Abstract
Objectives
The study aimed to estimate the burden of metastatic breast cancer (mBC) in Spain over 5 years.
Methods
An incidence-based cost-of-illness model was developed in which a cohort of patients with mBC was followed from the diagnosis of metastatic disease over 5 years or death. Resource use data were collected through a physician survey conducted with 10 clinical experts in Spain. The model distinguished patients according to HER2 and hormonal receptor (HR) status, and followed the patient cohort in monthly cycles.
Results
The incident cohort was estimated to be 2,923 patients with mBC, consisting of 1,575 HER2−/HR+, 520 HER2+/HR+, 324 HER2+/HR−, and 503 triple negative patients. The estimated mean survival over the 5-year time period was 2.51 years, on average, with longer survival of 3.36 years for HER2+/HR+, 2.41 years for HER2−/HR+, 2.82 years for HER2+/HR− and shortest mean survival of 1.74 years for triple negative patients. The total costs were €469,92,731 for the overall population, €190,079,787 for the HER2-/HR+, €151,045,260 for the HER2+/HR+, €80,827,171 for the HER2+/HR- and €47,540,512 for the triple negative subgroups over 5 years. Per patient total costs were €160,642 on average, €120,664 for HER2-/HR+, €290,346 for HER2+/HR+, €249,152 for HER2+/HR−and €94,572 for triple negative patients over 5 years.
Conclusions
The economic burden of mBC in Spain is significant, but differs by HER2 and HR status. HER2−/HR +patients account for the highest burden due to the prevalence of this category, but HER2+/HR +patients have the highest per patient costs.
Keywords: metastatic breast cancer, costs of illness, treatment patterns, direct costs, resource utilisation
Key messages.
What is already known on this subject
Breast cancer accounts for the highest healthcare costs among cancers.
The cost of metastatic breast cancer was evaluated in other countries, but not in Spain.
The appearance and increased usage of newer and costly targeted therapies in the past few years increased the expenditures related to the treatment of metastatic breast cancer.
What this study adds
This is the first study assessing the cost of metastatic breast cancer in Spain with an incidence-based cost of illness model taking into account the differing treatment practices by HER2 and hormonal receptor status.
These cost estimates may aid economic evaluations of strategies for the prevention of progression to metastatic disease allowing for differentiation by HER2 and hormonal receptor status.
Introduction
Breast cancer (BC) is the most common tumour among women in developed countries. In Spain, BC has the highest incidence, prevalence and mortality among all cancer types in the female population.1 In 2012, 25,215 Spanish women were diagnosed with BC.1
BC is a heterogeneous disease in terms of biological and clinical behaviour. The diversity of the disease can be characterised with molecular biomarkers.2 Human epidermal growth factor receptor 2 (HER2) is one of the most important predictive biomarkers, and hormonal receptor (HR) status is also crucial in disease management and prognosis. BC can be categorised into four subgroups based on the status of these biomarkers: HER2−/HR+ (HR+ being oestrogen receptor (ER)+ or progesterone receptor (PR); HER2+/hormonal− (ER− and PR−); HER2−/hormonal+; and triple negative. More than half of patients with mBC fall into the HER2−/HR +subgroup (54%), followed by the HER2+/HR+ (18%), triple negative (17%) and HER2−/HR+ (11%) subgroups.3
Metastatic BC (mBC) accounts for a small percentage of all BC cases. It can occur de novo, in around 6% to 10% of newly diagnosed cases, or it may present as a recurrence of earlier stage disease in 20% to 50% of patients.4 The median survival of mBC is around 21.8 months,5 although this varies significantly according to biomarker status.
In the past few years, the landscape of BC treatments has changed considerably with the introduction of novel targeted therapies, such as anti-HER2 monoclonal antibodies (trastuzumab or pertuzumab among others). Despite recent advancements in therapy, mBC is still an incurable disease, and the aim of the treatment is to maintain patients’ quality of life, alleviate symptoms and prolong survival.
