Abstract
Sleep disruption is a commonly reported consequence of cancer and its treatment in pediatric patients and survivors. This review summarizes common sleep concerns in this population and introduces a multidimensional framework of risk factors specific to childhood cancer that may interact to develop and maintain disrupted sleep. Based on the extant literature, parameters of the cancer and its treatment, physical and social environmental conditions both during and after treatment, changes to family behavior and norms, psychological factors and traumatic stress, and reduced physical activity are hypothesized to be the most pertinent risk factors for disrupted sleep in this population. Potential clinical assessment strategies and behavioral interventions relevant to these considerations are discussed, with reference to the behavioral model of insomnia. The review concludes by offering directions for research and clinical practice, including developing and testing comprehensive assessment tools, intervention effectiveness studies in both oncology and primary care clinics, and efforts to increase patient-provider communication about sleep in pediatric oncology.
Keywords: children, pediatric, cancer, oncology, sleep, insomnia, cognitive behavioral therapy for insomnia, CBT-I
‘One of the most chronic and disturbing side effects reported by pediatric cancer patients and survivors are problems with sleep . . . difficulties initiating and maintaining sleep.’
Approximately 1% of cancer cases diagnosed each year occur in children.1 Due to medical advances, the 5-year survival rate has increased to nearly 80% for some cancers, and it is estimated that there are upwards of 388 501 pediatric cancer survivors living in the United States.2 Of these, 150 074 (38.6%) are 5 to 20 years from their initial diagnosis, with 248 079 (63.8%) being survivors of ≥20 years. Because so many childhood patients reach long-term survivorship status, there has been an increased focus on lasting health and quality of life in this population. The disease and its treatment can lead to both early and late morbidities including physical limitations,3,4 pain,5 cognitive dysfunction,6 behavioral problems, social deficits,7,8 psychological distress, and other affective symptoms.7,9,10 One of the most chronic and disturbing side effects reported by pediatric cancer patients and survivors are problems with sleep, and in particular, difficulties initiating and maintaining sleep, suggesting that this is a major quality of life concern.6,11-15 Thus, it is highly important that health care providers who interact with this population be aware of the high prevalence of sleeping problems, unique risk factors in pediatric oncology, and the corresponding clinical practice implications.
Sleep disruption, which is a term that describes a range of difficulties related to getting enough quality sleep, is a considerable late effect of pediatric cancer that warrants empirical and clinical attention. Thus, the major goals of this review are to (1) identify and describe the scope of sleep disruption in pediatric oncology; (2) synthesize major findings related to predictors and correlates of disrupted sleep, and introduce a conceptual framework summarizing these factors; and (3) offer potential behavioral assessment and intervention strategies as suggestions for future research endeavors. Childhood is a diverse time period, from infancy to late adolescence, and there are unique developmental concerns for different age groups. Because the goal of the current review is to provide an overall context for this topic in pediatric oncology, it is important to note that developmental stage should be an additional consideration when applying research findings across this broad population.
Healthy Sleep and Sleep Disruption
High-quality sleep is crucial for healthy emotional, cognitive, physical, and behavioral development in childhood. Few studies have clearly defined “normal” sleep during childhood, but generally accepted definitions involve receiving a developmentally appropriate number of hours of sleep (sleep need declines from infancy to adolescence), few or no wakings during the night, and a subjective perception of feeling rested.16,17 Similarly, pediatric sleep disruption is fairly subjective, with symptom reporting often linked with the extent to which a child’s sleep affects a parent’s sleep.16 Disrupted sleep is a significant concern in childhood and adolescence, given that unconsolidated and short sleep has been shown to interfere with attention and concentration,18,19 learning, cognitive development,20,21 and performance in both social and academic domains.16,22-25 That is, sleep is a foundation for healthy growth and development, and poor sleep can have a wide-ranging impact on a child’s current and future well-being.
Although most parents report that their children have good sleep patterns, sleep disruption is not uncommon, affecting 20% to 30% of healthy children.26,27 However, more than half of pediatric cancer survivors report some combination of sleep-related symptoms even 9 to 15 years after their diagnosis.12,13 Good sleep is important for all children, but especially so for those who have been affected by cancer, given that disrupted sleep can affect inflammatory pathways and immune functioning, including natural killer cell activity.28,29 Moreover, sleep problems contribute to, and can exacerbate, many other morbidities common to survivorship.6,30
Insomnia
Pediatric insomnia is conceptualized as a constellation of symptoms representing repeated trouble with (1) sleep initiation, (2) sleep duration that is excessively short or long (hypersomnia), (3) poor sleep consolidation (fragmented sleep), or (4) a perception of low sleep quality “that occurs despite age-appropriate time and opportunity for sleep and results in daytime functional impairment for the child and/or family.”31 When making a diagnosis, the developmental stage of the child should be considered as sleep needs are greatest in early childhood and lessen as children age.17,32,33 Despite working definitions of pediatric insomnia, studies that have assessed prevalence and correlates of the clinical diagnoses are rare.11,34 None have been conducted in the context of pediatric oncology to our knowledge. Instead, sleep disruption in this population is commonly assessed and reported in terms of specific sleep domains that occur over an extended period of time. These include assessments of the quantity and quality of sleep, bedtime resistance, sleep onset delays, night wakings, sleep efficiency, sleep anxiety, and/or daytime sleepiness.
Fatigue and Sleepiness
Daytime fatigue and sleepiness are common concerns that may be concomitant with sleep disruption. Although the terms are often used interchangeably, they are distinct constructs. Fatigue is characterized by low energy and weariness, whereas sleepiness is the normative experience of feeling that one needs to sleep, and is only considered a disorder when it occurs too frequently (eg, excessive daytime sleepiness) or infrequently (eg, insomnia).35 Both fatigue and excessive daytime sleepiness occur in children and adolescents who have not been affected by cancer,16 but rates are higher among pediatric cancer patients11 and survivors.14,36
Behavioral Model of Insomnia and Pediatric Oncology
The Behavioral Model of Insomnia has been widely used to explain the process by which disruption to specific sleep domains become chronic and persistent.37 Within this model, sleep difficulties are theorized to develop from an interaction of predisposing and precipitating factors, which trigger an acute episode of symptoms. Predisposing factors refer to inherent characteristics of an individual, such as physical hyperarousal, hyperactivity, or a tendency to ruminate or worry. Precipitating factors are acute occurrences in an individual’s life that interact with predisposing factors and may include stress, psychological or physical illness, or even changes to the environment that interfere with normal sleep phases. In the context of pediatric oncology, a child may present with various levels of predisposing factors and preexisting sleep disruption. These predisposing factors are then theorized to interact with the stress of diagnosis, illness variables, and environmental and social changes during active treatment. These precipitating factors may also be retained into survivorship.
After an acute period of symptoms, perpetuating factors are theorized to contribute to the development and maintenance of chronic sleep disruption. Perpetuating factors prevent the reestablishment of normal sleep and may include maladaptive strategies that patients use to try to fix or adapt to their sleep and transient sleeping schedule. For example, a pediatric cancer patient may sustain neuroanatomical or physiological changes that affect their long-term sleep functioning, or they may maintain habits that began during treatment such as napping more frequently, which can negatively affect their sleep drive and ability to fall asleep that night. Additional factors may also include doing other activities in bed (eg, watching TV, reading) or spending an excessive amount of time in bed (eg, getting into bed earlier, or staying in bed until later), because the child feels fatigued or believes that these behaviors may assist with relaxation, falling asleep, or increasing the amount of time they are asleep. Paradoxically, such behaviors will maintain their sleep symptoms, particularly with repeated practice.
It follows that a child who has been affected by cancer may become increasingly frustrated, stressed, and anxious as their sleep deteriorates and that they may have these psychological experiences while lying in bed trying to sleep. Conditioned arousal, which is an individual’s association between wakefulness and arousal with cues that are normally associated with sleep (eg, being in bed), provides an explanatory mechanism for how the perpetuating factors become learned responses.38 Thus, conditioned arousal allows for the maintenance of symptoms even after a child has transitioned into the survivorship period. In children, conditioned arousal may also contribute to a constellation of nighttime symptoms including sleep anxiety, bedtime refusal, and difficulty falling asleep or maintaining sleep.
