Abstract
Background
Apelin receptor (APJ) is a G protein-coupled receptor (GPCR) activated by the endogenous peptide apelin. The apelin–APJ system has emerged as an important regulator of cardiovascular homeostasis. Recently, a potent benzimidazole-derived apelin peptidomimetic, CMF-019, was patented but without a comprehensive description of its synthesis and a complete spectroscopic characterization of the intermediates.
Objective
Here, a detailed preparation of CMF-019 through a modified and improved synthetic pathway is described.
Method
In particular, the benzimidazole ring in 7 was tailored by the condensation of methyl 3-amino-4-(pentan-3-ylamino)benzoate (4) with (thiophene-2-yl)acetimidate salt 6. Saponification of 7 and the subsequent condensation of the free acid 8 with the corresponding enantiopure β-amino acid methyl ester generated methyl (S)-5-methyl-3-{1-(pentan-3-yl)-2-(thiophen-2-ylmethyl)-1H-benzo[d]imidazole-5-carboxamido}hexanoate (9). Hydrolysis of the latter with KOH in THF/water, followed by HPLC-purification, afforded the desired product, CMF-019 (potassium salt) 10.
Results & Conclusion
The approach reported herein enables preparation of 10 at a total yield of 12% over seven linear steps. Additionally, it does not require applying expensive designated microwave reactors and high-pressure hydrogenators. Thus, the elaborate synthesis provides a latent availability of potent agonist 10 for further exploring the physiologically essential apelin-APJ system.
Keywords: CMF-019, peptidomimetics, apelin, apelin receptor, expedient synthesis, benzimidazole scaffold
1. INTRODUCTION
APJ was identified in 1993; [1] it displays the classic structure associated with GPCRs [1]. Apelin, the endogenous peptidic agonist of APJ, was discovered in 1998 by K. Tatemoto et al. [2]. The expression pattern of apelin correlates with the localization of the receptor in many human tissues [3]. On the other hand, apelin is also expressed in cells such as adipocytes, which lack the APJ receptor, and it has been detected in plasma at “hormone”-like levels [3]. These data support the hypothesis that apelin might act as a humoral regulator of numerous body functions including cardiovascular homeostasis [4]. A number of mechanisms beneficial to the cardiovascular system, which might be modulated by APJ, have been proposed: positive inotropy, angiogenesis, reduction of the mean arterial blood pressure, vasodilation by releasing nitric oxide, and blocking of cardiomyocyte apoptosis [4–6]. The potential therapeutic impact from using APJ as a target for the treatment of cardiovascular diseases might be very high. However, along with its scant availability from natural sources, apelin peptides are pharmacokinetically labile and are rapidly metabolized in vivo (half-life ca. 5 min) [7]. Thus, designing metabolically stable small-molecule agonists and antagonists for modulating the ARJ system is urgently needed.
Development of apelin like non-peptidic receptor modulators (peptidomimetics) is one of such options. In general, medicinal chemists use this approach (peptidomimetics development) very often [8]. In contrast to peptides, peptidomimetics can induce the desired biological effects without the disadvantages associated with peptides [9]. Recently, Sanofi-Aventis patented a set of small peptidomimetic agonists of APJ, including CMF-019 (compound number 107 in the original patent) as one of the most potent agonists [10]. CMF-019 exhibited impressive biological effects on the cardiovascular system via activation of APJ [11]. The availability of active peptidomimetic APJ-agonists such as CMF-019 could be helpful for elucidating vital physiological processes associated with an apelinergic signalling system. Currently, employment of APJ agonists as positive modulators of the cardiovascular system is under intense study and it has begun to attract the increasing attention of the scientific community. However, the earlier reported syntheses of CMF-019 and related compounds lack a detailed description of the experimental procedures and a spectroscopic characterization of the intermediates [10, 11]. Here, we unveil all the relevant details of the experimentally simplified and improved synthesis of CMF-019 (potassium salt), which largely follows the route briefly described by Read et al. [11]. We believe that the availability of reliable and reasonably yielding CMF-019 synthesis can be helpful towards clarifying the role of the apelin-APJ system in cardiovascular homeostasis and other vital physiological processes.
2. RESULTS AND DISCUSSION
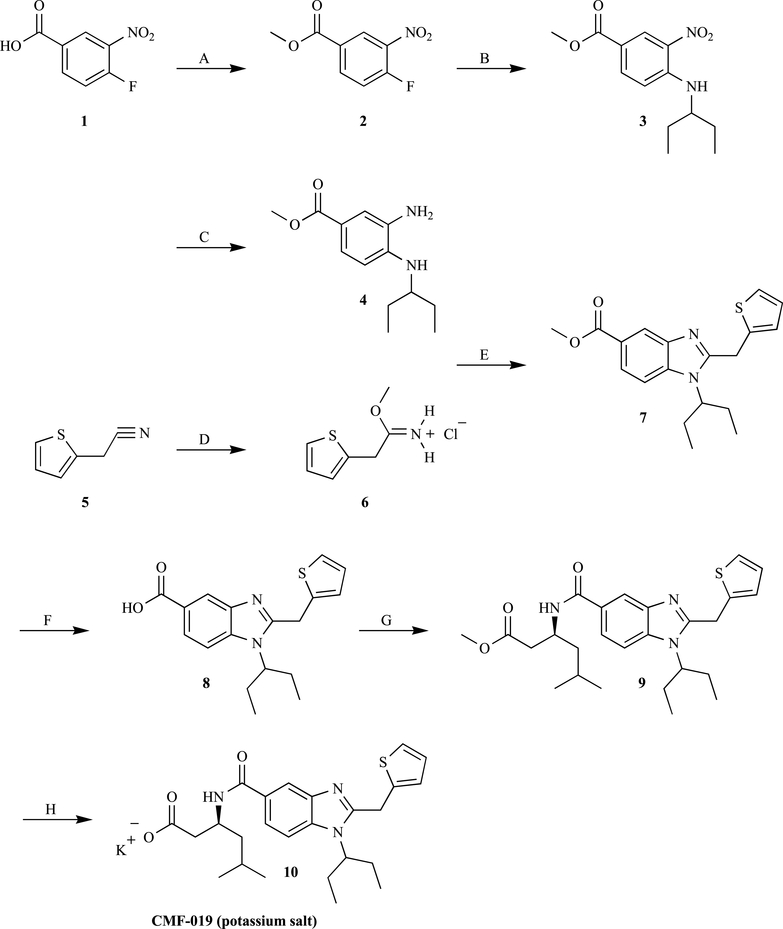
