Summary
Long-term potentiation and depression, inferred from analysis on brain slices, are considered the cellular processes underlying learning and memory formation. They have not so far been demonstrated in human stem cell-derived neurons. By expressing channelrhodopsin in hESCs-derived glutamate neurons and co-culturing them with GABA neurons, we found that blue light stimulation increased the frequency of miniature excitatory postsynaptic currents (mEPSCs) and decreased the ratio of paired pulse facilitation (PPF) in non-ChR2-expressing GABA neurons, indicating a facilitating action at the presynaptic terminals. When paired with postsynaptic depolarization, the repetitive stimulation significantly increased the amplitude of light-evoked EPSCs that persisted during the period, indicating long-term potentiation (LTP). In contrast, low-frequency light stimulation induced long-term depression (LTD). These effects were blocked by N-methyl-D-aspartic acid (NMDA) receptor antagonists, suggesting NMDA receptor-mediated synaptic plasticity in human neural networks. Furthermore, induced pluripotent stem cell (iPSC)-derived neurons of patient with Down syndrome showed absence of LTP or LTD. Thus, our platform offers a versatile model for assessing human neural plasticity under physiological and pathological conditions.
Subject Areas: Neuroscience, Cellular Neuroscience, Techniques in Neuroscience
Graphical Abstract
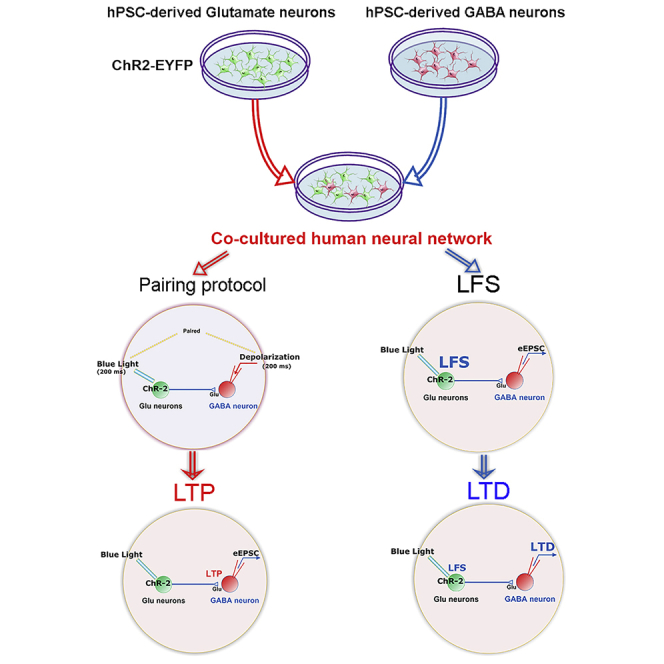
Highlights
-
•
Repetitive stimulation induces LTP in hPSC-derived neural networks
-
•
Low-frequency light stimulation induces LTD in hPSCs-derived neural networks
-
•
The LTP/LTD in human neural networks are NMDAR dependent
-
•
Down syndrome neural networks exhibit defective LTP/LTD
Neuroscience; Cellular Neuroscience; Techniques in Neuroscience
Introduction
Learning and memory are mental activities formed through modifications of synaptic strength among simultaneously active neurons (Tang et al., 1999). The electrophysiological basis is long-term potentiation and long-term depression (LTP/LTD), two forms of activity-dependent synaptic plasticity (Bliss and Collingridge, 1993, Nabavi et al., 2014). Underlying the physiochemical changes are the activation of AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptors followed by activation of postsynaptic N-methyl-D-aspartic acid (NMDA) receptors, which results in a rise in calcium concentration, a necessary trigger for LTP. Both presynaptic and postsynaptic neurons need to be stimulated simultaneously so that Ca2+ currents through the NMDA receptors are sufficient to activate the intracellular signaling cascades, including calmodulin-dependent protein kinase II (CaMKII), protein kinase C (PKC), and the tyrosine kinase Fyn (Dan and Poo, 2004), which ultimately alter the synaptic efficacy. Both LTP and LTD are best characterized in CA3 and CA1 glutamate neurons of the hippocampus in animals (Malenka, 1994), although they are also present in excitatory synapses in other brain regions, including the cerebral cortex, striatum, and amygdala (Daw et al., 2004, Lee and Kirkwood, 2011, Malenka and Bear, 2004, Sidorov et al., 2015, Suppa et al., 2015).
In humans, LTP/LTD is observed in surgically resected brain tissues (Beck et al., 2000, Chen et al., 1996). It is also observed indirectly through the long-lasting increase of the human auditory evoked potential or the rapid repetitive presentation of visual checkerboard-induced visual evoked potential (Clapp et al., 2005, Teyler et al., 2005). However, these are rare events and are difficult for mechanistic investigation. Given the availability of large collections of human stem cell lines, especially patient induced pluripotent stem cells (iPSCs), a simple and versatile model for analyzing the plasticity of human neuron networks will enable the understanding of changes in neural plasticity under physiological and pathological conditions.
We have engineered a simple yet versatile model for assessing neural plasticity by co-culturing channelrhodopsin (ChR2)-expressing glutamate neurons with non-ChR2-expressing GABA neurons, both of which are derived from human embryonic stem cells (ESCs). Repetitive light stimulation of glutamate neurons paired with postsynaptic depolarization persistently increased the amplitude of light-evoked EPSCs recorded in the GABA neurons, exhibiting LTP-like behavior. In contrast, low-frequency light stimulation decreased the amplitude of light-evoked EPSCs, signaling LTD. When the ChR2-glutamate neurons are from Down syndrome (DS) patient iPSCs, LTP/LTD was not induced.