Etiology and Maintenance of Sleep Disruption in Childhood Cancer
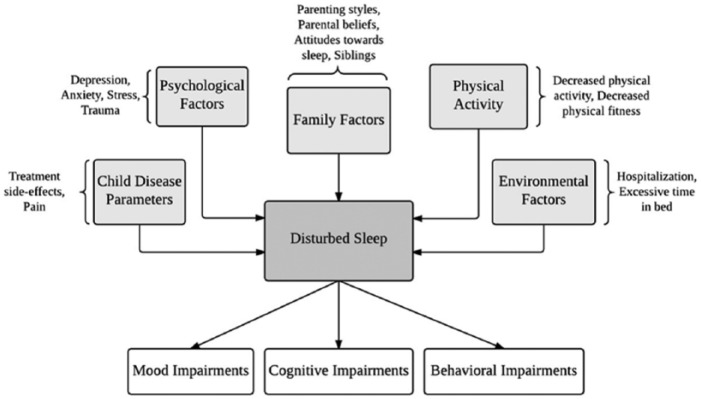
It is relatively common for children and adolescents to contend with biological (eg, shifting hormones), situational (eg, early school start times), and behavioral (eg, irregular sleep schedule) precipitating factors for sleep disruption.39 However, there are additional risk factors that are unique to the pediatric oncology population. It is well-documented that cancer patients are at increased risk for sleep disruption during the acute stresses of diagnosis and treatment,40 but childhood cancer patients may be particularly vulnerable because of their developmental stage and the fact that they likely do not yet have engrained healthy sleep habits.41,42 In the paragraphs below, we review factors related to childhood cancer and its treatment that may interact with predisposing individual characteristics, leading to disrupted sleep during treatment that continues into survivorship. Based on the extant literature, we propose a multidimensional framework describing the disease parameters, specific environmental conditions, psychological factors, family factors, and changes in physical activity that lead to the development and maintenance of sleep disruption in pediatric cancer patients and survivors (Figure 1). The temporal nature of how each variable contributes to the emergence and maintenance of disrupted sleep has yet to be definitively established. Thus, the proposed theoretical framework is presented within the behavioral model of insomnia and provides an outline for future research to clarify the underlying mechanisms.
Figure 1.
A proposed conceptual framework of sleep disruption in children impacted by cancer.
Disease Parameters
Cancer-related neuroanatomical and physiological changes may affect sleep, as has been suggested in adult oncology patients and survivors.43 In children, the most common types of cancer are leukemia (26% of cases) and malignancies of the brain and nervous system (18% of cases), with acute lymphocytic leukemia and acute myeloid leukemia comprising the majority of leukemia cases.1 Cancers with central nervous system involvement confer a high direct risk for insomnia and sleepiness, particularly when nuclei in sleep cycle regulation (eg, hypothalamus, pituitary gland, diencephalon, brainstem) are damaged by the tumor, the treatment, or both.11,44,45 Although treatment depends on the type of cancer and its stage, common strategies that may affect sleep in childhood patients include chemotherapy,46-48 bone marrow transplantation,49 and dexamethasone50,51 (a glucocorticoid frequently used to treat acute myeloid leukemia). High-dose cranial radiation has also been linked with sleep disruption among adult survivors of childhood cancer.52 Within the current framework, these factors may be precipitating (eg, changes that resolve after treatment has ended) and/or perpetuating (eg, long-term changes).
Pain and discomfort (eg, nausea) related to the disease process, medical procedures, and treatment side effects can initiate new sleep disruption symptoms53 and exacerbate existing ones, serving as precipitating factors for children in treatment.54-58 Aggressive pain management strategies including analgesic medication can mitigate the impact of cancer-related pain on sleep in hospitalized children.59 However, many commonly prescribed medications (ie, opioid analgesics such as morphine and fentanyl) have been associated with disrupted sleep in adults, and these effects need to be further evaluated in pediatrics.60,61
Environmental Factors
Changes to a child’s sleep environment, which may occur during hospitalization or at home, can be a precipitating factor that allows for the emergence of sleep disruption. Children in active treatment may be hospitalized for a period of time, significantly altering their daily environment and routines. For example, children who receive bone marrow transplants have prolonged hospitalizations where they are restricted to a room in order to reduce the risk of infection following the procedure. In the hospital, a child sleeps in an unfamiliar room and bed with sound and light pollution, recurrent entries and exits by parents and staff, nighttime medical procedures, and medication administration.42,46,49,54,62 These events have been associated with recurrent awakenings, fewer hours of sleep, and lower satisfaction with sleep in patients.42,46,54,62
Changing patterns of light exposure have also been shown to disrupt the circadian rhythm and sleep in both healthy63 and oncology64 populations and thus may serve as a precipitating factor for sleep disruption. For example, increased screen time (ie, time spent watching TV, or using a computer or handheld electronic device) before bed increases nighttime light exposure and has been shown to disrupt the sleep-wake cycle in healthy children and adolescents.65,66 Although this has not been specifically evaluated in pediatric oncology, it is plausible that this would be a significant risk factor given that clinically, it is common to see pediatric oncology patients watching TV or using tablets in bed, and some research has suggested that screen time may be increased among these patients.67 Additionally, if a child is hospitalized or not feeling well enough to leave the house, they may have restricted daytime light exposure, which is known to disrupt the sleep-wake cycle.63 Although studies of daytime sunlight underexposure in pediatric oncology have not been conducted, low levels of daytime light have been linked with fatigue and sleep impairment in other hospitalized populations.68
Excessive time in bed while in the hospital can also precipitate disrupted sleep, as has been demonstrated in hospitalized adults.69 During active treatment, a child may increase the amount of time they spend in bed, either at home or in the hospital, with some children even being temporarily restricted to bed.42,70,71 This can lead to sleeping at irregular hours during the day, which weakens the nighttime sleep drive.42,72 Moreover, the association between the bed and sleeping can be weakened if a child engages in activities other than sleep (eg, screen time, homework, visitation) while in bed. Over time, time in bed may also serve as a perpetuating factor, particularly if a child begins to do so in an attempt to achieve more or better sleep.
Family Factors
The impact of pediatric cancer cannot be understood in isolation from a child’s most important social relationships, which are typically within their immediate family. In families that are not affected by cancer, parental depressive symptoms73 and sleep disruption74 have been linked with children’s sleep disruption. In turn, healthy children’s sleep disruption has also been shown to impair maternal sleep.75 It has also been documented that siblings influence one another across a wide variety of behaviors,76 and there is an emerging literature suggesting that this is also true for sleep length in healthy adolescents.77 Together, these findings suggest that there may be some level of familial interdependence that serves as a precipitating factor for sleep disruption.
When a child has cancer, family members may feel shocked, guilty, sad, and anxious; roles and responsibilities may shift; and family functions and routines may change, putting significant stress on the entire family.78-80 Not surprisingly, parents of children with cancer report lower physical and mental well-being,81 and significantly disrupted sleep,54-56,79,81-83 particularly while staying with their child in the hospital.84 At least one study has also demonstrated that parent and patient sleep disruption is modestly associated.54 Siblings of childhood cancer patients and survivors also report psychosocial problems, sleep disruption, and fatigue at levels that are similar to the affected child.14,85 Although no studies have evaluated the possibility of mutual influence for sleep disruption among family members, it is plausible that such a system exists.
Another relevant precipitating factor may be parental presence during a patient’s sleep time. This practice, termed co-sleeping, may occur when a child is hospitalized and a parent stays overnight in the same room or sleeps on the same surface as the child (ie, bed sharing).86 Co-sleeping and bed sharing may also happen at home during treatment and/or into survivorship. While one study of children undergoing maintenance chemotherapy for acute myeloid leukemia found that parents often report their child sleeps better when someone sleeps near them at home,54 over time, co-sleeping may elicit a conditioned effect wherein a child may begin to have problems falling asleep or staying asleep independently if the parent is not present, because the child is expecting the parental cue in order to fall asleep.87,88 Although the goal of co-sleeping is often to facilitate better sleep, it may inadvertently lead to a child’s dependency on their parents for sleep-related support and cause problems when a parent does not co-sleep with their child (ie, co-sleeping can become a perpetuating factor as well).87,88 Co-sleeping may also shift parental attention toward their child’s sleep and increase their dysfunctional beliefs about sleep (eg, “my child will always have difficulty sleeping”), both of which have been linked with greater child-reported sleep disruption in children not affected by cancer.89
Parenting style during cancer treatment may also play a central role in the development (ie, precipitating factor) of sleep disruption in patients.88 During a child’s cancer treatment, many parents report having difficulty setting limits88 and being more likely to “spoil” their child.90 This may involve facilitating new bedtime practices (eg, TV, food, drink, or other activities in bed),54 and allowing for delayed bed times and wake times.46 When routines, rules, and sleep hygiene practices (activities, behaviors, and habits that encourage optimal sleep)91 such as having a regular bedtime, and only using the bed for sleep, that are normally in place are absent or inconsistent, new bedtime problems such as avoidance of bed, refusal to get into or stay in bed, frequent requests after lights are out, and impaired sleep, may arise.26 It follows that, if maintained, these could also serve as perpetuating factors into survivorship.