The expedient synthesis of CMF-019 is shown in Scheme 1. In the first step, esterification of commercially available acid 1 with methanol and a catalytic amount of concentrated H2SO4, following a slightly modified reported procedure, [12] provided crude NMR-pure ester 2 at 91% yield. Treatment of crude 2 with 3-aminopentane in the presence of K2CO3 in dry THF resulted in the efficient nucleophilic aromatic substitution of fluorine, affording aromatic secondary amine 3 (92%), which was found to be NMR-pure in a crude state. In our hands, the original procedure using DMF as a solvent [10] afforded a more complex reaction mixture that necessitated the tedious isolation of 3 [<80% yield after sequential flash chromatography (FC)]. According to the original procedure, the next high-yielding reduction of the nitro group in 3 to afford N-monosubstituted ortho-phenylenediamine 4 required 4 h of catalytic hydrogenation at 15 bar hydrogen pressure [10]. Since we are limited in high-pressure hydrogenation equipment, we successfully converted the crude nitroaniline 3 into phenylenediamine 4 (85% after FC purification) after 16 h of hydrogenation over 10% Pd/C catalyst (1.4 mol-% Pd loading) in ethanol under 5 bar hydrogen pressure in a regular Parr machine. Under these experimental conditions, a shortening of hydrogenation time to 4 h repeatedly led to the incomplete reduction and inferior yields of the intermediate 4 (60–64%). Nevertheless, a 4-time elongation of the hydrogenation time successfully compensated for a 3-time lowering of the hydrogen pressure.
Scheme 1.
Synthesis of CMF-019 (potassium salt) 10: a) MeOH, cone. H2SO4 (cat.), reflux, 3 h, 91%; b) 3-aminopentane, K2CO3, dry THF, room temperature, 4 h, 92%; c) 10% Pd/C, H2 (5 bar), EtOH, overnight, 85%; d) AcCl, dry MeOH, dry Et2O, 0 °C, 1 h, then at 4 °C, 3 days, 87%; e) 4 and 6, dry MeOH, reflux, 8 h, 57%; f) aq. 1M NaOH, MeOH, THF, room temperature, overnight, 87%; g) methyl (S)-5-methyl 3-aminohexanoate, HOBt, EDC, DIEA, dry DMF, room temperature, 3 days, 58%; h) aq. KOH, THF, at 0 °C, then room temperature, 21 h, 57%.
In the original approach, preparation of benzimidazole 7 relied on the acylation of 4 with 2-thiopheneacetyl chloride, followed by cyclization of the resulting amide in acetic acid at 140 °C under microwave irradiation [10, 11]. However, all attempts to accomplish such cyclization using a domestic microwave oven that was available in our lab were largely unsuccessful (<10% yield of 7 in the cyclization step). Perhaps the reported procedure [10, 11] could be reproduced by using scientific microwave reactors only [13]. Since our lab, like many other synthetic and medicinal chemistry laboratories, especially in developing countries, still lacks such an attractive, but rather expensive dedicated equipment, the microwave reactor-dependent step was replaced by a regular organic reaction. The direct cyclization of N-substituted ortho-phenylenediamine 4 with methyl 2-thiophenacetimidate hydrochloride 6 [14] was adopted. Indeed, iminoester salts are widely applied as one-carbon electrophiles and are generally more active than are carboxylic acids, in cyclizations with N-unsubstituted ortho-phenylenediamines affording N-unsubstituted benzimidazoles [15]. However, the set of non-patented examples describing the condensation of imidate salts with N-substituted ortho-phenylenediamines to afford the corresponding N-substituted benzimidazoles is minimal, varying considerably in both synthetic viabilities and in the reaction conditions [16]. In the current study, imidate hydrochloride 6 was synthesized at 87% yield, according to a further development of a “modified Pinner method” [17] from commercially available 2-thiophene acetonitrile (5), methanol, and acetyl chloride in dry ether at 0 °C. The hydrolytically labile crystalline imidate salt 6 was precipitated directly from the reaction mixture, which significantly simplified its isolation. Subsequent reflux of N-alkyl-substituted ortho-phenylenediamine 4 and imidate hydrochloride 6 in dry methanol for 8 h led to the condensation of the reactants, affording the desired 5-carbmethoxybenzimidazole 7 (57%). Although the overall yields of benzimidazole 7 from 4 in the previously reported [10, 11] and in our approach are comparable, the reported herein “microwave-free” method could be more practical for many scientific teams dealing with synthesis.
From a mechanistic viewpoint, condensation of imidate salt 6 with ortho-phenylenediamine 4, producing benzimidazole 7, consists of two single steps: (i) slow intermolecular aminolysis of 6 by 4 and replacing the alkoxy-group by a more nucleophilic ArNH2-group and the formation of an unstable N-(2-alkylaminophenyl)amidinium-type intermediate; [18] and (ii) the subsequent rapid intramolecular aminolysis of the positively charged amidinium system by the neighbouring alkylarylamino-group, resulting in benzimidazole 7 ring closure [19].
Basic hydrolysis of methyl ester 7, followed by acidification, afforded the free acid 8 in good yield [10]. The chiral β-amino ester residue was attached to the free carboxylic group of 8 by HOBt, and EDC induced condensation with commercially available enantiopure methyl (S)-3-amino-5-methyl hexanoate [20] in dry DMF. A pure direct precursor, 9, was isolated at 58% yield after FC on a softly basic Florisil absorbent. In contrast, FC on a slightly acidic silica gel led to considerable decomposition of product 9. This observation supports the conclusion that low yields of β-amidoacid CMF-019 (ca. 20%), obtained by the previously described condensation of 8 with (S)-3-amino-5-methylhexanoic acid, [10, 11] could have resulted from the inherent lability of the acidic form of CMF-019 rather than from insufficiency of the condensation process itself.
