Abstract
Background
Dexmedetomidine (DEX) is increasingly used intraoperatively in infants undergoing cardiac surgery. This phase 1 multicentre study sought to: (i) determine the safety of DEX for cardiac surgery with cardiopulmonary bypass; (ii) determine the pharmacokinetics (PK) of DEX; (iii) create a PK model and dosing for steady-state DEX plasma levels; and (iv) validate the PK model and dosing.
Methods
We included 122 neonates and infants (0–180 days) with D-transposition of the great arteries, ventricular septal defect, or tetralogy of Fallot. Dose escalation was used to generate NONMEM® PK modelling, and then validation was performed to achieve low (200–300 pg ml−1), medium (400–500 pg ml−1), and high (600–700 pg ml−1) DEX plasma concentrations.
Results
Five of 122 subjects had adverse safety outcomes (4.1%; 95% confidence interval [CI], 1.8–9.2%). Two had junctional rhythm, two had second-/third-degree atrioventricular block, and one had hypotension. Clearance (CL) immediately postoperative and CL on CPB were reduced by approximately 50% and 95%, respectively, compared with pre-CPB CL. DEX clearance after CPB was 1240 ml min−1 70 kg−1. Age at 50% maximum clearance was approximately 2 days, and that at 90% maximum clearance was 18 days. Overall, 96.1% of measured DEX concentrations fell within the 5th–95th percentile prediction intervals in the PK model validation. Dosing strategies are recommended for steady-state DEX plasma levels ranging from 200 to 1000 pg ml−1.
Conclusions
When used with a careful dosing strategy, DEX results in low incidence and severity of adverse safety events in infants undergoing cardiac surgery with cardiopulmonary bypass. This validated PK model should assist clinicians in selecting appropriate dosing. The results of this phase 1 trial provide preliminary data for a phase 3 trial of DEX neuroprotection.
Clinical trials registration
Keywords: anaesthesia, congenital heart surgery, dexmedetomidine, pharmacokinetics, tetralogy of Fallot, transposition of the great arteries, ventricular septal defect
Editor's key points.
-
•
Preclinical evidence suggests that dexmedetomidine is not neurotoxic and might be neuroprotective in young animals.
-
•
When used as an anaesthetic adjunct in humans, the drug reduces hypnotic and analgesic dose requirements.
-
•
In a cohort of 122 neonates and infants undergoing cardiac surgery with cardiopulmonary bypass, there was a low incidence of adverse effects associated with dexmedetomidine use.
-
•
The authors produced and validated a pharmacokinetic model for dexmedetomidine, and have shown that it can be used to guide drug dosing to achieve steady-state plasma concentrations.
Dexmedetomidine (DEX) is a novel sedative/hypnotic agent that acts at central nervous system α2-adrenergic receptor binding sites as a highly selective agonist. DEX produces hypnosis and anxiolysis by binding presynaptic α2 receptors in the locus coeruleus and analgesia by binding to α2 receptors in the spinal cord. DEX has gained widespread use in paediatric patients for intensive care sedation in cardiac and noncardiac populations, for procedural sedation, and as an adjunct to general anaesthesia for cardiac and noncardiac surgery.1, 2 DEX maintains normal respiratory patterns and can be used through and after the period of tracheal extubation after cardiac surgery.3, 4, 5 DEX can reduce doses of volatile anaesthetic agents, opioids, and benzodiazepines in the intraoperative and postoperative settings and acts as an adjunct to opioid analgesia.1 DEX has the ability to prevent and control atrial and junctional tachydysrhythmias, and ventricular dysrhythmias, in the intraoperative and postoperative period.6, 7, 8 DEX also controls blood pressure in the postoperative period, limiting hypertensive episodes in patients in whom this is a perioperative goal.3, 9
Data from neonatal rodent, fetal and neonatal sheep, and neonatal rhesus monkey models indicate that DEX does not produce neuroapoptosis at sedative doses.10, 11, 12 This is in direct contrast to anaesthetic and sedative agents that bind to γ-aminobutyric acid (GABA) and N-methyl-d-aspartate (NMDA) receptors, including all volatile anaesthetic agents, benzodiazepines, and ketamine.13 DEX also reduces neuroapoptosis and ameliorates longer-term neurobehavioural effects caused by volatile anaesthetics in neonatal animal models.14 Finally, DEX has intrinsic neuroprotective properties in neonatal animal models of hypoxia–ischaemia and inflammation.15, 16 Despite these advantages of DEX, it is associated with bradyarrhythmias including sinus arrest, atrioventricular block, and sinus or junctional bradycardia; hypotension with loading dose or prolonged infusion; and hypertension with rapid loading doses.1