Psychological Factors
A diagnosis of pediatric cancer can be conceptualized as a traumatic stressor, with substantial variability in psychosocial adjustment after the initial crisis has passed. Although there are differences across the developmental continuum in terms of a child’s ability to understand the meaning and implications of their disease, it is clear that the stress of a cancer diagnosis and its treatment has many consequences.92,93 Traumatic stress has been linked with at least some transient sleep disruption in many populations,94,95 and a relatively recent review has also suggested that childhood trauma (eg, abuse, family conflict, injuries, accidents, and other stressful events) is predictive of long-term sleep disruption in children.96 Thus, it is plausible that traumatic stress related to pediatric cancer may be a significant precipitating factor for sleep disruption.
Relatedly, the prevalence of clinically significant anxiety and depression is higher in pediatric cancer patients and survivors (~25%) than in unaffected children.97-99 Overlap between these disorders and sleep disruption has been well-established in many populations,100 and there is also empirical evidence of this in both pediatric cancer patients87 and survivors.101 Disrupted sleep has also been shown to predict the onset of depressive symptoms 1 week later among children and adolescents hospitalized for cancer treatment,62 suggesting that the relationship could be bidirectional. To the extent that children experience elevated levels of anxiety or depression prediagnosis, these could serve as predisposing factors for the development of sleep disruption. If mental health symptoms are untreated they may also be characterized as perpetuating factors that contribute to the development of conditioned arousal and maintenance of sleep disruption.
Physical Activity
It is well-established that physical activity is associated with better sleep quantity and quality in the general population,102 adult cancer patients,103 and adult cancer survivors.104 In pediatric cancer, it is likely a precipitating factor for acute sleep disruption as well, given that decreased physical activity has been linked with both increased number of wakings after sleep onset, decreased sleep time,105 and increased fatigue106 in pediatric cancer patients. Furthermore, some intervention research in pediatric oncology suggests that increasing levels of physical activity may enhance these outcomes,107 although another small study reported mixed evidence regarding the efficacy of a physical activity intervention on sleep efficiency.108
During cancer treatment, levels of physical activity and overall fitness decline due to changes in children’s daily routines, prolonged hospitalizations, altered physical functioning, side effect management,109 and increased screen time.67 It might be expected that a child would return to their prediagnosis levels of physical activity during survivorship, but several studies have indicated that pediatric cancer survivors are far more sedentary and have lower levels of physical fitness than their unaffected peers,109-112 suggesting that it may also serve as a perpetuating factor for disrupted sleep. While the maintenance of relatively lower physical activity in survivors is not completely understood, it has been suggested that the social (family and peer support) and environmental (access to resources, neighborhood characteristics) contexts are particularly important to maintaining these physical activity patterns in the long term, although cancer-related and demographic factors likely also contribute.113
Clinical Practice Implications
Although sleep disruption is a ubiquitous and debilitating consequence of childhood cancer and its treatment, there is a surprising lack of communication about sleep in clinical settings. In one study of pediatric cancer survivors (N = 75), medical notes suggested that 66.7% of all patients were not asked about sleep during routine visits at long-term survivorship clinics.13 Of the subsample with insomnia (n = 21), sleep was documented for 7 of the patients, and only 1 patient was provided with recommendations for their sleep.13
Evidently, there is a critical need for health care providers who work with children who have been affected by cancer to be aware of the increased risk of sleep disruption, the unique precipitating and perpetuating risk factors in this population, and the implications of those concerns for clinical practice, and be willing and able to open a dialogue about sleep. This is important for survivors, but also patients, given that the etiology of many behavioral aspects of sleep disruption begin during active treatment.
It should also be acknowledged that many health care practitioners receive little formal training to assess and intervene around child sleep disruption, and consultation with a hospital specialist (eg, psychologists, social workers) with expertise in sleep is likely warranted in many cases. Thus, in the absence of established consensus guidelines, the proposed framework offers multiple opportunities for the assessment and treatment of sleep disruption in childhood cancer patients and survivors that warrant attention in future clinical research.
Assessment of Sleep Disruption in Pediatric Oncology
Because not all children affected by cancer experience sleep disruption, an initial brief screening during regular clinic visits could be used to identify and triage individuals who would benefit from a more detailed assessment, as is the recommendation in adult oncology.114 This may be accomplished with a self-report measure of sleep quality for the child (age dependent) and/or parent to fill out while in the waiting room. There are several relatively short, valid, and reliable assessment tools of sleep disruption for children115 including the Children’s Sleep Habits Questionnaire,116 which is filled out by parents of children ages 4 to 10, or the Sleep Self Report,117 which is filled out by children ages 7 to 12. However, neither instrument has been validated for use specifically within pediatric oncology. During the appointment, providers may also ask open-ended questions regarding patient and parent perceptions of the child’s sleep quality.
For those who indicate some impairment, a provider may refer the patient to a sleep specialist, or gather further information during that visit. In the absence of a standardized tool to explore specific risks for sleep disruption in pediatric cancer, this information may be obtained by a review of the medical chart and clinical interview, and then integrated into the patient’s case conceptualization in order to identify the most appropriate route for treatment. Ideally, information should be reported from multiple sources including the child, parents, and, if applicable, other health care providers (eg, nurse in a hospital) who have observed the child’s sleep behavior.
Following general guidelines for pediatric sleep assessment, areas to be queried may include the following: the child’s prediagnostic sleep history, current pattern of sleep-wake habits via a 24-hour activity recall (including sleep/wake times, napping, daytime impairment, caffeine consumption, and daily activities), a 7-day sleep diary containing the elements of the 24-hour recall over the previous week, characteristics of the specific sleep-related symptoms (eg, falling asleep, wakings), medications or supplements that are being taken for sleep disruption (these are often used without knowledge of the medical team118), psychiatric symptoms (eg, mood), behavioral symptoms (eg, oppositional), child and parental beliefs regarding sleep, subjective impact of sleep disruption on functioning and quality of life (eg, performance in school), parenting strategies, cultural norms (eg, co-sleeping and bed sharing may be more common in collectivistic cultures), family history of sleep disorders, and goals for their sleep.91 Special considerations for disease parameters include the specific diagnosis and cancer stage, date of diagnosis, disease duration, past and present treatments, comorbidities, symptoms from the disease and/or treatment (eg, nausea, pain), and the amount of time in survivorship. Environmental factors to consider include changes to the amount of time a child spends in bed and whether this is due to medical recommendations or personal preference, nighttime disruptions that occurred during hospitalization, nighttime disruptions that are still occurring, and level of daytime and nighttime light exposure. Family factors to assess include changes in parenting strategies (eg, setting fewer limits) and resultant differences in the child’s sleep hygiene practices (eg, co-sleeping, screen time). Clinicians may also consider evaluating the psychological functioning of the parents and, if applicable, whether other family members (eg, siblings, parents) have disrupted sleep. Although psychological symptomatology is included in standard pediatric sleep assessments, providers may also want to evaluate changes in psychological functioning that may be specific to their cancer (eg, traumatic stress). Current physical activity and sedentary behavior, including screen time, should also be evaluated and contrasted with prediagnosis levels of these activities.
