Abstract
Thiscase report concerns a 34-year-old male with a hip prosthesis infection, under treatment with antidepressant and antihypertensive drugs, who presented with an increase in blood pressure after four days of treatment with oral tedizolid. Tedizolid was discontinued, and the dose of antihypertensive was increased. The patient progressively achieved the normalisation of blood pressure values, which allowed a reduction in the antihypertensive agent dose to its usual regimen. No cases of hypertension or serotonin toxicity are described in the initial tedizolid studies, where patients treated with other serotonergic drugs were excluded. However, this does not mean that these effects may not occur in clinical practice, especially in patients under concomitant treatment with this type of medication, because of the greater risk of drug interactions. The causality of this suspected drug reaction was analysed and it was considered as possible. The case has been reported to the pharmacovigilance system.
Keywords: adverse effects, tedizolid, pharmacological interactions, hypertensive crisis, serotonin syndrome
Background
Tedizolid is a next-generation oxazolidinone, which was approved by the FDA in 2014 and by the EMA in 2015. It is active against gram-positive pathogens, including methicillin-resistant Staphylococcus aureus, Enterococcus spp including vancomycin-resistant Enterococcus and Streptococcus spp in cutaneous, skin and soft tissues infections.1
Tedizolid phosphate is an inactive precursor that is converted in vivo by the action of serum phosphatases into tedizolid. Tedizolid differs from other antimicrobials of the same family, such as linezolid, by presenting a modified side chain at the C-5 position of the molecular ring. This variation provides the activity against some specific linezolid-resistant pathogens. However, tedizolid shares a similar mechanism of action with other oxazolidinones since bacteriostatic activity is mediated by the inhibition of bacterial protein synthesis.2
Tedizolid displays a high oral bioavailability (greater than 90%) and its 12-hour half-life allows a once-daily administration. The recommended dosage is 200 mg per day, either intravenous or oral, for a period of 6 days. The most commonly reported adverse effects of tedizolid are nausea, headache, diarrhoea and vomiting. This newer oxazolidinone offers some advantages compared with linezolid, such as a daily dosing and an improved safety profile with less gastrointestinal and haematological side effects.3 Referring to the pharmacological interactions, it must be noted that tedizolid inhibits monoamine oxidase (MAO), and it may present serotonergic effects like other drugs of the same antimicrobial family such as linezolid.4 Nevertheless, evidence on the safety profile and pharmacological interactions of tedizolid in clinical practice is scarce.
We report here the first case of a suspected adverse reaction consisting of a hypertensive crisis after 4 days of treatment with tedizolid.
Case presentation
A 34-year-old male with a total hip prosthesis due to osteonecrosis of the femur was admitted to the orthopaedics department for a total prosthesis removal and insertion of a temporary cement spacer because of a chronic infection due to a multidrug-resistant Staphylococcus epidermidis. The patient presented with a history of hypertension, depression and anxiety. Therefore, he was under treatment with enalapril 10 mg per day, amitriptyline 25 mg per day, paroxetine 40 mg per day and trazodone 100 mg per day. During hospitalisation, the patient received antibiotic therapy with vancomycin according to the outcome of the antibiogram. One month later, the signs of infection still persisted and a surgical debridement was performed. Intravenous antibiotic treatment with vancomycin continued for several weeks. During admission, he received analgesic treatment with paracetamol, metamizole and fentanyl for pain control. Once the patient was clinically stable and he was likely to be discharged in a few days, the physician suggested switching to oral antibiotic. The antibiotic proposed was oral tedizolid 200 mg per day. Linezolid was ruled out due to gastrointestinal intolerance presented when he was treated with this drug during previous admissions, and because of the possible interactions with the patient’s usual medication. Initially, the patient tolerated the first doses, but on the fourth day, an increase in blood pressure was detected, reaching values of 189/117 mmHg. Since the hypertensive crisis was suspected to be an adverse reaction to tedizolid, it was discontinued figure 1.
Figure 1.
