Abstract
Objectives
The main objective was to investigate Y-site compatibility of intravenous drugs with one standard total parenteral nutrition (TPN) admixture for preterm infants. Since micro-precipitation was observed in the water phase after addition of trace elements, the concentration effect on micro-precipitation formation developed as a sub-goal.
Methods
Seven drugs (ampicillin, ceftazidime, fluconazole, fosphenytoin, furosemide, metronidazole and paracetamol) were mixed in three mixing ratios with one preterm TPN admixture. Samples were investigated within 1 hour and again after 4 hours. Precipitation was studied in a lipid-free version called TPNaq by light obscuration, turbidimetry and visual examination. Emulsion stability data were assessed by light obscuration and laser diffraction. pH was measured to assess the theoretical risk of precipitation and emulsion destabilisation. The influence of different concentrations of trace elements on precipitation was investigated by visual examination, turbidimetry and light obscuration.
Results
Ampicillin, ceftazidime, fosphenytoin and furosemide led to precipitation after mixing with TPNaq. In some samples of TPN and fluconazole, metronidazole and paracetamol, the emulsion droplet size was above the acceptance limit, although this might also be inherent to the TPN admixture. An unexpected formation of micro-precipitate correlating with increasing amounts of added trace elements might be caused by an interaction of cysteine and copper, and complicated the compatibility assessment with drugs.
Conclusions
The micro-precipitate resulting from the addition of trace elements should be investigated further. This study did not provide sufficient evidence to recommend Y-site infusion of the tested drugs and the preterm admixture; however, it might offer some additional support to other compatibility data.
Keywords: Y-site compatibility, copper, cysteine, emulsion stability, precipitation, trace elements, TPN, total nutrition admixture
Introduction
Infants and children require varying amounts of nutrients at different stages due to their continuous growth and development.1 2 There has been an increased focus on standardised total parenteral nutrition (TPN) formulae, hospital-compounded and commercial admixtures, as they have been shown to be well tolerated, easy to use and reduce the risk of serious mistakes.3 4 Several benefits have been demonstrated also for preterm infants; recommended nutrition intake and weight gain can be obtained using standardised all-in-one (AIO) formulae.5
Neonates in intensive care units often receive complex therapy with many drugs in addition to TPN, so Y-site administration can be desirable. However, TPN admixtures contain more than 50 different components, and physicochemical interactions leading to formation of precipitates and/or emulsion destabilisation are quite possible if mixed with drugs. In the worst case scenario, particles and large oil droplets might cause blockage of blood vessels and even death if infused.6 7 Documented compatibility data for TPN and drugs in Y-site are important in order to provide safe care for the patients. Extrapolation of existing compatibility data of drugs and TPN admixtures for older children and adults should be done with care because of differences in TPN composition, drug concentrations, etc. The aim of this study was to obtain Y-site compatibility data for drugs and one standard TPN admixture used in preterm infants in Norway. Due to the observation of micro-precipitates in the admixture after addition of trace elements, investigation of the effect of different trace element concentrations on the risk of precipitation in TPN developed as a sub-goal.
Materials and methods
Materials
The TPN admixture was intended for peripheral or central administration to preterm infants from 4 days of age. This admixture can be ordered from Fresenius Kabi or compounded locally in the hospital pharmacy. Table 1 shows an overview of the ingredients of this admixture prepared in a local pharmacy in an ethyl vinyl acetate (EVA) monolayer bag (FrekaMix, Fresenius Kabi). Drugs and concentrations tested are also shown in table 1. Ceftazidime and fosphenytoin were reconstituted in glucose 50 mg/mL, and ampicillin and furosemide in NaCl 9 mg/mL. Fluconazole, metronidazole and paracetamol were used undiluted.
Table 1.
Overview of the ingredients constituting the total parenteral nutrition (TPN) admixture prepared at the local hospital pharmacy, and drugs and concentration tested in simulated Y-site
| Product type | Name | Manufacturer | Lot number |
| 3-in-1 TPN admixture for peripheral or central admini-stration | *Preterm regimen from 4 days of age containing: | – | |
| Vaminolac | Fresenius Kabi | 16HK0133; 16HB0237 | |
| Glucose 500 mg/mL | Fresenius Kabi | 121AH31; 12HKH17 | |
| Water for injection | Local pharmacy | 14L08BD; 15B24BH | |
| Glycophos | Fresenius Kabi | 12HKL28; 12HFL27 | |
| Magnesium sulfate 1 mmol/mL | B. Braun | 15035012; 14377012 | |
| Potassium chloride 1 mmol/mL | B. Braun | 144118091; 14423012; 14251013 | |
| Calcium chloride 1 mmol/mL | B. Braun | 15155036; 14412035; 13503035 | |
| Smoflipid* | Fresenius Kabi | 16HK0062 | |
| Trace elements | Peditrace | Fresenius Kabi | 12HFL07, 12HLL97 |
| Vitamins | Soluvit* | Fresenius Kabi | 10IB6649, 10HM4571 |
| water soluble | |||
| Vitamins lipid soluble | Vitalipid Infant* | Fresenius Kabi | 10HA2297; 10HK2215 |
| Drugs | Ampicillin sodium 50 mg/mL | Bristol-Myers Squibb | 3C02634, 4L02584, 5C03610, 3F02259, 3J01732 |
| Ceftazidime pentahydrate 40 mg/mL | Fresenius Kabi | 18H3210 | |
| Fluconazole 2 mg/mL | B. Braun | 13212418, 14384404 | |
| Fosphenytoin sodium 10 mg/mL (given in phenytoin sodium equivalents) | Pfizer | J76024, H74522, L58188 | |
| Furosemide 2 mg/mL | Nycomed, Takeda | 10820264 L1057442, 10992853 |
|
| Metronidazole 5 mg/mL | B. Braun | 143448131, 131218131 | |
| Paracetamol 10 mg/mL | B. Braun Fresenius Kabi |
14382407 16GL0200 |
*For precipitation testing the lipid emulsion was substituted with water for injection and vitamins were omitted.
