Abstract.
Timing of the intervention for intracranial hematomas is critical for its success, specifically since expansion of the hemorrhage can result in debilitating and sometimes fatal outcomes. Led by Britton Chance, we and an extended team from University of Pennsylvania, Baylor and Drexel universities developed a handheld brain hematoma detector for early triage and diagnosis of head trauma victims. After obtaining de novo Food and Drug Administration clearance, over 200 systems are deployed in all Marine battalion aid stations around the world. Infrascanner, a handheld brain hematoma detection system, is based on the differential near-infrared light absorption of the injured versus the noninjured part of brain. About 12 independent studies have been conducted in the USA, Canada, Spain, Italy, the Netherlands, Germany, Russia, Poland, Afghanistan, India, China, and Turkey. Here, we outline the background and design of the device as well as clinical studies with a total of 1293 patients and 203 hematomas. Infrascanner demonstrates high sensitivity (adults: 92.5% and children: 93%) and specificity (adults: 82.9% and children: 86.5%) in detecting intracranial hematomas in volume and from the surface of the brain. Infrascanner is a clinically effective screening solution for head trauma patients in prehospital settings where timely triage is critical.
Keywords: near-infrared spectroscopy, medical device, traumatic brain injury, epidural, subdural, hematoma
1. Introduction
Every year an estimated 1.5 million Americans incur head injuries, which represents only a fraction of the head injuries observed annually worldwide.1 While there are many aspects of a head injury that must be addressed by a physician, the most urgent of these is brain bleeding or intracranial hematoma. Failure to detect and treat such injuries within the first several hours significantly increases the possibility of severe neurological deficits and even death.2,3 Patients with intracranial hematomas often require surgery and therefore must be transported to a trauma center that has neurosurgical coverage. This requirement highlights the need for rapid and accurate initial screening for such injuries.
In rural and underdeveloped areas of the world as well as on a battlefield, timely identification of patients who require surgery can be difficult. Methods for identifying patients with hematomas in these settings primarily include a neurological exam and, when available, skull radiography. However, both of these modalities are notably unreliable.4–6 Limitations of neurological examination may stem from a variety of causes: (i) factors that alter the patient’s level of consciousness, such as alcohol intoxication, drug use, shock, hypoxemia, and metabolic disturbances interfere with the scale’s ability to accurately reflect the severity of a traumatic brain injury (TBI). (ii) Patients who are intubated with an endotracheal tube cannot talk, and the verbal component of the score will be unavailable. (iii) A patient with a spinal cord injury will make the motor scale invalid. (iv) Severe orbital trauma may make eye opening impossible to assess. (v) Finally, limited utility in children, particularly those months. A skull radiography can identify the presence of a skull fracture, which increases the probability of an intracranial hematoma. However, many of the patients with intracranial hematomas do not have a skull fracture. Moreover, fracture is not indicative of subdural or intracerebral bleeding.4 The computed axial tomography (CT) scan is the gold standard for identification and localization of traumatic intracranial hematomas; however, its high cost and limited availability restricts its use in many areas of the world, and its high x-ray radiation poses risks for repeated scans and especially for children and pregnant women.
To address this need, Britton Chance from University of Pennsylvania and Claudia Robertson from Baylor College of Medicine developed a near-infrared system for detection of brain hematomas and tested it successfully at Baylor. In collaboration with a Drexel University team, a medical product was developed, obtained United States Food and Drug Administration (FDA) clearance, and tested successfully around the world. This collaborative effort resulted in Infrascanner, a handheld brain hematoma detection system that is based on the differential near-infrared light (NIR) absorption of the injured versus the noninjured part of brain.7–10 Under normal circumstances, the brain’s absorption can be assumed to be symmetrical. However, when additional underlying extravascular blood is present, there is a greater local concentration of hemoglobin. Consequently, the light absorbance is significantly greater and the reflected component is commensurately less. This differential is detectable via sources and detectors placed on symmetrical sides of the skull.
Infrascanner development was supported initially by the Office of Naval Research and later by the Marine Corps Systems Command. So far, 12 studies have been published with over 1200 patients having over 200 hematomas. The studies were performed with diverse age populations and locations: USA, Canada, Spain, Italy, the Netherlands, Germany, Afghanistan, Russia, Poland, India, China, and Turkey.11–23 This paper outlines the development of the Infrascanner system, the first handheld device to aid in the detection of bleeding in the skull and reviews results of the clinical studies.
2. Brain Injury Overview
A TBI can result either from nonpenetrating injury (whereby the head suddenly and violently hits an object, but the skull remains intact) or a penetrating head injury. Other types of acquired brain injury such as stroke and meningitis are classified as nontraumatic.24 A highly individualized injury, TBI severity depends on the nature of the injury, strength of the force, area of the brain affected, as well as physical and genetic variations among patients. The damage from TBI can be localized (focal), confined to one area of the brain, or diffuse, involving more than one area of the brain.25,26
Types of focal brain injury include bruising of brain tissue (contusion) and rupture of blood vessels inside the skull, thereby resulting in heavy bleeding (intracranial hemorrhage or hematoma). Hemorrhaging can occur inside of the skull but outside of the brain (extra-axial) or within the brain itself (intra-axial). Extra-axial hemorrhages can be further divided into epidural hematoma, subdural hematoma, and subarachnoid hemorrhage. Intra-axial bleeding within the brain itself is called an intracerebral hematoma.
Diagnostic and treatment protocols mandate that a patient suffering from head trauma receive immediate medical assessment including a complete neurological examination.27 The severity of the head trauma and the responsiveness of the patient in a Glasgow Coma scale (GCS) evaluation will determine which diagnostic methods will be used for further evaluation.28 In a GCS evaluation, the patient is scored on his/her ability to open eyes, communicate verbally, and demonstrate motor skills. However, the GCS evaluation can be very subjective based upon the individual administering the test and can also be hampered if the patient is under sedation or has restrictions on his/her ability to verbally communicate (i.e., the patient has been intubated).