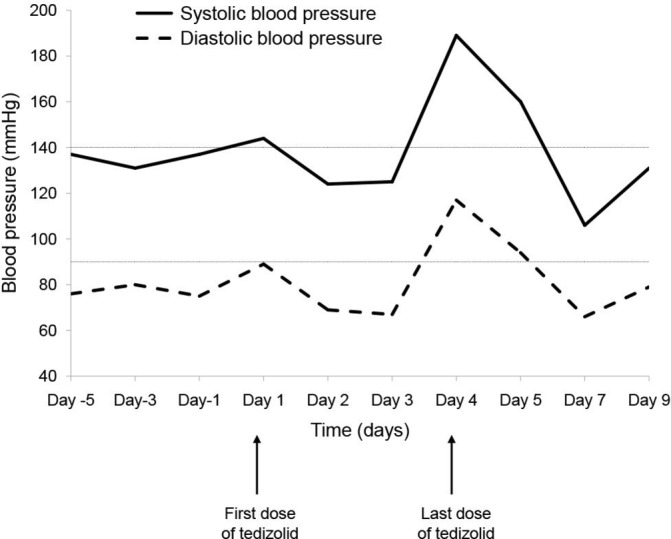
Evolution of patient’s blood pressure.
Investigations
The patient’s physician communicated to the hospital pharmacist the suspected adverse reaction and the pharmacist consulted the summary of product characteristics of tedizolid. During phase III clinical trials, no cases of hypertension or serotonergic effects had been reported for tedizolid, but they cannot be ruled out due to the exclusion of subjects treated with serotonergic drugs.4
Serotonin syndrome is an adverse drug reaction described for linezolid,5 6 a drug of the same family as tedizolid. Consequently, it cannot be excluded as an adverse effect shared with other oxazolidinones.
Treatment
Treatment with tedizolid was discontinued after the fourth dose due to hypertension, and the dose of enalapril was increased from 10 mg to 20 mg per day.
Outcome and follow-up
The patient progressively achieved the normalisation of blood pressure values, which allowed a reduction in the antihypertensive agent dose to its usual regimen. The suspicion of an adverse reaction was reported to the regional Catalan Pharmacovigilance Centre through the yellow card system. Antimicrobial therapy was resumed with dalbavancin.
Discussion
According to the European Public Assessment Report of tedizolid, the most frequent adverse effects (AEs) reported in phase III clinical trials were nausea, headache, diarrhoea and vomiting.7 The profile and incidence of AEs is similar to linezolid, although it was noted that nausea and vomiting are reported at lower incidence. It is noteworthy that in the studies mentioned, a great number of medications were prohibited by protocol, including selective serotonin re-uptake inhibitors, serotonin norepinephrine re-uptake inhibitors, tricyclic antidepressants, MAO inhibitors, triptans and other drugs with potential adrenergic or serotonergic activity. Thus, the potential serotonergic effect of tedizolid cannot be ruled out in patients receiving these medications.4
Serotonin syndrome is an iatrogenic disorder caused by drugs that increase the synaptic concentration of serotonin. The main symptoms are alteration of the autonomous nervous system, neuromuscular excitation and alteration of mental state. These nonspecific symptoms rarely occur simultaneously and may differ between patients depending on the severity of the serotonergic toxicity, making differential diagnosis difficult.8
The case that has been presented here describes a hypertensive crisis suspected to be caused by the pharmacodynamic interaction between tedizolid and the concomitant medication that the patient was taking: amitriptyline, paroxetine, trazodone and fentanyl.
Amitriptyline inhibits the reuptake of dopamine, norepinephrine and serotonin, while paroxetine and trazodone only inhibit the pre-synaptic reuptake of serotonin. This effect leads to an increase in serotonin concentration in the synaptic space. Likewise, synthetic opioids (including fentanyl) have shown, in vitro, inhibition of amine transporters at low concentrations.9 Opioids have been suggested as the cause of serotonergic syndrome in several case reports, fentanyl being the most frequently reported. Thus, the co-administration of fentanyl with serotonergic drugs may increase the risk of serotonin syndrome.10 11 Tedizolid is also a non-selective and reversible inhibitor of MAO and appears to be a more potent inhibitor in vitro of MAO-A than linezolid: however, these findings had no translation to in vivo studies.12
The causality of hypertension as an adverse drug reaction due to the co-administration of tedizolid and other serotonergic treatments was evaluated using the algorithm of Naranjo et al,13 obtaining a final score of 3. According to this value, the relationship between tedizolid and the hypertensive crisis should be classified as possible, as we were not able to rule out the involvement of other factors.