Methods
The full composition of two versions of the TPN admixtures used is shown in table 2. For the assessment of potential precipitation, the lipid emulsion was substituted with water for injection and no vitamins were added to the bag8 in order to avoid camouflage of particles by the white emulsion and strongly coloured vitamins. This version was referred to as TPNaq. For investigation of emulsion stability, the admixture including lipid and vitamins was compounded8 and this version is referred to as TPN. Additions of micronutrients were made in the highest recommended concentrations informed by Fresenius Kabi. However, in TPNaq used in drug compatibility assessments only 8 mL Peditrace per litre was added (see Results section).
Table 2.
Composition of the two versions of total parenteral nutrition (TPN) admixture: TPNaq, where the lipids are replaced by water for injection (contains no vitamins) and TPN containing all additives
| Ingredients | Per litre TPNaq | Per litre TPN |
| Lipids (g) | – | 23.6 |
| Olive oil | – | 25% |
| Soybean oil | – | 30% |
| MCT | – | 30% |
| Fish oil | – | 15% |
| Glucose anhydrous (g) | 56.4 | 54.2 |
| Amino acids total (g) | 27.5 | 26.4 |
| Alanine (g) | 2.7 | 2.6 |
| Arginine (g) | 1.7 | 1.7 |
| Aspartic acid (g) | 1.7 | 1.7 |
| Cysteine (g) | 0.4 | 0.4 |
| Glutamic acid (g) | 3.0 | 2.9 |
| Glycine (g) | 0.9 | 0.9 |
| Histidine (g) | 0.9 | 0.9 |
| Isoleucine (g) | 1.3 | 1.3 |
| Leucine (g) | 2.9 | 2.8 |
| Lysine (g) | 2.4 | 2.3 |
| Methionine (g) | 0.5 | 0.5 |
| Phenylalanine (g) | 1.1 | 1.1 |
| Proline (g) | 2.4 | 2.3 |
| Serine (g) | 1.6 | 1.5 |
| Taurine (g) | 0.1 | 0.1 |
| Threonine (g) | 1.5 | 1.5 |
| Tryptophan (g) | 0.6 | 0.6 |
| Tyrosine (g) | 0.2 | 0.2 |
| Valine (g) | 1.5 | 1.5 |
| Sodium (mmol) | 16.0 | 16.0 |
| Potassium (mmol) | 16.0 | 15.4 |
| Magnesium (mmol) | 2.0 | 1.9 |
| Calcium* (mmol) | 4.6 | 4.5 |
| Phosphate† (mmol) | 8.0 | 10.3 |
| Chloride (mmol) | 25.3 | 24.3 |
| Sulfate (mmol) | 2.0 | 1.9 |
| Peditrace‡(mL) | 8§ | 14.5§ |
| Zinc chloride (mg) | 4.1 | 7.4 |
| Copper chloride (2H2O) (mg) | 0.4¶ | 0.8** |
| Manganese chloride (4H2O) (mg) | 0.03 | 0.1 |
| Sodium selenite anhydrous (mg) | 0.03 | 0.1 |
| Sodium fluoride (mg) | 1.0 | 1.8 |
| Potassium iodide (mg) | 0.01 | 0.02 |
| Soluvit‡ (vials) | – | 2.9 |
| Vitalipid infant‡ (mL) | – | 33.4 |
*Calcium chloride as calcium source.
†From glycerophosphate, the emulsion and Vitalipid infant.
‡Micronutrient additives.
§Corresponds to 0.8 and 1.5 mL trace elements per 100 mL respectively.
¶Corresponds to 160 µg/L Cu2+.
**Corresponds to 290 µg/L Cu2+.
Some of the same drugs have been previously studied in combination with TPN admixtures for neonates and older children in our set-up.9 A range of relevant mixing ratios of drug plus TPN were calculated in the same way as described earlier9 to mimic different mixing ratios in the infusion line. Doses of drugs and TPN for preterm infants (weight 200 g to 2 kg) were used in the calculations. ESPEN/ESPGHAN and national guidelines were consulted in order to identify a relevant volume of TPN.1 10 Infusion times of 8 and 24 hours were used to calculate the infusion rate of TPN. Eight hours is probably too fast for most preterm infants, but was included to constitute an extreme. The BNF for Children, national guidelines, the Norwegian Medicines for Children Network’s reconstitution tables10–13 and summary of product characteristics (SmPC) were used to identify appropriate doses and infusion times of the drugs. Drug concentrations were chosen based on suggestions from clinicians and reconstitution tables.13 Finally, the infusion rate of the drug was divided by the infusion rate of TPN to obtain the mixing ratio. A mixing ratio of 1+1 plus the two most extremes (high drug:low TPN and low drug:high TPN) were chosen to best cover the full range of relevant mixing ratios. If no mixing ratio with excess drug was identified in this way, two mixing ratios with excess of TPN were chosen as an alternative.9
Samples of drug and TPN were mixed in a laminar airflow cabinet by addition of TPN to the drug in sterile 50 mL polypropylene centrifuge tubes (Corning, New York, USA). For visual examinations, clean and sterilised glass tubes were used (Scherf Präzision Europa GmbH, Meiningen, Germany). Drugs and TPNaq were filtered 0.22 µm before mixing. TPN (with lipids) was not filtered. The samples were tested as soon as possible (within 1 hour) and again 4 hours after mixing. In addition, the visual examinations were performed 24 hours after mixing.
The possible influence of adding trace elements on precipitation in pure TPNaq was investigated by adding an increasing amount of trace elements (zero to maximum amount stated by manufacturer).
A panel of test methods for assessment of precipitation and emulsion stability was employed (table 3).8 Before mixing with drug, characterisation of the drug-free TPNaq and TPN was performed to obtain baseline values. The experiments were conducted under ambient laboratory conditions.
Table 3.
