Abstract
Background
Despite simpler regimens than vitamin K antagonists (VKAs) for stroke prevention in atrial fibrillation (AF), adherence (taking drugs as prescribed) and persistence (continuation of drugs) to direct oral anticoagulants are suboptimal, yet understudied in electronic health records (EHRs).
Objective
We investigated (1) time trends at individual and system levels, and (2) the risk factors for and associations between adherence and persistence.
Methods
In UK primary care EHR (The Health Information Network 2011–2016), we investigated adherence and persistence at 1 year for oral anticoagulants (OACs) in adults with incident AF. Baseline characteristics were analysed by OAC and adherence/persistence status. Risk factors for non-adherence and non-persistence were assessed using Cox and logistic regression. Patterns of adherence and persistence were analysed.
Results
Among 36 652 individuals with incident AF, cardiovascular comorbidities (median CHA2DS2VASc[Congestive heart failure, Hypertension, Age≥75 years, Diabetes mellitus, Stroke, Vascular disease, Age 65-74 years, Sex category] 3) and polypharmacy (median number of drugs 6) were common. Adherence was 55.2% (95% CI 54.6 to 55.7), 51.2% (95% CI 50.6 to 51.8), 66.5% (95% CI 63.7 to 69.2), 63.1% (95% CI 61.8 to 64.4) and 64.7% (95% CI 63.2 to 66.1) for all OACs, VKA, dabigatran, rivaroxaban and apixaban. One-year persistence was 65.9% (95% CI 65.4 to 66.5), 63.4% (95% CI 62.8 to 64.0), 61.4% (95% CI 58.3 to 64.2), 72.3% (95% CI 70.9 to 73.7) and 78.7% (95% CI 77.1 to 80.1) for all OACs, VKA, dabigatran, rivaroxaban and apixaban. Risk of non-adherence and non-persistence increased over time at individual and system levels. Increasing comorbidity was associated with reduced risk of non-adherence and non-persistence across all OACs. Overall rates of ‘primary non-adherence’ (stopping after first prescription), ‘non-adherent non-persistence’ and ‘persistent adherence’ were 3.5%, 26.5% and 40.2%, differing across OACs.
Conclusions
Adherence and persistence to OACs are low at 1 year with heterogeneity across drugs and over time at individual and system levels. Better understanding of contributory factors will inform interventions to improve adherence and persistence across OACs in individuals and populations.
Keywords: atrial fibrillation, anticoagulant, adherence, persistence, stroke
Introduction
For 60 years, vitamin K antagonists (VKAs), mainly warfarin, dominated stroke prevention in atrial fibrillation (AF), the most common arrhythmia globally.1 2 Successive approval of four direct oral anticoagulants (DOACs: dabigatran,3 apixaban,4 rivaroxaban5 and edoxaban6) changed the landscape, with early adoption in guidelines7 8 and quality improvement initiatives.9 DOACs are often preferred over VKA due to reduced international normalised ratio (INR) monitoring, but only if OAC services are fully decommissioned and DOACs are taken appropriately. Paradoxically, removal of the need for INR monitoring also removes additional patient–clinician engagement that encourages adherence (taking drugs as prescribed) and persistence (continuation of therapy),10 both pertinent to oral anticoagulants (OACs) with a lifelong therapeutic indication.
Despite its importance in the context of population ageing, declining cognitive function, multimorbidity and polypharmacy, adherence was unreported in trials of DOACs,3–6 despite short half-lives, particularly dabigatran and apixaban which require dosing two times per day.11 Reported trial persistence was highest for dabigatran (79.3% at low dose) and lowest for edoxaban (65.6% at high dose)3–6 (online supplementary web table 1). All DOACs have proven efficacy compared with VKA, although at much lower time in therapeutic range (TTR) in trials than usual clinical practice, but ‘head-to-head’ DOAC trial comparisons are unlikely. However, several studies have shown suboptimal adherence and persistence for DOACs in different countries and settings, even compared with VKA, and effective interventions are lacking.12–14 Underlying causes include factors at social, economic, health system, clinician and patient levels. Although all patient-level factors are not captured, electronic health records (EHRs) allow population-level studies of adherence and persistence together across all DOACs in the same data set, which are rare.13
heartjnl-2019-315307supp001.pdf (227.4KB, pdf)
Only one study to date has considered all metrics of drug utilisation (‘initiation’, ‘implementation’ and ‘discontinuation’) together rather than ‘adherence’ or ‘persistence’ in isolation15 for OAC in AF.13 Steps in drug utilisation may be described as the ‘prescription-persistence cascade’ (from ‘recommendation’ to ‘persistence’/’continuation’), estimable from EHR. For OAC in AF, the steps are ‘ recommendation ’ (eligible for OAC), ‘ initial prescription’ (≥1 OAC prescription), ‘ dispensing ’ (no EHR data), ‘ initiation by patient’ (no EHR data), ‘ adherence’ / ‘ implementation ’ (adherent to OAC) and ‘ persistence’/‘ continuation’ (persistent to OAC). Interaction between adherence and persistence is often overlooked, for example, ‘persistent and non-adherent’ (ie, continuing medications but not taking as prescribed) versus ‘non-persistent and non-adherent’ (ie, discontinued medications and also not taking as prescribed).
The UK has universal primary healthcare, enabling large-scale, representative data sets where uptake, adherence and persistence for different DOACs can be studied. We used The Health Improvement Network (THIN) database in the UK to investigate adherence and persistence for OACs in individuals with AF, focusing on (1) time trends since DOAC introduction at health system level and after initiation in individuals; (2) relative impact of sociodemographic and baseline risk factors and treatment characteristics; and (3) associations between adherence and persistence.
Methods
The study conformed to the Strengthening the Reporting of Observational Studies in Epidemiology recommendations.16
Data source
The THIN database includes longitudinal, anonymised EHRs from over 500 UK general practices using Vision software (INPS, www.inps4.co.uk/), representative of the UK population.17
Study population
Our retrospective cohort included individuals aged ≥18 years with first-ever, non-valvular AF diagnosis between January 2011 and December 2016 and first prescription of VKA/DOAC on or after the date of AF diagnosis. The date of first prescription became the index date. For inclusion, patients needed ≥90 days of follow-up. Individuals with ≥1 prescription of VKA/DOAC were eligible for inclusion in adherence/persistence analyses. Exclusion criteria were taking OAC for other indications (eg, deep vein thrombosis and pulmonary embolism). Follow-up was until outcome event, death, the patient leaving the database or the most recent data upload.