For patients with mild-to-moderate injuries, further diagnostic tests may be limited to skull and neck x-rays to check for bone fractures.29 For patients that have demonstrated moderate-to-severe TBIs after undergoing a neurological examination, the gold standard imaging test is a computed tomography (CT) scan, which creates a series of cross-sectional x-ray images of the head and brain and can show bone fractures as well as the presence of hemorrhage, hematomas, contusions, brain tissue swelling, and tumors. Magnetic resonance imaging (MRI) uses magnetic fields to detect subtle changes in brain tissue content and with more detail than x-rays or CT and may be used after the initial assessment and treatment of the TBI patient.29
TBIs can cause a host of physical, cognitive, emotional, and social effects,30–32 and the eventual outcomes can be anything from complete recovery to permanent disability or death. An estimated 5.3 million individuals in the United States are living with long-term or life-long disability associated with a TBI that resulted in hospitalization. Unlike most causes of traumatic death, a large percentage of the people killed by brain trauma do not die right away but rather days to weeks after the traumatic event. In addition, rather than improving after being hospitalized, some 40% of TBI patients deteriorate. Primary injury (the damage that occurs at the moment of trauma when tissues and blood vessels are stretched, compressed, and torn) is not adequate to explain this degeneration. Rather, the deterioration is caused by secondary injury resulting from a complex set of biochemical cascades that occur in the minutes to days following the trauma.33–35 These biochemical cascades are instigated by brain swelling and inadequate flow of oxygen and blood to the brain resulting from brain compression by the expanding brain hematomas. The aim of the Infrascanner is to catch hematomas before they incur any brain damage and enable a much earlier intervention to evacuate the expanding brain hemorrhages.
3. Types of Intracranial Hematomas after Brain Injury
There are three major types of traumatic intracranial hematomas: (1) subdural, (2) epidural, and (3) intracerebral hematomas. Each of these lesions has characteristic clinical and CT scan findings, which can be present on admission to the hospital or can occur in a delayed fashion.36
3.1. Subdural Hematomas
The subdural hematoma is the most common focal intracranial lesion, occurring as the primary initial lesion in 24% of patients with severe closed head injuries in the traumatic coma data bank (TCDB) and occasionally as a delayed lesion.37 The hematoma is between the dura and the brain, usually resulting from a torn bridging vein between the cortex and the draining sinuses. An acute subdural hematoma typically appears on a CT scan as a high-density, homogenous crescent-shaped mass paralleling the inner surface of the skull.
Most acute subdural hematomas require surgery. Despite surgical evacuation, the mortality rate in patients with subdural hematomas was 50% in the TCDB series. The rapidity of surgical evacuation and the degree of associated brain damage are major determinants of outcome. Several studies report a decrease in the early mortality or morbidity in patients who underwent an early evacuation of subdural hematoma.
3.2. Epidural Hematomas
Epidural hematomas, or collections of blood between the skull and dura, are less common, occurring as the primary initial lesion in 6% of patients with severe closed head injuries in the TCDB series.38 Epidural hematomas can be present on the admission CT scan or less commonly may develop at some later time. In a consecutive series of 161 patients with epidural hematoma, 8% had delayed formation of the hematoma.39 The delayed epidural hematoma can develop after evacuation of a hematoma on the opposite side or after a hypotensive patient has been resuscitated. In addition, epidural hematomas can recur after surgical evacuation. In a study of 88 patients with postoperative hematomas, 47 patients with an epidural hematoma developed a postcraniotomy hematoma requiring a second surgical procedure.
Although patients with subdural hematomas are usually immediately comatose, only a third of patients with an epidural hematoma remain unconscious from the time of the injury. Of the remaining patients, one-third have a lucid interval, and one-third are never unconscious. An epidural hematoma is almost always associated with a skull fracture (91% in adults, and 75% in children).4 The blood comes from torn dural vessels (usually arterial) from the fractured skull bone, or occasionally from torn venous sinuses. On CT scan, an epidural hematoma is characterized by a biconvex, uniformly hyperdense lesion. Associated brain lesions are less common than with subdural hematomas.
Most epidural hematomas require surgery, and mortality and morbidity of surgical evacuation are low if the patient is operated upon early. The outcome of the patient with an epidural hematoma depends on the neurological status at the time of surgery. The mortality rate varies from 0% for patients who are not in coma, to 9% of obtunded patients, and up to 20% for patients in deep coma.
3.3. Intracerebral Hematomas
Intracerebral blood can take the form of a hematoma or a contusion. Intracerebral hematomas are more common, occurring as the primary lesion in 10% of the severe closed head injuries in the TCDB series.40 Most intracerebral hematomas are visualized on CT scan as hyperdense mass lesions. They are typically located in the frontal and temporal lobes and can be detected on a CT scan immediately after the trauma. However, delayed intracerebral hematomas may also be manifest during the hospital course. A delayed hematoma is one that is seen on a repeat CT scan within 24 to 48 h of the injury or operation but is not present on the initial CT scan. Commonly, a delayed hematoma is associated with clinical deterioration.
Hemorrhagic contusions were present as the primary lesion in 3% of severe closed head injuries in the TCDB series.40 Single contusions are located either below the region of the impact or opposite the region of impact. Contusions appear as heterogeneous areas of brain necrosis, hemorrhage, and infarct representing mixed-density lesions on CT scan. Multiple focal contusions have a “salt and pepper” appearance on CT scan.
The decision to operate on an intracerebral hematoma is based on the patient’s general condition, associated brain injuries, site and size of the hematoma, the intracranial pressure (ICP), and the magnitude of the mass effect. Generally accepted indications for surgery include (1) a hematoma associated with mass effect or one located in the anterior temporal lobe or in the cerebellum, (2) progressive neurological deterioration, or (3) refractory intracranial hypertension.
3.4. Delayed Intracranial Hematomas
Delayed intracranial hematomas are a treatable cause of secondary injury if identified early, but can cause significant disability or death if not promptly recognized and treated. CT scanning has revealed that delayed hematomas after head trauma are more common than had been previously suspected. Recurrent hematomas, postoperative epidural hematomas, and delayed traumatic intracerebral hematomas occur in up to 23% of patients with severe head injury. Mortality rates and the incidence of a poor neurological recovery are significantly increased in patients who develop delayed traumatic intracranial hematomas.39,40 Early identification prior to neurological deterioration is the key to successful surgical treatment.
