Abstract
Vascular graft infections are rare complications, usually associated with a monomicrobial pyogenic culture. We report a case of vascular graft co-infection with Streptococcus anginosus and Coxiella burnetii, complicated by an aorto-duodenal fistula. Screening for chronic C. burnetii co-infection in at-risk patients might prevent adverse long-term outcomes.
Keywords: Vascular graft, Intracellular, Pyogenic, Coxiella burnetii, Streptococcus anginosus
Introduction
Vascular graft infection is a rare but potentially devastating complication associated with high morbidity and mortality. The lifetime risk of infection, depending on the location of the graft, ranges from <1 to 6 % after implantation [1].
Early graft infections develop less than three months after surgery, usually present with acute symptoms and are caused by virulent microorganisms. Most are due to intra-operative microbial contaminations coming from the patient’s skin or bowel flora. Late graft infections usually develop years after surgery and either follow a relatively indolent course due to low virulence microorganisms, or rapidly progress when caused by virulent bacteria which have seeded the prosthesis typically through hematogenous spread. Other pathways of infection such as contiguous spread from the adjacent tissues or microbial colonisation of atherosclerotic plaques or thrombus are infrequent but possible [1].
The pathogens slightly vary according to the location of the graft and the available studies. Nevertheless, the most common are Staphylococcus aureus (ca. 30 %), coagulase-negative staphylococci (ca. 25 %), streptococci and enterococci (ca. 10 %), Pseudomonas aeruginosa (ca. 5 %) and Candida spp. (ca. 5 %). Polymicrobial infections occur in 10–15 % of cases and 2–15 % of cultures remain negative [2,3]. Culture negative vascular graft infections might be due to prior antimicrobial treatment, suboptimal sampling, fastidious growers or intracellular bacteria. In this last group, Coxiella burnetii, which has been reported as a cause of monomicrobial vascular infection can only be detected by indirect techniques such as serology or polymerase chain reaction (PCR) [4]. To the best of our knowledge, its association as a co-infectant in the setting of an aorto-enteric fistula has, never been reported.
Case report
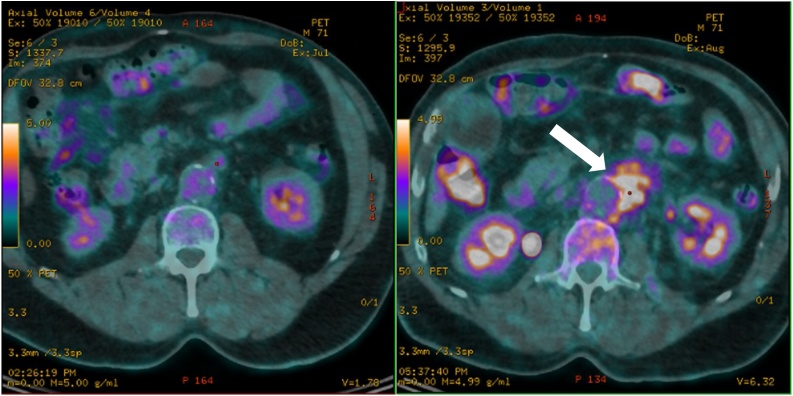
The patient was a 71-year-old polymorbid male known for hypertension, ischemic heart disease, dyslipidaemia and type 2 diabetes, who underwent an aorto-bi-iliac graft surgery in 2001, followed by a lower right limb amputation because of a vascular occlusion in 2002. In 2017, he suffered from a hip and lower left limb claudication for a few months, thought to be secondary to an internal iliac artery occlusion. An angio-computerized tomography (CT) in May 2017 revealed a thrombotic false aneurysm of the left femoral artery with a perianeurysmal infiltration. A first positron emission tomography (PET-CT) in July 2017 showed no suspicious signs of infection (Fig. 1, left panel) and an elective graft replacement surgery was scheduled. A few days before surgery, the patient suffered from several episodes of hematemesis and the gastroduodenoscopy revealed a Forrest IIb duodenal ulcer for which hemostasis was performed by endoscopic clipping. Simultaneously, he presented a rapidly increasing left iliac fossa pain, accompanied with fever, motivating a new imagery. A second PET-CT in August 2017 showed a new hypermetabolic infiltration of the aortic and proximal iliac parts of the prosthesis with a high suspicion of graft infection (Fig. 1, right panel). Blood cultures on the first day of fever were positive (3/8 bottles) for Streptococcus anginosus and a treatment of piperacillin-tazobactam was initiated just before surgery. A graft replacement and cure of the false aneurysm was performed. Intraoperatively, an aortoduodenal fistula was found to be responsible for the episodes of hematemesis. All (n = 5) intra-operative samples grew S. anginosus in scarce amounts.
Fig. 1.
Representative PET-CT images of the aorto-femoral prosthesis region at one month interval without (left panel) and with (right panel) a periprosthetic hypermetabolic fixation (white arrow) revealing the infectious foci.
Retrospectively, the patient reported a history of fatigue, weight loss and abdominal pain without fever for a few months. He lived in a rural area, with cattle around its habitation, but had no direct contact with the animals. He had not traveled outside of Switzerland during the previous fifteen years.
Because of this history, a C. burnetti serology was performed and the results indicated a chronic infection (IgG phase 1: 8192 ; IgG phase 2: 8192 ; IgM phase 1: <16 ; IgG phase 2: <16). C. burnetii PCR was therefore performed on the infected graft material and revealed positive (4/5 positive samples: 3/5 with a load of 50–500 copies/mL and 1/5 with a load of 58.000 copies/mL). Of note, C. burnetti PCR on blood was negative and a transoesophagal echocardiography did not reveal signs of endocarditis.