These recommendations are comprehensive, and empirical evaluation is needed to determine the most salient areas of assessment so that clinicians may tailor or shorten the interview based on an individual patient’s needs. Additionally, as with sleep evaluations in healthy children, this information should be considered within the child’s developmental context (eg, napping is more appropriate for a child in preschool than a child in middle school)91 and physical condition (eg, a child in active treatment may have increased sleep needs). Finally, it is worth noting that while objective measurement of sleep (ie, polysomnography, actigraphy) is the gold standard for sleep assessment in research, qualitative reports may be used in clinical settings because the criterion rests on the patient’s subjective perception of their sleep and functioning, and these are generally more accessible in health care contexts. If available, it would be ideal to also include objective assessments to use in combination with subjective reports to get a more precise measure of the actual amount of sleep a patient achieved, and how the patient is perceiving their sleep.
Behavioral Intervention in Pediatric Oncology
Sleep disruption is unlikely to remit without intervention,119 and longitudinal research shows that sleep disruption persists from childhood into adolescence for many children.120,121 Once a child has been identified as experiencing sleep disruption, there may be several intervention options, depending on the child’s age and current medical condition, which can help reestablish normal sleep patterns. To date, there have been no published trials using accepted guidelines from behavioral sleep medicine to treat sleep disruption in pediatric oncology patients or survivors. The intervention opportunities described below are based on results from clinical practice and the existing behavioral sleep medicine literature. Future research is needed to formally test the efficacy of such interventions within pediatric oncology.
Pharmacological Interventions
A significant number of children are prescribed medications to treat sleep disruption.122 These may be useful in conjunction with other chronobiotic treatments (eg, light therapy) when sleep-wake disruption is neurobiologically located,43 although it is worth noting that while hypnotic medications are effective for short-term treatment of insomnia, they are not recommended for long-term use, and there is little empirical evidence speaking to their safety or efficacy in children.118,123 A variety of medications are frequently prescribed in children not affected by cancer including barbiturates, benzodiazepines, α-agnostics, and tricyclic antidepressants.118,124 Over-the-counter medications such as antihistamines124 and melatonin118 are also often used. Notably, melatonin has been the subject of recent reviews with some concluding that it is helpful in reducing sleep onset latency and slightly extending total sleep time in children125,126 and others questioning its safety for use in children given its effects on multiple developing systems, specifically, reproductive, cardiovascular, immune, and metabolic.127,128
Although medication can be useful in the short term, it may not be the ideal solution in pediatric cancer patients and survivors who already have a complex schedule of treatments and medications. During active treatment, patients have access to a number of sleep-inducing medications. If habitual use of sleep medications is initiated during treatment and maintained into survivorship, this may even become a perpetuating factor, as continuous medication usage may disrupt a child’s natural sleep cycle and contribute to beliefs that medication is necessary for sleep. Additionally, medication fails to target long-term perpetuating factors (eg, conditioned arousal, sleep-wake cycle dysregulation) responsible for maintaining sleep disruption. In contrast, the cognitive, behavioral, and parenting intervention strategies outlined below target the factors that perpetuate sleep disruption in pediatric oncology. Providers and parents may also prefer these treatments to pharmacological options.26
Nonpharmacological Interventions
Sleep hygiene
Sleep hygiene involves the establishment of routines and habits that support healthy sleep behavior. Although sleep hygiene education should not be delivered as a primary intervention for clinically significant sleep disruption, it is often helpful in prevention, or as an adjunct to other empirically supported treatments.129 Sleep hygiene can be encouraged both during active treatment and in the survivorship period. Parents whose sleep has been disrupted are also encouraged to reestablish their own healthy sleep hygiene routines in conjunction with their children.
As with all children, heavy meals and high-energy play/exercise should also be avoided before bed, and caffeine consumption should be generally discouraged.129 It is also important to create a sleep environment that is as comfortable, dark, and quiet as possible, whether in the hospital or home. Adhering to a regular sleep schedule with a consistent sleep and wake time, maintaining rituals and behaviors that were in place prior to the cancer diagnosis (eg, brushing teeth and reading a book right before bedtime), and avoiding sleep-interfering activities (eg, eating in bed, relaxed rules about bed activities) also help create strong associations between the bed and sleep. Although parents may want to sleep in the same room as their child (ie, co-sleep) while in the hospital, this practice should be phased out on returning home. Another critical aspect of sleep hygiene is avoidance of all screen time in the bed and bedroom, which is being increasingly recognized as a key concern in children.66 For example, during active treatment and hospitalization, if a child is not restricted to bed, a small but helpful change might be to have screen time out of bed (eg, sit on a chair in the hospital room). After returning home and during survivorship, screen time should occur exclusively outside of the bedroom. Finally, within the recommendations of a child’s physicians, daytime physical activity may also be an opportunity to improve sleep quality.
Behavioral interventions
Recent systematic reviews and meta-analyses in general pediatric sleep medicine suggest there are 3 “well-established” interventions (parent education, standard extinction/unmodified extinction, graduated extinction) that target sleep disruption and can reduce negative nighttime behaviors (eg, spending extensive amounts of time in bed but not sleeping) and promote uninterrupted sleep in children.26,34 In clinical practice, these interventions are often combined to create multifaceted treatments that optimally address a given child’s concerns.26 Given this level of complexity, it is recommended that these interventions only be delivered by professionals who are well versed in these techniques.
The general sleep medicine literature provides a theoretical foundation for potential intervention in the pediatric oncology context, and treatment studies of behavioral interventions in this population are warranted, with several additional considerations. For example, one sleep-disrupting behavior may involve children leaving bed to seek their parents, complaining of illness. In the case of pediatric oncology, this may be a more prevalent and real concern. The need to critically evaluate complaints of illness should be taken seriously; however, repeated complaints over multiple nights without the presence of illness should still be considered a sleep-interrupting behavior. Managing this would necessitate a comprehensive discussion between the health care providers (eg, physician, psychologist, child life specialist) and the family to determine the medical, cognitive, and behavioral contributions to this behavior, and to plan behavioral strategies moving forward. Another nuanced consideration could be lingering cancer-related pain or trauma symptoms that interfere with sleep. These should be addressed either with pharmacotherapy or psychotherapy before, or in conjunction with, intervention for sleep disruption. With these considerations, the remaining paragraphs outline general principles that may be intervention targets to consider in future research.
Parent education and prevention interventions are typically delivered via written materials, individual coaching, or in group interventions, and have been shown to be effective in reducing symptoms of sleep disruption in healthy children.26 These programs involve teaching the parent behavioral skills (eg, reinforcement) to promote optimal sleep routines, discouraging sleep incompatible behavior, and setting appropriate boundaries around sleep.26,34 Parent stress reduction is also a common component to these interventions. In the case of pediatric oncology, part of the education may involve normalization of sleep disruptions that results from active treatment, and if sleep disruption was not present before diagnosis and treatment, an encouragement of the establishment of previously effective nighttime routines.
Two types of extinction interventions, called standard extinction/unmodified extinction and graduated extinction, are also effective for healthy young children whose difficulty falling asleep or staying asleep has led to a child’s inability to fall asleep independently, frequent attempts to get out of bed, or to avoid sleeping altogether.26,130 Clinically, extinction strategies to treat childhood sleep problems are best delivered when active monitoring for illness is no longer necessary and thus would likely be more appropriate during survivorship. Unmodified extinction therapy involves having a parent ignore a child’s maladaptive behaviors (ie, removing the reinforcement of parental attention) after a set bed time until a set wake time in order to reduce and eventually eliminate that behavior.130 Although there is good evidence for the efficacy of this strategy, many parents find it difficult to implement, and parents of cancer survivors should be counseled not to ignore a child’s report of serious illness or fever developing during the night. In contrast, graduated extinction is often more tolerable to parents and allows for periodic checking of the child.130 Similar to unmodified extinction, in gradual extinction there are set bed and wake times, but parents briefly check on their child (15-60 seconds) after the lights are out on a fixed or increasing time interval schedule.26 In this protocol, parents still deliberately ignore maladaptive behaviors, but the change is less abrupt.130 As with unmodified extinction, parents should be counseled to address reports of serious illness even during survivorship.
Several other interventions that may be applicable to pediatric oncology have also been identified as “probably efficacious” in healthy children.26,34 Bedtime fading (temporarily delaying bedtime to increase sleep drive and more rapid sleep initiation, and then gradually moving bedtime earlier) and stimulus control (removing the child from bed for a set period of time if a child does not fall asleep) both help reestablish an association between being in bed and sleeping. The positive routines technique (developing a set bedtime routine characterized by quiet activities that the child enjoys) may also help relax the child prior to bed and create conditioned cues for sleepiness.