Serial CT scans are the most reliable method for detecting a delayed hematoma. However, CT scans require that patients, many of whom are critically ill, be taken out of the intensive care unit, and the yield is relatively low if serial scans are obtained in all patients. A clinical monitoring technique for accurate selection of patients requiring follow-up CT scanning would improve the yield. Nevertheless, current clinical monitoring techniques are not ideal for detecting delayed hematomas. Patients with delayed hematomas may appear to be relatively normal only to undergo sudden neurological deterioration, or may not exhibit a change in their neurological examination. ICP may be normal in up to 20% of patients harboring delayed hematomas that require surgery.40
The ideal clinical monitor would be capable of making on-line continuous measurements in the intensive care unit and would identify the development of a hematoma prior to the onset of clinical neurological deterioration. Near-infrared spectroscopy (NIRS) is one methodology that may have these characteristics.
4. Near-Infrared Spectroscopy
NIRS has been increasingly used in human brain monitoring studies in adults since it was first described by Jobsis41 as an optical method for noninvasively assessing cerebral oxygenation changes. In the 1980s, Delpy et al.42–47 designed and tested an NIRS system for a clinical application with newborn infants. Further efforts improved the methodology and hardware and thus expedited the translation of NIRS-based techniques into a useful tool for brain function monitoring.47–53
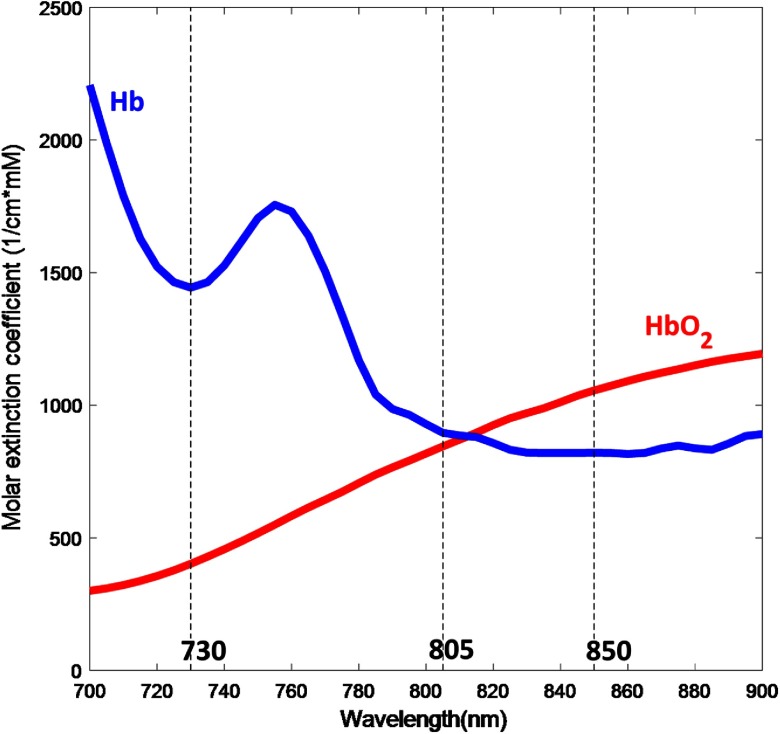
Typically, an optical apparatus for NIRS consists of at least one light source that shines light to the head and a light detector that receives light after it has interacted with the tissue. Photons that enter tissue undergo two different types of interaction: absorption and scattering. Most biological tissue is relatively transparent to light in the near-infrared range between 700 and 900 nm. This is largely because water, a major component of most tissues, absorbs very little energy at these wavelengths. Moreover, the hemoglobin molecule is the dominant chromophore (light-absorbing molecule) in this range of the spectrum. This spectral band is often referred to as the “optical window” for the noninvasive assessment of brain activation (See Fig. 1). Various types of brain activities, such as motor and cognitive activities, have been studied using this technique by making continuous measurement while subjects are engaged in an experimental protocol.52–55
Fig. 1.
Absorption of light by oxygenated and deoxygenated hemoglobin (blue and red lines) and common NIRS light source wavelengths (730 and 850 nm) as well as Infrascanner wavelength (805 nm) marked.
5. Method
5.1. Measurement
The principle used by Infrascanner to identify intracranial hematomas is that extravascular blood absorbs NIR light more than intravascular blood. This is because there is a greater concentration of hemoglobin in an acute hematoma than in normal brain tissue where blood is contained within vessels. Infrascanner compares the left and right side of the brain in four different areas. The absorbance of NIR light is greater (and therefore less light is detected) on the side of the brain containing a hematoma than on the uninjured side. Optical light source(s) or emitter(s) and a photodetector are placed at a distance, which allows proper NIRS absorption measurements in a desired volume of tissue. The wavelength of 805 nm is sensitive only to blood volume, not to oxygen saturation in the blood, as shown in the figure below.
A full head scan with Infrascanner involves measuring from eight head locations, two on each side of the head on frontal (F), parietal (P), temporal (T), and occipital (O) regions (Fig. 2). The difference in optical density () for different brain areas in F, P, T, and O regions is calculated using the following equation:
where is the intensity of reflected light on the normal side and is the intensity of reflected light on the hematoma side.
Fig. 2.

Head location of Infrascanner measurements.
5.2. System Design
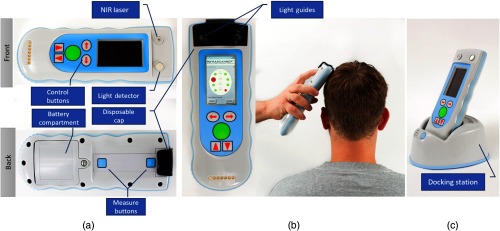
The Infrascanner system incorporates an 805-nm laser diode located 4 cm away from a silicon light detector, which is covered by an NIR optical filter and a disposable cap that contains fiber optic light guides (Fig. 3). Using this setup and the automated calibration algorithm, a full head scan can be acquired in .
Fig. 3.
Infrascanner 2000 handheld battery-operated sensor, front and back (a) disposable cap with light guides for NIR laser and detector positioned over scalp (b) an optional docking station (c) for data backup and/or battery recharge.