Baseline covariates
Baseline factors were assessed: demographics (age, sex, Townsend Deprivation Index quintile level 1—the least deprived category), comorbidities (heart failure, hypertension, diabetes mellitus, stroke/transient ischaemic attack, vascular disease, liver disease, hypercholesterolaemia, ie, on statin and/or had hypercholesterolaemia), social history (alcohol misuse, smoking status) and drug history (aspirin, statin, blood pressure-lowering drugs, and mean number of drugs including OAC, prescribed in ≤365 days until, but not including, the episode start date). CHA2DS2VASc (Congestive heart failure, Hypertension, Age≥75 years, Diabetes mellitus, Stroke, Vascular disease, Age 65-74 years, Sex category18) and ‘HASBLED-1’ (rather than HASBLED (Hypertension, Abnormal renal/liver function, Stroke, Bleeding, Labile INR, Elderly, Drugs or alcohol19), since INR and ‘labile INR’ were not available) scores were calculated from available variables and categorised based on current guidelines.
Outcomes
Outcomes were adherence to and persistence with OACs. Adherence was estimated by proportion of days covered (PDC) over the year following first prescription of VKA/DOAC, which more accurately reflects patient behaviour and treatment continuity than other adherence measures20:
Each prescription was assumed to last 30 days, unless a new prescription was issued within 30 days, in which case the original prescription’s duration was assumed to equal the gap between the two prescriptions. Patients with only one OAC prescription were classed as ‘missing’ and not included in the estimation. Impact of varying PDC threshold to 70% and 90% was examined for all OACs. Adherence was defined as PDC >80% like previous studies21 and produced more stable estimates. Individuals prescribed VKA/DOAC were deemed persistent until a prescription gap >90 days on that or an alternative OAC (‘switch’), in which case they were non-persistent, or there was no further longitudinal data (in which case persistence status was unknown beyond that time). For each DOAC, proportion of switching to VKA or another DOAC was analysed over 12 months. Impact of varying prescription gaps to 60 and 120 days was examined for all OACs. Like previous studies,22 the 90-day prescription gap was used, providing more stable estimates. As prescription gaps lengthened, persistence improved more with VKA than DOACs (online supplementary web table 1).
Statistical analysis
Baseline characteristics were analysed by OAC. If any OAC/DOAC group consisted of <100 individuals, sample size was deemed too low to undertake meaningful analysis. Persistence was estimated using Kaplan-Meier product-limit estimator. Crude persistence for different OACs was estimated through survival life tables (adopting different prescription gaps) and ascertaining the number and percentage (95% CI) of patients still in the study (ie, persistent or uncensored) after 1 year. After stratification by adherence/persistence status at 12 months, baseline characteristics were determined. χ2 test and analysis of variance test were used for categorical and continuous covariates, respectively. Relative effects of OACs on non-adherence and non-persistence were modelled using univariable and multivariable logistic regression and Cox proportional hazard regression (simple and multiple), respectively. For multivariable analyses, we adjusted for date of first OAC prescription (relative to study start date), CHA2DS2VASc, HASBLED-1, Townsend Deprivation Index quintile and number of drugs. Optimal adjustments for CHA2DS2VASc, HASBLED-1, number of drugs and date of first OAC prescription were investigated using continuous variables (including potential quadratic effects) or clinically appropriate categorisation. Models were compared using Bayesian information criterion (BIC) and the optimal model chosen based on the lowest BIC. For Cox regression, the proportional hazards assumption was investigated by adding interactions with ‘time in study’. Interactions were included if they improved the model (by BIC criterion). For non-adherence, a sensitivity analysis was performed in those who had ≥6 months’ potential OAC coverage (ie, ≥6 months between date of first prescription and date of last prescription plus 30 days) and ≥12 months’ OAC potential coverage to reduce potential bias in estimated adherence in short treatment periods, leading to an overestimate of PDC (online supplementary web tables 2a and 2b). For non-persistence, two sensitivity analyses were based around the chosen Cox regression model by (1) adding interactions (linear; linear and quadratic) between OAC and timing of first OAC prescription (relative to study initiation); and (2) reclassifying those switching to another OAC as censored (cessation of observation) rather than non-persistent to first OAC prescribed.
Results
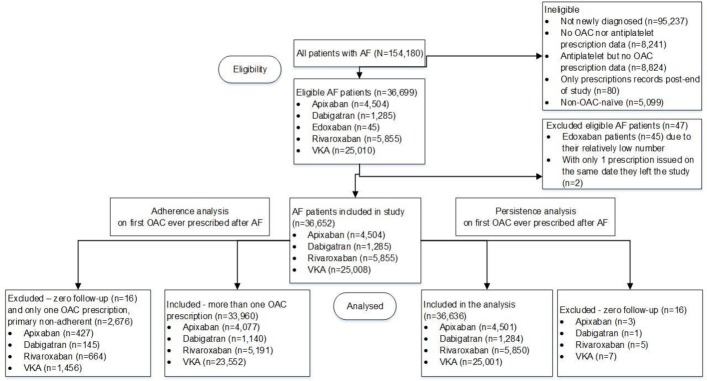
Among 4 354 740 individuals, 154 180 with AF were identified, of whom 36 652 met the inclusion criteria for analysis: VKA (n=25 008, 68.2%), dabigatran (n=1285, 3.5%), rivaroxaban (n=5855, 16.0%) and apixaban (n=4504, 12.3%) (figure 1). Major exclusions were no new AF diagnosis (n=95 237) and absence of OAC prescription data (n=17 065). Edoxaban was UK-approved in September 2015, leading to inadequate sample size for analysis (n=45) during the study period.
Figure 1.
Flow diagram of study population for adherence and persistence analyses. AF, atrial fibrillation; OAC, oral anticoagulant; VKA, vitamin K antagonist.