Overview of test methods for assessment of physical compatibility between total parenteral nutrition (TPN) and parenteral drugs and the acceptance criteria applied8
| Methods for detection of potential precipitates in mixed samples (drug+TPNaq) |
Acceptance criteria/points to consider |
| Sub-visual particle counting by light obscurationa | Particle counts <1000–2000/mL ≥0.5 µm,8 and large particles not exceeding Ph.Eur. limits for large volume parenterals14 |
| Turbidity measured by turbidimeterb | Turbidity <0.20–0.30 FNU (taking into consideration background turbidity of unmixed samples)8 |
| Visual examination against black background with Tyndall beamsc | No signs of visible particles or Tyndall effect8 15 |
| pH measured by pH meterd | Evaluation of risk of precipitation of drug and/or calcium phosphate. |
| Methods for assessment of emulsion stability in mixed samples (drug+TPN) |
Acceptance criteria/points to consider |
| MDD measurements; laser diffractione | V.W. MDD should be <500 nm Size fraction (%) >5 µm should be zero16 |
| PFAT5 calculated based on droplet size measurements from light obscurationa | PFAT5 <0.40%16 17 |
| pH measured by pH meterd | pH <5.5 might be an indication of increased risk of emulsion destabilisation17 |
a, Accusizer 780 Optical Particle Sizer, Nicomp PSS, Santa Barbara, USA.
b, 2100Qis Turbidimeter, Hach Lange GmbH, Düsseldorf, Germany.
c, Fibreoptic light source (Schott KL 1600 LED, Mainz, Germany) and red pocket laser pointer (630–650 nm, max output <1 mW).
d, Metrohm 744 pH meter, Metrohm AG, Herisau, Switzerland.
e, Mastersizer 2000 and Hydro 2000G sample dispersion unit, Malvern Instruments, Worcestershire, UK.
PFAT5, volume weighted percentage of fat droplets above 5 µm.
FNU, Formazin nephelometry units.
V.W. MDD, volume weighted mean droplet size.
Sub-visual particles were counted using light obscuration (Accusizer 780 Optical Particle Sizer, Nicomp PSS, Santa Barbara, USA). The sensor type was LE-400–05 set in summation mode, measuring particles from 0.5 to 400 µm in 15 mL of undiluted sample.8 The total particle count/mL ≥0.5 µm and the amount of particles ≥10 and 25 µm per mL were determined.8 14 The background count of the centrifugation tubes was below 100 particles/mL ≥0.5 µm.8
The turbidity of the samples was measured in Formazin nephelometry units (FNU) using a Turbidimeter (2100Qis, Hach Lange GmbH, Düsseldorf, Germany). The sample was gently inverted a few times before measurements.8
The samples were studied visually against a black background with two light sources, a fibreoptic light source (Schott KL 1600 LED, Mainz, Germany) and a red pocket laser pointer (630–650 nm, max output <1 mW). The samples were gently inverted to set possible particles in motion.8 15
The pH of samples was measured with a pH meter (Metrohm AG, Herisau, Switzerland) calibrated with buffers of pH 4.00, 7.00 and 10.00. Compatibility was theoretically evaluated based on pH values.8
The volume weighted mean droplet diameter and volume weighted percent of particles below 500 nm and 1 µm were estimated using laser diffraction (Mastersizer 2000 and Hydro 2000G sample dispersion unit, Malvern Instruments, Worcestershire, UK). The dispersion unit was filled with Milli-Q-water and the samples (≈2 mL aliquot) were added to this. The sonication was turned off to avoid breaking up large droplets. The absorbance was set to 0.001 and the refractive index to 1.46.8
Light obscuration was used to estimate the PFAT5% of the fat emulsion, that is the percent of fat droplets >5 μm in the large diameter tail.16 17 The sensor was set in extinction mode and the detection threshold at 1.80 µm. A 40 mL glass beaker was used to dilute the samples and Milli-Q-water as the dilution medium. Samples were collected with a micropipette and diluted to concentrations below the instrument’s coincidence limit of 9000 particles/mL using dilution factors of 1:300–1200 (sample:water). The samples were stirred for 60 s prior to measurements and during measurements with a magnetic stirrer embedded in the instrument. The sample withdrawal from the diluted emulsions was 15 mL. The counts were distributed over 128 channels and the equivalent spherical volumes of the oil droplets were calculated. The density of oil used in calculations was 0.92 g/mL and the final fat composition 0.027 g/mL (including fat from Vitalipid Infant).8 The following equation was used to calculate PFAT517:
TSV (total spherical volume) = number of particles counted x ESV (equivalent spherical volume)
Density = density of oil used in the emulsion
Sample volume = the amount of diluted sample measured, here 15 mL
Final fat composition = the amount of lipid in the TPN admixture (including added lipid soluble vitamins)
Statistical evaluations
Calculations of means and SD were performed. Compatibility was evaluated theoretically (pH/physicochemical properties of drugs/TPN) and according to stated acceptance criteria and negative controls (baseline). An overall assessment of these factors was considered more appropriate than isolated statistical analysis.