The economic burden of BC is high in developed countries.6 A recent European study found that among cancers, BC accounted for the highest healthcare costs, and estimated the healthcare cost of BC to be €518 million for the overall BC population in Spain in the year 2009.7 The study by Sorensen et al 8 found that the total cost of mBC was $12.2 billion over 5 years for an incident cohort in the US. However, patient characteristics, treatment patterns and healthcare costs may differ greatly even between developed countries. Furthermore, the appearance and increased usage of newer and costly targeted therapies in the past few years can further increase the expenditures related to the treatment of mBC.
Economic evaluations of strategies for the prevention or early detection of BC, the effective treatment of early disease or the prevention of progression to metastatic disease all require a good understanding of the costs associated with metastatic disease. Therefore, the aim of our study was to assess the total cost of mBC in Spain with an incidence-based cost of illness model.
Methods
Model overview and design
The model extended the works of Sorensen et al.8 An incidence-based cost-of-illness model was implemented in Microsoft Excel 2013 to evaluate the burden of mBC in Spain through calculating the 5-year costs of the disease for a cohort of patients with mBC.
The cohort consisted of patients with newly diagnosed or recurrent mBC diagnosed over 1 year. The cohort was stratified into subgroups according to HER2 and HR status. The model followed the subgroups in monthly cycles from the time of mBC diagnosis over 5 years. Patients could receive one to three lines of active treatment (please see list of treatments in the online supplementary Appendix). Proportions of patients receiving a given line of treatment were determined based on the survival of the cohort and the estimated percentage of patients starting the given line of treatment. Patients still alive, but not receiving active treatment, were assumed to receive palliative/best supportive care. In the active treatment phases, the cost of active treatment comprising drug and administration costs; the costs of treating the most prevalent type of metastases and bone metastases; and the costs of managing toxicities occurring during treatment, one-time cost of mBC diagnosis and cost of medical follow-up (comprising outpatient care and inpatient stays) were accrued. In the palliative care phase, patients incurred the cost of drugs and procedures utilised to palliate the symptoms of the disease, the costs of medical follow-up and the costs of social services required due to mBC. Indirect costs, measured by lost productivity due to missed days of work for the working population, were also taken into account.
ejhpharm-2017-001453supp001.pdf (488.4KB, pdf)
Model inputs
Patient cohort
The number of newly diagnosed mBC cases occurring during 1 year was estimated based on the size of the female population,9 the overall incidence of BC1 and the percentage of incident BC cases diagnosed at the metastatic stage10 in Spain. The number of patients who progressed from early stages to mBC during 1 year was derived using the distribution of mBC patients according to the stage of the disease at the detection of BC. Ganguli et al reported that almost 50% of patients with mBC were diagnosed with Stage IV BC,11 therefore it was assumed that patients diagnosed in the metastatic stage represent 50% of all patients presenting with mBC over a year (the other 50% progressed to metastatic stage from earlier disease stages).
Incident mBC cases were then divided into subgroups by biomarker status based on published data reporting the HER2 and HR status of patients with mBC3 (table 1). The number of patients suffering from bone metastases in each subgroup was also estimated based on the percentage of patients with mBC presenting with metastatic lesions in the skeletal system.3
Table 1.
Incident cohort of patients with metastatic breast cancer
| Percentage% | Number of patients | Source | |
| Incident cases of mBC | 100 | 2923 | 1 9–11 |
| HER2−/HR+ | 54 | 1575 | 3 |
| HER2+/HR+ | 18 | 520 | 3 |
| HER2+/HR− | 11 | 324 | 3 |
| Triple negative | 17 | 503 | 3 |
HER2, human epidermal growth factor receptor 2; HR, hormonal receptor; mBC, metastatic breast cancer.
Survival estimation
Monthly survival of the entire cohort was calculated based on 1- and 5-year BC-specific overall survival (OS) of patients with mBC.12 The survival of each biomarker subgroup was estimated using reported mortality hazard ratios5 to derive subgroup-specific mortality rates.
Medical resource utilisation and related costs
Since no published information of the resource use in mBC by biomarker status could be identified, a physician survey13 was conducted to obtain information on resource utilisation. Ten practising oncologists from different regions of Spain completed a questionnaire about the treatment patterns and resource use associated with mBC.
The experts estimated that approximately 95% of incident mBC patients received first-line treatment, 73% received second-line treatment and 53% received third-line treatment in Spain.