The study population had a mean age of 74.4 (SD 10.5) years and 45% were female. Cardiovascular comorbidities were common: hypertension (62.6%) and hypercholesterolaemia (71.8%), with a median CHA2DS2VASc of 3 (IQR 2–4). Polypharmacy was common (number of drugs: median 6, IQR 4–7). Individuals on dabigatran had lower CHA2DS2VASc scores, rates of hypertension and current smoking, with no other significant baseline differences across different OACs (table 1).
Table 1.
Baseline characteristics of study population
| Overall (N=36 652) | VKA (n=25 008) |
Dabigatran (n=1285) | Rivaroxaban (n=5855) | Apixaban (n=4504) | P value | |
| Characteristics, n (%) | ||||||
| Age, mean (SD) | 74.4 (10.5) | 74.3 (10.2) | 73.4 (11.2) | 74.8 (11.0) | 74.8 (11.0) | <0.001 |
| Female | 16 494 (45.0) | 11 186 (44.7) | 517 (40.2) | 2671 (45.6) | 2120 (47.1) | <0.001 |
| Townsend quintile, median (IQR) | 2 (1–4) | 2 (1–4) | 2 (1–3) | 3 (1–4) | 3 (1–4) | |
| 1 | 8862 (24.2) | 6124 (24.5) | 345 (26.9) | 1347 (23.0) | 1046 (23.2) | <0.001 |
| 2 | 8269 (22.6) | 5706 (22.8) | 321 (25.0) | 1300 (22.2) | 942 (20.9) | |
| 3 | 7330 (20.0) | 4964 (19.9) | 270 (21.0) | 1231 (21.0) | 865 (19.2) | |
| 4 | 5864 (16.0) | 4065 (16.3) | 185 (14.4) | 897 (15.3) | 717 (15.9) | |
| 5 | 3800 (10.4) | 2540 (10.2) | 98 (7.6) | 574 (9.8) | 588 (13.1) | |
| Missing | 2527 (6.9) | 1609 (6.4) | 66 (5.1) | 506 (8.6) | 346 (7.7) | |
| Comorbidities, n (%) | ||||||
| Heart failure | 2700 (7.4) | 1908 (7.6) | 79 (6.2) | 387 (6.6) | 326 (7.2) | 0.016 |
| Hypertension | 22 955 (62.6) | 15 824 (63.3) | 751 (58.4) | 3612 (61.7) | 2768 (61.5) | <0.001 |
| Diabetes mellitus | 6691 (18.3) | 4594 (18.4) | 210 (16.3) | 1058 (18.1) | 829 (18.4) | 0.31 |
| Stroke/transient ischaemic attack | 4622 (12.6) | 3096 (12.4) | 160 (12.5) | 735 (12.6) | 631 (14.0) | 0.026 |
| Vascular disease | 4793 (13.1) | 3316 (13.3) | 149 (11.6) | 717 (12.3) | 611 (13.6) | 0.052 |
| Alcohol misuse | 977 (2.7) | 589 (2.4) | 36 (2.8) | 175 (3.0) | 177 (3.9) | <0.001 |
| Chronic kidney disease | 7844 (21.4) | 5426 (21.7) | 221 (17.2) | 1229 (21.0) | 968 (21.5) | 0.002 |
| Liver disease | 100 (0.3) | 67 (0.3) | 4 (0.3) | 15 (0.3) | 14 (0.3) | 0.94 |
| Hypercholesterolaemia | 26 328 (71.8) | 17 979 (71.9) | 915 (71.2) | 4172 (71.3) | 3262 (72.4) | 0.56 |
| Smoking status | ||||||
| Current smoker | 3374 (9.2) | 2272 (9.1) | 103 (8.0) | 585 (10.0) | 414 (9.2) | <0.001 |
| Ex-smoker | 13 928 (38.0) | 9711 (38.8) | 484 (37.7) | 2125 (36.3) | 1608 (35.7) | |
| Never smoked | 18 484 (50.4) | 12 472 (49.9) | 666 (51.8) | 2993 (51.1) | 2353 (52.2) | |
| Not indicated | 866 (2.4) | 553 (2.2) | 32 (2.5) | 152 (2.6) | 129 (2.9) | |
| Risk scores, n (%) | ||||||
| CHA2DS2-VASc | ||||||
| 0–1 | 5856 (16.0) | 3887 (15.5) | 263 (20.5) | 984 (16.8) | 722 (16.0) | <0.001 |
| 2 | 7192 (19.6) | 4939 (19.8) | 279 (21.7) | 1106 (18.9) | 868 (19.3) | |
| 3–4 | 17 894 (48.8) | 12 324 (49.3) | 571 (44.4) | 2850 (48.7) | 2149 (47.7) | |
| 5–9 | 5710 (15.6) | 3858 (15.4) | 172 (13.4) | 915 (15.6) | 765 (17.0) | |
| HASBLED-1 | ||||||
| 0–2 | 28 279 (77.2) | 19 298 (77.2) | 1047 (81.5) | 4508 (77.0) | 3426 (76.1) | <0.001 |
| 3–8 | 8373 (22.8) | 5710 (22.8) | 238 (18.5) | 1347 (23.0) | 1078 (23.9) | |
| Drugs, n (%) | ||||||
| Aspirin | 20 510 (56.0) | 14 175 (56.7) | 683 (53.2) | 3116 (53.2) | 2536 (56.3) | <0.001 |
| Statin | 17 185 (46.9) | 11 803 (47.2) | 551 (42.9) | 2651 (45.3) | 2180 (48.4) | <0.001 |
| Blood pressure-lowering drugs | 29 136 (79.5) | 20 007 (80.0) | 961 (74.8) | 4563 (77.9) | 3605 (80.0) | <0.001 |
| Number of drugs, mean (SD) | 5.5 (2.2) | 5.5 (2.2) | 5.3 (2.2) | 5.5 (2.2) | 5.7 (2.2) | <0.001 |
CHA2DS2-VASc, Congestive heart failure; Hypertension, Age≥75 years; Diabetes mellitus; Stroke, Vascular disease; Age 65-74 years; Sex category; HASBLED-1, Hypertension; Abnormal renal/liver function; Stroke; Bleeding; Labile INR; Elderly; Drugs or alcohol; VKA, vitamin K antagonist.