DEX is increasingly used intraoperatively as part of a balanced anaesthetic technique in paediatric patients undergoing cardiac surgery with cardiopulmonary bypass (CPB).17, 18, 5, 19 However, reports of its use in the neonatal and young infant population are limited, and DEX pharmacokinetic (PK) data in this age group when used before, during, and after CPB have not been published.20 The aims of this phase 1 multicentre study were to: (i) determine the safety of DEX as part of a balanced anaesthetic and sedative strategy for corrective neonatal and infant cardiac surgery with CPB; (ii) determine PK parameters of DEX in this setting, including during CPB; (iii) create a PK model and dosing scheme to achieve steady-state DEX plasma concentrations throughout the perioperative course; and (iv) externally validate the PK model and dosing scheme to provide dosing guidance in this clinical setting.
Methods
Overall
This multicentre, open-label dose escalation safety and PK study was conducted in neonates (0–21 days) and infants (22–180 days) undergoing complete corrective surgery with CPB for D-transposition of the great arteries (arterial switch operation), ventricular septal defect, or tetralogy of Fallot. Specific age cut-offs were chosen based on previously published data demonstrating rapid increase in DEX clearance during the first 3 weeks of life.21 Exclusion criteria were prematurity (less than 37 weeks completed gestation for neonates and 36 weeks for infants); hepatic or renal dysfunction; preoperative administration of DEX or clonidine; major congenital anomalies outside of the cardiovascular system; preoperative central nervous system injury; planned deep hypothermic circulatory arrest; history of second- or third-degree heart block or sinus or junctional bradycardia; recent history of hypotension; or history of cardiac arrest or extracorporeal membrane oxygenation (ECMO) use. The study was approved by the Institutional Review Board for the Protection of Human Subjects at each of the enrolling sites, and written informed consent was obtained from a parent or legal guardian. DEX was used under a US Food and Drug Administration Investigational New Drug (IND) application (#118,058), and the study was registered with Clinical Trials.gov (NCT01915277). Four Pediatric Heart Network (PHN) centres in the USA enrolled participants. The study was overseen by the PHN's Data and Safety Monitoring Board, and adverse events (AEs) were reviewed and adjudicated by the PHN's independent Medical Monitor.
Overall, the study was divided into two parts. Part 1 included a fixed dose escalation protocol in infants then neonates. Part 2 of the study included model-driven dosing strategies to achieve predetermined low, medium, and high steady-state concentrations (Css) in infants and neonates, with an external validation of these doses through comparison of concentrations achieved with the dosing vs those concentrations that were predicted. Safety endpoints were evaluated throughout the study.
Anaesthetic and sedative protocol
All participants were subjected to a standardised general anaesthetic technique in the operating room (OR): volatile anaesthetic agent (sevoflurane, isoflurane, or desflurane) with any end-tidal concentration deemed necessary by the anaesthesiologist, and volatile agent in the sweep gas of the CPB circuit was allowed at any concentration deemed necessary by the anaesthesia/perfusion teams. In addition, fentanyl to a maximum dose of 10 μg kg−1 h−1, with induction dose of no more than 10 μg kg−1 was administered. DEX was initiated in the OR and continued for later cohorts during the postoperative period. Postoperative sedation and analgesia, for all cohorts, was standardised to use of any of the following agents alone or in combination per usual institutional protocol by either continuous infusion or intermittent bolus dosing: fentanyl, morphine, ketamine, midazolam, or lorazepam.