Results and discussion
Characterisation of TPNaq without added drug and investigation of the effect of added trace elements on precipitation
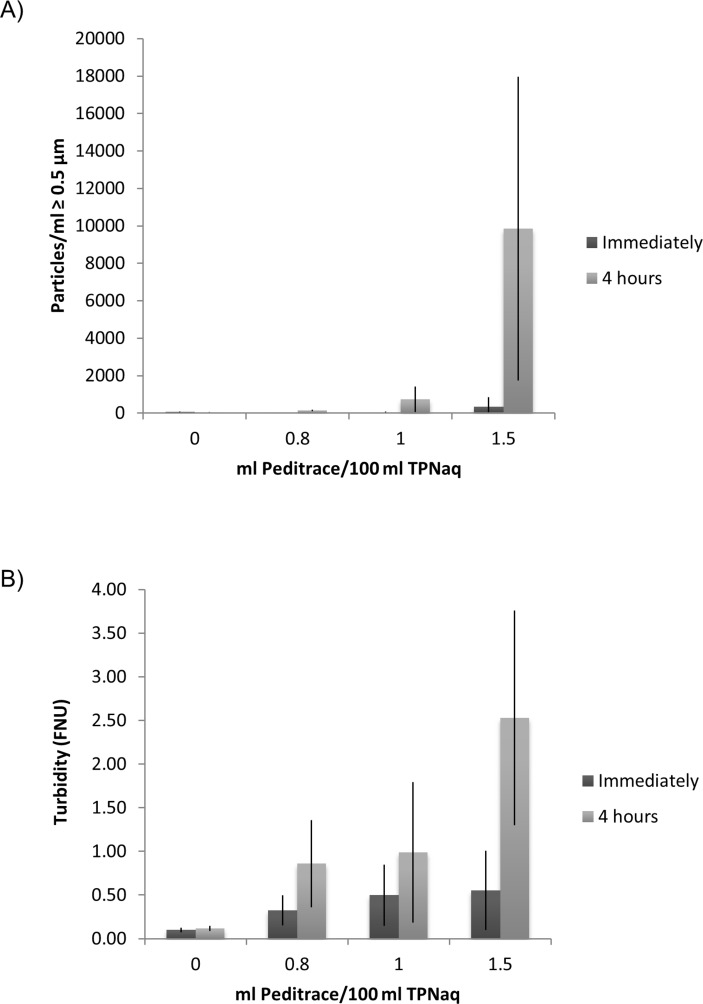
When the highest recommended addition of trace elements (1.5 mL Peditrace/100 mL) was added to TPNaq, fine powdery particles were seen using Tyndall light and both the sub-visual particle counts and the turbidity indicated ongoing precipitation in TPNaq (figure 1). Immediately after filtration of the TPNaq samples into the test tubes the sub-visual particle counts were ≈1000 particles/mL, but they increased dramatically in number (≈14 000 particles/mL) over the observation time of 4 hours. Particle sizes were mostly <1 µm and the particle concentrations of 10 and 25 µm particles were well below the Ph.Eur. limits.14 A correlation was observed between the amount of added trace elements and the extent of precipitation (figure 1). This was also the case for the turbidity measurements, although the FNU values were above the acceptance limit also immediately after filtration (figure 1). On visual examination small amounts of haze could be identified, increasing over 4 hours. After about 24 hours most of the haze seemed to have disappeared in the sample tubes. Furthermore, a brownish colour was noticed on the syringe filters used to filter the samples (figure 2), also disappearing over time. During the course of the shelf life of the mixture, the precipitation in the TPNaq bag seemed to gradually decrease. In an attempt to avoid precipitation, lower amounts of trace elements (1 mL/100 mL and 0.8 mL/100 mL) were added. 0.8 mL Peditrace/100 mL corresponds to ‘normal’ use instead of the maximum limits (table 2). The particle counts were much lower compared with the 1.5 mL/100 mL samples, however the turbidity was not acceptable and haze could still be seen in Tyndall light (figure 1).
Figure 1.
Increasing sub-visual particle counts (A) and turbidity (B) of TPNaq as a consequence of stepwise addition of trace elements (Peditrace) (n ≥3). Sample preparation and measurements were performed over several days within the shelf life of the TPNaq admixture.
Figure 2.

Appearance of filters after filtration of the TPNaq admixture; without addition of trace elements (left) and with ≈1.5 mL/100 mL of trace elements (right). A brown colour could be seen on filters that had been in contact with the admixture containing trace elements. The colour disappeared over time.
Detection of brown precipitates on in-line filters used during administration of TPN admixtures have been reported, possibly caused by an interaction between copper and cysteine.18–20 The preterm admixture contained cysteine, which is typically added as a semi-essential amino acid in paediatric TPN,1 and copper was introduced with the trace elements. Thibault suggests a limit of 157 µg copper per litre when using low pH, cysteine-containing amino acid solutions,19 which is in the same order of magnitude as in the current study. However, no similar precipitate was detected in our previous study with a TPN admixture containing higher concentrations of trace elements and a similar concentration of cysteine.9 Foinard et al observed a stronger colour on the filters after filtration of the complete TPN admixture compared with filters used for filtration of a solution containing only amino acids and trace elements, even though the latter mix contained a higher concentration of cysteine and trace elements.20 This suggests that the concentrations of trace elements and cysteine are not the only influencing factors. Additional factors such as pH, redox conditions, ion concentration, combination of metal ions, mixing order, temperature, glucose, derivate of cysteine, packaging (multilayer versus monolayer), light, presence of vitamins have been discussed.18 21–23
Fresenius Kabi performed a retest on this particular admixture in a multilayer bag without finding any precipitate (personal email correspondence, Hege Børringbo, Fresenius Kabi). The use of different packaging might have prevented the precipitation. On the other hand, Allwood and co-workers found the copper cysteinate (or copper sulfide) precipitate to occur more easily in multilayer bags.23 However, from consulting other authors describing this precipitation we learned that Foinard and colleagues20 used a multilayer bag (personal email correspondence, Dr Aurélie Foinard) and Thibault19 used a monolayer EVA bag (personal email correspondence, Dr Maxime Thibault), and both found this precipitate. Another aspect to consider is that studies have shown that TPN ingredients might be contaminated to different extents by trace elements,24 which could have influenced the outcomes in our study as well. Clearly, this is a complex matter and elucidating all influencing factors needs further research. Unfortunately, the nature of the precipitate and the actual copper content of the raw materials and final admixtures were not analysed. It should be noted that the admixture used in this study is not identical to the one delivered by Fresenius Kabi. The concentrations are the same but the raw materials and bag used are different.