Adherence
Adherence was 55.2% (54.6–55.7) overall and 51.2% (50.6–51.8), 66.5% (63.7–69.2), 63.1% (61.8–64.4) and 64.7 (63.2–66.1) for VKA, dabigatran, rivaroxaban and apixaban, respectively (online supplementary web table 1).
In univariable analysis, the likelihood of non-adherence with DOACs was lower than with VKA (OR 0.53, 0.47–0.60; 0.61, 0.58–0.65; and 0.57, 0.54–0.61 for dabigatran, rivaroxaban and apixaban, respectively) (table 2 and online supplementary web table 2d). In multivariable analysis, the likelihood of non-adherence was similar for dabigatran (0.54, 0.48–0.62), but higher for rivaroxaban (0.76, 0.71–0.82) and apixaban (0.77, 0.71–0.84). Increasing comorbidity (by CHA2DS2VASc) was associated with decreased likelihood of non-adherence (1.00, 0.93–1.08; 0.94, 0.88–1.01; and 0.81, 0.74–0.89 for CHA2DS2VASc scores 2, 3–4 and 5–9, respectively, compared with CHA2DS2VASc scores 0–1), but not for HASBLED-1 score (0.98, 0.93–1.05 for HASBLED-1 scores ≥3, compared with HASBLED-1 scores 0–2). The number of drugs and Townsend quintile were not associated with non-adherence (table 2). Age ≥75 years, diabetes, female gender and anaemia were associated with reduced risk of non-adherence, while hypertension and vascular disease were associated with increased risk (online supplementary web table 2c). Non-adherence was non-linearly associated with time since introduction of DOACs, increasing for approximately 2 years (to early 2013) before starting to decrease, returning to its original level by early 2015 (online supplementary web figure 1a) and then dropping below its original level. Baseline characteristics by adherence status are shown in online supplementary web table 4. Online supplementary web table 2e illustrates no important differences in the effect of time since introduction of DOACs between different OACs (online supplementary web figure 1b). Sensitivity analysis in only those who had at least 6 or 12 months of OAC prescriptions showed little impact on relative non-adherence for dabigatran and VKA; however, estimated ORs for rivaroxaban and apixaban both decreased to 0.65 (for 12-month restriction) (online supplementary web tables 2a and 2b).
Table 2.
Likelihood of non-adherence by oral anticoagulant
| n | Univariable OR (95% CI) |
Multivariable OR (95% CI) |
P value |
| 33 960 | 31 615 | ||
| VKA | 1.00 (–) | 1.00 (–) | |
| Dabigatran | 0.53 (0.47 to 0.60) | 0.54 (0.48 to 0.62) | <0.001 |
| Rivaroxaban | 0.61 (0.58 to 0.65) | 0.76 (0.71 to 0.82) | |
| Apixaban | 0.57 (0.54 to 0.61) | 0.77 (0.71 to 0.84) | |
| CHA2DS2VASc | |||
| 0–1 | 1.00 (–) | ||
| 2 | 1.00 (0.93 to 1.08) | <0.001 | |
| 3–4 | 0.94 (0.88 to 1.01) | ||
| 5–9 | 0.81 (0.74 to 0.89) | ||
| HASBLED-1 | |||
| 0–2 | 1.00 (–) | ||
| 3–9 | 0.98 (0.93 to 1.05) | 0.62 | |
| Number of drugs | |||
| Continuous/Linear | 0.99 (0.98 to 1.00) | 0.067 | |
| Townsend quintile | |||
| 1 | 1.00 (–) | ||
| 2 | 0.93 (0.87 to 0.99) | <0.001 | |
| 3 | 0.86 (0.80 to 0.91) | ||
| 4 | 0.91 (0.85 to 0.97) | ||
| 5 | 0.86 (0.80 to 0.94) | ||
| Date of first prescription* (years after 1 January 2011) | |||
| Continuous/Linear | 1.29 (1.22 to 1.37) | <0.001 | |
| Continuous/Quadratic | 0.94 (0.93 to 0.95) | ||
| BIC | 46 263.63 | 42 880.41 |
*Time difference (in years) between the date of the first ever OAC prescription for each patient and the start date of the study (1 January 2011). This suggests that the maximum effect of calendar time occurs at −ln(1.29)(2×/ln(0.94))=2.13 years.
BIC, Bayes information criterion; CHA2DS2-VASc, Congestive heart failure, Hypertension, Age≥75 years, Diabetes mellitus, Stroke, Vascular disease, Age 65-74 years, Sex category; HASBLED-1, Hypertension; Abnormal renal/liver function; Stroke; Bleeding; Labile INR; Elderly; Drugs or alcohol; OAC, oral anticoagulant; VKA, vitamin K antagonist.
Persistence
One-year persistence was 65.9% (65.4–66.5) overall and 63.4% (62.8–64.0), 61.4% (58.3–64.2), 72.3% (70.9–73.7) and 78.7% (77.1–80.1) for VKA, dabigatran, rivaroxaban and apixaban, respectively. Persistence reduced over 3 years for all OACs and was highest for apixaban and lowest for VKA and dabigatran (figure 2).
Figure 2.
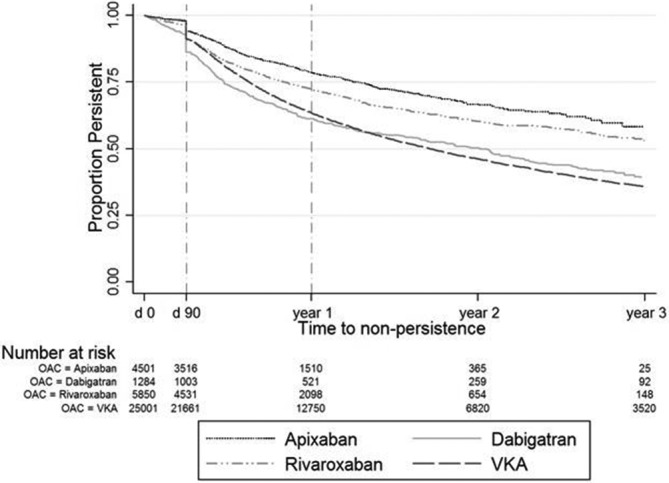
Kaplan-Meier analysis of persistence over time. OAC, oral anticoagulant; VKA, vitamin K antagonist.