Safety outcomes
The primary safety outcome was occurrence of any one of four types of haemodynamic or three additional safety events occurring within 4 h of the last DEX dosing, if they were determined to be related to DEX administration. Four hours was chosen as the cut-off as the elimination half-life of DEX is approximately 2 h.21, 22, 23 Haemodynamic effects included the following: bradycardia—(i) sinus bradycardia below 80 beats min−1 or (ii) junctional bradycardia below 80 beats min−1; (iii) second- or third-degree heart block; (iv) hypotension: mean arterial blood pressure (MAP) lower than 35 mm Hg for 0–21-day-old subjects in the Neonatal Group, and below 40 mm Hg for 22–180-day-old subjects in the Infant Group.24, 25, 26, 27 These haemodynamic changes met safety outcome criteria if they were sustained for longer than 15 min or required rescue treatments, and occurred before or after CPB. Additional safety outcomes included: (v) excessive sedation in the ICU: University of Michigan Sedation Scale (UMSS) score of 4 in the ICU, maintained for longer than 4 h despite no doses of opioids and benzodiazepines for 2 h or longer, and documented absence of neuromuscular block with nerve stimulator; (vi) cardiac arrest or ECMO cannulation during DEX infusion or within 4 h of the end of infusion; or (vii) other serious adverse events (SAE). Events determined to be possibly, probably, or definitely related to DEX administration were also classified as a dose-limiting toxicity (DLT). Secondary safety endpoints included: all-cause mortality through 30 days after the end of DEX infusion; adrenal insufficiency, suspected by catecholamine-resistant hypotension as defined by negative response to adrenocorticotropic hormone (ACTH) stimulation test28; and hypertension, defined as MAP above approximately the 95th percentile for age sustained for longer than 15 min. For neonates aged 0–21 days, hypertension was defined as MAP >60 mm Hg, and for infants, hypertension was defined as MAP >70 mm Hg.23, 24, 25, 26 AEs were recorded throughout the study period and for 30 days thereafter. AEs were adjudicated by an independent Medical Monitor for determination of primary safety outcomes.
Measurement of dexmedetomidine concentrations
Plasma DEX concentrations were determined using a validated high-performance liquid chromatography tandem mass spectrometry assay with a lower limit of quantitation of 5 pg ml−1. The intra- and inter-day coefficients of variation are 0.74–6.67% and 0.67–4.86%, respectively, for DEX concentrations in the range of 5–1200 pg ml−1.29 Samples were obtained by study personnel assisted by anaesthesiologists and bedside nurses, centrifuged at 760 g for 15 min to separate plasma, and plasma tubes were frozen at –70°C. Samples were batched and shipped overnight with dry ice packaging to the Children's Hospital of Philadelphia Center for Clinical Pharmacology (Philadelphia, PA, USA). Sampling times for all cohorts are reported in Supplementary Appendix 1.