The laser delivers NIR light to the tissue under the sensor via an optical fiber and the detector receives the signal via a separate optical fiber after the light has interacted with the tissue. The 4-cm separation between the light source and the detector allows measurement of NIR absorbance in a volume of tissue wide by 2- to 3-cm deep.
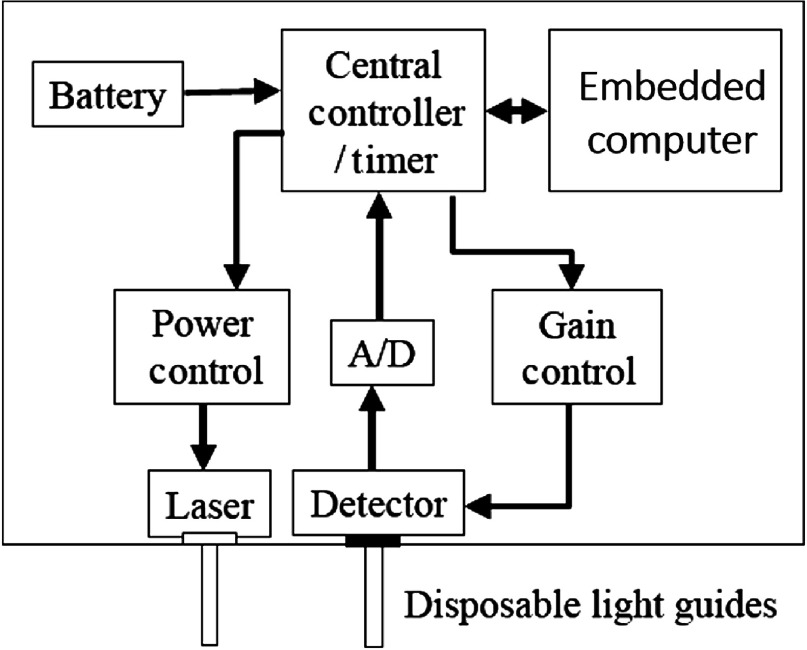
The detector signal is then digitized and processed at the computational unit (embedded computer) for quality control and additional measurements. The computational unit sets the hardware parameters for all measurements (laser power, gain, number of pulses, etc.) (Fig. 4).
Fig. 4.
Infrascanner hardware block diagram.
The measurement and control software that runs on the embedded computer was developed using Embedded Visual C++ and is compatible with any Microsoft Windows Mobile.
In measurement mode, the embedded computer receives the data from the sensor hardware and adjusts the sensor settings automatically until acceptable data quality is received within a predefined timeout period. Each received measurement set contains a series of multiple short scans to test reliability. An acceptable raw signal should be within low and high limits as either extremes indicate problematic measurement. Moreover, signal-to-noise ratio is calculated using mean and standard deviation of the current set and should be higher than a predefined stability threshold to guarantee proper measurement. Through an iterative algorithm (see Fig. 5), the embedded computer checks and corrects for detector saturation (too high gain and light intensity), signal-to-noise ratio, and stability of measurements.
Fig. 5.
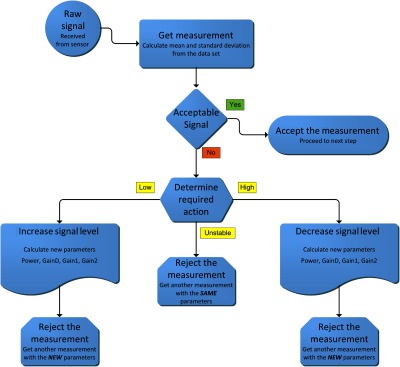
Block diagram depicting single iteration of measurement control algorithm.
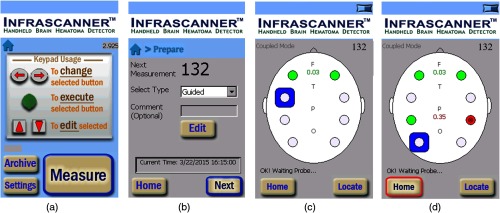
The data are further processed for the calculation of optical density, and the results are displayed on the screen to the operator. User interface also includes an auditory feedback to ensure the focus of the operator on the patient. Embedded computer assigns each measurement session a unique ID number and measurement date for later look-up. Figure 6 summarizes the measurement process and user interface that visualizes the data with a simulated head trauma generated by placing an optical attenuator between laser and detector light guides.
Fig. 6.
Representative screens with typical and simulated head trauma in the left parietal lobe. (a) Home screen, (b) preparation screen at the beginning of a session, (c) typical measurements completed at frontal lobe at two locations, and (d) measurements completed at parietal lobe with simulated head trauma.
6. Results
In a pilot study,10 an NIRS examination was obtained in the ER at the time of the admission CT scan. Using an early prototype of the Infrascanner, called the “Runman,” serial measurements of were obtained in 305 head-injured patients. The maximal among the various regions examined was recorded for each patient and was compared with the admission CT scan. on admission to the hospital was significantly elevated () in all but 4 (2%) patients with intracranial hematomas. was normal (0.00 to 0.05) in patients with diffuse brain injury (DBI). A single NIRS examination, therefore, reliably identified patients with an intracranial hematoma (98% had a ) and gave a suggestion of whether the hematoma was intracerebral (most had a ) or extracerebral (most had a ).
The pivotal double-blinded clinical study19 for FDA clearance was carried out in five different clinical sites, four of which are in the U.S.: (1) Baylor College of Medicine, (2) Hahnemann Hospital, (3) The Hospital of the University of Pennsylvania, and (4) University of Cincinnati. The fifth site was the Lokmanya Tilak Memorial General Hospital located in India. Clinical personnel in these five sites collected data using the Infrascanner and computer tomography (CT) scans (the gold standard in detecting hematomas). All the CT scans were examined by an independent expert radiologist at John’s Hopkins University Hospital. The evaluation of Infrascanner measurements in comparison to CT scans is based on a total of 431 patient data, where 122 of them were hematoma cases of various sizes, depths, and locations. Study results address the hematomas that clinicians may expect the Infrascanner to detect in the clinical practice setting. Consistent with preclinical testing, the Infrascanner demonstrated high sensitivity (88%) in detecting hematomas cc in volume and from the surface of the brain. Specificity in the per protocol population was 91%. The balance between specificity and sensitivity supports the utility of the Infrascanner to prioritize patients with suspected hematomas for CT scan. It should be noted that the device is indicated for use as a screening tool and as an adjunct to the standard diagnostic workup and will not be used in lieu of this workup.
