Abstract
Purpose
Persistent chemotherapy-related cognitive impairment (pCRCI) is commonly reported following cancer treatment and negatively affects quality of life; however, there is currently no pharmacological treatment indicated for pCRCI. This pilot study obtained preliminary data regarding the use of transdermal nicotine patches as a therapeutic strategy for women with pCRCI to(1) reduce subjective cognitive complaints and (2) enhance objective cognitive performance in breast, colon, lymphoma, or ovarian cancer survivors with pCRCI.
Methods
Participants were randomized to either placebo (n = 11) or transdermal nicotine (n = 11) for 6 weeks, followed by 2 weeks of treatment withdrawal for a total of 8 weeks. Participants were assessed using both subjective and objective measures of cognitive functioning at five visits before, during, and after treatment.
Results
Over the course of the study, women in both groups improved substantially in severity of self-reported cognitive complaints measured by Functional Assessment of Cancer Therapy-Cognitive Function Perceived Cognitive Impairments regardless of treatment arm. Additionally, objective cognitive performance measures improved in both groups; however, there was no significant difference in improvement between groups.
Conclusions
Due to a large placebo response, we were unable to determine if a drug effect was present. However, we did observe substantial improvement in self-reported cognitive symptoms, likely resulting from factors related to participation in the trial rather than specific drug treatment effects.
Trial registration
The study was registered with clinicaltrials.gov (trial registration: ).
Implications for Cancer Survivors
These results suggest that women with pCRCI can exhibit improvement in subjective cognition, with attention paid to symptoms and close follow-up over a short period of time.
Keywords: Chemotherapy-related cognitive impairment, Breast cancer, Survivorship, Cognitive impairment, Clinical trial
Introduction
Advances in cancer treatment are producing a growing number of cancer survivors, increasing the importance of quality of life issues during and following cancer treatment. Chemotherapy-related cognitive impairment (CRCI) is one such quality of life issue commonly reported following chemotherapy in patients with cancer [1]. Although cognitive impairments following chemotherapy have been reported in patients with non-central nervous system (non-CNS) cancers since the 1980s [2], the phenomenon commonly referred to as “chemo brain” or “chemo fog” is poorly understood and, until relatively recently, was largely unacknowledged [3]. CRCI can persist for months to years after finishing treatment [4]; therefore, the number of cancer survivors who will have to cope with CRCI is likely to increase, it is crucial to understand how CRCI presents clinically and to develop therapeutic interventions.
The American Cancer Society defines CRCI as increased forgetfulness, trouble concentrating and remembering details, difficulty with multitasking and word finding, and taking longer to finish tasks [5]. CRCI is associated with changes across multiple cognitive domains, with effects most prominently reported for attention, working memory, executive function, and processing speed [6]. Severity of CRCI is typically mild to moderate in nature, such that the level of impairment would not typically qualify for a diagnosis of mild cognitive impairment (MCI) [7] or dementia. However, even mild impairments in cognitive functioning can negatively influence quality of life [8]. Research examining CRCI has focused primarily on women with breast cancer [1, 9], who represent approximately 23% (3.6 million) of the 15.5 million US cancer survivors (January 2016 data) [10]. Although it is probable that patients who receive chemotherapy for any type of cancer may experience CRCI, research in populations beyond breast cancer is limited and preliminary [8]. However, research in patients with other types of cancer (e.g., ovarian cancer, lymphoma, colon cancer, leukemia, testicular cancer, multiple myeloma, and prostate cancer) reveal similar results [4, 11–19]. Estimates of the prevalence of CRCI in cancer patients vary across studies [8] but longitudinal studies suggest that approximately 40% of breast cancer patients have evidence of cognitive impairment prior to cancer treatment, up to 75% exhibit cognitive decline during treatment, and 35–60% exhibit cognitive decline following completion of chemotherapy [8]. For a more extensive review on cognitive effects of chemotherapy and cancer-related treatments, see Vega, Dumas, and Newhouse (2018) [20].
Most pharmacological treatment studies of cancer patients and survivors targeting chemotherapy side effects have focused on physical symptoms such as fatigue [21–24] and anemia [25, 26] rather than cognitive symptoms. Studies evaluating the efficacy of stimulants, such as methylphenidate, dexmethylphenidate, and modafinil, for the treatment of CRCI have yielded mixed results with respect to cognition; therefore, it remains unclear whether these medications are useful in treating CRCI [21–26]. Other studies evaluated donepezil, an acetylcholinesterase inhibitor approved for Alzheimer’s disease (AD) [27, 28]. Both open-label and placebo-controlled studies in glioma patients demonstrated improvements in cognitive performance [27, 28]. Additionally, a study in breast cancer survivors suggested donepezil improved verbal memory in those who had poorer cognitive functioning at baseline [29]. These studies support that the cholinergic system may be a therapeutic target for improving cognitive functioning in CRCI [27–29]. We propose that more selective cholinergic stimulation may potentially be useful for alleviating certain cognitive symptoms [20].
The cholinergic system has been studied extensively in cognitive aging and is the primary neurotransmitter system responsible for cognitive changes in both normal aging and dementia [30]. Cognitive domains such as attention, executive control, and memory rely heavily on cholinergic neurotransmitter system integrity, which modulates other neurotransmitter systems and overall cognition via nicotinic (and muscarinic) acetylcholine receptors [31]. The importance of the nicotinic cholinergic receptor system was first examined using temporary blockade studies. Acetylcholine receptor antagonist drugs such as mecamylamine result in deficits across several cognitive domains including learning, memory, psychomotor speed, and attention [32, 33]. In contrast, drugs that stimulate the nicotinic cholinergic system have the opposite effect, acting as cognitive enhancers [34]. A meta-analysis of over 41 double-blind placebo-controlled laboratory studies concluded that there are significant positive effects of nicotinic stimulation with nicotine on motor abilities, attention, and memory [34]. Nicotinic receptor agonists also improve cognitive performance in populations with impaired cognitive performance, including AD [35–39], MCI [40], and attention deficit hyperactivity disorder (ADHD) [41]. However, nicotine has not been explored as a potential treatment for CRCI.
There is substantial overlap between objective impairments and subjective cognitive complaints commonly reported in CRCI and cognitive functions modulated by the cholinergic system. As mentioned previously, effects have been reported most prominently in the domains of attention, working memory, executive function, and processing speed [42]—cognitive abilities that rely heavily on the cholinergic transmitter system [31]. Given the overlap between domains affected in CRCI and cholinergically modulated cognitive functions and the potential link between the nicotinic cholinergic system and CRCI [43], the nicotinic cholinergic system represents a potential therapeutic target for improving cognitive functioning in cancer patients with CRCI [20]. The purpose of this study was to gather preliminary data examining whether 6 weeks of transdermal nicotine treatment would (1) reduce subjective cognitive complaints and (2) enhance performance on laboratory tests of cognition in breast cancer, colon cancer, lymphoma, or ovarian cancer survivors with persistent chemotherapy-related cognitive impairment (pCRCI). We hypothesized that nicotine treatment would reduce subjective cognitive complaints and improve cognitive performance on measures of attention and processing speed.
Methods
Study design
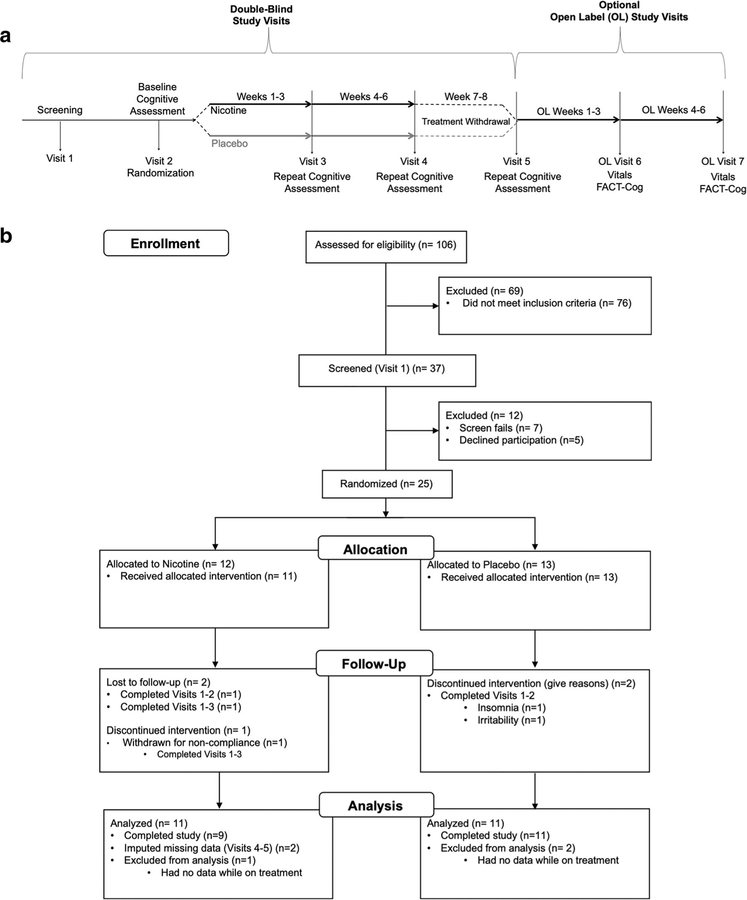
This was a randomized, placebo-controlled, parallel group pilot study () consisting of two phases, a double-blind phase and an optional open-label phase. See Fig. 1a for overall pCRCI study design. The initial double-blind phase consisted of five visits over 6 weeks. Prior studies examining the effects of nicotine on cognitive performance have shown benefit with 1 month of treatment [44]. Our experience with a number of other transdermal nicotine trials informed our choice of study length. After completing screening procedures for eligibility (visit 1; see Supplemental Materials for screening measures), at the baseline visit (visit2), participants completed cognitive testing and were randomized in equal allocations to receive either transdermal nicotine or placebo patches. Participants subsequently repeated cognitive assessment after 3 weeks (visit 3) and 6 weeks (visit 4) on patches. After completing the blinded trial period, participants stopped patches for 2 weeks, then repeated their baseline cognitive testing at their final study visit (visit 5). At the end of the double-blind portion of the study, participants could enter the optional open-label phase for an additional 6 weeks. During the open-label phase, only vital signs were collected at each visit and one subjective test was completed at the end of the 6 weeks.