Results
Stimulation of Glutamate Neurons Potentiates the Frequency of mEPSCs in GABA Neurons
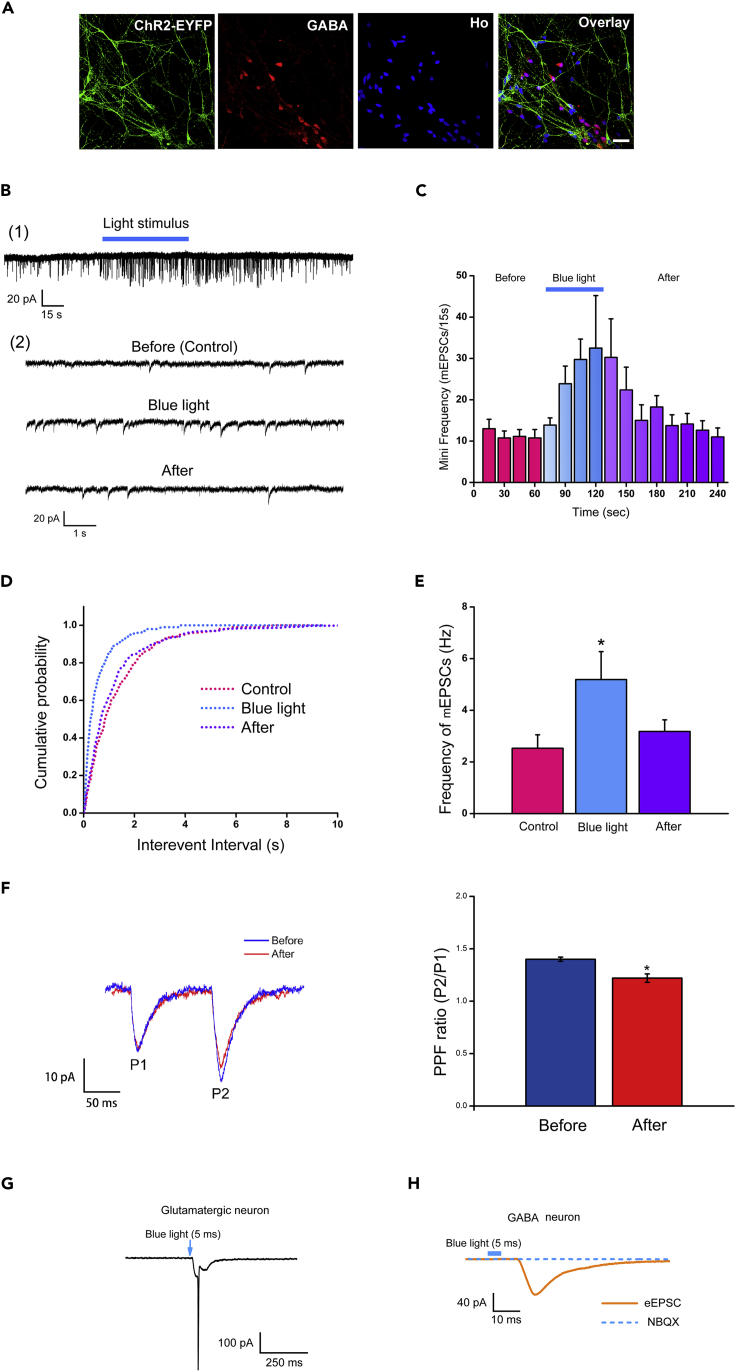
To enable precise stimulation of presynaptic neurons we established a ChR2-expressing human embryonic stem cells (hESC) line by inserting ChR2 and EYFP (for identification) in the AAVS1 locus under the CAG promoter by CRISPR (clustered regularly interspaced short palindromic repeats, Figure S1A). Genomic PCR confirmed the integration of the transgene in the resulting clonal hESCs (Figure S1B). Homozygous clones with two copies of the ChR2 gene were selected (Figure S1B). The ChR2-EYFP-expressing hESCs were differentiated to glutamatergic neurons (Li et al., 2009) in the presence of sonic hedgehog antagonist cyclopamine with 92.9 ± 3.3% of the total cells expressing glutamine synthase at day 36 (Figure S1D). The non-ChR2 hESCs were differentiated to medial ganglionic eminence progenitors and then to GABAergic neurons (Liu et al., 2013) with 81.9 ± 2.3% of total cells positive for GABA (Figure S1E). The ChR2-expressing cortical glutamate neurons were then co-cultured with cortical GABA interneurons derived from non-ChR2-expressing hESCs at a ratio of 2:1 (Figure 1A). Immunostaining of the co-cultures indicated that almost all the cells express a forebrain marker OTX2 but not the hippocampal marker Prox1 (Figures S1C and S1H), indicating the forebrain identity of the cells. The cultures were maintained for 5–7 weeks before recording.
Figure 1.
Effect of Light Stimulation on the Frequency of mEPSCs in non-ChR2-expressing GABA Neurons
(A) Immunostaining of co-cultures of forebrain glutamate neurons derived from ChR2-EYFP-expressing hESCs and GABA neurons derived from wild-type hESCs at 5 weeks after plating. Ho, Hoechst. Scale bar, 25 μm.
(B) Representative mEPSCs of a GABA neuron before, during, and after the light stimulation. (1) The 4 min of recording of mEPSCs. (2) Expanded timescale of the mEPSC recording before, during, and after the light stimulation.
(C) Time course of the average number of mEPSCs before, during, and after the light stimulation (the bin size is 15 s) (n = 8, mean ± SEM).
(D) The cumulative distributions of the inter-event intervals of mEPSCs before, during, and after the light stimulation.
(E) The averaged mEPSC frequency in GABA neurons before, during, and after the light stimulation (mean ± SEM, n = 8, paired t test, versus before [control]).
(F) Effect of light stimulation on PPF in non ChR2-expressing GABA neurons. Left, typical recordings of PPF before and after light stimulation. Holding potential, −70 mV. Right, Averaged PPF before and after light stimulation (p < 0.05, n = 6, mean ± SEM, t test).
(G) The action currents triggered by 5-ms pulses of blue light in ChR2-expressing glutamate neurons under voltage-clamp mode.
(H) eEPSC recording in a non-ChR2-expressing GABA neuron.
By plotting peak membrane potential (MP) response on current injection without action potential (AP) occurrence, the membrane input resistance (Rin) is 78.13 ± 3.43 MΩ (n = 7). The resting membrane potential is 58.25 ± 6.35 mV (n = 7). Under the blue light stimulation (2 s, 470 nm, 0.4 mW/mm2), robust action potentials (APs) were elicited in the glutamate neurons under the current-clamp mode (12.5 ± 0.49 Hz, n = 7, Figure S1F[1] [2]). In the voltage-clamp mode, at a holding potential of −70 mV, the same light stimulation elicited large inward currents with action currents (n = 7, Figure S1F[3]). Treatment with 1 μM tetrodotoxin (TTX) eliminated the action currents, suggesting that the remaining currents are ChR2-induced currents with two components: a large current with fast decay kinetics and a steady-state current (122.87 ± 8.79 pA at peak and 55.09 ± 2.65 pA at steady state, n = 7, Figure S1F[4]). Light stimulation had no effect on the GABA neurons that were differentiated from the non ChR2-expressing hESCs. Together, these data suggest that ChR2 is expressed in hESC-derived glutamatergic neurons and can induce rapid physiological responses with high fidelity and low “noise.”