Cognitive behavioral therapy for insomnia
For adolescent cancer survivors, who have more complex thinking, and whose sleep routines are less influenced by parental factors, cognitive behavioral therapy for insomnia (CBT-I) is a promising treatment option that warrants future study. CBT-I combines components of cognitive principles to challenge cognitions (eg, frustrated and anxious thoughts about sleep) and change the behaviors (eg, laying awake in bed) that contribute to the maintenance of sleep disruption. A large literature suggests that CBT-I is effective for treatment of insomnia in a range of populations,131,132 including adult cancer survivors.133 Initial studies have also shown its effectiveness in treating insomnia in adolescents,134,135 although there have not been studies of adolescent cancer patients or survivors.
Special considerations for CBT-I in pediatric oncology may involve a discussion with the parents about the rationale behind sleep consolidation procedures and ways to support their child’s establishment of healthy sleep routines. If both parents and children are experiencing disrupted sleep they may choose to implement the strategies from CBT-I together to support each other’s recovery. Siblings of the child affected by cancer may also participate in CBT-I interventions, which could directly benefit them and also provide support around reestablishment of healthy sleep routines for both sibling and patient.
Future Directions and Research Opportunities
There has been considerable progress in understanding sleep and sleep disruption in adults who have been affected by cancer, and this research is increasingly being extended to pediatric oncology. However, there remain significant gaps in research evidence, and in the translation of existing evidence into clinical practice for childhood cancer patients and survivors. Cumulative evidence from the developing literature suggests that parameters of the cancer and its treatment, the sleeping environment, family factors, changes in a child’s psychological status, and physical activity may contribute to short- and long-term sleep disruption, but there is not currently enough evidence to draw definitive conclusions about the relative magnitude of each. Studies with both objective (ie, polysomnography, actigraphy) and subjective (ie, standardized, validated measures) measures of sleep as they relate to these constructs are warranted. In particular, research on screen time, which may increase for pediatric cancer patients and survivors, and is a common factor for both reduced physical activity and increased nighttime light exposure, should be pursued. Promising studies on the benefits of physical activity on sleep and fatigue in adult and pediatric cancer patients also suggest that this could be another very promising area for future research.
Clinical research is also greatly needed. Objectives for this should include developing and evaluating brief assessment tools for use in oncology and primary care settings; validating existing pediatric sleep measures (eg, Children’s Sleep Habits Questionnaire, Sleep Self Report) specifically for pediatric oncology; and testing behavioral interventions and CBT-I tailored for this population. In the absence of such research, clinicians may extrapolate from findings in adult oncology and healthy pediatric samples with sleep disruption to meet the needs of this population. However, additional work that focuses directly on childhood cancer patients and survivors will undoubtedly be a stronger contribution. Notably, there have been several pilot studies of complementary approaches to treating sleep disruption in hospitalized pediatric cancer patients (eg, exercise intervention, massage), which are promising in terms of feasibility.108,136 Although sleep symptomatology was not significantly improved by these interventions, further exploration with larger samples is warranted.
Additionally, there needs to be discourse on prevention efforts such as educating stakeholders like parents and health care providers about the increased risk of sleep disruption in this population; sleep hygiene practices (eg, developing a sleep routine), and boundary setting; sleep health education for patients; and modifying nighttime hospital room environments to be more conducive to sleep. Such endeavors could potentially inhibit sleep disruption in this vulnerable population and should be considered.
Conclusion
This integrative review describes the problem and prevalence of sleep disruption in pediatric oncology, a theoretical framework for the development and maintenance of sleep disruption during childhood cancer treatment and survivorship, and a clinical summary intended to help guide future research, clinical assessment, and the tailoring of evidence-based behavioral treatments for sleep disruption. Because many children reach long-term survivorship, childhood is a critical time for developing habits that can affect sleep throughout the lifetime, and sleep is an important contributor to health and developmental outcomes, assessment and treatment of sleep disruption needs to be a routine component of standard care, not an auxiliary one.
Acknowledgments
We would like to thank Soeun Lee and Angelica Rivera for their help on this project.
References
- 1. American Cancer Society. Cancer facts & figures 2014 (Special Section: Childhood & Adolescent Cancers). http://www.cancer.org/acs/groups/content/@research/documents/webcontent/acspc-042151.pdf. Accessed November 22, 2016.
- 2. Phillips SM, Padgett LS, Leisenring WM, et al. Survivors of childhood cancer in the United States: prevalence and burden of morbidity. Cancer Epidemiol Biomarkers Prev. 2015;24:653-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rueegg CS, Michel G, Wengenroth L, et al. Physical performance limitations in adolescent and adult survivors of childhood cancer and their siblings. PLoS One. 2012;7(10):e47944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ness KK, Hudson MM, Ginsberg JP, et al. Physical performance limitations in the childhood cancer survivor study cohort. J Clin Oncol. 2009;27:2382-2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lu Q, Krull KR, Leisenring W, et al. Pain in long-term adult survivors of childhood cancers and their siblings: a report from the Childhood Cancer Survivor Study. Pain. 2011;152:2616-2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Clanton NR, Klosky JL, Li C, et al. Fatigue, vitality, sleep, and neurocognitive functioning in adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Cancer. 2011;117:2559-2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schultz KAP, Ness KK, Whitton J, et al. Behavioral and social outcomes in adolescent survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2007;25:3649-3656. [DOI] [PubMed] [Google Scholar]
- 8. Font-Gonzalez A, Feijen EL, Sieswerda E, et al. Social outcomes in adult survivors of childhood cancer compared to the general population: linkage of a cohort with population registers. Psychooncology. 2016;25:933-941. [DOI] [PubMed] [Google Scholar]
- 9. Patenaude AF, Kupst MJ. Psychosocial functioning in pediatric cancer. J Pediatr Psychol. 2005;30:9-27. [DOI] [PubMed] [Google Scholar]
- 10. Gianinazzi ME, Rueegg CS, Wengenroth L, et al. Adolescent survivors of childhood cancer. Psychooncology. 2013;22:2051-2058. [DOI] [PubMed] [Google Scholar]
- 11. Walter LM, Nixon GM, Davey MJ, Downie PA, Horne RSC. Sleep and fatigue in pediatric oncology: a review of the literature. Sleep Med Rev. 2015;24:71-82. [DOI] [PubMed] [Google Scholar]
- 12. Kaleyias J, Manley P, Kothare SV. Sleep disorders in children with cancer. Semin Pediatr Neurol. 2012;19:25-34. [DOI] [PubMed] [Google Scholar]
- 13. Zhou ES, Recklitis CJ. Insomnia in adult survivors of childhood cancer: a report from project REACH. Support Care Cancer. 2014;22:3061-3069. [DOI] [PubMed] [Google Scholar]