Part 1
Dose escalation
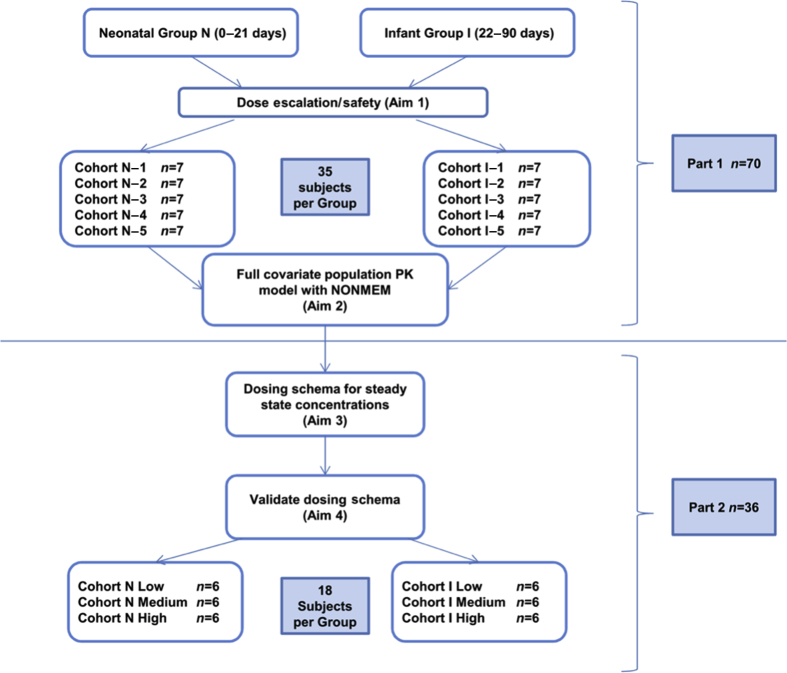
For the dose escalation, cohort size was determined a priori to provide a sample size sufficient to evaluate short-term safety and allow characterisation of the PK endpoints in this population, and to validate the PK model. The study was not powered to detect statistically significant differences in safety profiles for different doses. A planned seven participants were to be enrolled in each of five dosing cohorts in each of the neonatal and infant groups, for an initial target of a total of 70 subjects for Part 1 (Fig. 1). The first two cohorts in each age group (specifically N-1, N-2, I-1, and I-2) were designed for DEX dosing only in the CPB prime volume, with a portion of the dose to account for the CPB circuit volume in μg ml−1 of prime volume, and a second portion for the subject's weight in μg kg−1. Initial dosing was selected based on previously published data and in vitro data measuring DEX plasma levels with CPB circuit only.21, 22, 23 For cohorts N-1 and I-1, the loading dose was administered to the CPB circuit as a single bolus over 1–2 min, 10–30 min before the initiation of CPB to allow uniform drug distribution within the circuit before cannulation. Cohorts N-2 and I-2 were also dosed directly into the circuit, but instead had the CPB priming dose infused over 10 min directly into the CPB circuit within 5 min after the initiation of CPB in the subject. After the CPB-only cohorts, subsequent cohorts (N-3 through N-5; and I-3 through I-5) were planned to receive a loading dose administered to the subject over 10 min after induction of anaesthesia but before surgical incision, an infusion started immediately after the loading dose and the CPB priming dose infused over 10 min directly into the circuit within 5 min of initiation of CPB. The DEX infusion that was started after the bolus was continued through CPB and up to 6–12 h after the end of the operation.
Fig 1.
Dexmedetomidine phase 1 study enrolment plan. PK, pharmacokinetic; NONMEM®, non-linear mixed-effects modelling.
Dose escalation from lower to higher doses occurred only upon completion of data collection for a full dosing cohort (after all DEX PK samples had been obtained), an interim safety analysis for all subjects in that cohort, and the assessment of all DEX plasma concentrations. If the maximum tolerated dose (MTD; defined as the dose at which a second subject in a given cohort experienced an adjudicated safety outcome) was not achieved/exceeded, escalation to the next highest dose cohort within that age group occurred. This process was repeated for each cohort, through cohorts N-5 and I-5, or until the MTD had been achieved/exceeded. The original planned dosing cohorts are displayed in Figure 1.
PK modelling
After the completion of the dose escalation in all cohorts in both age groups, a compartmental population PK model using non-linear mixed-effects modelling to fit the combined cohorts was constructed. The population PK analysis was conducted using NONMEM® (ICON Development Solutions, Ellicott City, MD, USA) version 7, level 2.0 (ADVAN 3, TRANS 4). DEX plasma concentrations below the limit of quantitation were not included in the analysis if they were less than 10% of data points. All models were run with the first-order conditional estimation with interaction method. S-Plus version 6.2 (Insightful, Inc., Seattle, WA, USA) was used for goodness-of-fit diagnostics and graphical displays. The goodness-of-fit from each NONMEM® run was assessed by examining the following criteria: visual inspection of diagnostic scatter plots (observed vs predicted concentration, observed and predicted concentration vs time, and weighted residual vs predicted concentration or time), the precision of the parameter estimates as measured by asymptotic standard errors (se) derived from the covariance matrix of the estimates, successful minimisation with at least three significant digits in parameter estimates, changes in the minimum value of the objective function (MVOF), and changes in the estimates of inter-individual and residual variability for the specified model.