In univariable analysis, apixaban had the lowest (HR 0.53, 0.50–0.57) and dabigatran had the highest (HR 1.02, 0.93–1.11) risk of non-persistence at 1 year, relative to VKA. Table 3 illustrates the optimal multivariable model, where the effects of CHA2DS2VASc and OAC required an interaction term with time since first OAC prescription. The interaction between time and date of first prescription shows that immediately following first prescription, apixaban still had the lowest risk (0.53, 0.46–0.60) and dabigatran the highest (1.24, 1.08–1.42), relative to VKA. The risk of non-persistence did not change over time for apixaban (0.91, 0.78–1.06 per year), but reduced over time for dabigatran (0.75, 0.65–0.86 per year) and rivaroxaban (0.69, 0.62–0.77 per year). Immediately after the first prescription, increasing comorbidity, when measured by CHA2DS2VASc score, was associated with reduced risk of non-persistence (0.71, 0.66–0.76; 0.66, 0.62–0.71; and 0.69, 0.63–0.76 for CHA2DS2VASc scores 2, 3–4 and 5–9, respectively, compared with CHA2DS2VASc scores 0–1). However, this risk was lessened over time (1.05, 0.99–1.12 and 1.13, 1.06–1.20 per year for CHA2DS2VASc scores 2 and 5–9, respectively). There was no significant effect of HASBLED-1 (1.04, 0.99–1.08 for HASBLED-1 scores ≥3, compared with HASBLED-1 scores 0–2), nor for the number of drugs or Townsend quintile (table 3). Overall, risk of non-persistence increased from 2011 until 2016 (1.03, 1.01–1.05 per year). Heart failure, vascular disease, chronic kidney disease, prior bleeding and alcohol misuse were associated with increased risk of non-persistence, while hypertension and age >65 years were associated with reduced risk. Non-persistence was more likely for dabigatran soon after initiation, but the effect relative to VKA and to apixaban declined over the period of an individual’s prescription, although this did not happen relative to rivaroxaban (Online supplementary web tables 5a and 5b). Baseline characteristics are presented by 1-year persistence status in online supplementary web table 6.
Table 3.
Risk of non-persistence by oral anticoagulant
| n | Univariable HR (95% CI) |
Multivariable HR (95% CI) |
| 36 636 | 34 109 | |
| OAC (effect on first prescribing) | ||
| VKA | 1.00 (–) | 1.00 (–) |
| Dabigatran | 1.02 (0.93 to 1.11) | 1.24 (1.08 to 1.42) |
| Rivaroxaban | 0.71 (0.67 to 0.74) | 0.85 (0.77 to 0.93) |
| Apixaban | 0.53 (0.50 to 0.57) | 0.53 (0.46 to 0.60) |
| Time-dependent effect of OAC (per year of prescriptions) | ||
| VKA | 1.00 (–) | |
| Dabigatran | 0.75 (0.65 to 0.86) | |
| Rivaroxaban | 0.69 (0.62 to 0.77) | |
| Apixaban | 0.91 (0.78 to 1.06) | |
| CHA2DS2VASc (effect on first prescribing) | ||
| 0–1 | 1.00 (–) | |
| 2 | 0.71 (0.66 to 0.76) | |
| 3–4 | 0.66 (0.62 to 0.71) | |
| 5–9 | 0.69 (0.63 to 0.76) | |
| Time-dependent effect of CHA2DS2VASc (per year of prescriptions) | ||
| 0–1 | 1.00 (–) | |
| 2 | 1.05 (0.99 to 1.12) | |
| 3–4 | 1.12 (1.07 to 1.18) | |
| 5–9 | 1.13 (1.06 to 1.20) | |
| HASBLED-1 | ||
| 0–2 | 1.00 (–) | |
| 3–9 | 1.04 (0.99 to 1.08) | |
| Number of drugs | 1.00 (0.99 to 1.01) | |
| Townsend quintile | ||
| 1 | 1.00 (–) | |
| 2 | 0.99 (0.95 to 1.03) | |
| 3 | 0.95 (0.91 to 1.00) | |
| 4 | 0.97 (0.93 to 1.02) | |
| 5 | 0.97 (0.92 to 1.03) | |
| Date of first prescription* (years after 1 January 2011) | 1.03 (1.02 to 1.05) | |
*Time difference (in years) between the date of the first ever OAC prescription for each patient and the start date of the study (1 January 2011).
CHA2DS2-VASc, Congestive heart failure, Hypertension, Age≥75 years, Diabetes mellitus, Stroke, Vascular disease, Age 65-74 years, Sex category; HASBLED-1, Hypertension; Abnormal renal/liver function; Stroke; Bleeding; Labile INR; Elderly; Drugs or alcohol; OAC, oral anticoagulant; VKA, vitamin K antagonist.
Persistence and adherence
Of 36 652 individuals, 31.0% had <1 year of data, and of these 15.8% had primary non-adherence, 27.9% were non-adherent and 56.2% were adherent. Among 25 263 individuals with ≥1 year of data, primary non-adherence (3.5%) was less common than non-adherent, non-persistent (21.2%), adherent, non-persistent (8.6%), non-adherent, persistent (26.5%), and persistent, adherent (40.2%). Differences between OACs were significant (p<0.001). Primary non-adherence was highest with dabigatran (7.8%) and lowest (2.7%) with apixaban, while persistent adherence was highest with apixaban (50.7%) and lowest with VKA (38.2%). Non-adherent, non-persistence was greatest with VKA (23.4%) and least with apixaban (12.4%). Non-adherent persistence was highest with apixaban (29.0%) and lowest with dabigatran (19.5%) (table 4).
Table 4.