The possible clinical significance of the observed precipitate is not known. It has been discussed that such a precipitate might affect the availability of copper and cysteine and lead to symptoms of deficiency over time.20 It is also possible that infusion of the particles formed could have a harmful effect. The SmPC of the cysteine-containing amino acid solution Primene (Baxter) includes an instruction to use a final filter during administration of Primene and trace elements in order to remove particles that may form with, for example, copper, and further recommends to perform blood levels of copper (when medically relevant) if discoloration of filters is noted.25
Trace elements in the concentration 0.8 mL/100 mL, corresponding to a ‘normal’ amount of trace elements, was chosen for the compatibility testing with drugs. Baseline values for the TPNaq and TPN compositions outlined in table 2 are shown in table 4. Baseline values for the drugs in the same reconstituted concentrations were reported in a previous work.9 As can be seen in table 4, the sub-visual particle counts were low, but high turbidity and small amounts of visual micro-precipitates were still present in TPNaq. Since the test results after mixing with drug would be affected, this has to be kept in mind for the interpretation of the results. For tests on the emulsion stability, the maximum amounts of trace elements were added (table 2). It is not known whether the precipitate was present in the admixture containing lipid, since this version was not filtered and precipitates would be hidden by the white colour.
Table 4.
Results from the investigation of possible precipitation and emulsion stability following the mixing of drug and total parenteral nutrition (TPN) (n ≥3), V.W. MDD (volume weighted mean droplet diameter) and % size fractions: n=1 with multiple runs. Mix ratios denotes drug + TPNaq or drug + TPN, respectively. Shaded areas highlight values that might indicate an incompatible mix
| Drug | Mix ratio Drug+TPN/TPNaq | Investigation of possible formation of precipitation with TPNaq | Testing of emulsion stability with TPN | ||||||||||||||
| *Particles/ml ≥0.5 µm | Turbidity (FNU) | Visible particles and/or Tyndall effect (+/−) | pH | Light obscuration | Laser diffraction | pH | |||||||||||
| PFAT5 | % <500 nm | % < 1 µm |
V.W. MDD | ||||||||||||||
| 0h | 4h | 0h | 4h | 0h | 4h | 24 hours | 0h | 4h | 0h | 4h | 4h | 4h | 4h | 0h | 4h | ||
| Baseline (TPNaq/TPN) | – | 17±15 | 136±40 | 0.32±0.17 | 0.86±0.50 | +/− | +/− | − | 5.89 | 5.89 | 0.11±0.01 | 0.18±0.02 | 82 | 100 | 375 | 5.89 | 5.89 |
| Ampicillin 50 mg/mL† | 1+10 | 336±219 | 113±98 | 0.28±0.04 | 0.23±0.06 | + | + | + | 6.91 | 6.81 | 0.07±0.00 | 0.10±0.01 | 82 | 100 | 374 | 6.92 | 6.77 |
| 1+1 | 100±36 | 1932±200 | 0.45±0.17 | 0.67±0.06 | + | + | + | 7.95 | 7.92 | 0.08±0.01 | 0.04±0.01 | 83 | 100 | 373 | 8.03 | 7.92 | |
| 2+1 | 86±4 | 2287±591 | 0.96±0.10 | 1.03±0.16 | + | + | + | 8.16 | 8.19 | 0.07±0.00 | 0.04±0.01 | 85 | 100 | 370 | 8.23 | 8.16 | |
| Ceftazidime 40 mg/mL‡ |
1+10 | 55±16 | 12±6 | 0.10±0.02 | 0.20±0.02 | − | − | + | 6.04 | 6.00 | 0.09±0.01 | 0.23±0.02 | 83 | 100 | 369 | 6.00 | 6.04 |
| 1+1 | 19±5 | 18±2 | 0.12±0.02 | 0.12±0.03 | − | − | − | 6.48 | 6.42 | 0.12±0.07 | 0.04±0.03 | 87 | 100 | 360 | 6.63 | 6.71 | |
| 1+2 | 27±12 | 13±6 | 0.10±0.01 | 0.10±0.01 | − | − | +/− | 6.33 | 6.28 | 0.09±0.02 | 0.14±0.02 | 83 | 100 | 370 | 6.36 | 6.45 | |
| Fluconazole 2 mg/mL§ |
1+10 | 381±190 | 180±12 | 0.16±0.04 | 0.12±0.01 | + | +/− | − | 5.85 | 5.86 | 0.14±0.02 | 0.30±0.06 | 82 | 100 | 378 | 5.84 | 5.85 |
| 1+1 | 360±165 | 85±19 | 0.14±0.02 | 0.09±0.00 | + | +/− | − | 5.86 | 5.87 | 0.12±0.01 | 0.29±0.04 | 81 | 100 | 382 | 5.85 | 5.89 | |
| 9+1 | 92±4 | 77±26 | 0.08±0.02 | 0.07±0.01 | − | − | − | 5.85 | 5.87 | 0.10±0.01 | 0.32±0.28 | 80 | 100 | 385 | 5.88 | 5.90 | |
| Fosphenytoin 10 mg/mL‡ |
1+50 | 135±19 | 33±4 | 0.10±0.01 | 0.10±0.01 | − | − | − | 5.94 | 5.96 | 0.11±0.01 | 0.15±0.04 | 82 | 100 | 374 | 5.91 | 5.92 |
| 1+1 | 132±14 | 27±10 | 0.09±0.02 | 0.18±0.08 | +/− | +/− | + | 7.47 | 7.44 | 0.09±0.00 | 0.04±0.00 | 83 | 100 | 375 | 7.34 | 7.23 | |
| 5+1 | 54±20 | 52±25 | 0.08±0.01 | 0.11±0.01 | − | − | − | 8.21 | 8.25 | 0.09±0.01 | 0.08±0.01 | 82 | 100 | 378 | 8.14 | 8.07 | |
| Furosemide 2 mg/mL† |
1+100 | 436±215 | 105±31 | 0.14±0.05 | 0.10±0.01 | − | − | − | 5.87 | 5.90 | 0.13±0.03 | 0.24±0.02 | 83 | 100 | 373 | 5.84 | 5.85 |
| 1+1 | 561±319 | 39±16 | 0.13±0.04 | 0.08±0.00 | +/− | +/− | +/− | 5.94 | 5.98 | 0.10±0.01 | 0.04±0.01 | 83 | 100 | 372 | 5.90 | 5.93 | |
| 2+1 | 518±284 | 51±26 | 0.12±0.02 | 0.09±0.01 | +/− | +/− | +/− | 5.99 | 6.02 | 0.09±0.01 | 0.04±0.02 | 83 | 100 | 374 | 5.97 | 5.98 | |
| Metronidazole 5 mg/mL§ |
1+10 | 312±82 | 218±26 | 0.26±0.14 | 0.27±0.16 | +/− | +/− | − | 5.84 | 5.85 | 0.17±0.03 | 0.35±0.08 | 82 | 100 | 374 | 5.81 | 5.83 |
| 1+1 | 302±81 | 118±28 | 0.18±0.09 | 0.10±0.02 | +/− | − | − | 5.63 | 5.65 | 0.16±0.01 | 0.29±0.05 | 82 | 100 | 377 | 5.60 | 5.62 | |
| 5+1 | 252±25 | 109±85 | 0.10±0.01 | 0.10±0.01 | − | − | − | 5.29 | 5.28 | 0.15±0.05 | 0.27±0.10 | 82 | 100 | 377 | 5.27 | 5.29 | |
| Paracetamol¶ 10 mg/mL§ |
1+10 | 40±1 | 42±9 | 0.14±0.01 | 0.13±0.02 | − | − | − | 5.70 | 5.71 | 0.08±0.00 | 0.20±0.01 | 80 | 100 | 379 | 5.75 | 5.76 |
| 1+1 | 13±1 | 12±4 | 0.35±0.01 | 0.36±0.02 | + | + | + | 5.30 | 5.31 | 0.09±0.01 | 0.15±0.01 | 83 | 100 | 374 | 5.33 | 5.33 | |