- 14. Mulrooney DA, Ness KK, Neglia JP, et al. Fatigue and sleep disturbance in adult survivors of childhood cancer: a report from the childhood cancer survivor study (CCSS). Sleep. 2008;31:271-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Steur LMH, Kolk RHE, Mooij F, et al. The prevalence and risk factors of sleep problems in pediatric oncology: its effect on quality of life during and after cancer treatment. Expert Rev Qual Life Cancer Care. 2016;1:153-171. [Google Scholar]
- 16. Owens J. Classification and epidemiology of childhood sleep disorders. Sleep Med Clin. 2007;2:353-361. [Google Scholar]
- 17. Paruthi S, Brooks LJ, D’Ambrosio C, et al. Recommended amount of sleep for pediatric populations: a consensus statement of the American Academy of Sleep Medicine. J Clin Sleep Med. 2016;12:785-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bub KL, Buckhalt JA, El-Sheikh M. Children’s sleep and cognitive performance: a cross-domain analysis of change over time. Dev Psychol. 2011;47:1504-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Epstein R, Chillag N, Lavie P. Starting times of school: effects on daytime functioning of fifth-grade children in Israel. Sleep. 1998;21:250-256. [DOI] [PubMed] [Google Scholar]
- 20. Touchette E, Petit D, Séguin JR, Boivin M, Tremblay RE, Montplaisir JY. Associations between sleep duration patterns and behavioral/cognitive functioning at school entry. Sleep. 2007;30:1213-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vriend JL, Davidson FD, Corkum PV, Rusak B, Chambers CT, McLaughlin EN. Manipulating sleep duration alters emotional functioning and cognitive performance in children. J Pediatr Psychol. 2013;38:1058-1069. [DOI] [PubMed] [Google Scholar]
- 22. Wolfson AR, Carskadon MA. Sleep schedules and daytime functioning in adolescents. Child Dev. 1998;69:875-887. [PubMed] [Google Scholar]
- 23. Giannotti F, Cortesi F, Sebastiani T, Ottaviano S. Circadian preference, sleep and daytime behaviour in adolescence. J Sleep Res. 2002;11:191-199. [DOI] [PubMed] [Google Scholar]
- 24. Fallone G, Owens JA, Deane J. Sleepiness in children and adolescents: clinical implications. Sleep Med Rev. 2002;6:287-306. [DOI] [PubMed] [Google Scholar]
- 25. Bates JE, Viken RJ, Alexander DB, Beyers J, Stockton L. Sleep and adjustment in preschool children: sleep diary reports by mothers relate to behavior reports by teachers. Child Dev. 2002;73:62-74. [DOI] [PubMed] [Google Scholar]
- 26. Mindell JA, Kuhn B, Lewin DS, Meltzer LJ, Sadeh A. Behavioral treatment of bedtime problems and night wakings in infants and young children. Sleep. 2006;29:1263-1276. [PubMed] [Google Scholar]
- 27. Owens J. Classification and epidemiology of childhood sleep disorders. Prim Care. 2008;35:533-546. [DOI] [PubMed] [Google Scholar]
- 28. Wilder-Smith A, Mustafa FB, Earnest A, Gen L, Macary PA. Impact of partial sleep deprivation on immune markers. Sleep Med. 2013;14:1031-1034. [DOI] [PubMed] [Google Scholar]
- 29. Besedovsky L, Lange T, Born J. Sleep and immune function. Pflügers Arch. 2012;463:121-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Daniel L, Kazak AE, Li Y, et al. Relationship between sleep problems and psychological outcomes in adolescent and young adult cancer survivors and controls. Support Care Cancer. 2015;24:539-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mindell JA, Emslie G, Blumer J, et al. Pharmacologic management of insomnia in children and adolescents: consensus statement. Pediatrics. 2006;117:e1223-e1232. [DOI] [PubMed] [Google Scholar]
- 32. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 33. Sateia MJ. International classification of sleep disorders—third edition. Chest. 2014;146:1387-1394. [DOI] [PubMed] [Google Scholar]
- 34. Meltzer LJ, Mindell JA. Systematic review and meta-analysis of behavioral interventions for pediatric insomnia. J Pediatr Psychol. 2014;39:932-948. [DOI] [PubMed] [Google Scholar]
- 35. Shen J, Barbera J, Shapiro CM. Distinguishing sleepiness and fatigue: focus on definition and measurement. Sleep Med Rev. 2006;10:63-76. [DOI] [PubMed] [Google Scholar]
- 36. Jóhannsdóttir IM, Hjermstad MJ, Moum T, et al. Increased prevalence of chronic fatigue among survivors of childhood cancers: a population-based study. Pediatr Blood Cancer. 2012;58:415-420. [DOI] [PubMed] [Google Scholar]
- 37. Spielman AJ, Caruso LS, Glovinsky PB. A behavioral perspective on insomnia treatment. Psychiatr Clin North Am. 1987;10:541-553. [PubMed] [Google Scholar]
- 38. Perlis ML, Jungquist C, Smith MT, Posner D. The conceptual framework for CBTI. In: Cognitive Behavioural Treatment of Insomnia: A Session-by-Session Guide. New York, NY: Springer; 2005:7-12. [Google Scholar]
- 39. Mindell JA, Meltzer LJ, Carskadon MA, Chervin RD. Developmental aspects of sleep hygiene: findings from the 2004 National Sleep Foundation Sleep in America Poll. Sleep Med. 2009;10:771-779. [DOI] [PubMed] [Google Scholar]
- 40. Savard J, Morin CM. Insomnia in the context of cancer: a review of a neglected problem. J Clin Oncol. 2001;19:895-908. [DOI] [PubMed] [Google Scholar]
- 41. Thiedke CC. Sleep disorders and sleep problems in childhood. Am Fam Physician. 2001;63:277-284. [PubMed] [Google Scholar]
- 42. Hinds PS, Hockenberry M, Rai SN, et al. Nocturnal awakenings, sleep environment interruptions, and fatigue in hospitalized children with cancer. Oncol Nurs Forum. 2007;34:393-402. [DOI] [PubMed] [Google Scholar]
- 43. Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol. 2008;26:971-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rosen G, Brand SR. Sleep in children with cancer: case review of 70 children evaluated in a comprehensive pediatric sleep center. Support Care Cancer. 2011;19:985-994. [DOI] [PubMed] [Google Scholar]
- 45. Verberne LM, Maurice-Stam H, Grootenhuis MA, Van Santen HM, Schouten-Van Meeteren AYN. Sleep disorders in children after treatment for a CNS tumour. J Sleep Res. 2012;21:461-469. [DOI] [PubMed] [Google Scholar]
- 46. Linder LA, Christian BJ. Nighttime sleep characteristics of hospitalized school-age children with cancer. J Spec Pediatr Nurs. 2013;18:13-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Baggott C, Dodd M, Kennedy C, et al. Changes in children’s reports of symptom occurrence and severity during a course of myelosuppressive chemotherapy. J Pediatr Oncol Nurs. 2010;27:307-315. [DOI] [PubMed] [Google Scholar]
- 48. Walker AJ, Gedaly-Duff V, Miaskowski C, Nail L. Differences in symptom occurrence, frequency, intensity, and distress in adolescents prior to and one week after the administration of chemotherapy. J Pediatr Oncol Nurs. 2010;27:259-265. [DOI] [PubMed] [Google Scholar]
- 49. Dandoy CE, Coleman KM, Petiniot L, et al. Prospective pilot study evaluating sleep disruption in children and young adults undergoing stem cell transplantation. Biol Blood Marrow Transplant. 2015;21(2):S216. [Google Scholar]
- 50. Rosen G, Harris AK, Liu M, Dreyfus J, Krueger J, Messinger YH. The effects of dexamethasone on sleep in young children with acute lymphoblastic leukemia. Sleep Med. 2015;16:503-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Vallance K, Liu W, Mandrell BN, et al. Mechanisms of dexamethasone-induced disturbed sleep and fatigue in paediatric patients receiving treatment for ALL. Eur J Cancer. 2010;46:1848-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Van Someren EJW, Swart-Heikens J, Endert E, et al. Long-term effects of cranial irradiation for childhood malignancy on sleep in adulthood. Eur J Endocrinol. 2004;150:503-510. [DOI] [PubMed] [Google Scholar]
- 53. Rosen GM, Shor AC, Geller TJ. Sleep in children with cancer. Curr Opin Pediatr. 2008;20:676-681. [DOI] [PubMed] [Google Scholar]
- 54. Zupanec S, Jones H, Stremler R. Sleep habits and fatigue of children receiving maintenance chemotherapy for ALL and their parents. J Pediatr Oncol Nurs. 2010;27:217-228. [DOI] [PubMed] [Google Scholar]
- 55. Boman KK, Viksten J, Kogner P, Samuelsson U. Serious illness in childhood: the different threats of cancer and diabetes from a parent perspective. J Pediatr. 2004;145:373-379. [DOI] [PubMed] [Google Scholar]