An adult population study20 in Seville, Spain, evaluated a total of 35 TBI patients, aged 17 to 76 years. There were 19 intracranial hematomas, confirmed by a CT scan completed on all subjects within 40 min of the Infrascanner test. The sensitivity in this population was 89.5% and specificity was 81.3%.
A study in Pittsburgh18 evaluated 28 patients of 0 to 14 years in pediatric ICU who underwent CT as part of the clinical care not necessarily triggered by trauma. There were 12 intracranial hematomas confirmed by a CT scan completed on all subjects within 24 h of the Infrascanner test. The sensitivity in this test was 100%, and specificity was 81.3%.
A study in Padua and Treviso, Italy,17 evaluated 110 children at intermediate or high risk for intracranial injury according to the PECARN rules (GCS 14 and 15). There was only one brain hematoma case in this group (it was successfully detected). The specificity in this test was 93%, and the negative predictive value (NPV) was 100%. The use of Infrascanner would have reduced the number of CT scans by 10 (58.8% reduction).
A study in Lublin, Poland,22 evaluated 155 children with mild TBI (GCS 14 and 15). The study aimed to propose a protocol of screening patients using Infrascanner as a complement to repeated neurological examination and medical history review. The results of this study led to the adoption of the Infrascanner as part of the standard of pediatric care in Poland.16
A study in Moscow, Russia,14 evaluated Infrascanner ability to detect ICH among 95 children having experienced mild traumatic head trauma. About forty-two children with associated medium-high risk (GCS 13 and 14) received an evaluation by neurosurgeon, Infrascanner, and CT. About fifty-three children with associated low risk (GCS 15) received a scan with the Infrascanner and were clinically monitored for 72 h. The sensitivity was 100%, and the specificity was 92%.
A study in Beijing, China,12 evaluated a total of 85 TBI patients, aged 8 to 89 years. There were 45 intracranial hematomas confirmed by a CT scan completed on all subjects within 30 min of the Infrascanner test. The sensitivity in this population was 95.6%, and specificity was 92.5%.
A study in a physician-staffed Helicopter Emergency Medical Service in Nijmegen, Netherlands, evaluated a total of 25 TBI patients.13 There were 15 intracranial hematomas confirmed by a CT scan completed on all subjects upon arrival to a trauma center. The sensitivity in this population was 93.3%, and specificity was 78.6%.
Tables 1 and 2 below outline all the studies with details and results including sensitivity, specificity, positive predictive value (PPV), and NPV.
Table 1.
Infrascanner clinical studies with adults. The total number of patients is 715, and the total number of hematomas is 160.
| Study | Type | Patients (N) | Method of Selection | NIRS | CT | ICH | Results |
|---|---|---|---|---|---|---|---|
| Robertson et al. (USA and India)19 | Multicenter study (four centers in USA and one in India) | 365 | Admitted to emergency room with TBI and were sent for a head CT | Model 1000 Evaluated within 40 min of CT |
All patients received CT | 50 |
|
| Leon-Carrion et al. (Spain) 20 | Single center study | 35 | Admitted to emergency room with TBI and were sent for a head CT | Model 1000 Evaluated within 0.5 to 14.5 h of CT (5.7 h avg.) |
All patients received CT | 19 |
|
| Coskun et al. (Turkey)21 | Single center study | 92 | Admitted to emergency service with TBI | Model 1000 Evaluated prior to CT. No repeats for positive results. |
All patients received CT. | 8 |
|
| Braun et al. (Germany) 15 | Single center study in Kunduz, Afghanistan | 11 | Admitted to field emergency service with TBI | Model 1000 Evaluated |
None of the patients received CT | 0 |
|
| Xu et al. (China)12 | Single center study in Beijing, China | 85 | Admitted to Neuro ICU with TBI and were sent for a head CT | Model 2000 Evaluated within 40 min of CT |
All patients received CT or MRI | 45 |
|
| Peters et al. (the Netherlands)13 | Helicopter prehospital study in the Netherlands | 25 | Picked up by helicopter with TBI and were sent for a head CT in a hospital | Model 2000 Evaluated before or during flight in the helicopter |
All patients received CT in a hospital | 14 |
|
| Liang et al. (China)11 | Single center study in Beijing, China | 102 | Admitted to emergency room with TBI and were sent for a head CT | Model 2000 Evaluated within 40 min of CT |
All patients received CT or MRI | 24 |
|
| Total |
|
Table 2.
Infrascanner clinical studies with children. The total number of patients is 578, and the total number of hematomas is 43.