Despite serotonin syndrome not being reported either in the phase III clinical trials or in the summary of product characteristics of tedizolid, the plausibility of the mechanism, especially when it is co-administered with serotonin agonists and reuptake inhibitors, led us to the suspicion that this case may be related to tedizolid and this information was communicated to the pharmacovigilance system.
Learning points.
Possible drug interactions should be considered when starting a new treatment.
Importance of pharmacovigilance, especially with drugs of recent commercialisation.
Pharmacists as part of the multidisciplinary team can provide additional information about the drugs, helping doctors make clinical decisions.
Footnotes
Contributors: MdCJ: literature search; clinical data collection; drafting of the manuscript; graph; revision of the manuscript. PMA: literature search; clinical data collection; analysis of the causality of the adverse reaction; drafting of the manuscript; revision of the manuscript. SOR: literature search; drafting of the manuscript; graph; revision of the manuscript. RV: analysis of the causality of the adverse reaction; clinical data collection; revision of the manuscript. LF: detection and report of the adverse reaction; revision of the manuscript; obtaining the patient’s informed consent. MG-V: literature search; drafting of the manuscript; revision of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; internally peer reviewed.
References
- 1. Zhanel GG, Love R, Adam H, et al. Tedizolid: a novel oxazolidinone with potent activity against multidrug-resistant gram-positive pathogens. Drugs 2015;75:253–70. 10.1007/s40265-015-0352-7 [DOI] [PubMed] [Google Scholar]
- 2. Ferrández O, Urbina O, Grau S. Critical role of tedizolid in the treatment of acute bacterial skin and skin structure infections. Drug Des Devel Ther 2017;11:65–82. 10.2147/DDDT.S84667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Roger C, Roberts JA, Muller L. Clinical pharmacokinetics and pharmacodynamics of oxazolidinones. Clin Pharmacokinet 2018;57:559–75. 10.1007/s40262-017-0601-x [DOI] [PubMed] [Google Scholar]
- 4. EMA (European Medicines Agency). Summary of product characteristics: Sivextro (tedizolid) [Internet]. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002846/WC500184802.pdf (accessed 13 Apr 2018).
- 5. AEMPS (Spanish Agency of Medicines and Medical Devices). Summary of product characteristics. Zyvoxid (linezolid) [Internet]. https://cima.aemps.es/cima/dochtml/ft/64109/FT_64109.html (accessed 13 Apr 2018).
- 6. Lawrence KR, Adra M, Gillman PK. Serotonin toxicity associated with the use of linezolid: a review of postmarketing data. Clin Infect Dis 2006;42:1578–83. 10.1086/503839 [DOI] [PubMed] [Google Scholar]
- 7. Bay-mattawa N, Area SP. European public assessment report (tedizolid). Sivextro 2011;44:82–102. [Google Scholar]
- 8. Wang RZ, Vashistha V, Kaur S, et al. Serotonin syndrome: preventing, recognizing, and treating it. Cleve Clin J Med 2016;83:810–7. 10.3949/ccjm.83a.15129 [DOI] [PubMed] [Google Scholar]
- 9. Rickli A, Liakoni E, Hoener MC, et al. Opioid-induced inhibition of the human 5-HT and noradrenaline transporters in vitro: link to clinical reports of serotonin syndrome. Br J Pharmacol 2018;175:532–43. 10.1111/bph.14105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. AEMPS (Spanish Agency of Medicines and Medical Devices). Summary of product characteristics. Fendivia (fentanyl). https://cima.aemps.es/cima/dochtml/ft/68493/FT_68493.html
- 11. Armitage MC, Woolfield KI, Page CB. Serotonin toxicity caused by the interaction of fentanyl and serotonergic medications. Emerg Med Australas 2016;28:119–20. 10.1111/1742-6723.12521 [DOI] [PubMed] [Google Scholar]
- 12. Flanagan S, Bartizal K, Minassian SL, et al. In vitro, in vivo, and clinical studies of tedizolid to assess the potential for peripheral or central monoamine oxidase interactions. Antimicrob Agents Chemother 2013;57:3060–6. 10.1128/AAC.00431-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981;30:239–45. 10.1038/clpt.1981.154 [DOI] [PubMed] [Google Scholar]