Fig. 1.
Study design and participants enrollment. a Overall pCRCI study design. The study consisted of two phases, a double-blind portion and an optional open-label portion. In the double-blind portion of the study, participants were first screened (visit 1) to determine study eligibility. Once cleared for the study, participants completed a baseline visit (visit2) and were then randomized (50/50) to receive either transdermal nicotine or placebo patches. Participants then repeated their baseline cognitive assessment at visits 3 and 4 following 3 weeks and 6 weeks on patches, respectively. After completing 6 weeks on patches, participants went off patches for 2 weeks, then repeated their baseline cognitive testing at their final double-blind study visit (visit 5). At the end of the double-blind, placebo-controlled 8-week study, participants had the option to take part in the open-label portion of the study for an additional 6 weeks. For all open-label visits (visits 6 and 7), only vitals were collected and one subjective test was completed. b Participant enrollment. Of the 106 people pre-screened for the study, 37 were screened at visit 1. Of the 37 screened at visit 1, 25 people passed screening criteria and were randomized to treatment after completing visit 2. Twelve were randomized to nicotine treatment (9 completers, 11 with usable data) and 13 were randomized to placebo treatment (11 completers)
Participants
Recruitment began in Fall of 2015 and ended in Fall of 2017. Participants were recruited through Vanderbilt University-affiliated clinics and the greater Nashville, TN community. Recruitment strategies included Facebook advertisements, the use of fliers in strategic locations and clinics, and recruitment databases such as ResearchMatch.org, the Vanderbilt Email Distribution List, news articles, and existing collaborations between our lab and the Vanderbilt Breast Center. The cancer types included in the study (breast cancer, colon cancer, lymphoma, or ovarian cancer) were chosen with the aid of an oncologist due to overlapping chemotherapy regimens these cancer types receive. See Fig. 1b for details regarding participant enrollment. Of the 106 people pre-screened for the study, 37 attended the initial screening visit (visit 1). Of the 37 screened at visit 1, 25 people passed screening criteria and were randomized to treatment after completing their baseline visit (visit 2), 12 to transdermal nicotine patches (9 completed all visits and 2 completed 3 visits), and 13 to placebo patches (11 completers). Data were included from all participants who had completed the baseline visit (visit 2) and at least one post baseline visit (visit 3); therefore, the intent to treat analyses included 22 women (nicotine n = 11, placebo n = 11).
Inclusion criteria were as follows: (1) ages of 35 to 80 years;(2) previously diagnosed with invasive breast cancer, ovarian cancer, or lymphoma; (3) systemic chemotherapy treatment within the last 1 to 5 years; (4) endorsement of pCRCI subjective complaints (as defined below); (5) current non-smokers (no nicotine use within the last 5 years); (6) fluent in English. Following initial pre-screening, a review of medical records was conducted to ensure good general health and to confirm that pCRCI participants met criteria for breast cancer, ovarian cancer, or lymphoma and had received systemic chemotherapy. Participants were cognitively and behaviorally screened to rule out dementia and active psychiatric disorders (see Supplemental Material for more details regarding visit 1 assessments). pCRCI participants were excluded for (1) any active neurologic or psychiatric disease, history of significant head trauma followed by persistent neurologic deficits, or known structural brain abnormalities; (2) current major depression or another major psychiatric disorder as described in DSM-5 (use of psychotropic medications (e.g., antidepressants) for past depression was permitted, provided dosing had been stable for at least 3 months); (3) any history of alcohol or substance abuse or dependence within the past 2 years; (4) any significant systemic illness or unstable medical condition which could lead to difficulty complying with the protocol;(5) use of any investigational drugs within 30 days or 5 half-lives, whichever was longer, prior to screening; and (6) use of any drugs with pro-cholinergic properties (e.g., donepezil).
Treatment assignment and management
Nicotine was delivered by a transdermal patch provided in doses of 7 mg and 14 mg per 24 h. Participants were randomized (50/50) to receive either blinded nicotine patches or placebo patches. The patch titration schedule was as follows: week 1, 3.5 mg (1/2 7-mg patch) per day; week 2, full 7-mg patch/day; weeks 3–4, 10.5 mg (3/4 14-mg patch) per day; weeks 5–6, full 14-mg patch/day; weeks 7–8, treatment dis-continuation. Patches were applied for 16 h/day and removed at bedtime. Participants were contacted by phone or email weekly to assess tolerability and answer questions. If a participant appeared to be suffering persistent side effects at any dose, the dose was reduced to the previous dose until they were free of side effects. Participants were only moved to a higher dose once free of side effects. At each visit, vital signs (blood pressure, pulse), weight, and adverse events were assessed. Compliance was assessed by counting empty patch sachets at visits 3 and 4.
Defining pCRCI
For the purposes of the study, pCRCI was defined as follows:(1) perceived self-reported change in cognitive functioning the participant directly links to chemotherapy treatment received in the last 1–5 years; (2) evidence of substantial subjective impairment on the Cognitive Complaint Index (described below); and (3) subjective complaints not better accounted for by presence of depression or other psychiatric or neurologic conditions.
The Cognitive Complaint Index (CCI; visit 1) [45] was used to operationalize breast cancer patients as having subjective complaints. The CCI was chosen as the screening measure because previous research has shown that CCI score correlates with underlying neurodegenerative changes even when unaccompanied by deficits on formal testing [46] and it has been used in previous studies by our group examining cognitive complaints in post-menopausal women [47]. The CCI consists of items from multiple scales including the Memory Functioning Questionnaire [48], Memory Self-Rating Questionnaire [49], the Neurobehavioral Function and Activities of Daily Living Rating Scale (ADL-self) [50], the Informant Questionnaire on Cognitive Decline in the Elderly (IQCDE) [51], the 30 items from the Geriatric Depression Scale (GDS) [52], 12 items from a telephone-based screening for MCI, and 20 items from the Memory Assessment Questionnaire adapted in part from the Functional Activities Questionnaire [53]. Only items relevant to cognitive functioning are included from the GDS. A CCI score was calculated as the percentage of all items endorsed. pCRCI participants were required to have a CCI that includes endorsement of at least 20% of all items to be considered as having persistent chemotherapy-related subjective complaints [45].
Subjective (self-report) measure
The primary measure used to assess subjective cognitive performance was the Functional Assessment of Cancer Therapy-Cognitive Function (FACT-Cog; visits 2–5) [54] scale. This instrument has been used to monitor change in CRCI subjective complaints in previous studies and exhibits good internal consistency, test-retest reliability, and discriminant and convergent validity [55–57]. The FACT-Cog consists of four sub-scales: PCI, Perceived Cognitive Impairments; PCA, Perceived Cognitive Abilities; QOL, Impact on quality of life; and CFO, Comments from Others and evaluates memory, concentration, mental acuity, verbal fluency, functional interference, and multitasking ability. At each double-blind visit (visits 2–5), participants rated on a 5-point Likert scale how they assessed various aspects of their cognitive functioning over the last 7 days. Higher scores indicate better ratings of cognitive functioning. A summary of all subjective (self-report) measures (visits 2–5) is listed in Supplemental Table 1.
Objective cognitive performance measures
A summary of all objective cognitive performance measures (visits 2–5) is listed in Supplemental Table 1. To characterize the effects of nicotine on objective cognitive functioning in patients with pCRCI, we utilized measures that targeted domains most likely to be endorsed by patients with pCRCI (i.e., attention, working memory, executive function, and processing speed) or exhibit a change in response to nicotinic stimulation or blockade in past nicotine studies [40]. The cognitive performance battery consisted of computerized and verbal tasks, as well as tasks from the CogState battery.