We then evaluated synaptic interactions between the ChR2-expressing cortical glutamate neurons and the non-ChR2-expressing cortical GABA neurons. The recording GABA neurons were first identified as non-fluorescent and non-responsive to light stimulation. GABA neurons had spontaneous action potentials, often with a “fast-spiking” pattern (Figure S1G[1]). The spikes of GABA neurons were narrower than those of glutamate neurons and had a sharper repolarization (Figure S1G[2]), consistent with the findings in mouse neurons (Bean, 2007). After recording, we verified the identity by their positive staining for GABA (Figure S1G[1] left panel).
Before light stimulation of the glutamatergic neurons, the mean frequency of miniature excitatory postsynaptic currents (mEPSCs) in GABA neurons was 2.53 ± 0.52 Hz (n = 8), suggesting a high degree of connectivity. mEPSCs were abolished by the AMPA receptor antagonist NBQX (20 μM). With blue light (60 s, 470 nm, 0.4 mW/mm2) stimulation, the mean frequency of mEPSCs in GABA neurons increased to 5.19 ± 1.08 Hz. The effect reached statistical significance during the stimulation (range, 60–120 s, p < 0.05, n = 8, versus before, Figure 1B) and was not accompanied by changes in mEPSC amplitude. Following the light stimulation (180–240 s), the frequency of mEPSCs recovered to 3.18 ± 0.45 Hz (p > 0.05, n = 8, versus before) (Figures 1C and 1E). This is also illustrated by the cumulative distribution of the inter-event intervals of mEPSCs during light stimulation, showing a leftward shift, or a shorter inter-event interval, compared with the control and post-light stimulation (Figure 1D, p < 0.01; Kolmogorov-Smirnov test). Thus, light stimulation of the ChR2-expressing glutamatergic neurons potentiates the frequency of mEPSCs recorded in GABA neurons. To further demonstrate a facilitating action at the presynaptic terminals, we recorded PPF (paired pulse facilitation) at the presynaptic site in response to light stimulation. As shown in Figure 1F, light stimulation decreased the PPF ratio (P2/P1) from 1.4 ± 0.02 to 1.22 ± 0.04 (n = 6, p < 0.05). Together, these results suggest that light stimulation of the ChR2-expressing glutamatergic neurons potentiates the presynaptic glutamate release to GABA neurons.
Besides presynaptic regulation, postsynaptic response is similarly important for regulating synaptic transmission. Under pulse stimulation of blue light (5 ms), action currents were triggered in ChR2-expressing glutamatergic neurons under the voltage-clamp mode (Figure 1G). At the holding potential of −70 mV with extracellular Mg2+ concentration of 2 mM, which blocks NMDA receptor-mediated currents, the pulse blue light stimulation induced a large evoked EPSC (eEPSC, ∼70pA) in postsynaptic GABA neurons (Figure 1H). The interval between the blue light stimulus and the postsynaptic potential is 6.87 ± 0.05 ms. The eEPSC was completely inhibited by the AMPA receptor antagonist NBQX (2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione, 20 μM, Figure 1H), suggesting that the eEPSC was induced by the presynaptic glutamatergic release and mediated by the activation of AMPA receptors in postsynaptic GABA neurons. Together, light stimulation regulates presynaptic release, which in turn evokes strong postsynaptic response, enabling regulation of synaptic transmission.
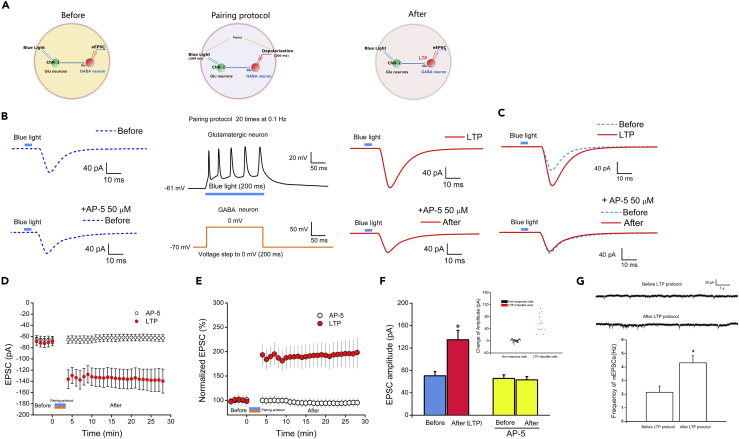
Repetitive Light Stimulation Induces LTP
With the ability to regulate synaptic transmission between cultured human neurons by light stimulation, we asked if it is possible to induce LTP between presynaptic glutamatergic neurons and postsynaptic GABA neurons. The typical protocol for LTP induction in brain slices involves high-frequency electric stimulation of presynaptic action potentials paired with postsynaptic depolarization (Chen et al., 1999). We have designed a new light-induction protocol consisting of a 200 ms light stimulation of ChR2-expressing glutamatergic neurons paired with postsynaptic depolarization (voltage step to 0 mV, 200 ms) of the patch-clamped GABA neuron (Figure 2A). Pairing was repeated 20 times at 0.1 Hz (Figure 2B). After the pairing protocol, the holding voltage was rapidly restored to −70 mV, thus establishing a stable baseline for eEPSC induction. Upon light stimulation, the eEPSC amplitudes increased significantly (Figure 2C top), from 70.36 ± 7.46 pA before to 134.74 ± 16.91 pA after the pairing protocol (p < 0.05, n = 13, Figure 2D). On average, the eEPSC amplitude was potentiated to 191.50 ± 24.61% of the control amplitude (p < 0.05, n = 13, Figure 2F). The increase in eEPSC amplitude persisted throughout the duration (30 min) of the recording (Figures 2D and 2E), indicating an LTP-like behavior. Besides the amplitude, the frequency of mEPSCs increased from 2.14 ± 0.46 to 4.31 ± 0.52 Hz after the LTP protocol (p < 0.05, n = 6, Figure 2G), indicating the increase in presynaptic glutamate release. Among the 59 GABA neurons that were responsive to light stimulation, i.e., synaptically connected (each from a different dish), 13 exhibited LTP-like behavior using our pairing protocol (22%).
Figure 2.
Induction of LTP in Cultured Human Neurons
(A) Cartoon of LTP induction. Left panel: blue light stimulates the ChR2-expressing glutamate neurons while recording eEPSCs in GABA neurons before pairing protocol. Middle panel: pairing protocol. Right panel: continued recording of eEPSC after pairing protocol.
(B) LTP induction protocol in the co-culture system. Five action potentials are induced by blue light stimulation (200 ms) of the ChR2-expressing glutamate neurons (Upper) when paired with a postsynaptic depolarization (voltage step to 0 mV, 200 ms) of the patch-clamped GABA neuron (Bottom). The protocol was repeated 20 times at 0.1 Hz.