The cancer treatments included in the survey were those that are considered by the clinical guideline of the Spanish Society of Medical Oncology14 for the management of mBC, and are available for prescription in Spain. The use of different anti-cancer drug classes and the average treatment durations were evaluated by the experts in each line of treatment for each biomarker subgroup as reported in the online supplementary Appendix. According to current guidelines,14 many treatments can be administered in combination, therefore summed share of drug classes could be greater than 100%. The share of different active ingredients within a drug class was also provided by the experts by biomarker subgroup. The dosing schedules were obtained from the Summary of Product Characteristics (SPCs). Based on experts’ opinions, intravenous (IV) active treatments are administered in the outpatient setting and, therefore, the administration cost of IV treatments was taken into account. It was also assumed that phial sharing is practised for each IV treatment.
Monthly costs of active treatments (consisting of drug and administration costs) were derived for each line of treatment and each biomarker subgroup separately. First, the monthly drug cost and administration cost of each active product were calculated based on the dosing schedule of the drug and the corresponding drug unit cost. Based on current treatment practice, as provided by the clinical experts, the costs of each product were combined into a weighted average for each drug class, and then the costs of drug classes were combined into a weighted average treatment cost.
The proportion of patients experiencing adverse events during active treatment were collected from the SPCs or, where not available, from representative published clinical trials. i Toxicities were assumed to happen evenly distributed over the treatment duration. Resource utilisation related to the management of toxicities was evaluated by the experts, and included diagnostic tests, drugs and procedures utilised to diagnose and treat the adverse events.
The physician survey also collected data on the resource use associated with medical follow-up of patients with mBC in the active treatment phases and during palliative/best supportive care. The survey included outpatient specialist visits, outpatient appointments with other healthcare professionals, outpatient imaging and laboratory tests, or other diagnostic procedures used to monitor patients with mBC. Inpatient hospital admissions due to mBC were also considered in all phases of the disease course. Drugs and medical procedures received and social services (eg, day care or residential care) required during palliative/best supportive care were also assessed.
To calculate monthly costs, unit costs for drugs were collected from publicly available sources.15 16 For other resource use items, costs were obtained from three different autonomous communities in Spain (Madrid,17 Andalusia18 19 and Catalonia20), and the average of the three unit costs was applied for the costing analysis. The costs applied in the model can be seen in table 2.
Table 2.
Cost inputs
| Parameter | Cost (€) | Source |
| Active cancer treatment | ||
| Weighted average monthly cost of treatment | ||
| First-line | ||
| HER2−/HR+ | 1184 € | 13 15–20 |
| HER2+/HR+ | 7404 € | |
| HER2+/HR− | 7491 € | |
| Triple negative | 2823 € | |
| Second-line | ||
| HER2−/HR+ | 1670 € | 13 15–20 |
| HER2+/HR+ | 6869 € | |
| HER2+/HR− | 6656 € | |
| Triple negative | 1534 € | |
| Third-line | ||
| HER2−/HR+ | 1967 € | 13 15–20 |
| HER2+/HR+ | 6534 € | |
| HER2+/HR− | 6349 € | |
| Triple negative | 1609 € | |
| Treatment of bone metastases | ||
| Weighted average monthly cost of bone metastasis treatment | 316 € | 13 15–20 |
| Toxicity management | ||
| Per-event toxicity management cost | ||
| Neutropaenia | 546 € | 13 15–20 |
| Febrile neutropaenia | 1875 € | |
| Leucopaenia | 390 € | |
| Lymphopaenia | 79 € | |
| Thrombocytopaenia | 1295 € | |
| Infection | 993 € | |
| Anaemia | 1794 € | |
| Haemorrhage | 3233 € | |
| All neuropathies | 644 € | |
| Nausea | 362 € | |
| Vomiting | 592 € | |
| Diarrhoea | 931 € | |
| Dehydration | 955 € | |
| Pain | 1255 € | |
| Hypersensitivity | 366 € | |
| Stomatitis/mucositis | 968 € | |
| Alopecia | 95 € | |
| Hepatobiliary – alanine aminotransferase | 358 € | |
| Hepatobiliary – aspartate aminotransferase | 358 € | |
| Hyperbilirubinaemia | 602 € | |
| Increased alkaline phosphatase | 200 € | |
| Hypertension | 417 € | |
| Dyspnea | 1241 € | |
| Fatigue | 895 € | |
| Skin reaction | 241 € | |
| Fluid retention | 687 € | |
| Hand and foot syndrome | 270 € | |
| Asthaenia | 953 € | |
| Diagnosis | ||
| One-time cost of diagnosis | 1191 € | 13 15–20 |
| Medical follow-up during active treatment | ||
| Monthly cost of outpatient care during active treatment | 436 € | 13 15–20 |
| Monthly cost of hospitalisations during active treatment | 635 € | |
| Palliative/best supportive care | ||
| One-time cost of medications during palliative/best supportive care | 391 € | 13 15–20 |
| Monthly cost of medications during palliative/best supportive care | 2656 € | |
| Monthly cost of outpatient care during palliative/best supportive care | 330 € | |
| Monthly cost of hospitalisations during palliative/best supportive care | 1668 € | |
| Monthly cost of community/social services during palliative/best supportive care | 602 € | |
| Indirect costs | ||
| Monthly lost income | 8 € | 1 21 |
HER2, human epidermal growth factor receptor 2; HR, hormonal receptor.