In the final step of the synthesis, deprotection of methyl ester 9 by directly converting to the potassium salt of CMF-019 (10) was accomplished. Formation of salt 10 probably improves the stability of CMF-019. Furthermore, the salt from 10 is critical for the compound’s solubility in water and for its administration either to cells growing in medium or to animals [11]. Initially, we attempted to convert ester 9 to the potassium salt of CMF-019 (10) using purified potassium trimethylsilanolate, which is widely recognized as a superior reagent for conversion of methyl esters to potassium salts of the corresponding carboxylic acids [21]. However, our continuous experiments with Me3SiOK in dry THF or ether gave insufficient yields of 10 (20–30%). To our delight, ester 9 simply treated with KOH in THF/water, followed by reverse-phase HPLC-purification, afforded the desired product, CMF-019 (potassium salt) (10) (57% yield), as a hydroscopic colorless solid. In summary, the procedures reported here expedite the synthesis of CMF-019 (potassium salt) (10) to a total of 12% yield following seven linear steps.
3. MATERIALS AND METHODS
1H and 13C NMR spectra were recorded at ambient temperatures at 400 and 100 MHz, respectively, using a Bruker Avance-400 NMR spectrometer, or at 700 and 175 MHz, respectively, using Bruker Avance-700 NMR spectrometer. The chemical shifts are reported in ppm units (δ) relative to TMS at 0 ppm. The residual hydrogen signals of the deuterated solvents (δH = 7.26 ppm in CDCl3, 7.16 ppm in C6D6, and 2.50 ppm in DMSO-d6) were used as internal references in the 1H NMR measurements [22]. In the 13C NMR measurements, the chemical shifts were referenced to the signals of CDCl3 (δC = 77.1 ppm), C6D6 (128.0 ppm) and DMSO-d6 (39.5 ppm) [22]. Coupling constants, J, are reported in Hertz. All the given assignments are based on a combination of 1D (1H and 13C/DEPT) and 2D (COSY, HMQC, and HMBC) NMR spectra. The splitting pattern and the assignment abbreviations are as follows: s, singlet; d, doublet; t, triplet; q, quartet; qui, quintet; m, unresolved multiplet; br., broad; BZM, benzimidazole; and Th, thiophene.
High-resolution mass spectra (HRMS) were obtained using a QTOF instrument (Agilent) or an LTQ Orbitrap XL (Thermo Scientific) mass spectrometer in electrospray ionization (ESI) mode. The specific light rotation was measured with a JASCO digital polarimeter (Model P-1010 λ = 589 nm, ±0.05° accuracy) using a cylindrical quartz cell (5 mL, L = 5 cm) at room temperature by dissolving the crystals in CHCl3. Melting points were measured with a Fisher-Johns melting point apparatus and are uncorrected. Column flash chromatography (FC) was performed on silica gel 60 (230–400 mesh ASTM) from Merck or on Florisil (100–200 mesh) from Fluka. Analytical TLC was carried out on pre-coated Silica gel 60F254 (Merck) sheets using UV illumination and charring with vanillin stain for visualization. Preparative HPLC-separation was performed with an HPLC column (Luna 10-micron C18(2) 100A AX 100X30.00 mm 10 micron), using a H2O/MeCN gradient system of solvents: (i) 0–20 min [100/0]; (ii) gradient of 20 min [100/0] - 100 min [0/100]. The flow rate was 5 mL/min.
Dry THF was prepared by refluxing over sodium/benzophenone ketyl, followed by distillation under nitrogen. Other commercially available reagents and solvents were purchased and used as received.
4. EXPERIMENTAL
4.1. Synthetic Procedures
4.1.1. Methyl 4-fluoro-3-nitrobenzoate (2) [12]
4-Fluoro-3-nitrobenzoic acid (5.55 g, 29.98 mmol) was dissolved in methanol (50 mL) and concentrated H2SO4 (6.40 mL) was added. The reaction mixture was refluxed for 3 h with protection from atmospheric moisture. The reaction mixture was cooled to ambient temperature and poured into crushed ice. The precipitated colorless solid was collected by suction, thoroughly washed with water, and dried in vacuum to afford 5.49 g (91%) of the NMR-pure ester 2. 1H, 13C/DEPT NMR spectra, and m.p. of the prepared 2 completely match the reported data [12].
4.1.2. Methyl 3-nitro-4-(pentan-3-ylamino)benzoate (3)
To a mixture of methyl 4-fluoro-3-nitrobenzoate (2) (5.0 g, 25.3 mmol) and K2CO3 (5.2 g, 38.0 mmol) in dry THF (25.0 mL) under nitrogen, 3-aminopentane (3.3 mL, 2.5 g, 28.6 mmol) was added. After 4 h stirring at room temperature, THF was removed under reduced pressure, and water (100 mL) was added to the residue. The aqueous phase was extracted with ethyl acetate (3 × 100 mL). The combined organic phases were followed by washing with additional water (150 mL), drying over sodium sulfate, filtering and evaporating to yield 6.17 g (92%) of the NMR-pure product 3 as an orange oil. 1H NMR (400 MHz, CDC13): δ = 8.87 (d, J = 2.0 Hz, 1 H, 2-HAr), 8.34 (br. d, J = 7.6 Hz, 1 H, NH), 8.00 (dd, J = 9.2, 2.0 Hz, 1 H, 6-HAr), 6.86 (d, J = 9.2 Hz, 1 H, 5-HAr), 3.88 (s, 3 H, CH3O), 3.58–3.50 (m, 1 H, NHCH), 1.77–1.56 (m, 4 H, 2 CH2CH3), 0.97 (t, J = 7.6 Hz, 6 H, 2 CH2CH3) ppm. 13C NMR (100 MHz, CDCl3): δ = 165.8 (CO2CH3), 148.0 (4-CAr), 136.3 (6-HCAr), 131.2 (3-CAr), 130.0 (2-HCAr), 116.8 (1-CAr), 114.0 (5-HCAr), 55.8 (NHCH), 52.1 (CO2CH3), 26.9 (2 CH2CH3), 10.2 (2 CH2CH3) ppm. HRMS (ESI): calcd. for C13H19N2O4+ [M + H+]: 267.1339; found: 267.1343.
