phagosome-, antigen processing- and antigen presenting-related genes. In particular, in the cauda region, more of these transcripts are upregulated compared to MPs from the other regions, indicating segment specific functions of MPs.
Key steps for the establishment of peripheral tolerance are antigen uptake, processing and presentation. We found that CX3CR1+ MPs in the three epididymal regions examined can both capture and process circulatory antigens. The antigen uptake occurs quickly, within 1 h, but the processing activity requires a longer period of time, similar to what has been shown in other systems (Chang et al., 2013). In addition, we previously demonstrated that epididymal MPs have antigen-presenting and cross-presenting capabilities in vitro (Da Silva et al., 2011). Interestingly, a higher percentage of CX3CR1+ MPs that had captured and processed antigens was observed in the IS compared to the other epididymal segments, in agreement with the enriched vascularised nature of the IS, which contains a large number of fenestrated blood vessels (Suzuki, 1982; Abe et al., 1984; Markey and Meyer, 1992; Guiton et al., 2019). In this region, both intraepithelial and interstitial MPs were positive for OVA and Pro, indicating their capture and processing activities. However, the MP intraepithelial projections were positive only for OVA, indicating that while capture has occurred in the cells, antigen processing may not take place within these structures. In contrast, in the cauda region, only MPs located in the interstitium were positive for OVA and Pro, indicating that they provide an efficient protective barrier by taking up and processing antigens in the bloodstream, thus preventing them from reaching the epithelial lining. Together, these results indicate that the epithelial lining of the proximal regions is more likely to be invaded by blood-borne pathogens, compared to the more distal epididymal regions.
Interestingly, in the corpus and cauda regions, most of the interstitial cells that captured OVA are CX3CR1-negative, but the majority of those cells are F4/80+ by immunofluorescence. In addition, we observed that most CX3CR1+ MPs that have the ability to capture and process circulatory antigens in all segments present a ‘macrophage-type’ phenotype (CD64-positive cells). A large fraction of the MPs that had internalised and processed antigens were CD11c+. Altogether, our results are in agreement with the role of CX3CR1+CD11b+CD11c+F4/80+ cells in the uptake of bloodstream antigens in the intestine (Chang et al., 2013). In addition, we observed that some CX3CR1+ cells that take up antigens are CD103+, a tolerogenic ‘dendritic cell’ marker. A higher percentage of these cells were detected in the caput/corpus segments. In contrast, in the intestine, the majority of CX3CR1+ cells that take up OVA lack CD103 (Chang et al., 2013; Mazzini et al., 2014), indicating that CX3CR1+ cells play tissue-specific roles (Lee et al., 2018).
As cellular uptake is known to be essential for the maintenance of self-tolerance (Elliott and Ravichandran, 2010; Tanaka et al., 2010), phagocytosis of sperm fragments may contribute to maintaining tolerance to luminal sperm antigens in this region. Our data, showing that epididymal MPs have the ability to capture and process blood-borne antigens, indicate that these cells may also take up luminal antigens and thus may play an active role in maintaining immune tolerance via luminal sampling similar to MPs in other tissues (Lee et al., 2018). However, further experiments are needed to elucidate the role of CX3CR1+ cells in luminal antigen uptake and sperm tolerance in the epididymis. In support of their potential role in regulating tolerance, we found that epididymal MPs express the enzyme Ido1 (indoleamine 2,3-dioxygenase 1), which plays a role in immune tolerance toward sperm (Jrad-Lamine et al., 2013). We also found a higher expression of anti-inflammatory transcripts such as Tgfbr1 and Tgfbr2 in IS MPs and of Tgfb1 cytokine in caput/corpus MPs. This result is in agreement with a recent report showing that dendritic cells maintain immunological homeostasis in the epididymis through TGFβ receptor type 2 (Pierucci-Alves et al., 2018).
In other tissues, such as the intestine, breakdown of the finely tuned balance between tolerance and immunogenicity has the potential to trigger autoimmune or inflammatory disorders (Niess and Reinecker, 2005). Such disorders may occur in the epididymis and be a significant cause of male infertility (McLachlan, 2002; Fijak et al., 2018). In addition, we recently found that MPs establish intimate contact with epididymal epithelial clear cells and they interact to establish and maintain the protective immune-privileged environment (Battistone et al., 2019b).