| 1+2 | 25±6 | 28±12 | 0.26±0.01 | 0.26±0.01 | + | + | + | 5.39 | 5.38 | 0.12±0.02 | 0.36±0.15 | 83 | 100 | 374 | 5.40 | 5.42 | |
*Particle counts above 10 and 25 µm are not shown as the Ph.Eur. limits were not exceeded in any of the samples.
†Diluted in 9 mg/mL NaCl.
‡Diluted in 50 mg/mL glucose.
§Undiluted.
¶All tests were performed with paracetamol from B. Braun, except for laser diffraction measurements where the paracetamol was from Fresenius Kabi.
Characterisation of TPN (with lipid) without added drug
The lipid droplet size was as expected within the acceptance limits (tables 3 and 4). The PFAT5 was below 0.40% and the V.W. MDD was well below 500 nm. Even though the admixture was judged to be stable, some creaming and/or flocculation was visible in the bag. Creaming can be reversed as opposed to coalescence, and the admixture might still be safe for infusion provided prior thorough mixing.
Physical Y-site compatibility of drugs and TPNaq (without lipids and vitamins)
All sub-visual particle counts were low after mixing with the different drugs, except for ampicillin where the particle count had increased considerably after 4 hours (table 4). This is also described in previous studies,8 9 and is probably caused by calcium phosphate precipitation occurring when the pH values increase above pKa2 of phosphoric acid at pH 7.2.26 Ampicillin has been found incompatible in some studies27 28 and compatible in others.29 30 Based on the current investigations, ampicillin and the Preterm mix should be regarded as incompatible.
The turbidity was above the acceptance limit (>0.20–0.30 FNU) for some mixing ratios of samples with ceftazidime, fosphenytoin, metronidazole and paracetamol (table 4). Ceftazidime had a slightly increased turbidity after 4 hours in the mixing ratio 1+10, which might be due to the background noise of TPNaq. However, a clear precipitation and colour darkening was observed in samples that were re-examined visually after 24 hours, suggesting that the increased turbidity also might be an initial warning of precipitation in progress due to the mixing of drug with a high volume of TPNaq. Co-administration might therefore be discouraged; however, ceftazidime has been reported to be compatible in studies with other TPN admixtures.9 28–30
For fosphenytoin, a somewhat high but variable turbidity (high SD) was measured 4 hours after mixing in a mixing ratio of 1+1. Although this in isolation could be explained by the background noise, particles were also detected by visual examination in some of the samples immediately and 4 hours after mixing. After 24 hours a precipitate was obvious. Since fosphenytoin is formulated with an alkaline pH (8.6)9 and buffered with trometamol (SmPC), the pH value was quite high (7.5) also after mixing with the Preterm mix. The precipitate might be calcium phosphate due to alkaline pH and/or degradation of the prodrug to the less soluble phenytoin.31 In a mixing ratio of 5+1 there were no signs of precipitation, although the pH was 8.2. An explanation might be the lower concentration of TPN and therefore more dilution of calcium phosphate causing less chance of precipitation.
The high turbidity observed in mixtures with metronidazole can presumably be explained by the background noise of the TPNaq. On visual examination the haze was very similar to the trace element-induced precipitate, and it seemed to diminish over time like the turbidity of the pure TPNaq stored in sample tubes. The paracetamol samples also showed increased turbidity and Tyndall effect in mixing ratios 1+1 and 1+2, but not in 1+10. In contrast to the above, these findings did not change over time and were also observed in the pure drug. Therefore, the opacity could be attributed to the drug itself and not a sign of incompatibility.8 9 Fluconazole showed some signs of particles/Tyndall effect during visual examination after mixing with TPNaq, but no other signs of precipitation were detected (table 4). The haze in fluconazole:TPNaq was similar to the background noise of TPNaq, and decreased over time and was not detectable after 24 hours. Therefore, disregarding the trace element-induced precipitations and background noise of pure drug, metronidazole, paracetamol and fluconazole were probably compatible with the TPNaq admixture. This is supported by studies with other admixtures.9 28–30 32 33
The appearance of the particles observed in TPNaq mixed with furosemide was different. Traces of particle formation were occasionally encountered during visual examination, especially in samples examined 4 and 24 hours after mixing. The pH after mixing was close to that of TPNaq and, since furosemide might precipitate in acidic solution, it is probably safest to avoid mixing with TPN. This corresponds with the findings with one TPN admixture for children (Numeta G16E) previously tested in our set-up,9 and also with a study by Trissel and colleagues.29 Other reports have concluded with compatibility,28 30 33 including the results for the other TPN admixtures for older children (OlimelN5E) tested in our previously mentioned report.9 The different conclusions might be explained by differences in pH of the TPN products. The more acidic pH of the admixtures for the smallest children could result in an increased risk of precipitating furosemide.