| Study | Type | Patients () | Method of selection | NIRS | CT | ICH | Results |
|---|---|---|---|---|---|---|---|
| Robertson et al. (USA and India)19 | Multicenter study (four centers in USA and one in India) | 36 | Admitted to emergency room with TBI and were sent for a head CT | Model 1000 Evaluated within 40 min of CT |
All patients received CT | 5 |
|
| Coskun et al. (Turkey)21 | Single center study | 161 | Admitted to emergency service with TBI | Model 1000 Evaluated prior to CT. No repeats for positive results. |
All patients received CT. | 14 |
|
| Salonia et al. (USA)18 | Single center, prospective, case–control study | 28 | Patient underwent CT as part of clinical care, not necessarily triggered by trauma | Model 1000 Evaluated within 24 h of CT |
All 28 patients received CT | 12 |
|
| Bressan et al. (Italy)17 | Dual center, prospective observational study | 103 | Minor head injury children presenting with intermediate or high risk for intracranial injury according to PECARN | Model 1000 | 18 The rest were followed on the phone 7 to 90 days later to exclude initially missed hematoma. |
1 |
|
| Semenova et al. (Russia)14 | Single center study | 95 | Presented with mild TBI (GCS 13 to 15) | Model 1000 | 42 medium-high risk patients | 8 |
|
| Lewartowska-Nyga et al. (Poland)22 | Single center study | 155 | Presenting with mild head injury (i.e., no focal or meningeal signs, and GCS score of 14 to 15) | Model 1000 Evaluated up to 72 h postinjury |
28 The rest were followed for 2 months’ postenrollment |
3 |
|
| Total |
|
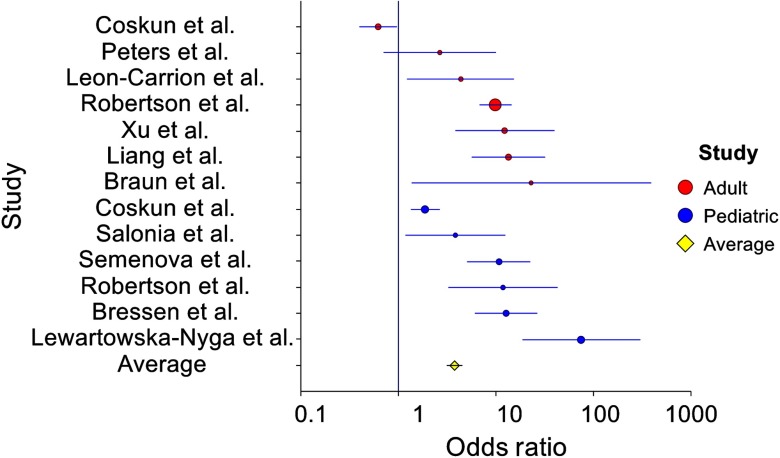
A forest plot of the studies listed in Tables 1 and 2 is included in Fig. 7. The combined odds ratio of adult and pediatric datasets was found to be 3.79 (95% confidence interval, 3.16 to 4.56), which indicated strong effect size of the NIRS measurements, , .
Fig. 7.
Forest plot of Infrascanner studies for both adult and pediatric groups totaling 1293 patients.
Several limitations for identifying intracranial hematomas with NIRS were also observed in the clinical studies. For example, the size, type, and location of the hematoma cannot be as precisely determined as with a CT scan. Additionally, because the NIRS examination relies on absorbance in the contralateral brain locations for comparison, bilateral lesions could be difficult to identify with this technology. An important confounding factor for NIRS technology with TBI patients is injury to the scalp. Blood contained within a scalp hematoma can also alter the OD and cause a false-positive result with this technology. These limitations could be addressed in future design and development of NIRS-based hematoma detection systems.
7. Conclusions
Infrascanner is a clinically effective screening solution for head trauma patients in prehospital settings, where timely triage is critical. It is intended to be used as an adjunct to the standard diagnostic workup to aid in the decision of choosing evacuation destination (a trauma center or the nearest hospital) and in prioritizing patients with suspected hematomas for urgent CT scans and surgical interventions.
Biographies
Hasan Ayaz is an associate professor of biomedical engineering at Drexel University. His research involves understanding the neural mechanisms related to human perceptual, cognitive, and motor functioning with a focus on real-world contexts, utilizing mobile neuroimaging and deploying neuroengineering approaches for neuroergonomics applications. His research aims to design, develop, and utilize (i.e., to measure->elucidate->enable) next-generation optical brain imaging for neuroergonomic applications over a broad spectrum including aerospace and healthcare.
Meltem Izzetoglu is an associate research professor of electrical and computer engineering at Villanova University, Villanova, PA. Her research is centered around design, development, and testing of functional near-infrared spectroscopy-based brain imaging systems using laboratory phantoms, animal models and human experimentation, signal processing and algorithm development for artifact cancellation, and biomarker extraction in biomedical applications specifically for cognitive activity assessment and physiological signal extraction in healthy, diseased, and disabled populations of all ages.
Kurtulus Izzetoglu is an associate research professor of biomedical engineering at Drexel University, Philadelphia, U.S.A. His research and teaching interests are in functional brain imaging, human performance, learning, training, medical sensor development, biomedical systems, and signal processing. He has background in both electrical and biomedical engineering, coupled with experience with developing a highly portable functional fNIRS system for use in field applications and translational neuroimaging.
Banu Onaral is the H. H. Sun Professor of biomedical engineering and electrical engineering at Drexel University. She received her PhD in biomedical engineering from the University of Pennsylvania. Her research is centered on information engineering with special emphases on complex systems and biomedical digital signal processing, and with a particular focus on functional optical brain imaging. She has led major research and development projects and founded several laboratories including the CONQUER (Cognitive Neuroengineering and Quantitative Experimental Research) Collaborative.
Baruch Ben Dor is the CEO of InfraScan. He received his PhD in physics from Technion and is a graduate of the Talpiot Program of the Israeli Air Force. He created InfraScan, a medical device company focused on handheld systems for detection of brain hematomas. He developed the company business plan, raised over $16M, got de novo FDA clearance, and launched sales. All battalion aid stations of the US Marines are equipped with the Infrascanner, saving lives.
Disclosures
InfraScan Inc. is the manufacturer of the Infrascanner systems. All authors own shares at the company due to their contributions to the development of the systems.