(C) Raw traces of an eEPSC recorded in a non ChR2-expressing GABA neuron. Top panel: raw traces before and after pairing protocol stimulation. Bottom panel: raw traces before and after pairing protocol stimulation in the presence of AP-5.
(D) Change in the amplitude of light-evoked EPSCs before and after pairing protocol in the absence and presence of AP-5 over 30-min recording.
(E) The percentage increase of light-evoked EPSCs before and after pairing protocol in the absence and presence of AP-5.
(F) Averaged amplitude of eEPSCs before and after pairing protocol in the absence (p < 0.05, n = 13, mean ± SEM, t test) and presence of AP-5 (p > 0.05, n = 14, mean ± SEM, t test). Inset: Full distribution of the change of eEPSC amplitude from 59 cells studied in the LTP-like protocol.
(G) The effect of LTP protocols on the frequency of mEPSCs in non ChR2-expressing GABA neurons. Upper, representative mEPSCs of a GABA neuron before and after the LTP protocol. Bottom, the averaged mEPSC frequency in GABA neurons before and after the LTP protocol (p < 0.05, n = 6, mean ± SEM, t test).
To examine the mechanism underlying the potentiation, we applied AP-5 (amino-5-phosphonovaleric acid), an NMDA receptor antagonist, during the paired recording. The presence of AP-5 (50 μM) completely blocked the potentiation of eEPSCs (65.69 ± 6.44 pA before versus 63.16 ± 5.98 pA during pairing in the presence of AP-5, p > 0.05, n = 14, Figures 2C bottom, and 2D–2F), suggesting an NMDA receptor-dependent event.
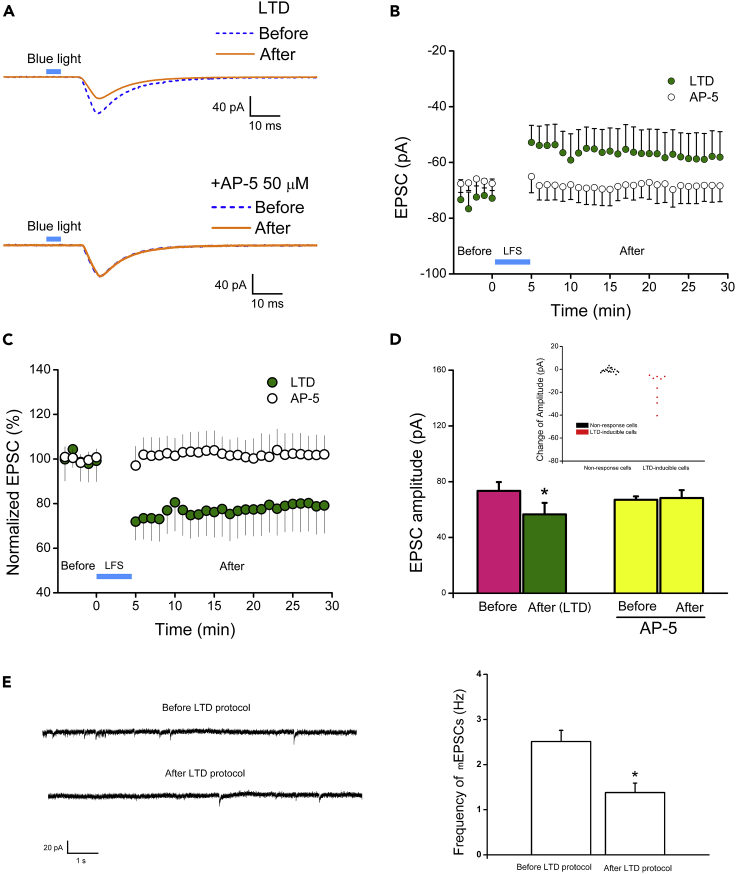
Low-Frequency Stimulation Induces LTD
Another form of synaptic plasticity is LTD. LTD is typically induced by low-frequency stimulation (LFS). We thus evaluated LTD in the above co-culture system by stimulating the ChR2-expressing glutamate neurons with low frequency (900 pulse, 3 Hz) of blue light and recording the non-ChR2-expressing GABA neurons. Under this condition, the eEPSC amplitudes decreased significantly, from 73.43 ± 6.32 pA before to 56.56 ± 8.27 pA after the LFS protocol (p < 0.05, n = 9, Figures 3A, 3B, and 3D). The normalized eEPSC amplitude was decreased to 77.02 ± 11.46% of the control (p < 0.05, n = 9, Figure 3C). The decrease in both the absolute value and the percentage change of eEPSC amplitude persisted throughout the duration (30 min) of the recording (Figures 3B and 3C), indicating an LTD-like behavior. In addition, the frequency of mEPSCs decreased from 2.51 ± 0.25 to 1.38 ± 0.21 Hz after the LTD protocol (p < 0.05, n = 6, Figure 3E), indicating the decrease in presynaptic glutamate release. Among the 26 synaptically connected GABA neurons, 9 exhibited LTD (35%, each from a different dish, Figure 3D inset). Again, blockade of NMDA receptors with AP-5 prevented the LTD (67.03 ± 2.47 pA before versus 68.33 ± 5.6 pA after stimulation, p > 0.05, n = 14, Figures 3B, 3C, and 3D), suggesting NMDA receptor-dependent LTD formation.
Figure 3.
Induction of LTD in Human Neurons
(A) Raw traces of eEPSCs recorded in a non-ChR2-expressing GABA neuron. Top panel: raw traces before and after LFS. Bottom panel: raw traces before and after LFS in the presence of AP-5.
(B) The amplitude of light-evoked EPSCs before and after LFS in the absence and presence of AP-5 during the 30-min recording.
(C) Time course of changes in percentage of eEPSCs before and after LFS in the absence and presence of AP-5.
(D) Averaged amplitude of eEPSCs before and after LFS in the absence (p < 0.05, n = 9, mean ± SEM, t test) and presence of AP-5 (p > 0.05, n = 14). Inset: Full distribution of the change of eEPSC amplitude from 26 cells studied in the LTD protocol.
(E) The effect of LTD protocols on the frequency of mEPSCs in non ChR2-expressing GABA neurons. Left, representative mEPSCs of a GABA neuron before and after the LTD protocol. Right, the averaged mEPSC frequency in GABA neurons before and after the LTD protocol (p < 0.05, n = 6, mean ± SEM, t test).