Computerized and verbal tasks
The primary objective cognitive outcome measure was the computerized Conners continuous performance test (CPT) [58, 59] that measures sustained attention and vigilance. Participants see a series of letters appearing one at a time on a computer screen and they press a button for every letter that appears on the screen, except for “X.” Lower scores indicate better performance. Secondary measures include the critical flicker fusion (CFF) task [60], another test of attention and vigilance. In an ascending trial, the participant presses a button indicating when the frequency of flashing lights (beginning at 12 Hz and increasing to 50 Hz) has increased to the point that the lights appear to be no longer flashing but rather appear continuously on (“fused”). In a descending trial, beginning at 50 Hz, the participant presses a button when the frequency of apparently fused lights is decreased such that lights begin to appear to be flashing. The participant needs to respond before the frequency hits the upper or lower limit in each trial. The outcome variable for CFF is frequency (Hz) for ascending and descending trials. The choice reaction time (CRT task) [61] was a secondary measure of attention and psychomotor speed. Outcome variables included the mean and median processing reaction time (RT) (time from stimulus onset to initiation of movement), the mean and median motor RT (time from initiation of movement to stimulus termination), and mean and median total reaction time, with lower scores indicating better performance. The Buschke selective reminding task (SRT) [62] assessed immediate and delayed memory recall. Participants are read a list of 16 words and must immediately recall the list across 8 trials. Upon completing the immediate recall portion of the SRT, and after a 20-min delay, participants complete a single delayed recall trial. See supplemental material for more details regarding these tasks.
CogState battery
The CogState battery (CogState Ltd., Melbourne, Australia) is comprised of various tests that measure a range of cognitive domains [63] specifically designed to assess the presence or absence of cognitive change. The tasks selected from this battery specifically target domains likely to be endorsed by breast cancer patients with persistent CRCI and because they can be repeated without eliciting practice effects [64, 65]. The detection task measures information processing speed with a well-validated simple reaction time paradigm using playing card stimuli; lower scores indicate better (i.e., faster) performance. The identification task measures visual attention with a well-validated choice reaction time paradigm using playing card stimuli; lower scores indicate better (i.e., faster) performance. The two-back memory task measures working memory with a well-validated n-back paradigm using playing card stimuli; higher scores indicate better performance. The set-shifting task is a measure of executive function; higher scores indicate better performance. The Groton maze-learning task (GMLT) is a measure of problem solving and reasoning and uses a well-validated maze-learning paradigm; lower scores indicate better performance. See supplemental material for more details regarding all CogState tasks.
Statistical analyses
Analyses were performed using IBM SPSS Statistics for Mac, version 25 (IBM Corp., Armonk, NY, USA). Group demographic differences were evaluated using independent samples t tests and chi-square tests. Group differences in screening and baseline cognitive test scores were evaluated using independent samples t tests. Data were included from all participants who had completed the baseline visit (visit 2) and one post baseline visit (visit 3). Two participants had missing data from visits 4–5; however, those participants completed at least one visit while on treatment; therefore, missing data was imputed for those participants (see Supplemental Material for more details regarding imputation) and was included in the intent to treat analysis. Only data from the double-blind portion of the study was included in analyses.
To assess change in subjective cognition, a mixed-models repeated measures ANOVA was used to assess the interaction of treatment group (nicotine, placebo) with time (visit), using change from baseline PCI FACT-Cog score (visits 3–5) as the dependent measure. t tests then examined post hoc pairwise differences. All pairwise comparisons were Sidak corrected for multiple comparisons at the p < 0.05 level. To assess change in objective cognitive performance, a mixed-models repeated measures ANOVA was used to assess the interaction of treatment group (nicotine, placebo) with time (visit), using change from baseline score for CPT reaction time standard error over interstimulus interval (a measure of variability of reaction time) (visits 3–5), as the dependent measure.
For secondary analyses, mixed-models repeated measures ANOVA was used to assess the interaction of treatment group (nicotine, placebo) with time (visit), using change from baseline scores (visits 3–5) for the CRT, CFF, SRT, and CogState tasks listed in Supplemental Table S1. t tests were used to look at post hoc pairwise differences. All pairwise comparisons were Sidak corrected for multiple comparisons at the p < 0.05 level. Differences for rates of adverse events or other safety abnormalities between groups were assessed using chi-square analysis. Mixed-models repeated measures ANOVA was used to assess treatment group differences (nicotine, placebo) in change from baseline systolic blood pressure (visits 3–5).
Results
Participant screening and baseline demographics
Of the 22 total participants included in the analysis, the majority were breast cancer survivors (n = 18), followed by lymphoma survivors (n = 2), and ovarian and colon cancer survivors (n = 1 each). The mean ages for the nicotine and placebo treated groups were 56.00 ± 11.58 and 52.55 ± 7.66, respectively. There was no difference between treatment arms in mean age, years since chemotherapy, cancer type, cancer stage, cancer treatment, current endocrine therapy use, whether they received targeted therapy, and menopausal status prior to chemotherapy (Table 1). See Supplemental Table S2 for demographics data for the full randomized sample (n = 25). Additionally, there was no difference between groups in screening assessments or the majority of baseline variables. However, there was a significant difference between treatment groups in baseline SRT total recall failure performance (t(20) = − 2.26 p = 0.04). At baseline, the placebo group had a greater number of recall failures (mean = 20.64) compared with the nicotine group (mean = 12.18).
Table 1.
pCRCI participant demographics
| Nicotine (n = 11) | Placebo (n = 11) | Group difference statistics | ||
|---|---|---|---|---|
| Age in years (mean ± S.D.) | 56.00 ± 11.58 | 52.55 ± 7.66 | t(20) = 4.22, p = 0.24 | |
| Years since completed chemotherapy (mean ± S.D.) | 2.49 ± 1.42 | 2.87 ± 1.73 | t(20) = − 0.56, p = 0.58 | |
| Cancer type | Breast | 8 | 10 | χ(3) = 4.22, p = 0.24 |
| Lymphoma | 2 | 0 | ||
| Ovarian | 0 | 1 | ||
| Colon | 1 | 0 | ||
| Cancer stage | I | 3 | 5 | χ(3) = 2.44, p = 0.48 |
| II | 3 | 4 | ||
| III | 4 | 1 | ||
| IV | 1 | 1 | ||
| Cancer treatment | Chemotherapy | 11 | 11 | – |
| Surgery | 10 | 11 | χ(1) = 1.05, p = 0.31 | |
| Radiation | 5 | 7 | χ(3) = 0.73 p = 0.39 | |
| Current endocrine therapy | Yes | 6 | 7 | χ(2) = 1.28, p = 0.53 |
| No | 5 | 4 | ||
| Received targeted therapy | Yes | 4 | 2 | χ(2) = 2.27, p = 0.32 |
| No | 7 | 9 | ||
| Menopausal status prior to chemotherapy | Pre-menopausal | 5 | 6 | χ(1) = 0.18, p = 0.67 |
| Post-menopausal | 6 | 5 |
Table only includes demographic data from the 22 participants that had usable data
Subjective cognition
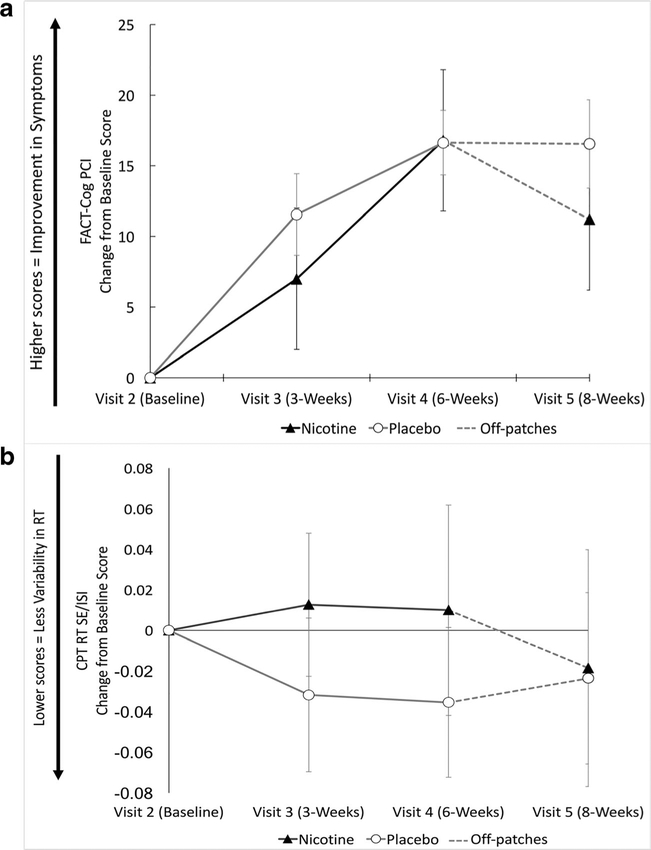
Data were analyzed using a mixed-models repeated measures ANOVA with a within-subjects factor of FACT-Cog PCI change from baseline score over time (visit 3, visit 4, and visit5) and a between-subject factor of drug treatment group (nicotine, placebo). Mauchly’s test indicated that the assumption of sphericity had been violated (χ2(2) = 12.22, p < 0.05); therefore, degrees of freedom were corrected using Greenhouse-Geisser estimates of sphericity (ε = 0.72). There was a main effect of FACT-Cog PCI change from baseline score (F(2.16,43.11) = 23.39, p < 0.001; Fig. 2a); however, there was no main effect of drug treatment group (F(1,20) =0.47, p = 0.50) or interaction between FACT-Cog PCI change from baseline score over time and drug treatment group (F(2.16,43.11) = 0.93, p = 0.41). Sidak corrected t tests were used to look at post hoc pairwise differences, revealing significant differences in FACT-Cog PCI change from baseline scores between visit 2 and all visits, as well as a significant difference between visit 3 and visit 4 (Supplemental Table S3).