In summary, by identifying distinct transcriptomic signatures and functional characteristics of MPs in the different epididymal regions, our results provide a deeper understanding of the role of MPs in the immune surveillance of the circulation based on their antigen sampling and processing ability during sperm maturation and storage. Identifying and dissecting segment-specific immunoregulatory mechanisms in the male excurrent duct enhances the possibility of developing new and targeted therapies for common disorders, such as male infertility and epididymitis, and of identifying potential targets for immunocontraception.
Supplementary Material
Acknowledgements
We thank the HSCI-CRM Flow Cytometry Facility (MGH, Boston, MA), in particular Maris Handley and Amy Galvin Watt, for their guidance and assistance in sorting and flow cytometry analysis.
Authors’ roles
M.A.B, A.V.N., D.B. and S.B. designed the study; M.A.B., R.G.S., A.C.M. and A.V.N. performed the experiments and analysed the data; M.A.B, D.B., A.V.N., and S.B. wrote the manuscript; M.A.B., R.G.S., A.V.N., A.C.M., D.B. and S.B. read and commented on various drafts of the manuscript.
Funding
Lalor Foundation Fellowship (to M.A.B.) and National Institutes of Health (HD040793 to S.B. and HD069623 to S.B.). The Microscopy Core facility of the Massachusetts General Hospital (MGH) Program in Membrane Biology receives support from Boston Area Diabetes and Endocrinology Research Center (DK57521) and Center for the Study of Inflammatory Bowel Disease (DK43351). The Zeiss LSM 800 microscope was acquired using an NIH Shared Instrumentation Grant S10-OD-021577-01. S.B. is the recipient of the Richard Moerschner Endowed MGH Research Institute Chair in Men’s Health.
Conflict of interest
Dr Breton is a co-founder of Kantum Pharma (previously ‘Kantum Diagnostic, Inc.’), a company developing a diagnostic and therapeutic combination to prevent and treat acute kidney injury. Dr Breton and her spouse own equity in the privately held company, and Dr Breton is an inventor on patents covering technology that has been licensed to the company through the MGH. Dr Breton’s interests were reviewed and are managed by MGH and Partners HealthCare in accordance with their conflict of interest policies. The remaining others have no conflicts of interest to declare.
References
- NICHD Infertility and Fertility updated 1/31/2017Available from: https://http://www.nichd.nih.gov/health/topics/infertility
- Abe K, Takano H, Ito T. Microvasculature of the mouse epididymis, with special reference to fenestrated capillaries localized in the initial segment. Anat Rec 1984;209:209–218. [DOI] [PubMed] [Google Scholar]
- Amit I, Winter DR, Jung S. The role of the local environment and epigenetics in shaping macrophage identity and their effect on tissue homeostasis. Nat Immunol 2016;17:18–25. [DOI] [PubMed] [Google Scholar]
- Anders S, Pyl PT, Huber W., HTSeq –a Python framework to work with high-throughput sequencing data. Bioinformatics 2015;31:166–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature 1998;392:245–252. [DOI] [PubMed] [Google Scholar]
- Battistone MA, Nair AV, Barton CR, Liberman RN, Peralta MA, Capen DE, Brown D, Breton S. Extracellular adenosine stimulates vacuolar ATPase-dependent proton secretion in medullary intercalated cells. J Am Soc Nephrol 2018;29:545–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battistone MA, Merkulova M, Park YJ, Peralta MA, Gombar F, Brown D, Breton S. Unraveling purinergic regulation in the epididymis: activation of V-ATPase-dependent acidification by luminal ATP and adenosine. J Physiol 2019a;597:1957–1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battistone MA, Spallanzani RG, Mendelsohn AC. Capen D Nair AV Brown D Breton S, Novel role of proton-secreting epithelial cells in sperm maturation and mucosal immunity. J Cell Sci 2019b;133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerovic V, Bain CC, Mowat AM, Milling SW. Intestinal macrophages and dendritic cells: what’s the difference? Trends Immunol 2014;35:270–277. [DOI] [PubMed] [Google Scholar]