LTP/LTD Induction Is Accompanied by Molecular Changes in Postsynaptic Cells
Molecular analysis on acutely prepared rodent brain slices following electrophysiological stimulation and recording has identified several signaling molecules to be differentially regulated at the transcriptional level accompanying the change of the postsynaptic strength (Nicoll, 2017). These include Ca2+/calmodulin-dependent protein kinase II (CAMKII) that plays the key role in the induction and maintenance of the long-term synaptic dynamic (Kim et al., 2016) and immediate-early genes (IEGs) that are activated following synaptic activation, including the inducible transcription factors (ITF) c-FOS (FOS), ZIF268 (EGR1), and CREB (CREB1) (Li et al., 2017, Minatohara et al., 2015). To determine if induction of LTP and LTD in our cultured human neurons is accompanied by similar molecular changes in the postsynaptic neurons to those observed in rodent brain slices, we aspirated off the intracellular components and performed single cell PCR assay. Strikingly, the expression level of CAMKII increased by 5-fold in cells after LTP induction compared with those synaptically connected but without LTP induction (Figure S2A left panel). In contrast, CAMKII decreased by-9 fold after LTD (Figure S2A right panel). IEGs, including c-FOS, EGR1, and CREB1, significantly increased after LTP induction but decreased after LTD except that the reduction in EGR1 expression was not statistically significant (Figure S2B). We further measured calcium influx at the presynaptic site in response to light stimulation, showing an increase in fluorescein intensity (IOD: 100 ± 10.99% before versus 203.52 ± 11.75% after, n = 4, p < 0.001) and area (100 ± 12.4% before versus 191.06 ± 11.49% after, n = 4, p < 0.001) (Figure S2C). Together, these results suggest that light stimulation of the ChR2-expressing glutamatergic neurons potentiates the presynaptic glutamate release to GABA neurons. They also suggest that the synaptic dynamics in our in vitro human neuronal network shares similar molecular processes to those revealed by ex vivo brain slices.
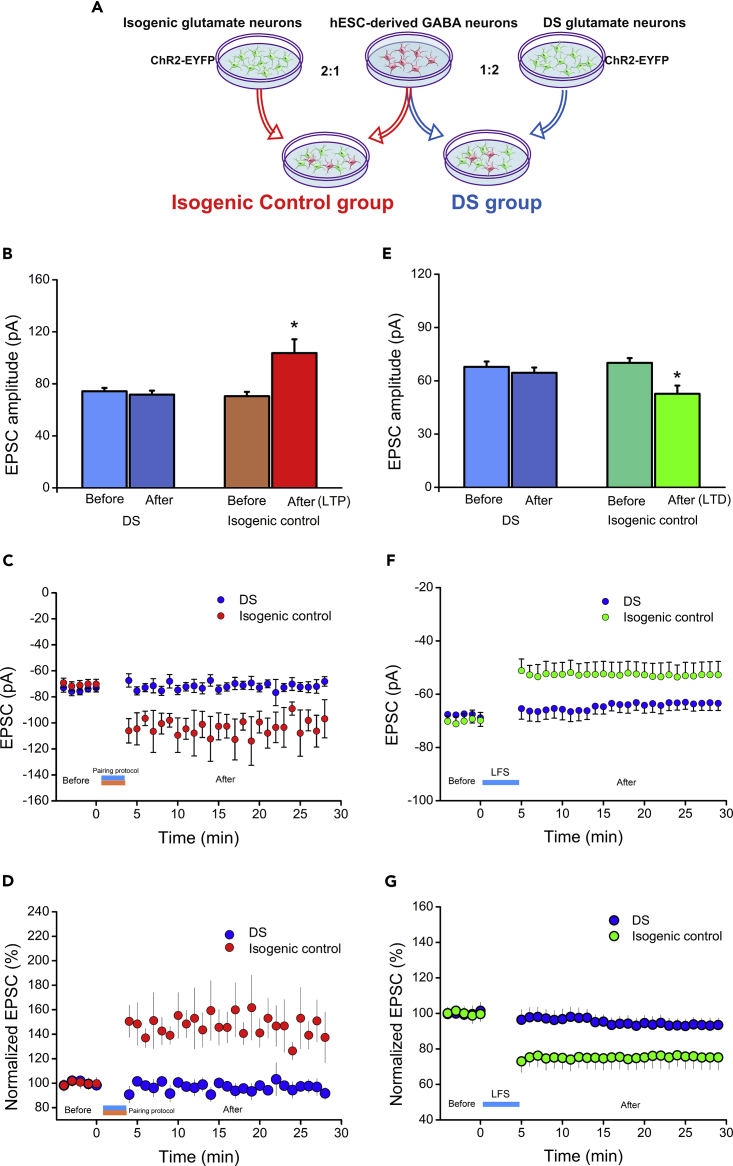
Down Syndrome Patient iPSC-Derived Neurons Show Absence of LTP/LTD
The ability to measure LTP and LTD from human stem cell-derived neuronal networks opens a possibility to interrogate if neuronal networks from patients with neurological and psychiatric disorders are altered. We have previously shown that cortical neurons derived from iPSCs of DS, a common developmental disease with mental retardation caused by an extra chromosome 21, show reduced synaptic currents (Weick et al., 2013). To validate the utility of our assay in iPSC-derived neuronal networks and to determine if DS cortical neurons exhibit defective LTP/LTD, we expressed ChR2-EYFP in DS patient iPSCs or isogenic control iPSCs by CRISPR and then co-cultured the ChR2-EYFP glutamate neurons (from DS or isogenic control iPSCs) with non-ChR2 GABA neurons (from H9 ESCs) by 2:1 (Figure 4A), similar to the ratio for non-DS cells mentioned above.
Figure 4.
Induction of LTP/LTD in DS Patient iPSC- and Isogenic iPSC-Derived Neural Networks
(A) Cartoon of the co-culture between DS/isogenic glutamate neurons and hESC-derived GABA neurons.
(B) Averaged amplitude of eEPSCs before and after pairing protocol in DS patient iPSC- (p > 0.05, n = 16, mean ± SEM, t test) and isogenic iPSC-derived neural network (p < 0.05, n = 7, mean ± SEM, t test).
(C) Change in the amplitude of light-evoked EPSCs before and after pairing protocol over 30-min recording.
(D) The percentage increase of light-evoked EPSCs before and after pairing protocol.
(E) Averaged amplitude of eEPSCs before and after LFS in DS patient iPSC- (p > 0.05, n = 8, mean ± SEM, t test) and isogenic iPSC-derived neural network (p < 0.05, n = 4, mean ± SEM, t test).
(F) Change in the amplitude of light-evoked EPSCs before and after LFS over 30-min recording.
(G) The percentage decrease of light-evoked EPSCs before and after LFS.