Fig. 2.
Results for primary analyses. a Study aim 1: FACT-Cog Perceived Cognitive Impairment (PCI) change from baseline scores. Positive change scores indicate improvement in symptoms. b Study aim 2: Conners’ continuous performance task (CPT), reaction time (RT), and standard error (SE) divided by interstimulus interval (ISI) change from baseline scores. Negative change scores indicate improved performance. In both graphs, treatment groups are distinguished by the following colors: nicotine (black triangles) and placebo (white circles). The gray dashed lines indicate that participants were off patches between visits 4 and 5. Error bars indicate SE
Objective cognitive performance
For analysis of CPT results, data were analyzed using a mixed-models repeated measures ANOVA with a within-subjects factor of time (visit), testing for treatment arm differences using change from baseline score for CPT performance, measured as reaction time standard error divided by interstimulus interval (a measure of variability of reaction time; visit 3, visit 4, and visit 5). No significant main effects were observed for CPT performance (F(3,60) = 0.18, p = 0.91) or drug treatment group (F(1,20) = 0.34, p = 0.57), and no interaction was observed between CPT performance and drug treatment group (F(3,60) = 0.35, p = 0.79).
For secondary objective cognitive outcomes, no significant differences were observed between treatment groups for CFF, CRT recognition reaction time, CRT total reaction time, SRT total consistency, SRT delayed recall, or on any CogState measures (Table 2). Within-subjects main effects of time were observed for FACT-Cog PCA, QOL, and total scores. Scores on the FACT-Cog PCA, QOL, and total scores improved in both groups over time (Supplemental Figure S1). A main effect of time was also observed for CRT motor reaction time, with CRT motor reaction times improving in both groups over time (Supplemental Figure S2). Time and group main effects were observed for SRT total recall change from baseline score and treatment group, where the placebo group improved more than the nicotine group. There was no significant interaction between SRT total recall score and drug treatment group; however, a trend was observed (Table 2; Supplemental Figure S3). Additionally, SRT total recall data were analyzed using an unpaired t test to conduct a pre-treatment/baseline (visit 2) and post-treatment (visit 4) comparison, and revealed a significant difference between groups (t(20) = − 2.49, p = 0.02) where the placebo group performed better at visit 4 (compared with visit 2) than the nicotine group.
Table 2.
Mixed-models repeated measures ANOVA results for primary and secondary outcome measures
| Measure | Task/outcome variable | Time main effect | Treatment group main effect | Time × treatment interaction effect |
|---|---|---|---|---|
| FACT-Cog | CFO score | F(3,60) = 2.18, p = 0.10 | F(1,20) = 0.18, p = 0.67 | F(3,60) = 0.96, p = 0.42 |
| PCA score | F(1.90,37.94) = 11.61, p <0.001*+ | F(1,20) = 0.75, p = 0.40 | F(1.90,37.94) =1.05, p = 0.38+ | |
| QOL score | F(2.18,43.62)= 17.66, p <0.001*+ | F(1,20) = 0.14, p = 0.72 | F(2.18,43.62) = 0.72, p = 0.72+ | |
| Total score | F(2.20,43.94) = 24.25, p <0.001*+ | F(1,20) = 0.66, p = 0.43 | F(2.20,43.94)= 1.18, p = 0.320+ | |
| SRT | Total recall | F(3,60) = 2.84, p = 0.04* | F(1,20) = 5.28, p = 0.03* | F(3,60) = 2.35, p = 0.08 |
| Total consistency | F(3,60) = 0.97, p = 0.41 | F(1,20) = 5.28, p = 0.31 | F(3,60) = 1.25, p = 0.30 | |
| Delayed recall | F(3,60) = 2.55, p = 0.06 | F(1,20) = 4.31, p = 0.06 | F(3,60) = 1.24, p = 0.30 | |
| CFF | Ascending mean (Hz) | F(1.88,37.57) = 0.58, p = 0.56+ | F(1,20) = 0.12, p = 0.73 | F(1.88,37.57) = 0.36, p = 0.78+ |
| Descending mean (Hz) | F(2.06,41.18) = 0.74, p = 0.49+ | F(1,20) = 0.74, p = 0.73 | F(2.06,41.18) = 0.73, p = 0.49+ | |
| CRT | Recognition reaction time (ms) | F(1.90,37.91) =1.67, p = 0.20+ | F(1,20) = 1.07, p = 0.31 | F(1.90,37.91) = 0.83, p = 0.44+ |
| Motor reaction time (ms) | F(1.92,38.39) = 3.68, p = 0.04*+ | F(1,20) = 0.12, p = 0.73 | F(1.92,38.39) = 0.46, p <0.63+ | |
| Total reaction time (ms) | F(1.73,34.58) = 3.16, p = 0.06+ | F(1,20) = 0.50, p = 0.49 | F(1.73,34.58) = 0.35, p = 0.68+ | |
| CogState | ID task (speed) | F(2.08,41.59) = 0.84, p = 0.44+ | F(1,20) = 0.11, p = 0.75 | F(2.08,41.59) = 0.51, p = 0.61+ |
| Detection task (speed) | F(3,60) = 1.18, p = 0.32 | F(1,20) = 0.32, p = 0.86 | F(3,60) = 0.50, p = 0.99 | |
| Two back (accuracy) | F(1.57,31.39) = 3.35, p = 0.06+ | F(1,20) = 0.09, p = 0.77 | F(1.57,31.39) = 0.45, p = 0.59+ | |
| Set-shifting task (errors) | F(2.02,40.48) = 3.07, p = 0.06+ | F(1,20) = 0.35, p = 0.56 | F(2.02,40.48) = 0.26, p = 0.78+ | |
| GMLT (total errors) | F(3,60) = 2.71, p = 0.05+ | F(1,20) = 1.79, p = 0.20 | F(3,60) = 1.78, p = 0.16+ |
FACT-Cog, Functional Assessment of Cancer Therapy-Cognitive Function; PCI, Perceived Cognitive Impairments; PCA, Perceived Cognitive Abilities; QOL, Impact on quality of life; CFO, Comments from Others; SRT, selective reminding task; CFF, critical flicker fusion task; CRT, choice reaction time task; GMLT, Groton maze-learning task
Adjusted for Greenhouse-Geisser estimates of sphericity
Significant at p < 0.05 level
To account for differences between treatment groups in baseline (visit 2) SRT total recall failure score, a mixed-models repeated measures ANCOVA with a within-subjects factor of raw SRT total recall failure scores (visit 3, visit 4, visit 5) and a between subjects factor of treatment group (nicotine, placebo), co-varied for raw baseline (visit 2) SRT total recall failure score, was used. After controlling for the effect of baseline (visit 2) SRT total recall failure score, there were no significant main or interaction effects observed.
Adverse events
Differences for rates of adverse events (AEs) were assessed using chi-square analysis (Supplemental Table S4). The total number of AEs for the double-blind treatment period was 11 for the nicotine group compared with 12 for the placebo group (p = 0.85). The majority of AEs experienced by both groups were mild in nature, with skin irritation being the most common AE (Supplemental Figure S4). There were no significant main or interaction effects between treatment group and mean systolic blood pressure change from baseline, weight (kg), or pulse (bpm; Supplemental Figure S5). No withdrawal symptoms were reported by participants nor did any participants report continuing nicotine use after the study was completed.
Discussion
Although there was a main effect of FACT-Cog PCI change from baseline score across subsequent visits, there was no main effect of treatment group, or interaction between FACT-Cog PCI change from baseline score and drug group. In other words, participants in both groups exhibited a reduction in self-reported cognitive complaints over the study regardless of treatment received. A previous study showed that the clinical important difference for the FACT-Cog subscale ranged from 3.1 to 8.8 points [66]; our study showed average FACT-Cog PCI change scores of ~ 5–15 points, indicating substantial improvement in self-reported cognitive complaints in both groups over time. No significant main effects or interaction effects were observed for our primary objective cognitive outcome measure (CPT reaction time standard error divided by interstimulus interval) and treatment group. In other words, there was no difference between treatment groups in CPT performance over the course of the study. We also did not observe any group effects or change in secondary cognitive outcome measures.