Indirect costs
Patient workdays missed were determined based on an analysis of temporary disability due to BC in Spain.21 The authors had investigated disability claims related to BC International Classification of Diseases (ICD) codes submitted in 2010, and had determined the number and average length of claims related to BC. For the purpose of the model, the number of claims per patient was calculated by dividing the reported total number of claims21 with the prevalence of BC in Spain.1 Multiplying by the average length of a claim allowed us to derive an average number of workdays lost per patient. The average of the three daily wage estimates reported in the above study21 was used to evaluate the costs of workdays lost.
All costs are in 2016 euros. Cost and health outcomes were discounted at 3% per annum based on the Spanish recommendations on economic evaluation of health technologies22 and guidelines on cost of illness studies.23
Analyses and outputs
The economic burden of mBC was evaluated in terms of total cost over 5 years for the overall population and for each biomarker subgroup, and in terms of average per patient costs.
A probabilistic sensitivity analysis, in which each input parameter was allowed to vary randomly according to a predefined distribution based on information about variability around the base-case estimate was performed to assess the uncertainty around the calculated burden of mBC. The probability distributions used for the different parameters were selected based on recommendations to correspond to the mathematical properties of the parameters;24 uncertainty in hazard ratios for OS was represented using log-normal distributions; and rates and probabilities were varied using a beta distribution, while the gamma distribution was used to represent uncertainty in treatment costs (cost of managing toxicities, one-time cost of mBC diagnosis, cost of medical follow-up during active treatment and cost of palliative/best supportive care). Drug acquisition costs were not varied, as they were considered certain. Probabilistic analysis mean results were validated against base case estimates, while face validity of predicted ranges was also assessed.
Results
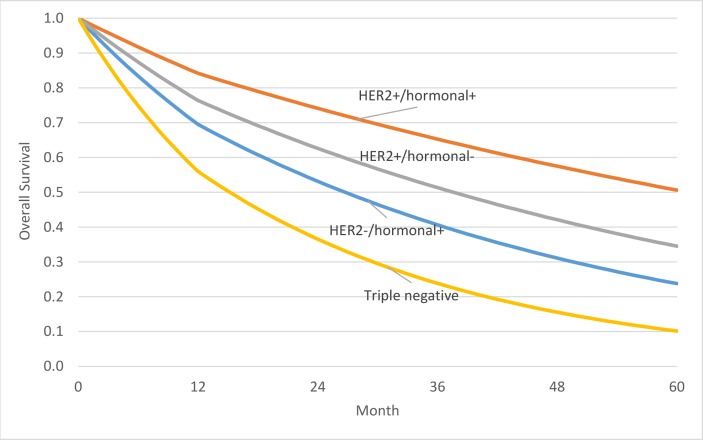
The incident cohort of patients with mBC consisted of 2,923 cases, from which 1,575 were HER2−/HR+, 520 HER2+/HR+, 324 HER2+/HR− and 503 triple negative. There are large differences in terms of survival between the subgroups, with 50.6% of patients predicted to be alive in the HER2+/HR +subgroup, but only 10.2% of patients predicted to be alive for the triple negative subgroup at the end of the 5-year period (see figure 1). Consequently, while estimated mean survival over this period was 2.51 years on average, it was 2.41 years for HER2-/HR+, 3.36 years for HER2+/HR+, 2.82 years for HER2+/HR− and only 1.74 years for triple negative patients.