4.1.3. Methyl 3-amino-4-(pentan-3-ylamino)benzoate (4)
A solution of methyl 3-nitro-4-(pentan-3-ylamino)benzoate (3) (6.2 g, 23.2 mmol) in ethanol (70 mL) was hydrogenated with molecular hydrogen at 5 bar over 10% Pd/C (0.35 g, 0.33 mmol Pd) for 16 h in a Parr machine. The reaction mixture was filtered via a plug of Celite, and ethanol was removed under reduced pressure to yield an oily residue. Purification of the residue by FC on silica gel (20% ethyl acetate in hexane) afforded 4.7 g (85%) of the title product as a brownish waxy solid, Rf = 0.22 (ethyl acetate/hexane, 1:4). 1H NMR (400 MHz, CDCl3): δ = 7.57 (dd, J = 8.4, 2 Hz, 1.0 H, 6-HAr), 7.41 (d, J = 2.0 Hz, 1 H, 2-HAr), 6.55 (d, J = 8.8 Hz, 1 H, 5-HAr), 3.84 (s, 3 H, CH3O), 3.32 (qui, J = 6.0 Hz, 1 H, CHN), 1.68–1.48 (m, 4 H, 2 CH2CH3), 0.94 (t, J = 7.6 Hz, 6 H, 2 CH2CH3) ppm. 13C NMR (100 MHz, CDCl3): δ = 167.5 (CO2CH3), 143.2 (4-CAr), 131.5 (3-CAr), 126.6 (6-HCAr), 118.9 (2-HCAr), 117.7 (1-CAr), 109.3 (5-HCAr), 55.1 (NHCH), 51.5 (CO2CH3), 26.7 (2 CH2CH3), 10.1 (2 CH2CH3) ppm. LRMS (ESI): calcd. for C13H21N2O2+ [M + H+]: 237.16; found: 237.2.
4.1.4. Methyl 2-(thiophen-2-yl)acetimidate Hydrochloride (6)
Acetyl chloride (8.0 mL, 8.8 g, 101.3 mmol) was added dropwise over 10 min to a cold (0 °C) well-stirred solution of 2-thiopheneacetonitrile (5) (3.4 mL, 4.0 g, 32.4 mmol) and dry methanol (6.6 mL, 5.2 g, 162.9 mmol) in dry diethyl ether (30 mL) under nitrogen. After the addition, the reaction mixture was stirred at 0 °C for an additional hour, then was closed well and kept in the cold (4 °C) without stirring. Colorless crystals precipitated after 3 days. The crystals were filtered via a glass sinter, washed with dry ether (60 mL), and then dried in vacuum to generate 5.44 g (87%) of a highly hydroscopic solid imidate salt 6. 1H NMR (400 MHz, CDC13): δ = 12.90 (br. s, 1 H, HaHbN+=), 11.94 (br. s, 1 H, HaHbN+=), 7.30–7.28 (m, 1 H, 5-HTh), 7.20 (d, J = 3.6 Hz, 1 H, 3-HTh), 7.02–7.00 (m, 1 H, 4-HTh), 4.33 (s, 5 H, CH2 + OCH3) ppm. 13C NMR (100 MHz, CDCl3): δ = 177.0 (OC=N+H2), 130.9 (2-CTh), 129.0 (3-HCTh), 127.5 (4-HCTh), 126.5 (5-HCTh), 61.2 (OCH3), 33.2 (CH2) ppm.
4.1.5. Methyl 1-(pentan-3-yl)-2-(thiophen-2-ylmethyl)-1H-benzo[d]imidazole-5-carboxylate (7)
A homogeneous solution of methyl 3-amino-4-(pentan-3-ylamino)benzoate (4) (3.0 g, 12.9 mmol) and methyl 2-thiopheneacetimidate hydrochloride (6) (2.5 g, 12.9 mmol) was refluxed in dry methanol (20.0 mL) for 8 h under nitrogen. The methanol was removed under reduced pressure, the residue was partitioned between ethyl acetate (200 mL) and water (100 mL), the organic layer was washed with additional water (2×100 mL) and brine (100 mL), and dried over sodium sulfate. Filtration, then evaporation followed by FC on silica gel (gradient elution with ethyl acetate - hexane) yielded 2.5 g (57%) of the title product 7, Rf = 0.45 (ethyl acetate - toluene, 3:7). A beige solid, m.p. 101–103 °C. 1H NMR (400 MHz, CDCl3): δ = 8.48 (d, J = 1.2 Hz, 1 H, 4-HBZM), 7.92 (dd, J = 8.8, 1.6 Hz, 1 H, 6-HBZM), 7.45 (d, J = 8.4 Hz, 1 H, 7-HBZM), 7.18 (dd, J = 5.2, 1.2 Hz, 1 H, 5-HTh), 6.94–6.90 (m, 2 H, 3-+ 4-HTh), 4.51 (s, 2 H, CH2C), 4.19–4.11 (m, 1 H, CHN), 3.94 (s, 3 H, CH30), 2.04–1.85 (m, 4 H, 2 CH2CH3), 0.61 (t, J = 7.6 Hz, 6 H, 2 CH2CH3) ppm. 13C NMR (100 MHz, CDCl3): δ = 167.6 (CO2CH3), 155.0 (2-CBZM), 142. 8 (9-CBZM), 138.3 (2-CTh), 136.8 (8-CBZM), 127.0 (4-HCTh), 126.3 (3-HCTh), 124.9 (5-HCTh), 124.0 (5-CBZM), 123.7 (6-HCBZM), 122.3 (4-HCBZM), 111.3 (7-HCBZM), 61.0 (NCH), 52.0 (CH3O), 29.7 (CH2C), 26.7 (2 CH2CH3), 10.8 (2 CH2CH3) ppm. HRMS (ESI): calcd. for C19H23N2O2S+ [M + H+]: 343.1475; found: 343.1473.