Adherence and persistence by oral anticoagulant
| Overall | VKA | Dabigatran | Rivaroxaban | Apixaban | |
| Total, n (%) | 36 652 | 25 008 | 1285 | 5855 | 4504 |
| Zero follow-up | 16 (0.04) | 7 (0.03) | 1 (0.08) | 5 (0.09) | 3 (0.07) |
| Total <1 year of data | 11 373 (31.0) | 5307 (21.2) | 441 (34.3) | 3014 (51.5) | 2611 (58.0) |
| Primary non-adherence | 1800 (15.8) | 818 (15.4) | 78 (17) | 531 (17.6) | 373 (14.3) |
| Non-adherent | 3178 (27.9) | 1654 (31.2) | 94 (21.3) | 771 (25.6) | 659 (25.2) |
| Adherent | 6395 (56.2) | 2835 (53.4) | 269 (61.0) | 1712 (56.8) | 1579 (60.5) |
| Total ≥1 year of data | 25 263 (68.9) | 19 694 (78.8) | 843 (65.6) | 2836 (48.4) | 1890 (42.0) |
| Primary non-adherence | 876 (3.5) | 631 (3.2) | 66 (7.8) | 128 (4.5) | 51 (2.7) |
| Non-adherent, non-persistent | 5352 (21.2) | 4616 (23.4) | 124 (14.7) | 378 (13.3) | 234 (12.4) |
| Adherent, non-persistent | 2173 (8.6) | 1711 (8.7) | 132 (15.7) | 232 (8.2) | 98 (5.2) |
| Non-adherent, persistent | 6699 (26.5) | 5221 (26.5) | 164 (19.5) | 766 (27.0) | 548 (29.0) |
| Persistent, adherent | 10 163 (40.2) | 7515 (38.2) | 357 (42.3) | 1332 (47.0) | 959 (50.7) |
VKA, vitamin K antagonist.
Switching
In non-persistent individuals, switching rates were 20.3%, 18.8%, 40.3%, 27.0% and 18.5% for all OACs, VKA, dabigatran, rivaroxaban and apixaban, respectively. In primary non-adherent individuals, the corresponding rates were 45.2%, 40.9%, 69.1%, 51.2% and 44.7%, respectively (online supplementary web table 7). When ‘switching’ was censored rather than ‘non-persistent’, differences between OACs were more consistent over time (since first prescription) and non-persistence was generally lower for dabigatran than VKA, with an increasing trend in the difference with time on OAC. The effect was less for other DOACs relative to VKA (online supplementary web figure 1 and web table 8).
Discussion
In this study of long-term persistence and adherence across all OACs in AF, we have four findings. First, primary non-adherence is uncommon (3.5%), and over time on a DOAC the likelihood of non-adherence and non-persistence increases. Second, the proportion of individuals on OACs who are both adherent and persistent at 1 year is low (40.2%), with heterogeneity across different OACs. Third, population-level time trends in adherence and persistence exist after new drugs (DOACs in this case) are introduced. Fourth, increased comorbidities were associated with reduced risk of non-adherence and non-persistence for DOACs, but the number of drugs was not.
Observed rates of primary non-adherence for OAC are comparable with a recent Spanish study using large-scale regional EHR.12 Primary non-adherence varies across different drugs, but our estimates appear lower than other chronic disease medications (≤20.8% for lipid-lowering drugs23). Rates are generally lower in European populations23 than in North America, probably due to greater provision of prescription medication in public-funded health systems. Our results suggest that improved adherence and persistence requires longer-term monitoring, rather than current strategies emphasising drug adherence postinitiation. Greater switching with dabigatran than other OACs may reflect greater discontinuation (due to dyspepsia or other side effects3) or prescription patterns favouring other DOACs.24
For drugs to be effective, both adherence and persistence are prerequisites. It is therefore concerning that adherent persistence at 1 year after initial prescription ranged from only 38.2% to 50.7% for VKA and apixaban, respectively. Non-adherent, non-persistent individuals constituted 23.4% and 12.4% for users of VKA and apixaban, respectively (table 4). Adherence and persistence should be considered in combination, yet this is rare in both research and clinical practice.25 Furthermore, observations that non-adherent, persistence is more common than adherent, non-persistence and that these proportions vary by type of OAC highlight the need for measurement of both metrics and potential for personalising approaches to improved drug utilisation. Factors which influence choice of drug in the same class include pharmacodynamics, pharmacokinetic, tolerability and cost,26 to which adherence and persistence may be added.
DOACs are unusual for several new drugs in the same class entering the market in a short timeframe. Other examples are statins, antihypertensives and novel hypoglycaemic agents, but four new drugs in a 5-year period is extraordinary. DOACs have proven efficacy and effectiveness over VKA, and appropriate prescribing of OACs in AF has improved in the UK between 2000 and 2016.27 However, there have been variations in prescription across DOACs over time.28 Our analyses add that when DOACs were first prescribed, persistence to all DOACs appears to have been initially higher than to VKA and in some cases increase further over time on the OAC, with clear differences between different OACs. Findings were sensitive to how switching of drugs was considered, with persistence to dabigatran, which had the highest rates of ‘switching’, appearing much lower than VKA when ‘switchers’ were classed as ‘non-persistent’ versus censored (‘no longer observed for that OAC’). Other possible reasons for differences between OACs include side effect profile, marketing strategies, and varying procurement and prescription practices. Adherence and persistence can have far-reaching implications on drug cost, effectiveness and policy at the population level29 and should be monitored at the population level.
Our findings are consistent with previous studies which have shown associations between polypharmacy12 30 and increased comorbidities25 and reduced risk of non-adherence or non-persistence for OACs and other cardiovascular medications. Understanding each of the multiple steps in the prescription-persistence cascade may aid design and implementation of better interventions to improve drug utilisation. In routine clinical practice, EHR-based methods may be used to highlight individuals at greater risk of non-adherence or non-persistence, for example, by suggesting that persistence should be more of a focus for improvement than adherence, or for monitoring long-term adherence/persistence.