Figure 1.
Modelled overall survival of patients with mBC, by biomarker subgroup.
The total cost of the overall incident cohort of patients with mBC in Spain was estimated to be €469,492, 731 over 5 years. The model considered each biomarker subgroup separately. Since it has the largest population, the HER2-/HR +mBC patient population contributed to the total expenditure with €190,079, 787 (40% of total costs). Although comprising less than 20% of the population, the HER2+/HR +mBC patients contributed to total expenditures with €151,045,260 (32% of total costs), while HER2+/HR- mBC patients with €80,827,171 (17%), and triple negative mBC patients with €47,540,512 (10%). The discrepancy between the proportion of the subgroups compared with the total population and their contribution to the total expenditures is due to the difference in the per patient treatment costs, with the cost per patient being €160,642 on average, but being €120,664 for HER2-/HR+, €290,346 for HER2+/HR+, €249,152 for HER2+/HR-and €94,572 for triple negative patients over 5 years.
Active treatment (drug and administration) costs contributed to the majority of mBC expenditures. In the overall population, 37% (€171,451,406) of the total costs was spent on anti-cancer agents. The per patient cost of active treatment was €58,664 on average, with the highest costs seen for HER2+ patients due to longer treatment duration and more expensive drugs used.
Among the non-drug-related other cost elements, the expenditures devoted to palliative/best supportive care were the highest, representing 49% of the total costs (€229,012,880). The contribution of other cost categories to the total spending was much lower within the overall mBC population (12% for cost of medical follow-up, 1.6% for diagnostic costs, 0.9% for cost of toxicity management and 0.15% for in
Table 3 Estimated total direct and indirect costs over 5 years
| HER2-/HR+ | HER2+/HR+ | HER2+/HR- | Triple negative | Overall population | Costs for overall population as % of total direct and indirect costs | |
| Direct costs | ||||||
| Active treatment | 32 082 923 € | 78 101 997 € | 47 942 420 € | 13 324 066 € | 171 451 406 € | 36.5% |
| Toxicity management | 1 970 091 € | 897 285 € | 552 383 € | 941 347 € | 4 361 107 € | 0.93% |
| Diagnostic | 4 010 272 € | 1 332 949 € | 825 517 € | 1 223 233 € | 7 391 971 € | 1.57% |
| Medical follow-up | 29 423 667 € | 12 716 285 € | 7 324 521 € | 7 119 424 € | 56 583 898 € | 12.05% |
| Palliative/BSC costs | 122 235 074 € | 57 831 745 € | 24 095 929 € | 24 850 132 € | 229 012 880 € | 48.78% |
| Total direct costs | 189 722 027 € | 150 880 260 € | 80 740 771 € | 47 458 202 € | 468 801 261 € | 99.85% |
| Indirect costs | 357 760 € | 165 000 € | 86 400 € | 82 310 € | 691 470 € | 0.15% |
| Total direct and indirect costs | 190 079 787 € | 151 045 260 € | 80 827 171 € | 47 540 512 € | 469 492 731 € | 100.00% |
BSC, best supportive care; HER2, human epidermal growth factor receptor 2; HR, hormonal receptor.
The probabilistic sensitivity analysis confirmed the base-case results, the estimated mean total cost per patient was €1 60 565 (SD €15 738) on average over 5 years.
Table 4.