4.1.6. 1-(Pentan-3-yl)-2-(thiophen-2-ylmethyl)-1H-benzo[d] imidazole-5-carboxylie Acid (8)
To a solution of methyl ester 7 (200 mg, 0.6 mmol) in methanol (2.0 mL) and THF (3.0 mL), 1 M aqueous solution of sodium hydroxide (1.2 mL) was added, and the reaction mixture was stirred at room temperature overnight. The solvents were removed under reduced pressure and water (50 mL) was added. The mixture was adjusted to pH 4 by the addition of 2.0 M aqueous HCl and the aqueous phase was extracted with ethyl acetate (3 × 100 mL). The combined organic phase was followed by washing with brine (100 mL), drying over sodium sulfate, filtering, evaporating, and vacuuming to afford 170 mg (87%) of carboxylic acid 8 as a light brown solid, mp 83–86 °C. 1H NMR (700 MHz, CDCl3): δ = 8.81 (s, 1 H, 4-HBZM), 8.05 (d, J = 8.4 Hz, 1 H, 6-HBZM), 7.51 (d, J = 8.4 Hz, 1 H, 7-HBZM), 7.18 (d, J = 4.9 Hz, 1 H, 5-HTh), 6.97 (d, J = 3.5 Hz, 1 H, 3-HTh), 6.94–6.93 (m, 1 H, 4-HTh), 4.78 (s, 2 H, CH2C), 4.25–4.20 (m, 1 H, CHN), 2.05–1.91 (m, 4 H, 2 CH2CH3), 0.64 (t, J = 7.0 Hz, 6 H, 2 CH2CH3) ppm. 13C NMR (175 MHz, CDCl3): δ = 170.4 (CO2H), 155.1 (2-CBZM), 141.6 (9-CBZM), 138.3 (2-CTh), 136.4 (8-CBZM), 127.3 (4-HCTh), 126.6 (3-HCTh), 125.5 (5-CBZM), 125.0 (5-HCn), 124.6 (6-HCBZM), 122.4 (4-HCBZM), 111.7 (7-HCBZM), 61.2 (NCH), 29.1 (CH2C), 26.8 (CH2CH3), 11.0 (CH2CH3) ppm. HRMS (ESI): calcd. for C18H21N2O2S+ [M + H+]: 329.1318; found: 329.1316.
4.1.7. Methyl (S)-5-methyl-3-{(1-(pentan-3-yl)-2-(thiophen-2-ylmethyl)-1H-benzo[d]imidazole-5-carboxamido)}hexanoate (9)
To a well-stirred solution of carboxylic acid 8 (500 mg, 1.5 mmol) in dry DMF (10 mL) at 0 °C under nitrogen, HOBt (310 mg, 2.3 mmol), EDC (450 mg, 2.89 mmol), and DIEA (0.60 mL, 452 mg, 3.9 mmol) were added consecutively. After 15 min stirring at 0 °C, a solution of methyl (S)-3-amino-5-methylhexanoate (850 mg, 5.3 mmol) and DIEA (0.6 mL, 452 mg, 3.9 mmol) in dry DMF (2 mL) was added. The reaction mixture was stirred at ambient temperature for 3 days and poured into water (125 mL). The solution was acidified to pH 3 by the addition of 2.0 M aqueous HCl and extracted with ethyl acetate (2 × 250 mL). The combined organic phase was washed with saturated sodium bicarbonate (2 × 200 mL) and brine (200 mL), dried over sodium sulfate, filtered, and evaporated. Purification of the residue by FC on Florisil (20% ethyl acetate in hexane) yielded 420 mg (58%) of the title product 9 as a pale brown oil, Rf = 0.36 (ethyl acetate/toluene, 1:1); [α]D25 = 23.7 (c = 0.002, CHCl3). 1H NMR (400 MHz, C6D6): δ = 8.58 (s, 1 H, 4-HBZM), 8.16 (d, J = 8.4 Hz, 1 H, 6-HBZM), 7.17–7.13 (m, 2 H, NH + 7-HBZM), 6.78 (d, J = 5.2 Hz, 1 H, 5-HTh), 6.70 (d, J = 2.0 Hz, 1 H, 3-HTh), 6.66–6.64 (m, 1 H, 4-HTh), 4.87–4.81 (m, 1 H, CONHCH), 4.24 (s, 2 H, CBZMCH2CTh), 3.76–3.70 (m, 1 H, NBZMCH), 3.32 (s, 3 H, CH3O), 2.54–2.45 (m, 2 H, CH3O2CCH2), 1.74–1.66 (m, 1 H, CH(CH3)2), 1.63–1.54 [m, 3 H, CHaHbCH(CH3)2 + CH2CH3], I. 41–1.31 (m, 2 H, CH2CH3), 1.28–1.21 [m, 1 H, CHaHbCH(CH3)2], 0.95 [d, J = 6.4 Hz, 3 H, CH(CH3)CH3], 0.84 [d, J = 6.4 Hz, 3 H, CH(CH3)CH3], 0.34 (t, J = 7.2 Hz, 6 H, 2 CH2CH3) ppm. 13C NMR (100 MHz, C6D6): δ = 172.4 (CO2CH3), 167.0 (CONH), 154.7 (2-CBZM), 143.7 (9-CBZM), 139.3 (2-CTh), 136.0 (8-CBZM), 129.7 (5-CBZM), 127.1 (4-HCTh), 126.5 (3-HCTh), 124.9 (5-HCTh), 122.8 (6-HCBZM), 119.1 (4-HCBZM), 111.9 (7-HCBZM), 60.7 (NBZMCH), 51.2 (CH3O), 45.2 (CONHCH), 43.9 [CH2CH(CH3)2], 39.8 (CH3O2CCH2), 29.6 (CThCH2), 26.7 (2 CH2CH3), 25.5 [CH(CH3)2], 23.3 [CH(CH3)CH3], 22.3 [CH(CH3)CH3], 10.8 (2 CH2CH3) ppm. HRMS (ESI): calcd. for C26H36N3O3S+[M+ H+]: 470.2472; found: 470.2479.