Following the same LTP-induction protocol described above, we found that, in the isogenic control group, the eEPSC amplitudes changed significantly, from 70.48 ± 3.38 pA before to 103.64 ± 10.64 pA (p < 0.05, n = 7 [of 34 connected cells], Figure 4B) after the same light stimulation protocol. On average, the eEPSC amplitude was potentiated to 139.20 ± 14.44% of the control amplitude (Figure 4D). The increase in eEPSC amplitude also persisted throughout the duration (30 min) of the recording (Figures 4C and 4D), indicating an LTP-like behavior. Among the 34 neurons that were responsive to light stimulation, i.e., synaptically connected, 7 exhibited LTP-like behavior using our pairing protocol (20.5%), indicating that our protocol is reproduced in iPSC-derived neurons with a similar efficiency. In contrast, among the 16 DS iPSC-derived neurons that were responsive to light stimulation, i.e., synaptically connected (each from a different dish), none exhibited LTP-like behavior (0%). The eEPSCs in DS patient iPSC-derived neural network did not show obvious changes (74.30 ± 2.55 pA before versus 71.78 ± 3.01 pA after the LTP-induction protocol, p > 0.05, n = 16, Figures 4B, 4C, and 4D), indicating an absence of LTP.
We then asked if LTD is affected in DS neurons. Under the same LTD-induction condition described above, we found that the eEPSC amplitudes decreased significantly in the isogenic group, from 70.10 ± 2.67 pA before to 52.70 ± 4.61 pA (p < 0.05, n = 4 [of 12 connected cells], Figure 4E) after the same LFS protocol. On average, the eEPSC amplitude was decreased to 79.32 ± 6.14% of the control amplitude (Figure 4G). The decrease in eEPSC amplitude also persisted throughout the duration (30 min) of the recording (Figures 4F and 4G), indicating an LTD-like behavior. Among the 12 neurons that were responsive to light stimulation, i.e., synaptically connected, 4 exhibited LTD-like behavior (33.3%), indicating that LTD is replicated in isogenic iPSC-derived neurons with a similar efficiency. In contrast, in the DS group, none exhibited LTD-like behavior (0%) among the eight neurons that were responsive to light stimulation, i.e., synaptically connected (each from a different dish). The eEPSCs in DS patient iPSC-derived neural network did not show obvious changes (67.84 ± 3.01 pA before versus 64.51 ± 2.96 pA after the LFS protocol, p > 0.05, n = 8, Figure 4E), signaling the lack of LTD induction.
Discussion
We have created a platform to enable analysis of neural plasticity in cultured human neurons. This is made possible by co-culturing hESC-derived inhibitory (GABA) neurons with excitatory (glutamate) neurons that are engineered with an optogenetic control (Ge et al., 2006, Li et al., 2012, Steinbeck et al., 2015, Weick et al., 2011). With such a model, we have now shown that LTP and LTD can be efficiently elicited in human neuronal networks by precisely regulating the activity of excitatory neurons and that the induction of LTP/LTD is NMDA receptor dependent. This assay is reproduced using iPSC-derived neuronal networks and in different laboratories. Furthermore, we found that LTP/LTD induction is defective in DS iPSC-derived cortical neuronal networks, highlighting the utility of such an in vitro platform for examining the plasticity of human neural networks under physiological and pathological conditions.
LTP/LTD is measured by stimulating the presynaptic components with electric stimulation and recording changes of synaptic strength at the postsynaptic end typically in well-defined structures, such as the hippocampus (Bliss and Collingridge, 2013). Even so, recording of LTP/LTD remains a rare event experimentally (Buonomano and Merzenich, 1998, Chen et al., 1994). It is nearly impossible to induce direct LTP/LTD in cultured neurons because the electric stimulation only activates the adjacent neurons owing to the attenuation of an electric field. Because of these difficulties, the change in mEPSC amplitude in cultured mouse hippocampal neurons is used to replace the evoked EPSCs in slices (Lu et al., 2001, Man et al., 2003, Ninan et al., 2006). Such a low efficiency places a substantial technical burden on recording LTP/LTD in “randomly” connected human neurons. In addition, electric stimulation activates both presynaptic and postsynaptic neurons at the same time because of the randomness in co-cultured neural circuits, making it difficult to interpret results. To overcome these technical hurdles, we have established a ChR2-expressing hESC line by CRISPR so that the activity of the derived neurons can be precisely regulated. Indeed, expression of ChR2 enables not only precise regulation of presynaptic human neuronal activity but also postsynaptic neuronal response (Weick et al., 2011). More importantly, simultaneous stimulation of large numbers of presynaptic glutamate neurons not only increases the probability of synaptic inputs but also synchronizes the inputs onto the recording postsynaptic neurons, thus amplifying outputs. Such a strategy enables us to achieve LTP/LTD in a substantial proportion (20%–30%) of synaptically connected neurons.
Induction of LTP/LTD requires coordinated changes at both the pre- and postsynaptic ends. After series of testing, we have identified a set of light-stimulation parameters that can effectively alter the strength of the presynaptic release. In particular, the repetitive light stimulation (200-ms light stimulation pairing with postsynaptic depolarization 20 times at 0.1 Hz) augments the presynaptic release of glutamate, as indicated by increased amplitude and frequency of eEPSCs, elevated calcium influx, and increased expression of CAMKIIA. This is very much similar to the changes induced by HFS in brain slices (Sokolov et al., 2003). When coupled with postsynaptic depolarization, it effectively induces LTP. The changes at the postsynaptic end induced by light stimulation are also similar to those seen in brain slices induced by electric stimulation, including changes of activity-regulated genes (immediate-early genes) like FOS, EGR1, and CREB1. These genes are preferentially expressed in the neurons that encode long-term memory in rodent models and are thought to be the markers of the memory engram (Liu et al., 2014, Zhou et al., 2009). Interestingly, the expression level of CAMKIIA in our postsynaptic human GABA neurons is also positively correlated with the neural activity, similar to a previous report using rodents (Thiagarajan et al., 2002). Therefore, the LTP/LTD induced in our system resembles that in the classical brain slice model. Furthermore, we have shown that the LTP/LTD is mediated by the NMDA receptors, as the antagonist of NMDA receptors blocked the light-induced LTP/LTD. The NMDA-dependent LTP/LTD is a cellular substrate of memory (Pare, 2004). Therefore, the NMDA-dependent LTP/LTD induced in our cultured human neural networks suggests that the human brain may use a very similar mechanism in processing information.