Estimated total direct and indirect costs per patient over 5 years
| HER2−/HR+ | HER2+/HR+ | HER2+/HR− | Triple negative | Average | |
| Direct costs | |||||
| Active treatment | 20 366 € | 150 131 € | 147 784 € | 26 506 € | 58 664 € |
| Toxicity management | 1251 € | 1725 € | 1703 € | 1873 € | 1492 € |
| Diagnostic | 2546 € | 2562 € | 2545 € | 2433 € | 2529 € |
| Medical follow-up | 18 678 € | 24 444 € | 22 578 € | 14 163 € | 19 361 € |
| Palliative/BSC costs | 77 595 € | 111 167 € | 74 276 € | 49 434 € | 78 359 € |
| Total direct costs | 1 204 376 € | 290 029 € | 248 885 € | 94 409 € | 160 405 € |
| Indirect costs | 227 € | 317 € | 266 € | 164 € | 237 € |
| Total direct and indirect costs | 120 664 € | 290 346 € | 249 152 € | 94 572 € | 160 642 € |
BSC, best supportive care; HER2, human epidermal growth factor receptor 2; HR, hormonal receptor.
Discussion
The cost for the management of mBC in Spain for this incident cohort was over € 700 million over 5 years, with the HER2-/HR +mBC patient population contributing the most to this total due to the fact that more than half of patients fall into this category. The per patient cost over 5 years varied, ranging from € 94,572 for triple negative patients to €290,346 for HER2+/HR +patients. The differences between the per patient total cost results of the subgroups highlights the economic consequences of the different treatment patterns between the biomarker subgroups. The per patient costs of the HER2 +subgroup are much higher than the costs of the HER2- subgroup, mainly due to the increased usage of costly anti-HER2 therapies, as well as the longer survival and treatment duration in these subgroups.
A recent literature review focusing on any type of cost related to different types of cancer (prostate, breast, colorectal, lung, cervical and skin cancers) covering articles over 15 years (from 1998 to 2015)25 has confirmed that previously there has been no incidence-based mBC cost-of-illness model published for Spain, and our study is the first estimating the consequential stream of costs accrued over 5 years. Luengo-Fernandez et al published a study in 2013 that evaluated the economic burden of cancer across the EU for 2009 in a population-based cost analysis.7 They reported that the total healthcare cost was €518 million for the overall prevalent BC population over 1 year in Spain.7 Unfortunately, differences in study population, design and methods (incidence-based metastatic patients followed over 5 years versus prevalent cohort of all BC patients regardless of status in a single year) hinder the comparison with our results. Two economic evaluations were also identified which looked at the cost effectiveness of treatments in pre-treated metastatic breast cancer patients in Spain.26 27 Calculated treatment costs are similar across studies with, for example, Frias et al 27 estimating €1978 per cycle versus this study using treatment costs of €1910 for nanoparticle albumin-bound paclitaxel. Similar to this study, both economic evaluations relied on expert opinion to estimate resource use in this patient population. However, cost estimates used for patient monitoring and best supportive care in the two cost-effectiveness models are much lower (€490.43 for best supportive care for the entire post-progression period in Frias et al and only €226.76 per month for the post-progression period in Alba et al).26 Furthermore, this study included first-line treatment too, while the published studies looked at the time period starting from second-line treatment only. Therefore, the 5-year total costs are also lower (in the range of €10 to €20,000) compared with the results of this study where the average was around €160, 000.
The first and main limitation of our study is the lack of publicly available data on resource utilisation associated with the management of patients with mBC in Spain. Thus, our study relied on a survey of 10 clinicians from different regions of Spain. They provided information on current treatment practices across Spain in terms of types of drugs used, how patients are monitored during active treatment, what kinds of resources are available to provide supportive care to those patients no longer receiving active treatments and how adverse events are managed. None of this information is available from other sources, therefore the information provided by the clinicians was indispensable in building a picture of what happens to patients with mBC in Spain. To account for the variability in the estimates derived from the survey, a probabilistic sensitivity was performed. However, future research is needed to accurately quantify the medical resource use of patients with mBC directly from a representative sample.
Significant toxicities can frequently occur during active cancer treatment, which may lead to dose reductions or delays, therefore the dose intensity and ultimately the total cost of the treatment is reduced. Our model did not take into account the dose reductions and delays, therefore it may overestimate the cost of active treatment. On the other hand, it was assumed that phial sharing was applied for each IV treatment, that is, no wastage was generated, which may underestimate the cost of IV cancer treatments.