Physical Y-site compatibility of drugs and TPN (with lipid)
Regarding emulsion stability, there were only a few occasions where the PFAT5 values of drug + TPN mixtures were above the acceptance criteria of <0.40% (ie, if the SDs are included) (table 4). After mixing with fluconazole, metronidazole and paracetamol the PFAT5 limit was sometimes crossed. As mentioned, some creaming was observed in the bag immediately after compounding. Therefore, the occasional high PFAT5 values might be intrinsic to the admixture itself. Scrutinising the different mixing ratios of drug + TPN for all drugs, the PFAT5 was also high in mixing ratios containing a high volume of TPN and a low volume of drug. It is less likely that such a small amount of drug would destabilise the emulsion. Nevertheless, based on the current results, we cannot recommend co-administration of the Preterm mix with fluconazole, metronidazole or paracetamol.
What this paper adds.
What is already known on this subject
TPN admixtures are complex blends and Y-site infusion of incompatible combinations of drugs and TPN might cause precipitation of particles or destabilisation of the lipid emulsion, both presenting risk of emboli if infused into the blood circulation.
There is a lack of documented compatibility data for many drugs and TPN combinations, especially for doses, products and infusion regimes relevant for infants and children, and extrapolation of data generated for the adult population should be done with great care.
What this study adds
Preliminary compatibility data adopted for preterm infants for seven drugs (ampicillin, ceftazidime, fluconazole, fosphenytoin, furosemide, metronidazole and paracetamol) with a preterm infant TPN formulation.
The complexity of parallel infusion of drugs and TPN is emphasised by an unforeseen micro-precipitate generated by addition of increasing amounts of micronutrients, yet within the recommended range, to the TPN.
Acknowledgments
We would like to thank the Northern Norway Regional Health Authority (Helse Nord RHF, grant number SFP1055-12) and the Norwegian Medicines for Children Network, Bergen, Norway for funding the project. We would also like to express our gratitude to clinicians at the pediatric wards at University Hospital Northern Norway/Tromsø and Haukeland/Bergen, Frank Sundby at the Institute of Animal and Aqua-cultural Sciences, The Norwegian University of Life Sciences, Ås, Norway, to the Hospital Pharmacy of Oslo, Rikshospitalet, Oslo, Norway, School of Pharmacy, University of Oslo, Hege Børringbo at Fresenius Kabi and Margaret Aarag Antonsen, Hospital Pharmacy of North Norway Trust and the employees of the Hospital Pharmacy of Tromsø, Norway.
Footnotes
Contributors: VS, SW, IG and IT designed and planned the study. VS conducted the experiments. VS and IT had primary responsibility for drafting the manuscript, but all authors provided comments and contributed to the preparation and approved the final manuscript. IT is a senior author and the project leader.
Funding: This study was funded by Helse Nord RHF (grant no: SFP1055-12).
Competing interests: None declared.
Patient consent: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Koletzko B, Goulet O, Hunt J, et al. . 1. Guidelines on Paediatric Parenteral Nutrition of the European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) and the European Society for Clinical Nutrition and Metabolism (ESPEN), supported by the European Society of Paediatric Research (ESPR). J Pediatr Gastroenterol Nutr 2005;41:S1–S4. 10.1097/01.mpg.0000181841.07090.f4 [DOI] [PubMed] [Google Scholar]
- 2. Fusch C, Bauer K, Böhles HJ, et al. . Working group for developing the guidelines for parenteral nutrition of the German Society for Nutritional Medicine. Neonatology/Paediatrics – Guidelines on Parenteral Nutrition, Chapter 13. Germ Med Sci 2009;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Colomb V, Marlowe ML, Bonnot D, et al. . Practical use of a new three-chamber bag for parenteral nutrition in pediatric patients. Espen J 2012;7:e93–e99. 10.1016/j.clnme.2012.01.002 [DOI] [Google Scholar]
- 4. Meyer R, Timmermann M, Schulzke S, et al. . Developing and implementing all-in-one standard paediatric parenteral nutrition. Nutrients 2013;5:2006–18. 10.3390/nu5062006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rigo J, Marlowe ML, Bonnot D, et al. . Benefits of a new pediatric triple-chamber bag for parenteral nutrition in preterm infants. J Pediatr Gastroenterol Nutr 2012;54:210–7. 10.1097/MPG.0b013e318232f915 [DOI] [PubMed] [Google Scholar]
- 6. Levene MI, Wigglesworth JS, Desai R. Pulmonary fat accumulation after intralipid infusion in the preterm infant. Lancet 1980;2:815–9. 10.1016/S0140-6736(80)90170-1 [DOI] [PubMed] [Google Scholar]
- 7. Bradley JS, Wassel RT, Lee L, et al. . Intravenous ceftriaxone and calcium in the neonate: assessing the risk for cardiopulmonary adverse events. Pediatrics 2009;123:e609–e613. 10.1542/peds.2008-3080 [DOI] [PubMed] [Google Scholar]
- 8. Staven V, Wang S, Grønlie I, et al. . Development and evaluation of a test program for Y-site compatibility testing of total parenteral nutrition and intravenous drugs. Nutr J 2016;15:29 10.1186/s12937-016-0149-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Staven V, Iqbal H, Wang S, et al. . Physical compatibility of total parenteral nutrition and drugs in Y-site administration to children from neonates to adolescents. J Pharm Pharmacol 2017;69:448–62. 10.1111/jphp.12647 [DOI] [PubMed] [Google Scholar]
- 10. Klingenberg C. Metodebok i Nyfødtmedisin. 4th edn, 2015. [Google Scholar]
- 11. BNF for Children. Volume 10 London: BMJ Group, The Royal Pharmaceutical Society of Great Britain, and RCPCH Publications, 2015. http://www.medicinescomplete.com [Google Scholar]
- 12. Akuttveileder i pediatri. Status epilepticus. Norsk barnelegeforening, Den Norske Legeforening. Revised in 2015. https://www.helsebiblioteket.no/retningslinjer/akuttveileder-i-pediatri/nevrologi/status-epilepticus/konvulsiv-status-epilepticus (accessed Jan 2015).