Our in vitro plasticity model is simple and versatile. We first developed the parameters using hESC-derived neurons, but the protocol is reproduced using iPSC-derived neurons. This is also reproduced when the lead authors moved to a new institution to set up a new laboratory. Although we chose cortical glutamate and GABA neurons to mimic cortical circuitry, one can use a combination of any other neural cell types to examine the transmission and plasticity. When these cells are from patient or disease stem cells, the model will enable assaying synaptic plasticity under psychiatric or neurological conditions, as shown by the defective LTP and LTD in DS iPSC-derived neuronal networks. It should be noted that the inability to induce LTP/LTD in the DS neuronal culture may also be associated with reduced synaptic activity (Weick et al., 2013). Nevertheless, our observation in DS patient iPSC-derived neuronal network is very similar to the deficit or even complete lack of LTP reported in hippocampal CA1 synapses of adult Ts65Dn mice, a model for DS (Costa and Grybko, 2005, Kleschevnikov et al., 2004, Siarey et al., 1997, Siarey et al., 1999). Since our model is based on precise control of synaptic transmission by light and intracellular recording, it offers a new way to assess human neural plasticity at the single-synapse level when combined with engineering tools. Theoretically, such a model could provide a research platform for studying human learning and memory and building a computational model for circuit learning and memory.
Limitations of the Study
The LTP/LTD phenomena in the current paper are based on hESC-derived human neural networks. Although they are similar to those recorded in animal brain slices, they may involve different mechanisms, which requires further investigation.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This study was supported in part by the NIH-NIMH (MH099587, MH100031), NIH-NINDS (NS076352, NS086604), and the NICHD (HD076892, U54 HD090256). S.-C.Z. acknowledges the Steenbock Professorship. S.-C.Z. is co-founder of BrainXell, Inc.
Author Contributions
S.-C.Z. and Y.D. designed the experiments and wrote the manuscript with input from M.X. and Y.C. Y.D. and M.X. processed the differentiation and histology experiments. Y.D. performed the electrophysiology experiments. Y.C. did the cell line construction experiments. Y.T. and X.L. helped with some of the experiments. Y.D. and M.X. collected and analyzed data. S.-C.Z. supervised the project.
Declaration of Interests
The authors declare no competing interests.
Published: February 21, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.100829.
Contributor Information
Yi Dong, Email: brainstein@fudan.edu.cn.
Su-Chun Zhang, Email: suchun.zhang@wisc.edu.
Supplemental Information
References
- Bean B.P. The action potential in mammalian central neurons. Nat. Rev. Neurosci. 2007;8:451–465. doi: 10.1038/nrn2148. [DOI] [PubMed] [Google Scholar]
- Beck H., Goussakov I.V., Lie A.L., Helmstaedter C., Elger C.E. Synaptic plasticity in the human dentate gyrus. J. Neurosci. 2000;20:7080–7086. doi: 10.1523/JNEUROSCI.20-18-07080.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss T.V., Collingridge G.L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bliss T.V., Collingridge G.L. Expression of NMDA receptor-dependent LTP in the hippocampus: bridging the divide. Mol. Brain. 2013;6:5. doi: 10.1186/1756-6606-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonomano D.V., Merzenich M.M. Cortical plasticity: from synapses to maps. Annu. Rev. Neurosci. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- Chen H.X., Otmakhov N., Lisman J. Requirements for LTP induction by pairing in hippocampal CA1 pyramidal cells. J. Neurophysiol. 1999;82:526–532. doi: 10.1152/jn.1999.82.2.526. [DOI] [PubMed] [Google Scholar]
- Chen W., Hu G.Y., Zhou Y.D., Wu C.P. Two mechanisms underlying the induction of long-term potentiation in motor cortex of adult cat in vitro. Exp. Brain Res. 1994;100:149–154. doi: 10.1007/BF00227287. [DOI] [PubMed] [Google Scholar]
- Chen W.R., Lee S.H., Kato K., Spencer D.D., Shepherd G.M., Williamson A. Long-term modifications of synaptic efficacy in the human inferior and middle temporal cortex. Proc. Natl. Acad. Sci. U S A. 1996;93:8011–8015. doi: 10.1073/pnas.93.15.8011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp W.C., Kirk I.J., Hamm J.P., Shepherd D., Teyler T.J. Induction of LTP in the human auditory cortex by sensory stimulation. Eur. J. Neurosci. 2005;22:1135–1140. doi: 10.1111/j.1460-9568.2005.04293.x. [DOI] [PubMed] [Google Scholar]
- Costa A.C., Grybko M.J. Deficits in hippocampal CA1 LTP induced by TBS but not HFS in the Ts65Dn mouse: a model of Down syndrome. Neurosci. Lett. 2005;382:317–322. doi: 10.1016/j.neulet.2005.03.031. [DOI] [PubMed] [Google Scholar]
- Dan Y., Poo M.M. Spike timing-dependent plasticity of neural circuits. Neuron. 2004;44:23–30. doi: 10.1016/j.neuron.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Daw N., Rao Y., Wang X.F., Fischer Q., Yang Y. LTP and LTD vary with layer in rodent visual cortex. Vis. Res. 2004;44:3377–3380. doi: 10.1016/j.visres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Ge S., Goh E.L., Sailor K.A., Kitabatake Y., Ming G.L., Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K., Saneyoshi T., Hosokawa T., Okamoto K., Hayashi Y. Interplay of enzymatic and structural functions of CaMKII in long-term potentiation. J. Neurochem. 2016;139:959–972. doi: 10.1111/jnc.13672. [DOI] [PubMed] [Google Scholar]
- Kleschevnikov A.M., Belichenko P.V., Villar A.J., Epstein C.J., Malenka R.C., Mobley W.C. Hippocampal long-term potentiation suppressed by increased inhibition in the Ts65Dn mouse, a genetic model of Down syndrome. J. Neurosci. 2004;24:8153–8160. doi: 10.1523/JNEUROSCI.1766-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.K., Kirkwood A. AMPA receptor regulation during synaptic plasticity in hippocampus and neocortex. Semin. Cell Dev. Biol. 2011;22:514–520. doi: 10.1016/j.semcdb.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Aimone J.B., Xu X., Callaway E.M., Gage F.H. Development of GABAergic inputs controls the contribution of maturing neurons to the adult hippocampal network. Proc. Natl. Acad. Sci. U S A. 2012;109:4290–4295. doi: 10.1073/pnas.1120754109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., You Q.L., Zhang S.R., Huang W.Y., Zou W.J., Jie W., Li S.J., Liu J.H., Lv C.Y., Cong J. Satb2 ablation impairs hippocampus-based long-term spatial memory and short-term working memory and immediate early genes (IEGs)-Mediated hippocampal synaptic plasticity. Mol. Neurobiol. 2017 doi: 10.1007/s12035-017-0531-5. [DOI] [PubMed] [Google Scholar]