The major strength of our analysis is consideration of the relationship between adherence and persistence together across all DOACs and VKA in the same population. As well as sociodemographic, health and medication characteristics, the influence of time was also analysed. Even in large-scale data sets with prescription data, there are several limitations. First, we did not have dispensing data, and therefore used previously validated methods to estimate adherence/persistence from prescription data. Our methods may be more uncertain for VKA than for DOACs (eg, differential impact of varying prescription gaps on persistence), possibly due to patients on warfarin often having longer duration prescriptions than DOACs. Second, missing prescription data meant that not all eligible individuals could be included due to incompleteness of follow-up. On the other hand, these are real-world data, which are routinely available and nationally representative. Third, relatively small numbers of patients could be included for DOACs, but numbers were comparable with other studies. Fourth, TTR would be a better measure of adherence for VKA but could not be estimated in our analysis due to lack of INR data (which also limited our HASBLED analysis). Finally, we focused on initial OAC prescription in OAC-naive patients to minimise bias in adherence/persistence based on previous OAC use, but did not focus on second and subsequent OACs used, where there would be more bias and greater consideration of overall treatment pattern which requires consideration of multidrug, multidisease adherence/persistence over time.
Conclusions
Our study shows changes in adherence and persistence for DOACs over time in AF. Since these are usually lifelong therapies, more emphasis should be placed on long-term adherence and persistence in clinical practice and research. Standardisation is required for EHR methods of adherence and persistence estimation across drugs, diseases and data sets. Persistence and adherence may have different determinants and should be studied together in EHR. Better understanding of these factors will lead to interventions which are more likely to improve adherence and persistence at individual and population levels across OACs and other drugs. Postmarketing surveillance should take into account adherence and persistence particularly for multiple drugs in the same class where head-to-head trials are unlikely.
Key messages.
What is already known on this subject?
Despite proven efficacy for stroke prevention in atrial fibrillation, adherence and persistence are suboptimal for oral anticoagulants (OACs).
Adherence and persistence are rarely studied together in the same population across all anticoagulants, taking into account all baseline factors in electronic health records.
What might this study add?
This is the first study evaluating the time trends, predictive factors and associations between adherence and persistence of anticoagulants in atrial fibrillation in a population-based study in electronic health records.
Persistence and adherence to OACs are relatively low at 1 year and there is heterogeneity across different OACs.
There are significant variations over time that a patient is on a direct oral anticoagulant (DOAC), and population-level time trends in adherence and persistence after new drugs (DOACs in this case) are introduced.
How might this impact on clinical practice?
Interventions should focus on improving adherence and persistence together and across drugs.
These data may help to better understand the determinants of adherence and persistence, and to design and target interventions.
Footnotes
Correction notice: Since this article was first published online, open access has been selected.
Contributors: The study was conceived by AB. VB, PG, JB, AB and CJS wrote the statistical analysis plan, carried out the analysis, collected the data and produced the initial draft of the manuscript. AB was guarantor. All authors contributed to the revision of the manuscript and have accepted the final version.
Funding: The research leading to these results has received funding from the European Research Council under the European Union’s Seventh Framework Programme (FP/2007-2013)/ERC Grant Agreement no. 339239. WDS was supported by the NIHR Exeter Clinical Research Facility and the NIHR Collaboration for Leadership in Applied Health Research and Care (CLAHRC) for the South West Peninsula. CW and VB were supported by the NIHR CLAHRC North West Coast. TM is supported by the NIHR CLAHRC West Midlands.
Competing interests: AB reports personal fees from AstraZeneca, Boehringer Ingelheim, Pfizer and Novo Nordisk. SA reports personal fees from Bayer, Boehringer Ingelheim, Pfizer/BMS and Daiichi Sankyo. RJS reports grants and personal fees from Boehringer Ingelheim; grants and personal fees from Daiichi Sankyo; grants, personal fees and non-financial support from Medtronic; grants, personal fees and non-financial support from Boston Scientific; grants, personal fees and non-financial support from Abbott Medical; and grants, personal fees and non-financial support from Biosense Webster. WDS reports grants and personal fees from Boehringer Ingelheim; grants and personal fees from Daiichi Sankyo; grants, personal fees and non-financial support from Medtronic; grants, personal fees and non-financial support from Boston Scientific; grants, personal fees and non-financial support from Abbott Medical; and grants, personal fees and non-financial support from Biosense Webster. The remaining authors have no competing interests.
Patient consent for publication: Not required.
Ethics approval: Research carried out using The Health Improvement Network data was approved by the NHS South-East Multicentre Research Ethics Committee (MREC) in 2003, subject to independent scientific approval. Approval for this analysis was obtained in 2015 (SRC reference number 15THIN002).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: THIN data are available upon application after Scientific Review Committee (SRC) approval through a licenced organisation. Data are not publicly available.