- 13. Norwegian Medicines for Children Network. Reconstitution tables. 2015. https://www.legemidlertilbarn.no/helsepersonell/blandekort/Sider/Blandekortliste.aspx (accessed: 10.2015).
- 14. European Pharmacopoeia. 2.9.19. Particulate Contamination: Sub-visible Particles. 8th edn Supplement 8.5, 2015. [Google Scholar]
- 15. Staven V, Waaseth M, Wang S, et al. . Utilization of the tyndall effect for enhanced visual detection of particles in compatibility testing of intravenous fluids: validity and reliability. PDA J Pharm Sci Technol 2015;69:270–83. 10.5731/pdajpst.2015.01020 [DOI] [PubMed] [Google Scholar]
- 16. United States Pharmacopeia. General chapters: <729> Globule size distribution in lipid injectable emulsions USP 38–NF 33, 2015. [Google Scholar]
- 17. Driscoll DF, Bhargava HN, Li L, et al. . Physicochemical stability of total nutrient admixtures. Am J Health Syst Pharm 1995;52:623–34. [DOI] [PubMed] [Google Scholar]
- 18. Yamaoka K, Yamaoka H, Nakajima Y, et al. . Coloring and blocking of in-line filters when total parenteral nutrition solutions are supplemented with vitamins and trace elements. Iryo Yakugaku 2005;31:620–4. 10.5649/jjphcs.31.620 [DOI] [Google Scholar]
- 19. Thibault M. Possible incompatibility between amino acids and copper in solutions for pediatric parenteral nutrition. Can J Hosp Pharm 2014;67:160–4. 10.4212/cjhp.v67i2.1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Foinard A, Perez M, Barthélémy C, et al. . In vitro assessment of interaction between amino acids and copper in neonatal parenteral nutrition. JPEN J Parenter Enteral Nutr 2016;40 10.1177/0148607115571967 [DOI] [PubMed] [Google Scholar]
- 21. Barnett MI, Cosslett AG, Duffield JR, et al. . Parenteral nutrition. Pharmaceutical problems of compatibility and stability. Drug Saf 1990;5(Suppl 1):101–6. [DOI] [PubMed] [Google Scholar]
- 22. Minton AR, Barnett MI, Cosslett AG. Detection of particulate material in parenteral nutrition admixtures. Nutrition 1998;14:251–2. 10.1016/S0899-9007(97)00442-5 [DOI] [PubMed] [Google Scholar]
- 23. Allwood MC, Martin H, Greenwood M, et al. . Precipitation of trace elements in parenteral nutrition mixtures. Clin Nutr 1998;17:223–6. 10.1016/S0261-5614(98)80063-0 [DOI] [PubMed] [Google Scholar]
- 24. Pluhator-Murton MM, Fedorak RN, Audette RJ, et al. . Trace element contamination of total parenteral nutrition. 1. Contribution of component solutions. JPEN J Parenter Enteral Nutr 1999;23:222–7. 10.1177/0148607199023004222 [DOI] [PubMed] [Google Scholar]
- 25. SmPC Primene. 2018. 02 https://www.gov.uk/government/organisations/medicines-and-healthcare-products-regulatory-agency [Google Scholar]
- 26. Newton DW, Driscoll DF. Calcium and phosphate compatibility: revisited again. Am J Health Syst Pharm 2008;65:73–80. 10.2146/ajhp070138 [DOI] [PubMed] [Google Scholar]
- 27. Watson D. Piggyback compatibility of antibiotics with pediatric parenteral nutrition solutions. JPEN J Parenter Enteral Nutr 1985;9:220–4. 10.1177/0148607185009002220 [DOI] [PubMed] [Google Scholar]
- 28. Veltri M, Lee CK, Ckk L. Compatibility of neonatal parenteral nutrient solutions with selected intravenous drugs. Am J Health Syst Pharm 1996;53:2611–3. [DOI] [PubMed] [Google Scholar]
- 29. Trissel LA, Gilbert DL, Martinez JF, et al. . Compatibility of parenteral nutrient solutions with selected drugs during simulated Y-site administration. Am J Health Syst Pharm 1997;54:1295–300. [DOI] [PubMed] [Google Scholar]
- 30. Trissel LA, Gilbert DL, Martinez JF, et al. . Compatibility of medications with 3-in-1 parenteral nutrition admixtures. JPEN J Parenter Enteral Nutr 1999;23:67–74. 10.1177/014860719902300267 [DOI] [PubMed] [Google Scholar]
- 31. Valentino SJ. A case for prodrugs: Fosphenytoin. Adv Drug Deliv Rev 1996;19:311–30. [Google Scholar]
- 32. Fox LM, Wilder AG, Foushee JA. Physical compatibility of various drugs with neonatal total parenteral nutrient solution during simulated Y-site administration. Am J Health Syst Pharm 2013;70:520–4. 10.2146/ajhp110715 [DOI] [PubMed] [Google Scholar]
- 33. Bouchoud L, Fonzo-Christe C, Klingmüller M, et al. . Compatibility of intravenous medications with parenteral nutrition: in vitro evaluation. JPEN J Parenter Enteral Nutr 2013;37:416–24. 10.1177/0148607112464239 [DOI] [PubMed] [Google Scholar]