Using nicotinic receptor agonists for cognitive enhancement is not in itself novel, but to our knowledge, the idea of using nicotine treatment for non-smoking individuals with pCRCI has not been explored. While previous pharmacological treatment studies in CRCI have yielded mixed results [21–26], a strong placebo effect similar to what we observed has not been previously reported for this population. A number of factors that occurred in the current study (discussed below) could be considered when designing a future clinical trial aimed at using transdermal nicotine to treat pCRCI.
Study design
The study that provided a template for the current study design was a 6-month randomized clinical trial evaluating transdermal nicotine as a treatment for mild cognitive impairment (MCI) [40]. The study by Newhouse et al. observed improvements in attention, memory, psychomotor speed, and subjective ratings of cognition after 6 months of treatment with transdermal nicotine compared with placebo. For the current study, the duration of 6 weeks on treatment and 8 weeks total was chosen to facilitate feasibility in order to gather preliminary data in a population we had not previously studied with transdermal nicotine. Our experience with a number of other transdermal nicotine trials informed our choice of study length. Prior studies examining the effects of nicotine on cognitive performance have shown benefit in 1 month of treatment [44]. Although we were able to detect a change/improvement in self-reported cognitive complaints, the treatment duration of 6 weeks may not have been enough time to distinguish between a drug and placebo response. For example, when FACT-Cog PCI change from baseline scores from open-label study visits was included in a graph with the double-blind data (Supplemental Figure S6), the open-label scores for the group that received nicotine during both the double-blind portion and open-label portions of the study (i.e., participants who received 12 weeks of nicotine vs 6 weeks) start to rise above those that received placebo. It may be the case that placebo effects are strong early-on in the study, but plateau or dissipate over longer study lengths. This suggests that, in future, longer treatment duration could potentially help separate the drug response from the placebo response.
Placebo responses are commonly reported in treatment studies across a wide range of medical and psychiatric conditions [67–70]. A number of potential factors may contribute to observed placebo response in clinical trials [71] including high levels of expectancy, the frequency of patient–clinician interactions [72], clinician/researcher attributes (e.g., personality, interaction style) [73], route of treatment administration [74], the dosing regimen, the color of pills, and the technological sophistication of the treatment procedures [75]. These treatment factors may be moderated by participant characteristics, including personality, demographics, self-efficacy, stress, previous experiences/personal history of patient–clinician/researcher interactions, and shared experiences of the patient and clinician/researcher [69, 71, 76, 77]. Measurement factors, which represent sources of bias and error inherent in measuring subjective symptoms, and natural history factors, such as spontaneous improvement or worsening in condition, provide additional sources of placebo effects [71]. The sum effects and the interactions of the aforementioned treatment, patient characteristic, measurement, and natural history factors result in the placebo response observed in clinical trials. The factors that likely contributed to the placebo response observed in the current study were the frequency of patient–clinician interactions over a short study length and clinician/researcher attributes (e.g., personality, interaction style).
As placebo responses can be affected by the frequency of patient–clinician interactions [72], one important consideration that could be made when extending a future trial length would be reducing the number of in-person study visits. One solution could be to minimize the number of in-person study visits, thereby limiting benefits of patient–clinician/researcher interactions, by using ecological momentary assessment (EMA) or web-based testing to collect data. EMA, also referred to as experience sampling, permits the repeated sampling of a research participant’s current behaviors and experiences in real time (e.g., self-report, actigraphy, psychophysiological variables), in their natural environments [78]. Although not required, EMA often uses mobile technology such as tablets and cell phones to collect these data [78]. EMA aims to capture more accurate self-reports by asking people about their experiences closer to the time and the context they occur [79].
Study dosing
Additionally, the maximum dose for the previous 6-month nicotine treatment study in MCI done by Newhouse was higher than the current study [40]. However, in non-smokers, high levels of nicotine can result in significant side effects including nausea, vomiting, dizziness, and lightheadedness. When the study was designed, it was judged that a maximum dose of 14 mg would be most tolerable (in terms of side effects) given the shorter study length and younger age of the participants. In terms of AEs, the study medication was very well tolerated. The majority of AEs experienced by both groups were mild in nature, with skin irritation being the most common AE. There was no difference between groups in the number of adverse events experienced, and the skin irritation was thus most likely the result of the adhesive rather than the nicotine. Rates of AEs in the current study were fewer compared with past studies using transdermal nicotine [40], but comparable considering the shorter study length. The only participants to withdraw due to AEs during the double-blind portion of the study were in the placebo group and there was no significant difference in number of AEs between groups in any body system. Thus, 14 mg may not have been a sufficient dose to fully test this hypothesis. The fact that substantial weight changes in the nicotine group were not observed also supports that the participants were under dosed since weight changes were observed in the MCI study conducted by Newhouse and colleagues. Importantly, we do not know about potential long-term risks with transdermal nicotine patches, although in our 6-month study, we did not observe any nicotine cravings, withdrawal, or other long-term negative effects [40]. No withdrawal symptoms were reported by participants nor did any participants report continuing nicotine use after the current study was completed.
Despite chemotherapy treatment being a known risk factor for cognitive decline, only limited information exists regarding negative cognitive effects of specific chemotherapy agents. The question of whether or not certain chemotherapy types contribute more than others to CRCI is difficult to answer for several reasons. The primary reason is that chemotherapy regimens often differ not only in the types of medications received but also the duration of treatment and number of cycles. Studies have shown that anthracyclines, taxanes, and cumulative chemotherapy exposure are associated with poorer cognitive functioning [80–82]. The 22 participants included in the analysis received 10 different treatment regimens, ranging anywhere from 4 to 18 cycles. With this het-erogeneity in a small sample, the cell sizes are too small and too numerous to make an adequate comparison. However, given that we were unable to detect group treatment differences, it is unlikely that this had any effect on the study outcome.
Another important limitation to consider is that this study only included women as participants, which limits the generalizability of these results for men with pCRCI. While the study did not specifically exclude for men, the sample consisted of primarily breast cancer survivors. Although breast cancer can occur in men, it is considerably less common (~ 1% of breast cancer cases per year) [83]. Our study is not unique by including exclusively women as the vast majority of CRCI research has been done primarily in breast cancer survivors [1, 9]; however, more research is needed to determine if the results of the current study would generalize to men.
Conclusion
In conclusion, we did not observe an effect of transdermal nicotine on subjective or cognitive performance due to a large placebo response. However, we were able to observe substantial improvement in self-reported cognitive symptoms, likely resulting from participation in the trial itself. This may suggest that women with pCRCI could benefit from the incorporation of not only support and validation but also cognitive rehabilitation/therapies that enhance the patient–clinician relationship, into their post-cancer care. Cognitive rehabilitation refers to a clinic-based, therapeutic program aimed at improving cognitive abilities, functional capacity, and real-world skills [8]. There is some evidence that suggests that nonpharmacological interventions such as cognitive behavioral therapy, cognitive brain training, mindfulness-based stress reduction, and physical activity may be beneficial for patients with CRCI [84, 85]. For example, two pilot studies examining cognitive behavioral therapy in breast cancer patients demonstrated improvement on both objective and subjective (self-report) measures of cognitive function [86, 87]. Computerized cognitive brain-training studies suggest improvement in executive functioning [88] and yoga may reduce subjective memory complaints [89]. It may also be the case that for a syndrome such as pCRCI, for which no current treatment exists and is only now becoming increasingly recognized and accepted, that the intensive nature of clinical management that these participants received (which is likely beyond the level of individual attention they might have received in a typical clinical setting) may have provided benefits such as stress reduction, decreased anxiety, and improvement of mood, thus contributing to the strong placebo response observed.
Supplementary Material
Acknowledgments
We wish to acknowledge the invaluable contributions of our research volunteers for their dedication to clinical research.
Funding Preparation of this work was supported by 1 R01 AG047992-01A1 to PN, Vanderbilt Institute for Clinical and Translational Research (VICTR) CTSA Grant (UL1TR000445) from the National Center for Advancing Translational Sciences to JNV, K24 MH110598 to WDT, and T32-AG058524 (JNV) and the Vanderbilt Memory & Alzheimer’s Center.
Footnotes
Conflict of interest JN Vega, KM Albert, PA Newhouse, WD Taylor, and Paul A. Newhouse declare no conflicts of interest. IA Mayer has received consultancy/advisory fees from AstraZeneca, Novartis, Genentech, Eli Lilly, Immunomedics, MacroGenics, and GlaxoSmithKline and received research funding from Novartis, Genentech, and Pfizer.