Another limitation of our study is that some of the estimates are slightly outdated. The survival data used to estimate survival in our model was collected from patients diagnosed between 1992 and 2005.12 Due to the rapidly changing treatment landscape, survival patterns are changing, therefore the survival and per patient costs may be slightly underestimated in our model. At the same time, the study informing the proportion of patients diagnosed with metastatic BC was based on information gathered between 2005 and 2010.11 With improvements in BC awareness and diagnostics, the proportion of patients only diagnosed in the metastatic stage may have decreased since, therefore the population size in the model may also be underestimated.
Lastly, only the cost of workdays lost were taken into account, while indirect costs related to premature mortality and caregiver costs were not. Therefore, the societal burden arising from the indirect costs of mBC are underestimated.
The economic burden of mBC in Spain is significant. Our study provided an estimate of the per patient cost of treatment by hormonal receptor status as well as the total cost of treatment of metastatic patients. Economic evaluations of interventions aimed at the prevention of BC or the prevention of progression to metastatic disease such as the use of adjuvant trastuzumab with HER2+ early breast cancer or the addition of cyclin-dependent kinase inhibitors to standard adjuvant hormonal therapy for HR+ early breast cancer can utilise these estimates to assess the impact of delaying progression to offset some of the cost of the interventions themselves.
Footnotes
See Appendix for full references
Contributors: ER and MB planned the study, developed the economic model, developed and analysed the physician questionnaires, and drafted the manuscript. Doctors BBdlH, JCRC, EGC, JdlHR, JGM, FMA, IPF, AR-L, CARS and MR-B reviewed, modified and completed the physician questionnaire; reviewed model results and reviewed the manuscript. MR and JSA planned the study, reviewed the physician questionnaire, reviewed model results and reviewed the manuscript.
Funding: Funding for the development of the model was provided by Pfizer S.A. to Evidera Inc. Authors who are employees of Pfizer S.A. were involved in study design, review of study outcomes and the decision to submit the paper for publication.
Competing interests: None declared.
Patient consent: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. International Agency for Research on Cancer. GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalance Worldwide in 2012: Population Fact Sheets, 2012. http://globocan.iarc.fr/Pages/fact_sheets_population.aspx (accessed 7 Nov 2016). [Google Scholar]
- 2. Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature 2000;406:747–52. 10.1038/35021093 [DOI] [PubMed] [Google Scholar]
- 3. DeKoven M, Bonthapally V, Jiao X, et al. Treatment pattern by hormone receptors and HER2 status in patients with metastatic breast cancer in the UK, Germany, France, Spain and Italy (EU-5): results from a physician survey. J Comp Eff Res 2012;1:453–63. 10.2217/cer.12.43 [DOI] [PubMed] [Google Scholar]
- 4. Mestres JA, iMolins AB, Martinez LC, et al. Defining the optimal sequence for the systemic treatment of metastatic breast cancer: Clinical & Translational Oncology: official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lobbezoo DJA, van Kampen RJW, Voogd AC, et al. Prognosis of metastatic breast cancer subtypes: the hormone receptor/HER2-positive subtype is associated with the most favorable outcome. Breast Cancer Res Treat 2013;141:507–14. 10.1007/s10549-013-2711-y [DOI] [PubMed] [Google Scholar]
- 6. Foster TS, Miller JD, Boye ME, et al. The economic burden of metastatic breast cancer: a systematic review of literature from developed countries. Cancer Treat Rev 2011;37:405–15. 10.1016/j.ctrv.2010.12.008 [DOI] [PubMed] [Google Scholar]
- 7. Luengo-Fernandez R, Leal J, Gray A, et al. Economic burden of cancer across the European Union: a population-based cost analysis. Lancet Oncol 2013;14:1165–74. 10.1016/S1470-2045(13)70442-X [DOI] [PubMed] [Google Scholar]
- 8. Sorensen SV, Goh JW, Pan F, et al. Incidence-based cost-of-illness model for metastatic breast cancer in the United States. Int J Technol Assess Health Care 2012;28:12–21. 10.1017/S026646231100064X [DOI] [PubMed] [Google Scholar]
- 9. Instituto Nacional de Estadística (INE). Población (españoles/extranjeros) por edad (grupos quinquenales), sexo y año. 2015. http://www.ine.es/jaxi/tabla.do?path=/t20/e245/p08/l0/&file=02002.px&type=pcaxis&L=0 (accessed 3 Mar 2015).