One-, two-, and three-compartment models were investigated. A two-compartment disposition model was deemed optimal to describe the DEX plasma concentration profile based on results from the model building process (goodness-of-fit as described above) and supported by previously published data.21, 23, 30, 31, 32 Models were parameterised by clearance (CL, ml min−1), inter-compartmental clearance (Q, ml min−1), central volume of distribution (V1, L), and peripheral volume of distribution (V2, L). Parameters were estimated for the time course during CPB, and again during the post-CPB period. As such, each parameter is represented by two estimates (i.e. CLcpb and CLpost). The final model also included parameter estimates for the time course prior to the initiation of CPB (CLpre, V2pre, Qpre, and V2pre).
An exponential variance model was used to describe the unexplained random variability of PK parameters across individuals in the form: Pi=θkexp(ηki), where Pi is the estimated parameter value for the individual subject i, θk is the typical population value of parameter k, and ηki (eta values) are the inter-individual random effects for individual i and parameter k. Models were explored using various inter-individual random-effect covariance structures. Inter-individual variability was initially estimated for clearance, and then subsequently for the remaining PK parameters. Additive, proportional, and combined (additive and proportional) residual error models were considered during the model building process. Ultimately, a combined additive and proportional error model was used to describe random residual variability.
The impact of weight on all PK parameters was investigated using an allometric model: TVP=θTVP×(WTi/WTref)θallometric, where θallometric is an allometric power parameter based on physiologic consideration of size impact on metabolic processes and is fixed at a value of 0.75 for clearances, and a value of 1 for volumes.33 A reference weight of 70 kg was used. Visual inspection of the base model in exploratory graphics for associations between covariates of interest with parameter estimates and non-linear relationships of eta values with covariates was performed to guide further testing if physiologically plausible. As such, total CPB time was evaluated as a covariate on CLpost, V1cpb, and V1post using a power model normalised to the median total bypass time. The effect of temperature on CLpost, V1cpb, and V1post was investigated as a power model normalised to 37°C. Age was evaluated as a covariate on clearance using the Hill equation, after the inclusion of weight in the model. Ultrafiltration was also assessed as a covariate on intraoperative parameters. The final model from this analysis was deemed Model 1.
Part 2
External validation and target concentration attainment
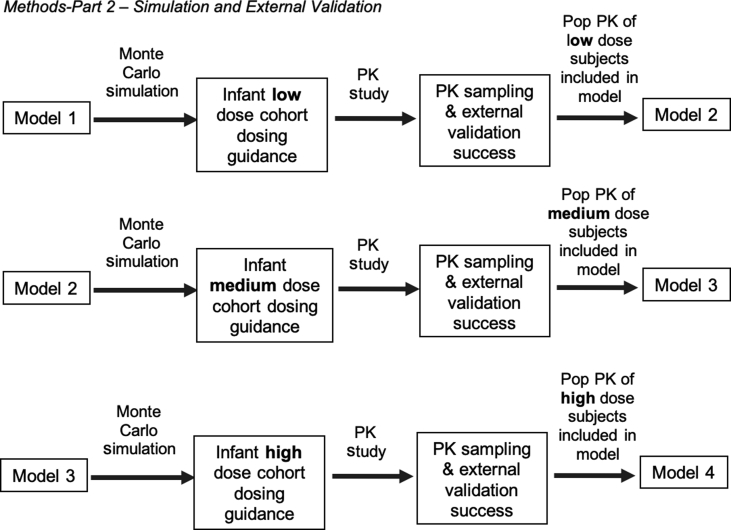