4.1.8. Potassium (S)-5-methyl-3-{1-(pentan-3-yl)-2-(thiophen-2-ylmethyl)-1H-benzo[d]imidazole-5-carboxamido} hexanoate - CMF-019 (potassium salt) (10)
To a solution of methyl ester 9 (50 mg, 0.1 mmol) in THF (1.0 mL) an aqueous solution of KOH (7 mg, 0.12 mmol in 0.6 mL of H20) was added slowly at 0 °C and the reaction mixture was stirred at room temperature for 21 h. THF and water were removed under reduced pressure to afford an oily residue. The residue was treated with ethyl ether (10 mL), and the mixture was stirred for 30 min to afford a pale insoluble precipitate. The solution was decanted off the solid and discarded. The solid residue was purified using reverse phase preparative HPLC to afford, after thorough lyophilization for 5 days, 30 mg (57%) of the target CMF-019 (potassium salt) (10). The product is a colorless hydroscopic powder, m.p. 116–120 °C; [α]D25 = −16.9 (c = 0.002, CHCl3). 1H NMR (400 MHz, DMSO-d6): δ = 9.81 (d, J = 8.0 Hz, 1 H, CONH), 8.02 (d, J = 0.8 Hz, 1 H, 4-HBZM), 7.66–7.60 (m, 2 H, 6 + 7-HBZM), 7.38 (dd, J = 5.2, 1.2 Hz, 1 H, 5-HTh), 7.07 (dd, J = 3.2, 1.2 Hz, 1 H, 3-HTh), 6.96 (dd, J = 5.2, 3.2 Hz, 1 H, 4-HTh), 4.54 (s, 2 H, CBZMCH2CTh), 4.35–4.27 (m, 1 H, NBZMCH), 4.18–4.10 (m, 1 H, CONHCH), 2.10 (d, J = 4.4 Hz, 2 H, CH3O2CCH2), 1.99–1.82 (m, 4 H, 2 CH2CH3), 1.65–1.55 [m, 1 H, CHaHbCH(CH3)2], 1.54–1.47 [m, 1 H, CHaHbCH(CH3)2], 1.35–1.28 [m, 1 H, CHaHbCH(CH3)2], 0.90 (d, J = 6.4 Hz, 3 H, CH(CH3)CH3], 0.86 [d, J = 6.4 Hz, 3 H, CH(CH3)CH3], 0.50 (t, J = 7.2 Hz, 6 H, 2 CH2CH3) ppm. 13C NMR (100 MHz, DMSO-d6): δ = 174.3 (CO2−), 164.8 (CON), 155.0 (2-CBZM), 142.4 (9-CBZM), 139.2 (2-CTh), 134.7 (8-CBZM), 129.0 (5-CBZM), 126.7 (4-HCTh), 126.4 (3-HCTh), 125.4 (5-HCTh), 120.9 (6-HCBZM), 117.7 (4-HCBZM), 111.5 (7-HCBZM), 59.5 (NBZMCH), 45.1 (CONHCH), 43.9 [CH2CH(CH3)2], 41.8 (CH2CO2−), 28.5 (CThCH2), 25.8 (2 CH2CH3), 24.8 [CH(CH3)2], 22.9 [CH(CH3)CH3], 22.7 [CH(CH3)CH3], 10.3 (2 CH2CH3) ppm. HRMS (ESI): calcd. for C25H33KN3O3S+ [M + H+]: 494.1874; found: 494.1870.
CONCLUSION
An improved, convenient synthesis of CMF-019 (potassium salt) (10) was developed and reported here with detailed synthetic procedures and spectroscopic data. The elaborated synthesis avoids the use of high-pressure hydrogenation and a dedicated microwave reactor. Since 10 is a potent APJ receptor agonist, a description of its expedient synthesis could benefit investigating the apelin-APJ system and potential treatment of the system’s dysfunction-associated disorders.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Gila Levy for her assistance with the polarimetric measurements and Steve Manch for English editing. This study was supported by a Bar-Ilan University new faculty grant (AG), by the Israel Ministry of Immigration and Integration through Kamea fellowship (EEK), and by EY024864 and EY027283 grants (KP).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No Animals/Humans were used for studies that are the basis of this research.
CONSENT FOR PUBLICATION
Not applicable.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher’s web site along with the published article.