- 10. Pfizer Spain. Data on file. Disease Insights. Breast Cancer–Spain, 2014. [Google Scholar]
- 11. Ganguli A, DeKoven M, Bonthapally V, et al. P1-08-21: Demographic and clinical characteristics of metastatic breast cancer patients and biomarker -based prevalence in the UK, Germany, France, Spain and Italy (EU-5). Cancer Res 2011;71:P1-08-21 10.1158/0008-5472.SABCS11-P1-08-21 [DOI] [Google Scholar]
- 12. Macià F, Porta M, Murta-Nascimento C, et al. Factors affecting 5- and 10-year survival of women with breast cancer: an analysis based on a public general hospital in Barcelona. Cancer Epidemiol 2012;36:554–9. 10.1016/j.canep.2012.07.003 [DOI] [PubMed] [Google Scholar]
- 13. Pfizer Spain. Data on file. Physician survey for metastatic breast cancer resource use collection in Spain, 2016. [Google Scholar]
- 14. Gavilá J, Lopez-Tarruella S, Saura C, et al. SEOM clinical guidelines in metastatic breast cancer 2015. Clin Transl Oncol 2015;17:946–55. 10.1007/s12094-015-1476-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sanidad M. Servicios Sociales e Igualdad,. Información sobre los productos incluidos en la prestación farmacéutica del SNS (dispensables a través de oficinas de farmacia). Nomenclátor de Facturación de Junio 2016;2016 http://www.msssi.gob.es/profesionales/nomenclator.do (accessed 30 Jun 2016). [Google Scholar]
- 16. Colegio de Faramacéuticos de Pontevedra. Consulta de Precios de Medicamentos, 2016. Actualizado: 04/05/2016 (accessed 30 Jun 2016). [Google Scholar]
- 17. Boletin Oficial de la Comunidad de Madrid, Martes 10 de Septiembre de 2013 – B.O.C.M. Núm. 215.
- 18. Boletin Oficial de la Junta de Andalucia (BOJA), 27 de Octubre 2005 – Núm. 210.
- 19. Boletin Oficial de la Junta de Andalucia (BOJA), 24 de Noviembre 2015 – Núm. 228.
- 20. Diari Oficial de la Generalitat de Catalunya - Núm. 6326 – 1.3. 2013.
- 21. Vicente-Herrero MT, Terradillos-García MJ, Ramírez-Iñiguez De La Torre MV, et al. Breast cancer in Spain. Economic cost approach to temporary disability in 2010. Gaceta Mexicana de Oncologia 2012;11:351–7. [Google Scholar]
- 22. López-Bastida J, Oliva J, Antoñanzas F, et al. Spanish recommendations on economic evaluation of health technologies. Eur J Health Econ 2010;11:513–20. 10.1007/s10198-010-0244-4 [DOI] [PubMed] [Google Scholar]
- 23. Larg A, Moss JR. Cost-of-illness studies: a guide to critical evaluation. PharmacoEconomics 2011;29:653–71. [DOI] [PubMed] [Google Scholar]
- 24. Briggs A, Claxton K, Schulper M. Decision Modelling for Health Economic Evaluation. Oxford University Press 2006. [Google Scholar]
- 25. Paul A. The economic burden of cancer in Spain: a literature review. Health Econ & Out Res 2017;3:125. [Google Scholar]
- 26. Alba E, Ciruelos E, López R, et al. Cost-utility analysis of nanoparticle albumin-bound paclitaxel versus paclitaxel in monotherapy in pretreated metastatic breast cancer in Spain. Expert Rev Pharmacoecon Outcomes Res 2013;13:381–91. 10.1586/erp.13.18 [DOI] [PubMed] [Google Scholar]
- 27. Frías C, Cortés J, Seguí Miguel Ángel, et al. Cost-effectiveness analyses of docetaxel versus paclitaxel once weekly in patients with metastatic breast cancer in progression following anthracycline chemotherapy, in Spain. Clinical & Trans Oncol 2010;12:692–700. 10.1007/s12094-010-0579-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ejhpharm-2017-001453supp001.pdf (488.4KB, pdf)