- 56. Wright M. Children receiving treatment for cancer and their caregivers: a mixed methods study of their sleep characteristics. Pediatr Blood Cancer. 2011;56:638-645. [DOI] [PubMed] [Google Scholar]
- 57. Lewin DS, Dahl RE. Importance of sleep in the management of pediatric pain. J Dev Behav Pediatr. 1999;20:244-252. [DOI] [PubMed] [Google Scholar]
- 58. Kazak AE, Kassam-Adams N, Schneider S, Zelikovsky N, Alderfer MA, Rourke M. An integrative model of pediatric medical traumatic stress. J Pediatr Psychol. 2006;31:343-355. [DOI] [PubMed] [Google Scholar]
- 59. Jacob E, Hesselgrave J, Sambuco G, Hockenberry M. Variations in pain, sleep, and activity during hospitalization in children with cancer. J Pediatr Oncol Nurs. 2007;24:208-219. [DOI] [PubMed] [Google Scholar]
- 60. Lewandowski AS, Ward TM, Palermo TM. Sleep problems in children and adolescents with common medical conditions. Pediatr Clin North Am. 2011;58:699-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Collins JJ, Stevens MM, Berde CB. Pediatric cancer pain. In: Macintyre PE, Walker SM, Rowbotham DJ, eds. Clinical Pain Management. London, England: Hodder Arnold; 2008. [Google Scholar]
- 62. Hockenberry MJ, Hooke MC, Gregurich M, McCarthy K, Sambuco G, Krull K. Symptom clusters in children and adolescents receiving cisplatin, doxorubicin, or ifosfamide. Oncol Nurs Forum. 2010;37:E16-E27. [DOI] [PubMed] [Google Scholar]
- 63. Dumont M, Beaulieu C. Light exposure in the natural environment: relevance to mood and sleep disorders. Sleep Med. 2007;8:557-565. [DOI] [PubMed] [Google Scholar]
- 64. Innominato PF, Roche VP, Palesh OG, Ulusakarya A, Spiegel D, Lévi FA. The circadian timing system in clinical oncology. Ann Med. 2014;46:191-207. [DOI] [PubMed] [Google Scholar]
- 65. Hale L, Guan S. Screen time and sleep among school-aged children and adolescents: a systematic literature review. Sleep Med Rev. 2015;21:50-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cain N, Gradisar M. Electronic media use and sleep in school-aged children and adolescents: a review. Sleep Med. 2010;11:735-742. [DOI] [PubMed] [Google Scholar]
- 67. Bogg TF, Shaw PJ, Cohn RJ, et al. Physical activity and screen-time of childhood haematopoietic stem cell transplant survivors. Acta Paediatr. 2015;104:e455-e459. [DOI] [PubMed] [Google Scholar]
- 68. Bernhofer EI, Higgins PA, Daly BJ, Burant CJ, Hornick TR. Hospital lighting and its association with sleep, mood and pain in medical inpatients. J Adv Nurs. 2014;70:1164-1173. [DOI] [PubMed] [Google Scholar]
- 69. Bano M, Chiaromanni F, Corrias M, et al. The influence of environmental factors on sleep quality in hospitalized medical patients. Front Neurol. 2014;5:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Winter C, Müller C, Hoffmann C, Boos J, Rosenbaum D. Physical activity and childhood cancer. Pediatr Blood Cancer. 2010;54:501-510. [DOI] [PubMed] [Google Scholar]
- 71. Winter C, Müller C, Brandes M, et al. Level of activity in children undergoing cancer treatment. Pediatr Blood Cancer. 2009;53:438-443. [DOI] [PubMed] [Google Scholar]
- 72. Hockenberry-Eaton M, Hinds PS. Fatigue in children and adolescents with cancer: evolution of a program of study. Semin Oncol Nurs. 2000;16:261-272. [DOI] [PubMed] [Google Scholar]
- 73. El-Sheikh M, Kelly RJ, Bagley EJ, Wetter EK. Parental depressive symptoms and children’s sleep: the role of family conflict. J Child Psychol Psychiatry. 2012;53:806-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Brand S, Gerber M, Hatzinger M, Beck J, Holsboer-Trachsler E. Evidence for similarities between adolescents and parents in sleep patterns. Sleep Med. 2009;10:1124-1131. [DOI] [PubMed] [Google Scholar]
- 75. Meltzer LJ, Mindell JA. Relationship between child sleep disturbances and maternal sleep, mood, and parenting stress: a pilot study. J Fam Psychol. 2007;21:67-73. [DOI] [PubMed] [Google Scholar]
- 76. Kramer L, Conger KJ. What we learn from our sisters and brothers: for better or for worse. New Dir Child Adolesc Dev. 2009;2009(126):1-12. [DOI] [PubMed] [Google Scholar]
- 77. Mednick SC, Christakis NA, Fowler JH. The spread of sleep loss influences drug use in adolescent social networks. PLoS One. 2010;5:e9775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Marcus J. Psychosocial issues in pediatric oncology. Ochsner J. 2012;12:211-215. [PMC free article] [PubMed] [Google Scholar]
- 79. Pollock EA, Litzelman K, Wisk LE, Witt WP. Correlates of physiological and psychological stress among parents of childhood cancer and brain tumor survivors. Acad Pediatr. 2013;13:105-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Pai ALH, Greenley RN, Lewandowski A, Drotar D, Youngstrom E, Peterson CC. A meta-analytic review of the influence of pediatric cancer on parent and family functioning. J Fam Psychol. 2007;21:407-415. [DOI] [PubMed] [Google Scholar]
- 81. Klassen AF, Klaassen R, Dix D, et al. Impact of caring for a child with cancer on parents’ health-related quality of life. J Clin Oncol. 2008;26:5884-5889. [DOI] [PubMed] [Google Scholar]
- 82. Matthews EE, Neu M, Cook PF, King N. Sleep in mother and child dyads during treatment for pediatric acute lymphoblastic leukemia. Oncol Nurs Forum. 2014;41:599-610. [DOI] [PubMed] [Google Scholar]
- 83. Neu M, Matthews E, King NA. Exploring sleep-wake experiences of mothers during maintenance therapy for their child’s acute lymphoblastic leukemia. J Pediatr Nurs. 2014;29:410-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. McLoone JK, Wakefield CE, Yoong SL, Cohn RJ. Parental sleep experiences on the pediatric oncology ward. Support Care Cancer. 2013;21:557-564. [DOI] [PubMed] [Google Scholar]
- 85. Lähteenmäki PM, Sjöblom J, Korhonen T, Salmi TT. The siblings of childhood cancer patients need early support: a follow up study over the first year. Arch Dis Child. 2004;89:1008-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Daniel LC, Schwartz LA, Mindell JA, Tucker CA, Barakat LP. Initial validation of the sleep disturbances in pediatric cancer model. J Pediatr Psychol. 2016;41:588-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Rosen GM. Sleep in children who have cancer. Sleep Med Clin. 2007;2:491-500. [Google Scholar]
- 88. McCarthy MC, Bastiani J, Williams LK. Are parenting behaviors associated with child sleep problems during treatment for acute lymphoblastic leukemia? Cancer Med. 2016;5:1473-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ng AS, Dodd HF, Gamble AL, Hudson JL. The relationship between parent and child dysfunctional beliefs about sleep and child sleep. J Child Fam Stud. 2013;22:827-835. [Google Scholar]
- 90. Long KA, Keeley L, Reiter-Purtill J, Vannatta K, Gerhardt CA, Noll RB. Child-rearing in the context of childhood cancer: perspectives of parents and professionals. Pediatr Blood Cancer. 2014;61:326-332. [DOI] [PubMed] [Google Scholar]
- 91. Mindell J, Owen J. A Clinical Guide to Pediatric Sleep Diagnosis and Management of Sleep Problems in Children and Adolescents. 3rd ed. Philadelphia, PA: Wolters Kluwer; 2003. [Google Scholar]
- 92. Erickson SJ, Steiner H. Trauma and personality correlates in long-term pediatric cancer survivors. Child Psychiatry Hum Dev. 2001;31:195-213. [DOI] [PubMed] [Google Scholar]
- 93. Stuber ML, Christakis DA, Houskamp B, Kazak AE. Posttrauma symptoms in childhood leukemia survivors and their parents. Psychosomatics. 1996;37:254-261. [DOI] [PubMed] [Google Scholar]
- 94. Lavie P. Sleep disturbances in the wake of traumatic events. N Engl J Med. 2001;345:1825-1832. [DOI] [PubMed] [Google Scholar]
- 95. Kim E-J, Dimsdale JE. The effect of psychosocial stress on sleep: a review of polysomnographic evidence. Behav Sleep Med. 2007;5:256-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Bader K, Schäfer V. Sleep disturbances following traumatic experiences in childhood and adolescence: a review. Somnologie. 2007;11:101-110. [Google Scholar]
- 97. Shah SS, Dellarole A, Peterson EC, et al. Long-term psychiatric outcomes in pediatric brain tumor survivors. Childs Nerv Syst. 2015;31:653-663. [DOI] [PubMed] [Google Scholar]