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the Declaration of Helsinki. This study was approved by the Vanderbilt-Ingram Cancer Center (VICC BRE 1692) and the Vanderbilt University Institutional Review Boards (IRB 141584).
Informed consent All participants gave written informed consent in accordance with the Declaration of Helsinki.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s11764-019-00786-6) contains supplementary material, which is available to authorized users.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Janelsins MC, Kohli S, Mohile SG, Usuki K, Ahles TA, Morrow GR. An update on cancer- and chemotherapy-related cognitive dysfunction: current status. Semin Oncol. 2011. [cited 2014 Jan 22];38: 431–8. Available from: http://www.sciencedirect.com/science/article/pii/S0093775411000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silberfarb PM. Chemotherapy and cognitive defects in cancer patients. Annu Rev Med. 1983. [cited 2014 Feb 26];34:35–46. Available from: http://www.ncbi.nlm.nih.gov/pubmed/6344764. [DOI] [PubMed] [Google Scholar]
- 3.Ahles TA. Brain vulnerability to chemotherapy toxicities. Psychooncology. 2012. [cited 2014 Feb 24];21:1141–8. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3788849&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahles TA, Saykin AJ, Furstenberg CT, Cole B, Mott LA, Skalla K, et al. Neuropsychologic impact of standard-dose systemic chemotherapy in long-term survivors of breast cancer and lymphoma. J Clin Oncol. 2002. [cited 2014 Feb 18];20:485–93. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11786578. [DOI] [PubMed] [Google Scholar]
- 5.Craig CD, Monk BJ, Farley JH, Chase DM. Cognitive impairment in gynecologic cancers: a systematic review of current approaches to diagnosis and treatment. Support Care Cancer. 2014. [cited 2014 Apr 4];22:279–87. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24212261. [DOI] [PubMed] [Google Scholar]
- 6.Mandelblatt JS, Hurria A, McDonald BC, Saykin AJ, Stern RA, VanMeter JW, et al. Cognitive effects of cancer and its treatments at the intersection of aging: what do we know; what do we need to know? Semin Oncol. 2013. [cited 2015 Jun 17];40:709–25. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3880205&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vega JN, Newhouse PA. Mild cognitive impairment: diagnosis, longitudinal course, and emerging treatments. Curr Psychiatry Rep. 2014. [cited 2014 Aug 30];16:490.Available from: http://www.ncbi.nlm.nih.gov/pubmed/25160795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wefel JS, Kesler SR, Noll KR, Schagen SB. Clinical characteristics, pathophysiology, and management of noncentral nervous system cancer-related cognitive impairment in adults. CA Cancer J Clin. 2015. [cited 2016 Jun 3];65:123–38. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4355212&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wefel JS, Schagen SB. Chemotherapy-related cognitive dysfunction. Curr Neurol Neurosci Rep. 2012. [cited 2014 Feb 21];12:267–75. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22453825. [DOI] [PubMed] [Google Scholar]
- 10.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016. [cited 2016 Jun 7]; Available from: http://doi.wiley.com/10.3322/caac.21349 [DOI] [PubMed] [Google Scholar]
- 11.Pedersen AD, Rossen P, Mehlsen MY, Pedersen CG, Zachariae R, von der Maase H. Long-term cognitive function following chemotherapy in patients with testicular cancer. J Int Neuropsychol Soc. 2009. [cited 2016 May 5];15:296–301. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19203434. [DOI] [PubMed] [Google Scholar]
- 12.Wefel JS, Vidrine DJ, Marani SK, Swartz RJ, Veramonti TL, Meyers CA, et al. A prospective study of cognitive function in men with non-seminomatous germ cell tumors. Psychooncology. 2014. [cited 2016 Jun 3];23:626–33. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4066616&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones D, Vichaya EG, Wang XS, Sailors MH, Cleeland CS, Wefel JS. Acute cognitive impairment in patients with multiple myeloma undergoing autologous hematopoietic stem cell transplant. Cancer. 2013. [cited 2016 Jun 3];119:4188–95. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3834212&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vardy J, Tannock I. Cognitive function after chemotherapy in adults with solid tumours. Crit Rev Oncol Hematol.2007. [cited 2014 Mar7];63:183–202. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17678745. [DOI] [PubMed] [Google Scholar]
- 15.Correa DD, Zhou Q, Thaler HT, Maziarz M, Hurley K, Hensley ML. Cognitive functions in long-term survivors of ovarian cancer. Gynecol Oncol. 2010. [cited 2014 Mar 10];119:366–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20630576. [DOI] [PubMed] [Google Scholar]
- 16.Chao HH, Uchio E, Zhang S, Hu S, Bednarski SR, Luo X, et al. Effects of androgen deprivation on brain function in prostate cancer patients - a prospective observational cohort analysis. BMC Cancer. 2012. [cited 2016 Jun 3];12:371 Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3502584&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahles TA, Saykin AJ. Breast cancer chemotherapy-related cognitive dysfunction. Clin Breast Cancer. 2002. [cited 2014 Feb 27];3 Suppl 3:S84–90. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12533268. [DOI] [PubMed] [Google Scholar]
- 18.Scheibel RS, Valentine AD, O’Brien S, Meyers CA. Cognitive dysfunction and depression during treatment with interferon-alpha and chemotherapy. J Neuropsychiatry Clin Neurosci. 2004. [cited 2019 Jun 7];16:185–91. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15260370. [DOI] [PubMed] [Google Scholar]
- 19.Hodgson KD, Hutchinson AD, Wilson CJ, Nettelbeck T. A meta-analysis of the effects of chemotherapy on cognition in patients with cancer. Cancer Treat Rev. 2013. [cited 2014 Feb 27];39:297–304. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23219452. [DOI] [PubMed] [Google Scholar]
- 20.Vega JN, Dumas J, Newhouse P. Cognitive effects of chemotherapy and cancer-related treatments in older adults. Am J Geriatr Psychiatry. 2017. [cited 2017 Apr 13];25:1415–26. Available from: http://www.sciencedirect.com/science/article/pii/S1064748117302786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lower EE, Fleishman S, Cooper A, Zeldis J, Faleck H, Yu Z, et al. Efficacy of dexmethylphenidate for the treatment of fatigue after cancer chemotherapy: a randomized clinical trial. J Pain Symptom Manage. 2009. [cited 2014 Mar 10];38:650–62. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19896571. [DOI] [PubMed] [Google Scholar]
- 22.Mar Fan HG, Clemons M, Xu W, Chemerynsky I, Breunis H, Braganza S, et al. A randomised, placebo-controlled, double-blind trial of the effects of d-methylphenidate on fatigue and cognitive dysfunction in women undergoing adjuvant chemotherapy for breast cancer. Support Care Cancer. 2008. [cited 2014 Mar 10];16: 577–83. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17972110. [DOI] [PubMed] [Google Scholar]
- 23.Kohli S, Fisher SG, Tra Y, Adams MJ, Mapstone ME, Wesnes KA, et al. The effect of modafinil on cognitive function in breast cancer survivors. Cancer. 2009. [cited 2014 Mar 10];115:2605–16. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2796482&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lundorff LE, Jønsson BH, Sjøgren P. Modafinil for attentional and psychomotor dysfunction in advanced cancer: a double-blind, randomised, cross-over trial. Palliat Med. 2009. [cited 2014 Mar10];23:731–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19648224. [DOI] [PubMed] [Google Scholar]
- 25.O’Shaughnessy JA. Effects of epoetin alfa on cognitive function, mood, asthenia, and quality of life in women with breast cancer undergoing adjuvant chemotherapy. Clin Breast Cancer. 2002. [cited 2014 Mar 10];3 Suppl 3:S116–20. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12533272. [DOI] [PubMed] [Google Scholar]
- 26.Fan HGM, Park A, Xu W, Yi Q-L, Braganza S, Chang J, et al. The influence of erythropoietin on cognitive function in women following chemotherapy for breast cancer. Psychooncology. 2009. [cited 2014 Feb 16];18:156–61. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18561284. [DOI] [PubMed] [Google Scholar]
- 27.Shaw EG, Rosdhal R, D’Agostino RB, Lovato J, Naughton MJ, Robbins ME, et al. Phase II study of donepezil in irradiated brain tumor patients: effect on cognitive function, mood, and quality of life. J Clin Oncol. 2006. [cited 2016 Dec 5];24:1415–20. Available from: http://www.jco.org/cgi/doi/10.1200/JCO.2005.03.3001 [DOI] [PubMed] [Google Scholar]