- Li X.J., Zhang X., Johnson M.A., Wang Z.B., Lavaute T., Zhang S.C. Coordination of sonic hedgehog and Wnt signaling determines ventral and dorsal telencephalic neuron types from human embryonic stem cells. Development. 2009;136:4055–4063. doi: 10.1242/dev.036624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Ramirez S., Redondo R.L., Tonegawa S. Identification and manipulation of memory engram cells. Cold Spring Harb. Symp. Quant. Biol. 2014;79:59–65. doi: 10.1101/sqb.2014.79.024901. [DOI] [PubMed] [Google Scholar]
- Liu Y., Weick J.P., Liu H., Krencik R., Zhang X., Ma L., Zhou G.M., Ayala M., Zhang S.C. Medial ganglionic eminence-like cells derived from human embryonic stem cells correct learning and memory deficits. Nat. Biotechnol. 2013;31:440–447. doi: 10.1038/nbt.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W., Man H., Ju W., Trimble W.S., MacDonald J.F., Wang Y.T. Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron. 2001;29:243–254. doi: 10.1016/s0896-6273(01)00194-5. [DOI] [PubMed] [Google Scholar]
- Malenka R.C. Synaptic plasticity in the hippocampus: LTP and LTD. Cell. 1994;78:535–538. doi: 10.1016/0092-8674(94)90517-7. [DOI] [PubMed] [Google Scholar]
- Malenka R.C., Bear M.F. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Man H.Y., Wang Q., Lu W.Y., Ju W., Ahmadian G., Liu L., D'Souza S., Wong T.P., Taghibiglou C., Lu J. Activation of PI3-kinase is required for AMPA receptor insertion during LTP of mEPSCs in cultured hippocampal neurons. Neuron. 2003;38:611–624. doi: 10.1016/s0896-6273(03)00228-9. [DOI] [PubMed] [Google Scholar]
- Minatohara K., Akiyoshi M., Okuno H. Role of immediate-early genes in synaptic plasticity and neuronal ensembles underlying the memory trace. Front. Mol. Neurosci. 2015;8:78. doi: 10.3389/fnmol.2015.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabavi S., Fox R., Proulx C.D., Lin J.Y., Tsien R.Y., Malinow R. Engineering a memory with LTD and LTP. Nature. 2014;511:348–352. doi: 10.1038/nature13294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll R.A. A brief history of long-term potentiation. Neuron. 2017;93:281–290. doi: 10.1016/j.neuron.2016.12.015. [DOI] [PubMed] [Google Scholar]
- Ninan I., Liu S., Rabinowitz D., Arancio O. Early presynaptic changes during plasticity in cultured hippocampal neurons. EMBO J. 2006;25:4361–4371. doi: 10.1038/sj.emboj.7601318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare D. Presynaptic induction and expression of NMDA-dependent LTP. Trends Neurosci. 2004;27:440–441. doi: 10.1016/j.tins.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Siarey R.J., Carlson E.J., Epstein C.J., Balbo A., Rapoport S.I., Galdzicki Z. Increased synaptic depression in the Ts65Dn mouse, a model for mental retardation in Down syndrome. Neuropharmacology. 1999;38:1917–1920. doi: 10.1016/s0028-3908(99)00083-0. [DOI] [PubMed] [Google Scholar]
- Siarey R.J., Stoll J., Rapoport S.I., Galdzicki Z. Altered long-term potentiation in the young and old Ts65Dn mouse, a model for Down Syndrome. Neuropharmacology. 1997;36:1549–1554. doi: 10.1016/s0028-3908(97)00157-3. [DOI] [PubMed] [Google Scholar]
- Sidorov M.S., Kaplan E.S., Osterweil E.K., Lindemann L., Bear M.F. Metabotropic glutamate receptor signaling is required for NMDA receptor-dependent ocular dominance plasticity and LTD in visual cortex. Proc. Natl. Acad. Sci. U S A. 2015;112:12852–12857. doi: 10.1073/pnas.1512878112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolov M.V., Rossokhin A.V., M Kasyanov A., Gasparini S., Berretta N., Cherubini E., Voronin L.L. Associative mossy fibre LTP induced by pairing presynaptic stimulation with postsynaptic hyperpolarization of CA3 neurons in rat hippocampal slice. Eur. J. Neurosci. 2003;17:1425–1437. doi: 10.1046/j.1460-9568.2003.02563.x. [DOI] [PubMed] [Google Scholar]
- Steinbeck J.A., Choi S.J., Mrejeru A., Ganat Y., Deisseroth K., Sulzer D., Mosharov E.V., Studer L. Optogenetics enables functional analysis of human embryonic stem cell-derived grafts in a Parkinson's disease model. Nat. Biotechnol. 2015;33:204–209. doi: 10.1038/nbt.3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suppa A., Li Voti P., Rocchi L., Papazachariadis O., Berardelli A. Early visuomotor integration processes induce LTP/LTD-like plasticity in the human motor cortex. Cereb. Cortex. 2015;25:703–712. doi: 10.1093/cercor/bht264. [DOI] [PubMed] [Google Scholar]
- Tang Y.P., Shimizu E., Dube G.R., Rampon C., Kerchner G.A., Zhuo M., Liu G., Tsien J.Z. Genetic enhancement of learning and memory in mice. Nature. 1999;401:63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- Teyler T.J., Hamm J.P., Clapp W.C., Johnson B.W., Corballis M.C., Kirk I.J. Long-term potentiation of human visual evoked responses. Eur. J. Neurosci. 2005;21:2045–2050. doi: 10.1111/j.1460-9568.2005.04007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiagarajan T.C., Piedras-Renteria E.S., Tsien R.W. alpha- and betaCaMKII. Inverse regulation by neuronal activity and opposing effects on synaptic strength. Neuron. 2002;36:1103–1114. doi: 10.1016/s0896-6273(02)01049-8. [DOI] [PubMed] [Google Scholar]
- Weick J.P., Held D.L., Bonadurer G.F., 3rd, Doers M.E., Liu Y., Maguire C., Clark A., Knackert J.A., Molinarolo K., Musser M. Deficits in human trisomy 21 iPSCs and neurons. Proc. Natl. Acad. Sci. U S A. 2013;110:9962–9967. doi: 10.1073/pnas.1216575110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weick J.P., Liu Y., Zhang S.C. Human embryonic stem cell-derived neurons adopt and regulate the activity of an established neural network. Proc. Natl. Acad. Sci. U S A. 2011;108:20189–20194. doi: 10.1073/pnas.1108487108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Won J., Karlsson M.G., Zhou M., Rogerson T., Balaji J., Neve R., Poirazi P., Silva A.J. CREB regulates excitability and the allocation of memory to subsets of neurons in the amygdala. Nat. Neurosci. 2009;12:1438–1443. doi: 10.1038/nn.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.