The patient S. anginosus graft infection was treated with one-stage replacement procedure and twelve weeks of antimicrobial therapy. Piperacillin-tazobactam was replaced by ceftriaxone after culture results and oral rifampin introduced. After 6 weeks, ceftriaxone was replaced by oral amoxicillin and the bitherapy continued for 6 more weeks. For the C. burnetii infection, doxycyline and hydroxychloroquine were introduced for a minimum of 18 months, with therapeutic drug monitoring and serologic follow-up.
Discussion
Q fever is a worldwide zoonosis caused by C. burnetii, a strictly intracellular gram-negative coccobacillus. The main reservoir is ticks but the most commonly identified sources of human infection are farm animals and occasionally pets. Shedding occurs in urine, milk, feces and birth products. Air samples are positive for up to 2 weeks after parturition, and viable organisms are present in the soil for periods of up to 150 days. For these reasons, humans are mostly infected by inhalation of contaminated aerosols and even though most cases concern those with direct contact with infected animals, infection can occur without such contact [5].
Primary infection is symptomatic in ca. 40 % of cases (flu-like illness, interstitial pneumonia, acute hepatitis) after an incubation period of 9–39 days. A chronic form occurs in 1–5 % of all infections and can manifest itself months or even years after exposure, with a localization depending on host risk factors. Endocarditis is the main manifestation of chronic Q fever, followed by vascular infections, bone infections and chronic hepatitis. The basis for the serodiagnosis of acute or chronic Q fever is the two antigenic variations, called “phase variation” of Coxiella burnetii [6].
In Switzerland, mandatory reporting of new Q fever cases was instigated in 2012, and surveillance indicates that ca. 40–60 new cases are reported every year, with an incidence of 0.4-0.6 per 100 000 inhabitants and outbreaks of acute Q fever have been reported [7]. In the Netherlands, a large outbreak of acute Q fever occurred between 2007–2010, with over 4000 cases and resulted in the creation of a national database. Its analysis revealed that among 284 patients with proven or probable chronic Q fever, 122 (42 %) were diagnosed with vascular chronic Q fever. Most of the patients had a vascular history with aneurysm or graft. Almost half of the 122 patients showed complications that needed surgery either for acute or late complications (new- progressive- or ruptured aneurysms, aortoduodenal fistula, infected vascular prosthesis). The overall mortality among the 122 patients was nearly 25 % amounted essentially due to aneurysm-related complications, including fistula [8].
The histological characteristics of the vascular wall of patients suffering from vascular chronic Q fever show inflammation and sometimes necrotizing granulomas, thought to weaken the aneurysm structure, leading to increased risk of rupture or fistula [9]. Our clinical suspicion for this patient is that the chronic C. burnetii graft infection induced the development of the aortoduodenal fistula, leading to the secondary S. anginosus superinfection and bacteremia.
Conclusion
Given the poor prognosis of unrecognized chronic C. burnetii infection, regardless of whether another etiological agent has been identified as causal of the vascular graft infection, we propose to screen at-risk patients for underlying vascular chronic Q fever.
Funding
No funding source to declare.
Consent
Ethics committee approval was not applicable.
Author contribution
SD: data collection, analysis and writing.
AB: data collection, analysis and writing.
Declaration of Competing Interest
All authors have nothing to declare.
References
- 1.Hasse B., Husmann L., Zinkernagel A., Weber R., Lachat M., Mayer D. Vascular graft infections. Swiss Med Wkly. 2013;143 doi: 10.4414/smw.2013.13754. w13754. [DOI] [PubMed] [Google Scholar]
- 2.Erb S., Sidler J.A., Elzi L. Surgical and antimicrobial treatment of prosthetic vascular graft infections at different surgical sites: a retrospective study of treatment outcomes. PLoS One. 2014;9 doi: 10.1371/journal.pone.0112947. e112947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siracuse J.J., Nandivada P., Giles K.A. Prosthetic graft infections involving the femoral artery. J Vasc Surg. 2013;57:700–705. doi: 10.1016/j.jvs.2012.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fournier P.E., Casalta J.P., Piquet P., Tournigand P., Branchereau A., Raoult D. Coxiella burnetii infection of aneurysms or vascular grafts: report of seven cases and review. Clin Infect Dis. 1998;26:116–121. doi: 10.1086/516255. [DOI] [PubMed] [Google Scholar]
- 5.Raoult D., Marrie T., Mege J. Natural history and pathophysiology of Q fever. Lancet Infect Dis. 2005;5:219–226. doi: 10.1016/S1473-3099(05)70052-9. [DOI] [PubMed] [Google Scholar]
- 6.Fournier P.E., Marrie T.J., Raoult D. Diagnosis of Q fever. J Clin Microbiol. 1998;36:1823–1834. doi: 10.1128/jcm.36.7.1823-1834.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.2019. Swiss Federal Office of Public Health. Figures and statistics for Q fever.https://www.bag.admin.ch/bag/fr/home/zahlen-und-statistiken/zahlen-zu-infektionskrankheiten.html Available at: [Google Scholar]
- 8.Broos P.P.H.L., Hagenaars J.C.J.P., Kampschreur L.M. Vascular complications and surgical interventions after world’s largest Q fever outbreak. J Vasc Surg. 2015;62:1273–1280. doi: 10.1016/j.jvs.2015.06.217. [DOI] [PubMed] [Google Scholar]
- 9.JCJP Hagenaars, Koning O.H.J., van den Haak R.F.F. Histological characteristics of the abdominal aortic wall in patients with vascular chronic Q fever. Int J Exp Pathol. 2014;95:282–289. doi: 10.1111/iep.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]