References
- 1.Thurman D. J., et al. , Traumatic Brain Injury in the United States: A Report to Congress, Centers for Disease Control and Prevention; (1999). [Google Scholar]
- 2.Cowley R. A., “Trauma center. A new concept for the delivery of critical care,” J. Med. Soc. N J 74(11), 979–987 (1977). [PubMed] [Google Scholar]
- 3.Melton J., et al. , “Helicopter Emergency Ambulance Service (HEAS) transfer: an analysis of trauma patient case-mix, injury severity and outcome,” Ann. Royal Coll. Surg. Engl. 89(5), 513–516 (2007). 10.1308/003588407X202074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jennett B., et al. , “Severe head injuries in three countries,” J. Neurol. Neurosurg. Psychiatry 40(3), 291–298 (1977). 10.1136/jnnp.40.3.291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perel P., et al. , “Intracranial bleeding in patients with traumatic brain injury: a prognostic study,” BMC Emergency Med. 9(1), 15 (2009). 10.1186/1471-227X-9-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stein S. C., et al. , “Limitations of neurological assessment in mild head injury,” Brain Injury 7(5), 425–430 (1993). 10.3109/02699059309029685 [DOI] [PubMed] [Google Scholar]
- 7.Gopinath S., et al. , “Near-infrared spectroscopic localization of intracranial hematomas,” J. Neurosurg. 79(1), 43–47 (1993). 10.3171/jns.1993.79.1.0043 [DOI] [PubMed] [Google Scholar]
- 8.Ayaz H., et al. , “Infrascanner: cost effective, mobile medical imaging system for detecting hemotomas,” J. Med. Devices 5(2), 027540 (2011). 10.1115/1.3591407 [DOI] [Google Scholar]
- 9.Onaral B., et al. , “Update on a handheld brain hematoma detector project,” in Adv. Technol. Appl. for Combat Casualty Care (ATACCC) (2011). [Google Scholar]
- 10.Robertson C. S., Gopinath S., Chance B., “Use of near infrared spectroscopy to identify traumatic intracranial hemotomas,” J. Biomed. Opt. 2(1), 31–42 (1997). 10.1117/12.261680 [DOI] [PubMed] [Google Scholar]
- 11.Liang C.-Y., et al. , “Chinese military evaluation of a portable near-infrared detector of traumatic intracranial hematomas,” Mil. Med. 183(7–8), e318–e323 (2018). 10.1093/milmed/usx088 [DOI] [PubMed] [Google Scholar]
- 12.Xu L., et al. , “Portable near-infrared rapid detection of intracranial hemorrhage in Chinese population,” J. Clin. Neurosci. 40, 136–146 (2017). 10.1016/j.jocn.2017.02.056 [DOI] [PubMed] [Google Scholar]
- 13.Peters J., et al. , “Near-infrared spectroscopy: a promising prehospital tool for management of traumatic brain injury,” Prehosp. Disaster Med. 32(4), 414–418 (2017). 10.1017/S1049023X17006367 [DOI] [PubMed] [Google Scholar]
- 14.Semenova Z. B., et al. , “Infrascanner™ in the diagnosis of intracranial lesions in children with traumatic brain injuries,” Brain Injury 30(1), 18–22 (2016). 10.3109/02699052.2014.989401 [DOI] [PubMed] [Google Scholar]
- 15.Braun T., et al. , “Nahinfrarotspektroskopie zur Detektion intrakranieller traumatischer Hirnblutungen,” Der Unfallchirurg 118(8), 693–700 (2015). 10.1007/s00113-013-2549-0 [DOI] [PubMed] [Google Scholar]
- 16.Skotnicka-Klonowicz G., Godziński J., Hermanowicz A., “Management of minor to moderate head trauma in children-guidelines of the polish association of pediatric surgeons,” Standardy Medyczne/Problemy Chirurgii Dziecięcej 4(1), 42–50 (2014). [Google Scholar]
- 17.Bressan S., et al. , “The use of handheld near-infrared device (Infrascanner) for detecting intracranial haemorrhages in children with minor head injury,” Child’s Nerv. Syst. 30(3), 477–484 (2014). 10.1007/s00381-013-2314-2 [DOI] [PubMed] [Google Scholar]
- 18.Salonia R., et al. , “The utility of near infrared spectroscopy in detecting intracranial hemorrhage in children,” J. Neurotrauma 29(6), 1047–1053 (2012). 10.1089/neu.2011.1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robertson C. S., et al. , “Clinical evaluation of a portable near-infrared device for detection of traumatic intracranial hematomas,” J. Neurotrauma 27(9), 1597–1604 (2010). 10.1089/neu.2010.1340 [DOI] [PubMed] [Google Scholar]
- 20.Leon-Carrion J., et al. , “The Infrascanner, a handheld device for screening in situ for the presence of brain haematomas,” Brain Injury 24(10), 1193–1201 (2010). 10.3109/02699052.2010.506636 [DOI] [PubMed] [Google Scholar]
- 21.Coskun F., et al. , “An assessment on the use of Infrascanner for the diagnosis of the brain hemotoma by using support vector machine,” Sci. Res. Essays 5(14), 1911–1915 (2010). [Google Scholar]
- 22.Lewartowska-Nyga D., Nyga K., Skotnicka-Klonowicz G., “Can Infrascanner be useful in hospital emergency departments for diagnosing minor head injury in children?” Dev. Period Med. 21(1), 51–59 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tyzo B., et al. , “Algorithm of initial management of mild head injury using the portable near-infrared spectroscope,” Neurol Praktyczna 2, 13–20 (2014). [Google Scholar]
- 24.Brazinova A., et al. , “Epidemiology of traumatic brain injury in Europe: a living systematic review,” J. Neurotrauma 33, 1–30 (2016). 10.1089/neu.2015.4126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Russo M. V., McGavern D. B., “Inflammatory neuroprotection following traumatic brain injury,” Science 353(6301), 783–785 (2016). 10.1126/science.aaf6260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walker P., et al. , “Traumatic brain injury in combat casualties,” Curr. Trauma Rep. 4(2), 149–159 (2018). 10.1007/s40719-018-0133-3 [DOI] [Google Scholar]
- 27.Carney N., et al. , “Guidelines for the management of severe traumatic brain injury,” Neurosurgery 80(1), 6–15 (2017). 10.1227/NEU.0000000000001432 [DOI] [PubMed] [Google Scholar]
- 28.Zuercher M., et al. , “The use of Glasgow Coma Scale in injury assessment: a critical review,” Brain Injury 23(5), 371–384 (2009). 10.1080/02699050902926267 [DOI] [PubMed] [Google Scholar]