- 98. Kunin-Batson AS, Lu X, Balsamo L, et al. Prevalence and predictors of anxiety and depression after completion of chemotherapy for childhood acute lymphoblastic leukemia: a prospective longitudinal study. Cancer. 2016;122:1608-1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Muffly LS, Hlubocky FJ, Khan N, et al. Psychological morbidities in adolescent and young adult blood cancer patients during curative-intent therapy and early survivorship. Cancer. 2016;122:954-961. [DOI] [PubMed] [Google Scholar]
- 100. Alvaro PK, Roberts RM, Harris JK. A systematic review assessing bidirectionality between sleep disturbances, anxiety, and depression. Sleep. 2013;36:1059-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Gordijn MS, van Litsenburg RR, Gemke RJ, et al. Sleep, fatigue, depression, and quality of life in survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2013;60:479-485. [DOI] [PubMed] [Google Scholar]
- 102. Loprinzi PD, Cardinal BJ. Association between objectively-measured physical activity and sleep, NHANES 2005-2006. Ment Health Phys Act. 2011;4:65-69. [Google Scholar]
- 103. Humpel N, Iverson DC. Sleep quality, fatigue and physical activity following a cancer diagnosis. Eur J Cancer. 2010;19:761-768. [DOI] [PubMed] [Google Scholar]
- 104. Mishra SI, Scherer RW, Geigle PM, et al. Exercise interventions on health-related quality of life for cancer survivors. Cochrane Database Syst Rev. 2012;(8):CD007566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Orsey AD, Wakefield DB, Cloutier MM. Physical activity (PA) and sleep among children and adolescents with cancer. Pediatr Blood Cancer. 2013;60:1908-1913. [DOI] [PubMed] [Google Scholar]
- 106. Spathis A, Booth S, Grove S, Hatcher H, Kuhn I, Barclay S. Teenage and young adult cancer-related fatigue is prevalent, distressing, and neglected: it is time to intervene. A systematic literature review and narrative synthesis. J Adolesc Young Adult Oncol. 2015;4:3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Huang TT, Ness KK. Exercise interventions in children with cancer: a review. Int J Pediatr. 2011;2011:461512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Hinds PS, Hockenberry M, Rai SN, et al. Clinical field testing of an enhanced-activity intervention in hospitalized children with cancer. J Pain Symptom Manage. 2007;33:686-697. [DOI] [PubMed] [Google Scholar]
- 109. Gilliam MB, Schwebel DC. Physical activity in child and adolescent cancer survivors: a review. Health Psychol Rev. 2013;7:92-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Stolley MR, Restrepo J, Sharp LK. Diet and physical activity in childhood cancer survivors: a review of the literature. Ann Behav Med. 2010;39:232-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Hocking MC, Schwartz LA, Hobbie WL, et al. Prospectively examining physical activity in young adult survivors of childhood cancer and healthy controls. Pediatr Blood Cancer. 2013;60:309-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. van Brussel M, Takken T, Lucia A, van der Net J, Helders PJM. Is physical fitness decreased in survivors of childhood leukemia? A systematic review. Leukemia. 2005;19:13-17. [DOI] [PubMed] [Google Scholar]
- 113. Gilliam MB, Madan-Swain A, Whelan K, Tucker DC, Demark-Wahnefried W, Schwebel DC. Social, demographic, and medical influences on physical activity in child and adolescent cancer survivors. J Pediatr Psychol. 2012;37:198-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. PDQ Supportive and Palliative Care Editorial Board. Sleep Disorders (PDQ): Health Professional Version. Bethesda, MD: National Cancer Institute; 2002. [PubMed] [Google Scholar]
- 115. Lewandowski AS, Toliver-Sokol M, Palermo TM. Evidence-based review of subjective pediatric sleep measures. J Pediatr Psychol. 2011;36:780-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Owens JA, Spirito A, McGuinn M. The Children’s Sleep Habits Questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children. Sleep. 2000;23:1043-1051. [PubMed] [Google Scholar]
- 117. Owens JA, Spirito A, McGuinn M, Nobile C. Sleep habits and sleep disturbance in elementary school-aged children. J Dev Behav Pediatr. 2000;21:27-36. [DOI] [PubMed] [Google Scholar]
- 118. Owens JA, Rosen CL, Mindell JA. Medication use in the treatment of pediatric insomnia: results of a survey of community-based pediatricians. Pediatrics. 2003;111:e628-e635. [DOI] [PubMed] [Google Scholar]
- 119. Moore M, Meltzer LJ, Mindell JA. Bedtime problems and night waking in children. Sleep Med Clin. 2007;2:377-385. [Google Scholar]
- 120. Smaldone A, Honig JC, Byrne MW. Sleepless in America: inadequate sleep and relationships to health and well-being of our nation’s children. Pediatrics. 2007;119:S29-S37. [DOI] [PubMed] [Google Scholar]
- 121. Fricke-Oerkermann L, Plück J, Schredl M, et al. Prevalence and course of sleep problems in childhood. Sleep. 2007;30:1371-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Stojanovski SD, Rasu RS, Balkrishnan R, Nahata MC. Trends in medication prescribing for pediatric sleep difficulties in US outpatient settings. Sleep. 2007;30:1013-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Pelayo R, Dubik M. Pediatric sleep pharmacology. Semin Pediatr Neurol. 2008;15:79-90. [DOI] [PubMed] [Google Scholar]
- 124. Reed MD, Findling RL. Overview of current management of sleep disturbances in children: I—Pharmacotherapy. Curr Ther Res. 2002;63:B18-B37. [Google Scholar]
- 125. Bruni O, Alonso-Alconada D, Besag F, et al. Current role of melatonin in pediatric neurology: clinical recommendations. Eur J Paediatr Neurol. 2015;19:122-133. [DOI] [PubMed] [Google Scholar]
- 126. Gitto E, Aversa S, Reiter RJ, Barberi I, Pellegrino S. Update on the use of melatonin in pediatrics. J Pineal Res. 2011;50:21-28. [DOI] [PubMed] [Google Scholar]
- 127. Kennaway DJ. Potential safety issues in the use of the hormone melatonin in paediatrics. J Paediatr Child Health. 2015;51:584-589. [DOI] [PubMed] [Google Scholar]
- 128. Andersen LPH, Gögenur I, Rosenberg J, Reiter RJ. The safety of melatonin in humans. Clin Drug Investig. 2016;36:169-175. [DOI] [PubMed] [Google Scholar]
- 129. Denlinger CS, Ligibel JA, Are M, et al. Survivorship: sleep disorders, version 1.2014. J Natl Compr Canc Netw. 2014;12:630-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Morgenthaler TI, Owens J, Alessi C, et al. Practice parameters for behavioral treatment of bedtime problems and night wakings in infants and young children. Sleep. 2006;29:1277-1281. [PubMed] [Google Scholar]
- 131. Savard J, Simard S, Ivers H, Morin CM. Randomized study on the efficacy of cognitive-behavioral therapy for insomnia secondary to breast cancer, part I: sleep and psychological effects. J Clin Oncol. 2005;23:6083-6096. [DOI] [PubMed] [Google Scholar]
- 132. Edinger JD, Wohlgemuth WK, Radtke RA, Marsh GR, Quillian RE. Cognitive behavioral therapy for treatment of chronic primary insomnia: a randomized controlled trial. JAMA. 2001;285:1856-1864. [DOI] [PubMed] [Google Scholar]
- 133. Johnson JA, Rash JA, Campbell TS, et al. A systematic review and meta-analysis of randomized controlled trials of cognitive behavior therapy for insomnia (CBT-I) in cancer survivors. Sleep Med Rev. 2016;27:20-28. [DOI] [PubMed] [Google Scholar]
- 134. de Bruin EJ, Oort FJ, Bögels SM, Meijer AM. Efficacy of internet and group-administered cognitive behavioral therapy for insomnia in adolescents: a pilot study. Behav Sleep Med. 2014;12:235-254. [DOI] [PubMed] [Google Scholar]
- 135. de Bruin EJ, Bogels SM, Oort FJ, Meijer AM. Efficacy of cognitive behavioral therapy for insomnia in adolescents: a randomized controlled trial with internet therapy, group therapy and a waiting list condition. Sleep. 2015;38:1913-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Jacobs S, Mowbray C, Cates LM, et al. Pilot study of massage to improve sleep and fatigue in hospitalized adolescents with cancer. Pediatr Blood Cancer. 2016;63:880-886. [DOI] [PubMed] [Google Scholar]