References
- 1. Chugh SS, Roth GA, Gillum RF, et al. . Global burden of atrial fibrillation in developed and developing nations. Glob Heart 2014;9:113–9. 10.1016/j.gheart.2014.01.004 [DOI] [PubMed] [Google Scholar]
- 2. Gage BF, Cardinalli AB, Albers GW, et al. . Cost-Effectiveness of warfarin and aspirin for prophylaxis of stroke in patients with nonvalvular atrial fibrillation. JAMA 1995;274:1839–45. 10.1001/jama.1995.03530230025025 [DOI] [PubMed] [Google Scholar]
- 3. Connolly SJ, Ezekowitz MD, Yusuf S, et al. . Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139–51. 10.1056/NEJMoa0905561 [DOI] [PubMed] [Google Scholar]
- 4. Granger CB, Alexander JH, McMurray JJV, et al. . Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981–92. 10.1056/NEJMoa1107039 [DOI] [PubMed] [Google Scholar]
- 5. Patel MR, Mahaffey KW, Garg J, et al. . Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883–91. 10.1056/NEJMoa1009638 [DOI] [PubMed] [Google Scholar]
- 6. Giugliano RP, Ruff CT, Braunwald E, et al. . Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093–104. 10.1056/NEJMoa1310907 [DOI] [PubMed] [Google Scholar]
- 7. Kirchhof P, Curtis AB, Skanes AC, et al. . Atrial fibrillation guidelines across the Atlantic: a comparison of the current recommendations of the European Society of Cardiology/European heart rhythm Association/European association of cardiothoracic surgeons, the American College of cardiology Foundation/American heart Association/Heart rhythm Society, and the Canadian cardiovascular Society. Eur Heart J 2013;34:1471–4. 10.1093/eurheartj/ehs446 [DOI] [PubMed] [Google Scholar]
- 8. January CT, Wann LS, Alpert JS, et al. . 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American heart association Task force on practice guidelines and the heart rhythm Society. J Am Coll Cardiol 2014;64:e1–76. 10.1016/j.jacc.2014.03.022 [DOI] [PubMed] [Google Scholar]
- 9. Lewis WR, Piccini JP, Turakhia MP, et al. . Get with the guidelines AFIB: novel quality improvement Registry for hospitalized patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes 2014;7:770–7. 10.1161/CIRCOUTCOMES.114.001263 [DOI] [PubMed] [Google Scholar]
- 10. Raebel MA, Schmittdiel J, Karter AJ, et al. . Standardizing terminology and definitions of medication adherence and persistence in research employing electronic databases. Med Care 2013;51(8 Suppl 3):S11–21. 10.1097/MLR.0b013e31829b1d2a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Clemens A, Noack H, Brueckmann M, et al. . Twice- or once-daily dosing of novel oral anticoagulants for stroke prevention: a fixed-effects meta-analysis with predefined heterogeneity quality criteria. PLoS One 2014;9:e99276 10.1371/journal.pone.0099276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rodriguez-Bernal CL, Peiró S, Hurtado I, et al. . Primary nonadherence to oral anticoagulants in patients with atrial fibrillation: real-world data from a population-based cohort. J Manag Care Spec Pharm 2018;24:440–8. 10.18553/jmcp.2018.24.5.440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mueller T, Alvarez-Madrazo S, Robertson C, et al. . Use of direct oral anticoagulants in patients with atrial fibrillation in Scotland: applying a coherent framework to drug utilisation studies. Pharmacoepidemiol Drug Saf 2017;26:1378–86. 10.1002/pds.4272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Paquette M, Riou França L, Teutsch C, et al. . Persistence With Dabigatran Therapy at 2 Years in Patients With Atrial Fibrillation. J Am Coll Cardiol 2017;70:1573–83. 10.1016/j.jacc.2017.07.793 [DOI] [PubMed] [Google Scholar]
- 15. Vrijens B, De Geest S, Hughes DA, et al. . A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol 2012;73:691–705. 10.1111/j.1365-2125.2012.04167.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gallo V, Egger M, McCormack V, et al. . STrengthening the Reporting of OBservational studies in Epidemiology--Molecular Epidemiology (STROBE-ME): an extension of the STROBE Statement. PLoS Med 2011;8:e1001117 10.1371/journal.pmed.1001117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Blak BT, Thompson M, Dattani H, et al. . Generalisability of the health improvement network (thin) database: demographics, chronic disease prevalence and mortality rates. Inform Prim Care 2011;19:251–5. 10.14236/jhi.v19i4.820 [DOI] [PubMed] [Google Scholar]
- 18. Lip GYH, Nieuwlaat R, Pisters R, et al. . Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro heart survey on atrial fibrillation. Chest 2010;137:263–72. 10.1378/chest.09-1584 [DOI] [PubMed] [Google Scholar]
- 19. Pisters R, Lane DA, Nieuwlaat R, et al. . A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro heart survey. Chest 2010;138:1093–100. 10.1378/chest.10-0134 [DOI] [PubMed] [Google Scholar]
- 20. Forbes CA, Deshpande S, Sorio-Vilela F, et al. . A systematic literature review comparing methods for the measurement of patient persistence and adherence. Curr Med Res Opin 2018;34:1613–25. 10.1080/03007995.2018.1477747 [DOI] [PubMed] [Google Scholar]
- 21. Karve S, Cleves MA, Helm M, et al. . Good and poor adherence: optimal cut-point for adherence measures using administrative claims data. Curr Med Res Opin 2009;25:2303–10. 10.1185/03007990903126833 [DOI] [PubMed] [Google Scholar]
- 22. Cataldo N, Pegoraro V, Ripellino C, et al. . Non-persistence risk and health care resource utilization of Italian patients with non-valvular atrial fibrillation. Recenti Prog Med 2018;109:113–21. 10.1701/2865.28904 [DOI] [PubMed] [Google Scholar]
- 23. Lemstra M, Nwankwo C, Bird Y, et al. . Primary nonadherence to chronic disease medications: a meta-analysis. Patient Prefer Adherence 2018;12:721–31. 10.2147/PPA.S161151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Loo SY, Dell'Aniello S, Huiart L, et al. . Trends in the prescription of novel oral anticoagulants in UK primary care. Br J Clin Pharmacol 2017;83:2096–106. 10.1111/bcp.13299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Manzoor BS, Lee TA, Sharp LK, et al. . Real-World adherence and persistence with direct oral anticoagulants in adults with atrial fibrillation. Pharmacotherapy 2017;37:1221–30. 10.1002/phar.1989 [DOI] [PubMed] [Google Scholar]
- 26. Brown MJ. A rational basis for selection among drugs of the same class. Heart 2003;89:687–94. 10.1136/heart.89.6.687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Adderley NJ, Ryan R, Nirantharakumar K, et al. . Prevalence and treatment of atrial fibrillation in UK general practice from 2000 to 2016. Heart 2019;105:27–33. 10.1136/heartjnl-2018-312977 [DOI] [PubMed] [Google Scholar]
- 28. Zhu J, Alexander GC, Nazarian S, et al. . Trends and variation in oral anticoagulant choice in patients with atrial fibrillation, 2010-2017. Pharmacotherapy 2018;38:907–20. 10.1002/phar.2158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cutler RL, Fernandez-Llimos F, Frommer M, et al. . Economic impact of medication non-adherence by disease groups: a systematic review. BMJ Open 2018;8:e016982 10.1136/bmjopen-2017-016982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ofori-Asenso R, Ilomäki J, Tacey M, et al. . Predictors of first-year nonadherence and discontinuation of statins among older adults: a retrospective cohort study. Br J Clin Pharmacol 2019;85:227–35. 10.1111/bcp.13797 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
heartjnl-2019-315307supp001.pdf (227.4KB, pdf)