- 28.Castellino SM, Tooze JA, Flowers L, Hill DF, McMullen KP, Shaw EG, et al. Toxicity and efficacy of the acetylcholinesterase (AChe) inhibitor donepezil in childhood brain tumor survivors: a pilot study. Pediatr Blood Cancer. 2012. [cited 2016 Dec 5];59:540–7. Available from: http://doi.wiley.com/10.1002/pbc.24078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lawrence JA, Griffin L, Balcueva EP, Groteluschen DL, Samuel TA, Lesser GJ, et al. A study of donepezil in female breast cancer survivors with self-reported cognitive dysfunction 1 to 5 years following adjuvant chemotherapy. J Cancer Surviv [Internet]. 2016. [cited 2016 Jul 20];10:176–84. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26130292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dumas JA, Newhouse PA. The cholinergic hypothesis of cognitive aging revisited again: cholinergic functional compensation. Pharmacol Biochem Behav [Internet]. 2011. [cited 2014 Jan 30];99:254–61. Available from: http://www.sciencedirect.com/science/article/pii/S0091305711000682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ellis JR, Ellis KA, Bartholomeusz CF, Harrison BJ, Wesnes KA, Erskine FF, et al. Muscarinic and nicotinic receptors synergistically modulate working memory and attention in humans. Int J Neuropsychopharmacol [Internet]. 2006. [cited 2014 Feb 20];9: 175–89. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15877932. [DOI] [PubMed] [Google Scholar]
- 32.Newhouse PA, Potter A, Corwin J, Lenox R. Age-related effects of the nicotinic antagonist mecamylamine on cognition and behavior. Neuropsychopharmacology [Internet]. 1994. [cited 2014 Feb 20];10:93–107. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8024677. [DOI] [PubMed] [Google Scholar]
- 33.Vitiello B, Martin A, Hill J, Mack C, Molchan S, Martinez R, et al. Cognitive and behavioral effects of cholinergic, dopaminergic, and serotonergic blockade in humans. Neuropsychopharmacology [Internet]. 1997. [cited 2014 Feb 20];16:15–24. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8981385. [DOI] [PubMed] [Google Scholar]
- 34.Heishman SJ, Kleykamp BA, Singleton EG. Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology (Berl) [Internet]. 2010. [cited 2014 Feb 25];210:453–69. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3151730&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dawkins L, Powell JH, West R, Powell J, Pickering A. A double-blind placebo-controlled experimental study of nicotine: II–effects on response inhibition and executive functioning. Psychopharmacology (Berl) [Internet]. 2007. [cited 2014 Apr 9];190:457–67. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17205318. [DOI] [PubMed] [Google Scholar]
- 36.Engeland C, Mahoney C, Mohr E, Ilivitsky V, Knott V. Nicotine and sensory memory in Alzheimer’s disease: an event-related potential study. Brain Cogn [Internet]. 2002. [cited 2014 Apr 9];49: 232–4. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15259398. [PubMed] [Google Scholar]
- 37.Howe MN, Price IR. Effects of transdermal nicotine on learning, memory, verbal fluency, concentration, and general health in a healthy sample at risk for dementia. Int Psychogeriatr [Internet]. 2001. [cited 2014 Apr 9];13:465–75. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12003253. [DOI] [PubMed] [Google Scholar]
- 38.Newhouse PA, Sunderland T, Tariot PN, Blumhardt CL, Weingartner H, Mellow A, et al. Intravenous nicotine in Alzheimer’s disease: a pilot study. Psychopharmacology (Berl) [Internet]. 1988. [cited 2014 Apr 9];95:171–5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/3137593. [DOI] [PubMed] [Google Scholar]
- 39.Wilson AL, Langley LK, Monley J, Bauer T, Rottunda S, McFalls E, et al. Nicotine patches in Alzheimer’s disease: pilot study on learning, memory, and safety. Pharmacol Biochem Behav [Internet]. 1995. [cited 2014 Apr 9];51:509–14. Available from:http://www.ncbi.nlm.nih.gov/pubmed/7667377. [DOI] [PubMed] [Google Scholar]
- 40.Newhouse P, Kellar K, Aisen P, White H, Wesnes K, Coderre E, et al. Nicotine treatment of mild cognitive impairment: a 6-month double-blind pilot clinical trial. Neurology [Internet]. 2012. [cited 2014 Feb 5];78:91–101. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3466669&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Potter AS, Bucci DJ, Newhouse PA. Manipulation of nicotinic acetylcholine receptors differentially affects behavioral inhibition in human subjects with and without disordered baseline impulsivity. Psychopharmacology (Berl) [Internet]. 2012. [cited 2014 25];220: 331–40. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3288699&tool=pmcentrez&rendertype= abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mandelblatt JS, Hurria A, McDonald BC, Saykin AJ, Stern RA, VanMeter JW, et al. Cognitive effects of cancer and its treatments at the intersection of aging: what do we know; what do we need to know? Semin Oncol [Internet]. 2013. [cited 2014 Mar 17];40:709–25. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24331192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahles TA, Li Y, McDonald BC, Schwartz GN, Kaufman PA, Tsongalis GJ, Moore JH SA. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: the impact of APOE and smoking. Psychooncology [Internet]. 2014. [cited 2014 Jul 23]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/24789331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rezvani AH, Levin ED. Cognitive effects of nicotine. Biol Psychiatry [Internet]. Elsevier; 2001. [cited 2019 31];49:258–67. Available from: https://www.sciencedirect.com/science/article/pii/S0006322300010945?via%3Dihub [DOI] [PubMed] [Google Scholar]
- 45.Saykin AJ, Wishart HA, Rabin LA, Santulli RB, Flashman LA, West JD, et al. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology [Internet]. 2006. [cited 2014 May 6];67:834–42. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3488276&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saykin AJ, Wishart HA, Rabin LA, Santulli RB, Flashman LA, West JD, et al. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology [Internet]. 2006. [cited 2016 May 9];67:834–42. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16966547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dumas JA, Kutz AM, Naylor MR, Johnson JV, Newhouse PA. Estradiol treatment altered anticholinergic-related brain activation during working memory in postmenopausal women. Neuroimage [Internet]. 2012. [cited 2015 Jun 17];60:1394–403. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3303937&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gilewski MJ, Zelinski EM, Schaie KW. The Memory Functioning Questionnaire for assessment of memory complaints in adulthood and old age. Psychol Aging [Internet]. 1990. [cited 2014 May 6];5: 482–90. Available from: http://www.ncbi.nlm.nih.gov/pubmed/2278670. [DOI] [PubMed] [Google Scholar]
- 49.Squire LR, Wetzel CD, Slater PC. Memory complaint after electroconvulsive therapy: assessment with a new self-rating instrument. Biol Psychiatry [Internet]. 1979. [cited 2014 May 6];14:791–801. Available from: http://www.ncbi.nlm.nih.gov/pubmed/497304. [PubMed] [Google Scholar]
- 50.Saykin AJ. Neurobehavioral function and activities of daily living rating scale (NBFADL-63 item version). Hanover, NH: Dartmouth Medical School. [Google Scholar]
- 51.Jorm AF, Christensen H, Henderson AS, Korten AE, Mackinnon AJ, Scott R. Complaints of cognitive decline in the elderly: a comparison of reports by subjects and informants in a community survey. Psychol Med [Internet]. 1994. [cited 2014 May 6];24:365–74. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8084932. [DOI] [PubMed] [Google Scholar]
- 52.Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res [Internet]. [cited 2014 May 6];17:37–49. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7183759. [DOI] [PubMed] [Google Scholar]
- 53.Pfeffer RI, Kurosaki TT, Harrah CH, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol [Internet]. 1982. [cited 2014 May 6];37:323–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7069156. [DOI] [PubMed] [Google Scholar]
- 54.Jacobs SR, Jacobsen PB, Booth-Jones M, Wagner LI, Anasetti C. Evaluation of the Functional Assessment of Cancer Therapy Cognitive Scale with hematopoietic stem cell transplant patients. J Pain Symptom Manage [Internet]. 2007. [cited 2014 May 6];33:13–23. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17196903. [DOI] [PubMed] [Google Scholar]
- 55.Lai J-S, Butt Z, Wagner L, Sweet JJ, Beaumont JL, Vardy J, et al. Evaluating the dimensionality of perceived cognitive function. J Pain Symptom Manage [Internet]. NIH Public Access; 2009. [cited 2017 Dec 19];37:982–95. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19500722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sanford SD, Beaumont JL, Butt Z, Sweet JJ, Cella D, Wagner LI. Prospective longitudinal evaluation of a symptom cluster in breast cancer. J Pain Symptom Manage [Internet]. 2014. [cited 2014 May 20];47:721–30. Available from: http://www.sciencedirect.com/science/article/pii/S0885392413003254 [DOI] [PubMed] [Google Scholar]
- 57.Wagner LI, Sweet J, Butt Z, Lai J-S, Cella D. Measuring patient self-reported cognitive function: development of the Functional Assessment of Cancer Therapy-Cognitive Function instrument. J Support Oncol. 2009;7:W32–9. [Google Scholar]
- 58.The Conners continuous performance test. Toronto, Canada: multi-health systems; 1994. [Google Scholar]
- 59.The continuous performance test. Toronto, Canada: multi-health systems; 1995. [Google Scholar]