REFERENCES
- [1].O’Dowd BF; Heiber M; Chan A; Heng HH; Tsui LC; Kennedy JL; Shi X; Petronis A; George SR; Nguyen T A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene, 1993, 136, 355–360. [DOI] [PubMed] [Google Scholar]
- [2].Tatemoto K; Hosoya M; Habata Y; Fujii R; Kakegawa T; Zou MX; Kawamata Y; Fukusumi S; Hinuma S; Kitada C; Kurokawa T; Onda H; Fujino M Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem. Biophys. Res. Commun, 1998,251,471–476. [DOI] [PubMed] [Google Scholar]
- [3].Japp AG; Newby DE The apelin-APJ system in heart failure pathophysiologic relevance and therapeutic potential. Biochem. Pharmacol, 2008, 75, 1882–1892. [DOI] [PubMed] [Google Scholar]
- [4].Narayanan S; Harris DL; Maitra R; Runyon SP Regulation of the apelinergic system and its potential in cardiovascular disease: peptides and small molecules as tools for discovery. J. Med. Chem, 2015, 58, 7913–7927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Japp AG; Cruden NL; Amer DA; Li VK; Goudie EB; Johnston NR; Sharma S; Neilson I; Webb DJ; Megson IL; Flapan AD; Newby DE Vascular effect of apelin in vivo in man. J. Am. Coll. Cardiol, 2008, 52, 908–913. [DOI] [PubMed] [Google Scholar]
- [6].Brame AL; Maguire JJ; Yang P; Dyson A; Torella R; Cheriyan J; Singer M; Glen RC; Wilkinson LB.; Davenport, A.P. Design, characterization, and first-in-human study of the vascular actions of a novel biased apelin receptor agonist. Hypertension, 2015, 65, 834–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Murza A; Parent A; Besserer-Offroy E; Tremblay H; Karadereye F; Beaudet N; Leduc R; Sarret P; Marsault E Elucidation of the structure-activity relationships of apelin: influence of unnatural amino acids on binding, signaling, and plasma stability. ChemMedChem, 2012, 7, 318–325. [DOI] [PubMed] [Google Scholar]
- [8].a) Agyei D; Ahmed I; Akram Z; Iqbal HMN; Danquah MK Protein and peptide biopharmaceuticals: An Overview. Protein Peptide Lett, 2017, 24, 94–101; [DOI] [PubMed] [Google Scholar]; b) D’Addio SM; Bothe JR; Neri C; Walsh PL; Zhang J; Pierson E; Mao Y; Gindy M; Leone A; Templeton AC New and evolving techniques for the characterization of peptide therapeutics. J. Pharm. Sci, 2016, 105, 2989–3006; [DOI] [PubMed] [Google Scholar]; c) Xiao Y-F; Jie M-M; Li B-S; Hu C-J; Xie R; Tang B; Yang S-M Peptide-based treatment: a promising cancer therapy. J. Immunol. Res, 2015, 2015, 761820; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Khafagy E-S; Morishita M Oral biodrug delivery using cell-penetrating peptide. Adv. DrugDeliv. Rev, 2012, 64, 531–539. [DOI] [PubMed] [Google Scholar]
- [9].a) Qvit N; Rubin SJS; Urban TJ; Mochly-Rosen D; Gross ER Peptidomimetic therapeutics: scientific approaches and opportunities. Drug Discov. Today, 2017, 22, 454–462; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Royo Gracia S; Gaus K; Sewald N Synthesis of chemically modified bioactive peptides: recent advances, challenges and developments for medicinal chemistry. Future Med. Chem, 2009, 1, 1289–1310; [DOI] [PubMed] [Google Scholar]; c) Fabbrizzi P; Menchi G; Guama A; Trabocchi A Use of click-chemistry in the development of peptidomimetic enzyme inhibitors. Curr. Med. Chem, 2014,21, 1467–1477. [DOI] [PubMed] [Google Scholar]
- [10].a) Hachtel S; Wohlfart P; Weston J; Mueller M; Defossa E; Mertsch K; Weng J-H; Binnie RA; Abdul-Latif F; Bock WJ; Walser A Preparation of benzimidazolecarboxylic acid amino acid amides as apelin receptor modulators. WO2014,044738 Al, March 27, 2014;; b) Hachtel S; Wohlfart P; Weston J; Mueller M; Defossa E; Mertsch K; Weng J-H; Binnie RA; Abdul-Latif F; Bock WJ; Walser A Benzoimidazole-carboxylic acid amide derivatives as APJ receptor modulators. U.S. Patent 2014,0094450 Al, April 3,2014.
- [11].Read C; Fitzpatrick CM; Yang P; Kuc RE; Maguire JJ; Glen RC; Foster RE; Davenport AP Cardiac action of the first G protein biased small molecule apelin agonist. Biochem. Pharmacol., 2016,116, 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Dietrich SA; Lindauer R; Stierlin C; Gertsch J; Matesanz R; Notararigo S; Femando D; Altmann KH Epothilone analogues with benzimidazole and quinoline side chains: chemical synthesis, antiproliferative activity, and interactions with tubulin. Chem. Eur. J, 2009,15,10144–10157. [DOI] [PubMed] [Google Scholar]
- [13].a) For a brief discussion on efficiency and reproducibility of microwave-assisted reactions, see, e.g.:Lidström P; Tierney J; Wathey B; Westman J Microwave assisted organic synthesis – a review. Tetrahedron, 2001, 57, 9225–9283; [Google Scholar]; b) Kappe CO Controlled microwave heating in modem organic synthesis. Angew. Chem., Int. Ed, 2004, 43, 6250–6284; [DOI] [PubMed] [Google Scholar]; c) Gising J; Odell LR; Larhed M Microwave-assisted synthesis of small molecules targeting the infectious diseases tuberculosis, HTV/AIDS, malaria and hepatitis C. Org. Biomol. Chem, 2012,10, 2713–2729; [DOI] [PubMed] [Google Scholar]; d) Baña P; Greiner B Comparison of conventional and microwave heating for evaluation of microwave effects. Austral. J. Chem, 2016, 69, 865–871. [Google Scholar]
- [14].The imidate hydrochloride 6 was previously prepared according to the standard Pinner method and reported without spectral characterization. See:Leschke C; Elz S; Garbarg M; Schunack W Synthesis and histamine Hi receptor agonist activity of a series of 2-phenylhistamines, 2-heteroarylhistamines, and analogues. J. Med. Chem, 1995, 38, 1287–1294. [DOI] [PubMed] [Google Scholar]