- Chang SY, Song JH, Guleng B, Cotoner CA, Arihiro S, Zhao Y, Chiang HS, O'Keeffe M, Liao G, Karp CL et al. Circulatory antigen processing by mucosal dendritic cells controls CD8(+) T cell activation. Immunity 2013;38:153–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes JL, Powrie F. Dendritic cells in intestinal immune regulation. Nat Rev Immunol 2008;8:435–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med 2007;204:1757–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwall GA. New insights into epididymal biology and function. Hum Reprod Update 2009;15:213–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva N, Barton CR. Macrophages and dendritic cells in the post-testicular environment. Cell Tissue Res 2016;363:97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva N, Cortez-Retamozo V, Reinecker HC, Wildgruber M, Hill E, Brown D, Swirski FK, Pittet MJ, Breton S. A dense network of dendritic cells populates the murine epididymis. Reproduction 2011;141:653–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva N, Smith TB. Exploring the role of mononuclear phagocytes in the epididymis. Asian J Androl 2015;17:591–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR, STAR: ultrafast universal RNA-seq aligner. Bioinformatics 2013;29:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott MR, Ravichandran KS. Clearance of apoptotic cells: implications in health and disease. J Cell Biol 2010;189:1059–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fijak M, Pilatz A, Hedger MP, Nicolas N, Bhushan S, Michel V, Tung KSK, Schuppe HC, Meinhardt A. Infectious, inflammatory and ‘autoimmune’ male factor infertility: how do rodent models inform clinical practice? Hum Reprod Update 2018;24:416–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flickinger CJ, Bush LA, Howards SS, Herr JC. Distribution of leukocytes in the epithelium and interstitium of four regions of the Lewis rat epididymis. Anat Rec 1997;248:380–390. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Gordon S, Hume DA, Mowat AM, Randolph GJ. Unravelling mononuclear phagocyte heterogeneity. Nat Rev Immunol 2010;10:453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S, Pluddemann A, Martinez Estrada F. Macrophage heterogeneity in tissues: phenotypic diversity and functions. Immunol Rev 2014;262:36–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol 2005;5:953–964. [DOI] [PubMed] [Google Scholar]
- Gosselin D, Link VM, Romanoski CE, Fonseca GJ, Eichenfield DZ, Spann NJ, Stender JD, Chun HB, Garner H, Geissmann F et al. Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell 2014;159:1327–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiton R, Voisin A, Henry-Berger J, Saez F, Drevet JR. Of vessels and cells: the spatial organization of the epididymal immune system. Andrology 2019. [DOI] [PubMed] [Google Scholar]
- Hashimoto D, Miller J, Merad M. Dendritic cell and macrophage heterogeneity in vivo. Immunity 2011;35:323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedger MP. Immunophysiology and pathology of inflammation in the testis and epididymis. J Androl 2011;32:625–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helft J, Ginhoux F, Bogunovic M, Merad M. Origin and functional heterogeneity of non-lymphoid tissue dendritic cells in mice. Immunol Rev 2010;234:55–75. [DOI] [PubMed] [Google Scholar]
- Hulspas R, O'Gorman MR, Wood BL, Gratama JW, Sutherland DR. Considerations for the control of background fluorescence in clinical flow cytometry. Cytometry B Clin Cytom 2009;76:355–364. [DOI] [PubMed] [Google Scholar]
- Hume DA. Differentiation and heterogeneity in the mononuclear phagocyte system. Mucosal Immunol 2008;1:432–441. [DOI] [PubMed] [Google Scholar]
- Itoh M, Terayama H, Naito M, Ogawa Y, Tainosho S. Tissue microcircumstances for leukocytic infiltration into the testis and epididymis in mice. J Reprod Immunol 2005;67:57–67. [DOI] [PubMed] [Google Scholar]
- Jakubzick CV, Randolph GJ, Henson PM. Monocyte differentiation and antigen-presenting functions. Nat Rev Immunol 2017;17:349–362. [DOI] [PubMed] [Google Scholar]
- Jose-Miller AB, Boyden JW, Frey KA. Infertility. Am Fam Physician 2007;75:849–856. [PubMed] [Google Scholar]
- Jrad-Lamine A, Henry-Berger J, Damon-Soubeyrand C, Saez F, Kocer A, Janny L, Pons-Rejraji H, Munn DH, Mellor AL, Gharbi N et al. Indoleamine 2, 3-dioxygenase 1 (ido 1) is involved in the control of mouse caput epididymis immune environment. PLoS One 2013;8:e66494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR. Analysis of fractalkine receptor CX (3) CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol 2000;20:4106–4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavin Y, Winter D, Blecher-Gonen R, David E, Keren-Shaul H, Merad M, Jung S, Amit I. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell 2014;159:1312–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Lee Y, Song J, Lee J, Chang SY. Tissue-specific role of CX3CR1 expressing immune cells and their relationships with human disease. Immune Netw 2018;18:e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markey CM, Meyer GT. A quantitative description of the epididymis and its microvasculature: an age-related study in the rat. J Anat 1992;180(Pt 2):255–262. [PMC free article] [PubMed] [Google Scholar]
- Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep 2014;6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteoli G, Mazzini E, Iliev ID, Mileti E, Fallarino F, Puccetti P, Chieppa M, Rescigno M. Gut CD103+ dendritic cells express indoleamine 2, 3-dioxygenase which influences T regulatory/T effector cell balance and oral tolerance induction. Gut 2010;59:595–604. [DOI] [PubMed] [Google Scholar]
- Mazzini E, Massimiliano L, Penna G, Rescigno M. Oral tolerance can be established via gap junction transfer of fed antigens from CX3CR1(+) macrophages to CD103(+) dendritic cells. Immunity 2014;40:248–261. [DOI] [PubMed] [Google Scholar]
- McGovern N, Schlitzer A, Janela B, Ginhoux F. Protocols for the identification and isolation of antigen-presenting cells in human and mouse tissues. Methods Mol Biol 2016;1423:169–180. [DOI] [PubMed] [Google Scholar]
- McLachlan RI. Basis, diagnosis and treatment of immunological infertility in men. J Reprod Immunol 2002;57:35–45. [DOI] [PubMed] [Google Scholar]
- Niess JH, Reinecker HC. Lamina propria dendritic cells in the physiology and pathology of the gastrointestinal tract. Curr Opin Gastroenterol 2005;21:687–691. [DOI] [PubMed] [Google Scholar]
- Pierucci-Alves F, Midura-Kiela MT, Fleming SD, Schultz BD, Kiela PR. Transforming growth factor beta signaling in dendritic cells is required for immunotolerance to sperm in the epididymis. Front Immunol 2018;9:1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollanen P, Cooper TG. Immunology of the testicular excurrent ducts. J Reprod Immunol 1994;26:167–216. [DOI] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, Smyth GK., edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010;26:139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan YC, Wang Y, Da Silva N, Kim B, Diao RY, Hill E, Brown D, Chan HC. Breton S. CFTR interacts with ZO-1 to regulate tight junction assembly and epithelial differentiation through the ZONAB pathway. J Cell Sci 2014;127:4396–4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serre V, Robaire B.. Interactions of the immune system and the epididymis In: Robaire B, Hinton BT, (ed). The Epididymis: from Molecular to Clinical Practice A Comprehensive Survey of the Efferent Ducts, the Epididymis and the Vas Deferens, New York: Kluwer Academic/Plenum, 2002,219–231. [Google Scholar]
- Shum WW, Smith TB, Cortez-Retamozo V, Grigoryeva LS, Roy JW, Hill E, Pittet MJ, Breton S, Da Silva N. Epithelial basal cells are distinct from dendritic cells and macrophages in the mouse epididymis. Biol Reprod 2014;90:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature 2007;449:419–426. [DOI] [PubMed] [Google Scholar]
- Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol 2003;21:685–711. [DOI] [PubMed] [Google Scholar]
- Suzuki F. Microvasculature of the mouse testis and excurrent duct system. Am J Anat 1982;163:309–325. [DOI] [PubMed] [Google Scholar]
- Tamoutounour S, Henri S, Lelouard H, de Bovis B, de Haar C, van der Woude CJ, Woltman AM, Reyal Y, Bonnet D, Sichien D et al. CD64 distinguishes macrophages from dendritic cells in the gut and reveals the Th1-inducing role of mesenteric lymph node macrophages during colitis. Eur J Immunol 2012;42:3150–3166. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Asano K, Qiu CH. Immune regulation by apoptotic cell clearance. Ann N Y Acad Sci 2010;1209:37–42. [DOI] [PubMed] [Google Scholar]
- Voisin A, Whitfield M, Damon-Soubeyrand C, Goubely C, Henry-Berger J, Saez F, Kocer A, Drevet JR, Guiton R. Comprehensive overview of murine epididymal mononuclear phagocytes and lymphocytes: unexpected populations arise. J Reprod Immunol 2018;126:11–17. [DOI] [PubMed] [Google Scholar]
- Yanagimachi R. Fertility of mammalian spermatozoa: its development and relativity. Zygote 1994;2:371–372. [DOI] [PubMed] [Google Scholar]
- Yeung CH, Nashan D, Sorg C, Oberpenning F, Schulze H, Nieschlag E, Cooper TG. Basal cells of the human epididymis--antigenic and ultrastructural similarities to tissue-fixed macrophages. Biol Reprod 1994;50:917–926. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