- 60.Kupke T, Lewis R. Relative influence of subject variables and neurological parameters on neuropsychological performance of adult seizure patients. Arch Clin Neuropsychol [Internet]. 1989. [cited 2014 May 21];4:351–63. Available from: http://www.ncbi.nlm.nih.gov/pubmed/14591131. [PubMed] [Google Scholar]
- 61.Hindmarch I Psychological performance models as indicators of the effects of hypnotic drugs on sleep. Psychopharmacology Suppl [Internet]. 1984. [cited 2014 May 21];1:58–68. Available from: http://www.ncbi.nlm.nih.gov/pubmed/6382255. [DOI] [PubMed] [Google Scholar]
- 62.Buschke H, Fuld PA. Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology [Internet]. 1974. [cited 2014 Oct 2];24:1019–25. Available from: http://www.ncbi.nlm.nih.gov/pubmed/4473151. [DOI] [PubMed] [Google Scholar]
- 63.Maruff P, Thomas E, Cysique L, Brew B, Collie A, Snyder P, et al. Validity of the CogState brief battery: relationship to standardized tests and sensitivity to cognitive impairment in mild traumatic brain injury, schizophrenia, and AIDS dementia complex. Arch Clin Neuropsychol [Internet]. 2009. [cited 2014 Apr 1];24:165–78. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19395350. [DOI] [PubMed] [Google Scholar]
- 64.Darby D, Maruff P, Collie A, Mc Stephen M. Mild cognitive impairment can be detected by multiple assessments in a single day. Neurology [Internet]. 2002. [cited 2014 May 21];59:1042–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12370459. [DOI] [PubMed] [Google Scholar]
- 65.Tannock IF, Ahles TA, Ganz PA, Van Dam FS. Cognitive impairment associated with chemotherapy for cancer: report of a workshop. J Clin Oncol [Internet]. 2004. [cited 2014 Feb 18];22:2233–9. Available from: http://jco.ascopubs.org/content/22/11/2233.full [DOI] [PubMed] [Google Scholar]
- 66.Bell ML, Dhillon HM, Bray VJ, Vardy JL. Important differences and meaningful changes for the Functional Assessment of Cancer Therapy-Cognitive Function (FACT-Cog). J Patient-Reported Outcomes [Internet]. Springer; 2018. [cited 2019 23];2:48 Available from: https://jpro.springeropen.com/articles/10.1186/s41687-018-0071-4 [Google Scholar]
- 67.Peciña M, Bohnert ASB, Sikora M, Avery ET, Langenecker SA, Mickey BJ, et al. Association between placebo-activated neural systems and antidepressant responses: neurochemistry of placebo effects in major depression. JAMA psychiatry [Internet]. NIH Public Access; 2015. [cited 2018 Feb 27];72: 1087–94. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26421634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Agid O, Siu CO, Potkin SG, Kapur S, Watsky E, Vanderburg D, et al. Meta-regression analysis of placebo response in antipsychotic trials, 1970–2010. Am J Psychiatry [Internet]. 2013. [cited 2018 Feb 27];170:1335–44. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23896810. [DOI] [PubMed] [Google Scholar]
- 69.Colagiuri B, Schenk LA, Kessler MD, Dorsey SG, Colloca L. The placebo effect: from concepts to genes. Neuroscience [Internet]. NIH Public Access; 2015. [cited 2018 Feb 27];307: 171–90. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26272535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mestre TA, Shah P, Marras C, Tomlinson G, Lang AE. Another face of placebo: the lessebo effect in Parkinson disease: meta-analyses. Neurology [Internet]. American Academy of Neurology; 2014. [cited 2018 Feb 27];82:1402–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24658930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rutherford BR, Roose SP. A model of placebo response in antidepressant clinical trials. Am J Psychiatry [Internet]. NIH Public Access; 2013. [cited 2018 Mar 2];170:723–33. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23318413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Posternak MA, Zimmerman M. Therapeutic effect of follow-up assessments on antidepressant and placebo response rates in antidepressant efficacy trials. Br J Psychiatry [Internet]. 2007. [cited 2018 Feb 28];190:287–92. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17401033. [DOI] [PubMed] [Google Scholar]
- 73.Oken BS. Placebo effects: clinical aspects and neurobiology. Brain [Internet]. Oxford University Press; 2008. [cited 2018 Feb 28];131: 2812–23. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18567924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Khan A, Kolts RL, Thase ME, Krishnan KRR, Brown W. Research design features and patient characteristics associated with the outcome of antidepressant clinical trials. Am J Psychiatry [Internet]. 2004. [cited 2018 Mar 2];161:2045–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15514405. [DOI] [PubMed] [Google Scholar]
- 75.Benedetti F Mechanisms of placebo and placebo-related effects across diseases and treatments. Annu Rev Pharmacol Toxicol [Internet]. 2008. [cited 2018 Mar 2];48:33–60. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17666008. [DOI] [PubMed] [Google Scholar]
- 76.Brody H The placebo response. Recent research and implications for family medicine. J Fam Pract [Internet]. 2000. [cited 2018 Feb 28];49:649–54. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10923577. [PubMed] [Google Scholar]
- 77.Di Blasi Z, Harkness E, Ernst E, Georgiou A, Kleijnen J. Influence of context effects on health outcomes: a systematic review. Lancet (London, England) [Internet]. 2001. [cited 2018 Feb 28];357:757–62. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11253970. [DOI] [PubMed] [Google Scholar]
- 78.Armey MF, Schatten HT, Haradhvala N, Miller IW. Ecological momentary assessment (EMA) of depression-related phenomena. Curr Opin Psychol [Internet]. Elsevier; 2015. [cited 2018 Apr 24];4: 21–5. Available from: https://www.sciencedirect.com/science/article/pii/S2352250X15000494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annu Rev Clin Psychol [Internet]. 2008. [cited 2018 Apr 20];4:1–32. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18509902. [DOI] [PubMed] [Google Scholar]
- 80.Kesler SR, Blayney DW. Neurotoxic effects of anthracycline- vs nonanthracycline-based chemotherapy on cognition in breast cancer survivors. JAMA Oncol [Internet]. 2016. [cited 2016 Jul 20];2: 185–92. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26633037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cerulla N, Arcusa À, Navarro J-B, Garolera M, Enero C, Chico G, et al. Role of taxanes in chemotherapy-related cognitive impairment: a prospective longitudinal study. Breast Cancer Res Treat [Internet]. 2017. [cited 2018 Jun 5];164: 179–87. Available from:http://www.ncbi.nlm.nih.gov/pubmed/28421379. [DOI] [PubMed] [Google Scholar]
- 82.Collins B, MacKenzie J, Tasca GA, Scherling C, Smith A. Cognitive effects of chemotherapy in breast cancer patients: a dose-response study. Psychooncology [Internet]. 2013. [cited 2016 Oct 14];22:1517–27. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22936651. [DOI] [PubMed] [Google Scholar]
- 83.American Cancer Society. Cancer facts & figures. Atlanta; 2013. [Google Scholar]
- 84.Janelsins MC, Kesler SR, Ahles TA, Morrow GR. Prevalence, mechanisms, and management of cancer-related cognitive impairment. Int Rev Psychiatry [Internet]. Informa Healthcare USA; 2014. [cited 2014 6];26:102–13. Available from: http://informahealthcare.com/doi/abs/10.3109/09540261.2013.864260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Joly F, Giffard B, Rigal O, De Ruiter MB, Small BJ, Dubois M, et al. Impact of cancer and its treatments on cognitive function: advances in research from the Paris International Cognition and Cancer Task Force Symposium and Update Since 2012. J Pain Symptom Manage [Internet]. 2015. [cited 2016 Dec 3];50:830–41. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26344551. [DOI] [PubMed] [Google Scholar]
- 86.Ferguson RJ, McDonald BC, Rocque MA, Furstenberg CT, Horrigan S, Ahles TA, et al. Development of CBT for chemotherapy-related cognitive change: results of a waitlist control trial. Psychooncology [Internet]. 2012. [cited 2016 5];21:176–86. Available from: http://doi.wiley.com/10.1002/pon.1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ferguson RJ, Ahles TA, Saykin AJ, McDonald BC, Furstenberg CT, Cole BF, et al. Cognitive-behavioral management of chemotherapy-related cognitive change. Psychooncology [Internet]. 2007. [cited 2016 Dec 5];16:772–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17152119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kesler S, Hadi Hosseini SM, Heckler C, Janelsins M, Palesh O, Mustian K, et al. Cognitive training for improving executive function in chemotherapy-treated breast cancer survivors. Clin Breast Cancer [Internet]. 2013. [cited 2016 Dec 5];13:299–306. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1526820913000499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Janelsins MC, Peppone LJ, Heckler CE, Kesler SR, Sprod LK, Atkins J, et al. YOCAS©® yoga reduces self-reported memory difficulty in cancer survivors in a nationwide randomized clinical trial: investigating relationships between memory and sleep. Integr Cancer Ther [Internet]. 2015. [cited 2016 Jun 1]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/26621521. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.