- [15].a) For selected recent reviews on synthesis of benzimidazoles, see:Grimmett MR Product class 4: benzimidazoles In: Science of Synthesis’, Neier R, Ed.; Georg Thieme: New York, 2002, Vol. 12, pp. 529–612; [Google Scholar]; b) Carvalho LCR; Fernandes E; Marques MMB Developments towards regioselective synthesis of 1,2-disubstituted benzimidazoles. Chem. Eur. J, 2011,17, 12544–12555; [DOI] [PubMed] [Google Scholar]; c) Arulmurugan S; Kavitha HP.; Sathishkumar S; Arurmozhi R Biologically active benzimidazole derivatives. Mini-Rev. Org. Chem, 2015,12, 178–195; [Google Scholar]; d) Rajasekhar S; Mati B; Balamurali MM; Chanda K Synthesis and medicinal applications of benzimidazoles: an overview. Curr. Org. Synth, 2017,14,40–60. [Google Scholar]
- [16].a) King FE; Acheson RM Synthesis of benzimidazoles from o-phenylenediamines and imino ethers. J. Chem. Soc, 1949, 1396–1400; [Google Scholar]; b) Chapman DD; Elwood JK; Heseltine DW; Hess HM; Kurtz DW Annelation of pyridinium rings onto nitrogen heterocycles. J. Org. Chem, 1977, 42, 2474–2480; [Google Scholar]; c) Musser JH; Kubrak DM; Chang J; DiZio SM; Hite M; Hand JM; Lewis AJ Leukotriene D4 antagonists and 5-lipoxygenase inhibitors. Synthesis of benzoheterocyclic [(methoxyphenyl)amino] oxoalkanoic acid esters. J. Med. Chem, 1987, 30, 400–405; [DOI] [PubMed] [Google Scholar]; d) Caron S; Jones BP; Wei L Preparation of substituted benzimidazoles and imidazopyridines using 2,2,2-trichloroethyl imidates. Synthesis, 2012, 3049–3054; [Google Scholar]; e) Bandarage U; Hare B; Parsons J; Pham L; Marhefka C; Bemis G; Tang Q; Moody CS; Rodems S; Shah S; Adams C; Bravo J; Charonnet E; Savic V; Come JH; Green J 4-(Benzimidazol-2-yl)-l,2,5-oxadiazol-3-ylamine derivatives: Potent and selective p70S6 kinase inhibitors. Bioorg. Med. Chem. Lett, 2009, 19, 5191–5194; [DOI] [PubMed] [Google Scholar]; f) Burgeson JR; Moore AL; Gharaibeh DN; Larson RA; Cerruti NR; Amberg SM; Hruby DE; Dai D Discovery and optimization of potent broad-spectrum arenavirus inhibitors derived from benzimidazole and related heterocycles. Bioorg. Med. Chem. Lett, 2013, 23, 750–756; [DOI] [PubMed] [Google Scholar]; g) Tonelli M; Novelli F; Tasso B; Vazzana I; Sparatore A; Boido V; Sparatore F; La Colla P; Sanna G; Giliberti G; Busonera B; Farci P; Ibba C; Loddo R Antiviral activity of benzimidazole derivatives. HI. Novel anti-CVB-5, anti-RSV and anti-Sb-1 agents. Bioorg. Med. Chem, 2014, 22, 48934909. [DOI] [PubMed] [Google Scholar]
- [17].a) For the originally reported “modified Pinner method”, consisting of reaction of nitrile with excess alcohol and acetyl chloride as HC1-sourse without an inert diluting solvent, see:Yadav VK; Babu KG A remarkably efficient Markovnikov hydrochlorination of olefins and transformation of nitriles into imidates by use of AcCl and an alcohol. Eur. J. Org. Chem, 2005, 2005, 452–456; [Google Scholar]; Kumar Y; Shaw M; Thakur R; Kumar A Copper(II)-mediated aerobic oxidation of benzylimidates: Synthesis of primary α-ketoamides. J. Org. Chem, 2016, 81, 6617–6625. [DOI] [PubMed] [Google Scholar]
- [18].a) Reactions of imidates and their salts with anilines represent a classical synthesis of N-arylamidines. For a review, see:Dunn PJ In Comprehensive Organic Functional Group Transformations II; Elsevier: Oxford, UK, 2005; Vol. 5, pp. 655–699. For selected more recent examples, see: [Google Scholar]; b) Caron S; Wei L; Douville J; Ghosh A Preparation and utility of trihaloethyl imidates: useful reagents for the synthesis of amidines. J. Org. Chem, 2010, 75, 945–947; [DOI] [PubMed] [Google Scholar]; c) Causey CP; Jones JE; Slack JL; Kamei D; Jones LE Jr.; Subramanian V; Knuckley B; Ebrahimi P; Chumanevich AA; Luo Y; Hashimoto H; Sato M; Hofseth LJ; Thompson PR The development of N-α-(2-carboxyl)benzoyl-N5-(2-fluoro-l-iminoethyl)-L-omithine amide (o-F-amidine) and N-α-(2-carboxyl)benzoyl-N5-(2-chloro-1-iminoethyl)-L-omithine amide (o-Cl-amidine) as second generation protein arginine deiminase (PAD) inhibitors. J. Med. Chem, 2011, 54, 6919–6935; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Annedi SC; Maddaford SP; Ramnauth J; Renton P; Speed J; Rakhit S; Andrews JS; Porreca F 3,5-Disubstituted indole derivatives as selective human neuronal nitric oxide synthase (nNOS) inhibitors. Bioorg. Med. Chem. Lett, 2012, 22, 1980–1984; [DOI] [PubMed] [Google Scholar]; e) Weigel LF; Nitsche C; Graf D; Bartenschlager R; Klein CD Phenylalanine and phenylglycine analogues as arginine mimetics in dengue protease inhibitors. J. Med. Chem, 2015, 58, 7719–7733. [DOI] [PubMed] [Google Scholar]
- [19].a) Intentionally prepared or unambiguously generated N-(ortho-aminophenyl)amidines are spontaneously cyclised to benzimidazoles even at ambient temperature. See:Hölljes Jr., Wagner EL, E.C. Some reactions of nitriles as acid anammonides. J. Org. Chem, 1944, 9, 31–49; [Google Scholar]; b) Gupta S; Agarwal PK; Kundu B Catalyst/ligand-free synthesis of benzimidazoles and quinazolinones from amidines via intramolecular transamination reaction. Tetrahedron Lett, 2010, 51,1887–1890. [Google Scholar]
- [20].Enantiopure methyl (S)-3-amino-5- methylhexanoate, [α]D25 = +2.4 (c = 0.002, CHCl3), was purchased from Aquila Pharmatech LLC (USA).
- [21].Bennet CE Potassium tremethylsilanolate In: e-EROS Encyclopedia of Reagents for Organic Synthesis; Wiley: Chichester, 2009, pp. 1–2. [Google Scholar]
- [22].Gottlieb HE; Kotlyar V; Nudelman A NMR chemical shifts of common laboratory solvents as trace impurities. J. Org. Chem, 1997, 62, 7512–7515. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.