- 29.Korley F. K., et al. , “Emergency department evaluation of traumatic brain injury in the united states, 2009–2010,” J. Head Trauma Rehabil. 31(6), 379–387 (2016). 10.1097/HTR.0000000000000187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silver J. M., McAllister T. W., Arciniegas D. B., “Depression and cognitive complaints following mild traumatic brain injury,” Am. J. Psychiatry 166(6), 653–661 (2009). 10.1176/appi.ajp.2009.08111676 [DOI] [PubMed] [Google Scholar]
- 31.McCauley S. R., et al. , “Postconcussional disorder following mild to moderate traumatic brain injury: anxiety, depression, and social support as risk factors and comorbidities,” J. Clin. Exp. Neuropsychol. 23(6), 792–808 (2001). 10.1076/jcen.23.6.792.1016 [DOI] [PubMed] [Google Scholar]
- 32.Hoofien D., et al. , “Traumatic brain injury (TBI) 10? 20 years later: a comprehensive outcome study of psychiatric symptomatology, cognitive abilities and psychosocial functioning,” Brain Injury 15(3), 189–209 (2001). 10.1080/026990501300005659 [DOI] [PubMed] [Google Scholar]
- 33.Narayan R. K., et al. , “Clinical trials in head injury,” J. Neurotrauma 19(5), 503–557 (2002). 10.1089/089771502753754037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiong Y., Lee C., Peterson P., “Mitochondrial dysfunction following traumatic brain injury,” Head Trauma: Basic, Preclin. Clin. Dir., Miller L. P., Newcomb R. L. H. J. K., Eds., pp. 257–280, Wiley-Liss, New York: (2001). [Google Scholar]
- 35.Maas A. I. R., Stocchetti N., Bullock R., “Moderate and severe traumatic brain injury in adults,” Lancet Neurol. 7(8), 728–741 (2008). 10.1016/S1474-4422(08)70164-9 [DOI] [PubMed] [Google Scholar]
- 36.Silver J. M., McAllister T. W., Yudofsky S. C., Textbook of Traumatic Brain Injury, 2nd ed, American Psychiatric Publishing, Arlington, Virginia: (2011). [Google Scholar]
- 37.Vos P. E., et al. , “Evaluation of the traumatic coma data bank computed tomography classification for severe head injury,” J. Neurotrauma 18(7), 649–655 (2001). 10.1089/089771501750357591 [DOI] [PubMed] [Google Scholar]
- 38.Seelig J. M., et al. , “Traumatic acute subdural hematoma: major mortality reduction in comatose patients treated within four hours,” N. Engl. J. Med. 304(25), 1511–1518 (1981). 10.1056/NEJM198106183042503 [DOI] [PubMed] [Google Scholar]
- 39.Rivas J. J., et al. , “Extradural hematoma: analysis of factors influencing the courses of 161 patients,” Neurosurgery 23(1), 44–51 (1988). 10.1227/00006123-198807000-00010 [DOI] [PubMed] [Google Scholar]
- 40.Foulkes M. A., et al. , “The traumatic coma data bank: design, methods, and baseline characteristics,” J. Neurosurg. 75(1S), S8–S13 (1991). 10.3171/sup.1991.75.1s.00s8 [DOI] [Google Scholar]
- 41.Jobsis F. F., “Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters,” Science 198(4323), 1264–1267 (1977). 10.1126/science.929199 [DOI] [PubMed] [Google Scholar]
- 42.Wyatt J., et al. , “Quantification of cerebral oxygenation and haemodynamics in sick newborn infants by near infrared spectrophotometry,” Lancet 328(8515), 1063–1066 (1986). 10.1016/S0140-6736(86)90467-8 [DOI] [PubMed] [Google Scholar]
- 43.Delpy D., et al. , “Estimation of optical pathlength through tissue from direct time of flight measurement,” Phys. Med. Biol. 33, 1433–1442 (1988). 10.1088/0031-9155/33/12/008 [DOI] [PubMed] [Google Scholar]
- 44.Wray S., et al. , “Characterization of the near infrared absorption spectra of cytochrome aa3 and haemoglobin for the non-invasive monitoring of cerebral oxygenation,” Biochim. Biophys. Acta-Bioenerg. 933(1), 184–192 (1988). 10.1016/0005-2728(88)90069-2 [DOI] [PubMed] [Google Scholar]
- 45.Cope M., “The development of a near infrared spectroscopy system and its application for non invasive monitoring of cerebral blood and tissue oxygenation in the newborn infant,” University College London, London (1991). [Google Scholar]
- 46.Elwell C., et al. , “Quantification of adult cerebral hemodynamics by near-infrared spectroscopy,” J. Appl. Physiol. 77(6), 2753–2760 (1994). 10.1152/jappl.1994.77.6.2753 [DOI] [PubMed] [Google Scholar]
- 47.Chance B., et al. , “A novel method for fast imaging of brain function, non-invasively, with light,” Opt. Express 2(10), 411–423 (1998). 10.1364/OE.2.000411 [DOI] [PubMed] [Google Scholar]
- 48.Hoshi Y., Tamura M., “Near-infrared optical detection of sequential brain activation in the prefrontal cortex during mental tasks,” Neuroimage 5(4 Pt 1), 292–297 (1997). 10.1006/nimg.1997.0270 [DOI] [PubMed] [Google Scholar]
- 49.Obrig H., et al. , “Near-infrared spectroscopy: does it function in functional activation studies of the adult brain?” Int. J. Psychophysiol. 35(2–3), 125–142 (2000). 10.1016/S0167-8760(99)00048-3 [DOI] [PubMed] [Google Scholar]
- 50.Strangman G., Boas D. A., Sutton J. P., “Non-invasive neuroimaging using near-infrared light,” Biol. Psychiatry 52(7), 679–693 (2002). 10.1016/S0006-3223(02)01550-0 [DOI] [PubMed] [Google Scholar]
- 51.Villringer A., et al. , “Near infrared spectroscopy (NIRS): a new tool to study hemodynamic changes during activation of brain function in human adults,” Neurosci. Lett. 154(1–2), 101–104 (1993). 10.1016/0304-3940(93)90181-J [DOI] [PubMed] [Google Scholar]
- 52.Ayaz H., et al. , “Optical brain monitoring for operator training and mental workload assessment,” NeuroImage 59(1), 36–47 (2012). 10.1016/j.neuroimage.2011.06.023 [DOI] [PubMed] [Google Scholar]
- 53.Izzetoglu M., et al. , “Functional near-infrared neuroimaging,” IEEE Trans. Neural. Syst. Rehabil. Eng. 13(2), 153–159 (2005). 10.1109/TNSRE.2005.847377 [DOI] [PubMed] [Google Scholar]
- 54.Ayaz H., et al. , “Using Mazesuite and functional near infrared spectroscopy to study learning in spatial navigation,” J. Vis. Exp. 8(56), 3443 (2011). 10.3791/3443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Izzetoglu K., et al. , “The evolution of field deployable fNIR spectroscopy from bench to clinical settings,” J. Innovative Opt. Health Sci. 4(3), 239–250 (2011). 10.1142/S1793545811001587 [DOI] [Google Scholar]