Part 2 of the study targeted 36 additional subjects for external validation and trial simulations to achieve predetermined steady-state concentrations (Fig. 1). Six cohorts of six subjects each were planned to be enrolled: three neonatal and three infant cohorts—resulting in an original planned total number of 106 subjects for both Parts 1 and 2. Dosing schemes in each age group were validated for their ability to achieve target low (200–300 pg ml−1), then medium (400–500 pg ml−1), and lastly high (600–700 pg ml−1) DEX plasma steady-state concentrations21, 30, 31, 32, 34 (Table 1). The final covariate model from Part 1 (Model 1) developed after Part 1 dose escalation was complete was used to simulate expected concentration–time profiles in subjects previously enrolled in Part 1 under various dosing scenarios using 500 Monte Carlo simulation replicates, incorporating inter-individual and residual random variability and covariate effects, to achieve a Css of 200 pg ml−1 in the infant population. The 5th–95th percentile prediction interval was determined for each subject's model-predicted concentration–time profile. Six infants in each age group received the model-based dosing strategy and underwent PK sampling. Each subject's measured PK concentrations were compared with their model-predicted concentrations. Model prediction for an individual subject was considered successful if at least 80% of an individual's measured DEX concentrations fell within the 5th–95th percentile prediction interval of their model-predicted concentration–time profile. This external validation provided a robust approach to evaluate the predictive performance of the model.35, 36, 37 Once the model was deemed successful, it was updated with each new cohort's data and then used to simulate the dosing for the next respective cohort (Fig. 2) in an iterative fashion.
Table 1.
Dexmedetomidine dosing scheme for Part 2. *Infusion dose 1 to subject before and during CPB, to be given in OR through 2 h 13 min CPB. †Infusion dose 2 to subject restarted or decreased (if less than 2 h 13 min CPB) in the OR after CPB before ICU admission. ‡Infusion dose 1 to subject before and 1 h during CPB. ¶Infusion dose 2 to subject after infusion dose 1 completed during CPB through hour 4 (omitted if CPB 1 h or less). §Postoperative infusion dose 3 to subject 2 h after CPB. ||Infusion dose 2 to subject after infusion dose 1 completed during CPB (omitted if CPB 1 h or less). #Postoperative infusion dose 3 to subject 1 h after CPB. I, infant; N, Neonatal; DEX, dexmedetomidine; CPB, cardiopulmonary bypass; OR, operating room.
| Cohort | Target steady-state DEX plasma level (pg ml−1) | Age (days), number of subjects | Loading dose to subject (μg kg−1) | Infusion to subject (μg kg−1 h−1) | Loading dose to CPB prime volume (μg ml−1) |
|---|---|---|---|---|---|
| I-Low | 200–300 | 22–180, n = 6 | 0.5 | Dose 1*: 0.4 Dose 2†: 0.25 |
0.0006 |
| I-Medium | 400–500 | 22–180, n = 6 | 0.9 | Dose 1‡: 0.8 Dose 2¶: 0.2 Dose 3§: 0.5 |
0.0024 |
| I-High | 600–700 | 22–180, n = 6 | 1.1 | Dose 1‡: 1.4 Dose 2||: 0.15 Dose 3#: 0.6 |
0.014 |
| N-Low | 200–300 | 0–21, n = 6 | 0.4 | Dose 1‡: 0.3 Dose 2||: 0.1 Dose 3#: 0.25 |
0.006 |
| N-Medium | 400–500 | 0–21, n = 6 | 0.8 | Dose 1‡: 0.6 Dose 2||: 0.2 Dose 3#: 0.5 |
0.012 |
| N-High | 600–700 | 0–21, n = 6 | 1.0 | Dose 1‡: 0.8 Dose 2||: 0.25 Dose 3#: 0.7 |
0.016 |
Fig 2.
Iterative approach for model development and simulation strategy for Part 2. PK, pharmacokinetic; Pop, population.
Final model
After completion of the three dosing schemes in both cohorts, the data were pooled from Part 1 and Part 2 to create the final PK model. Covariates were evaluated as described above. The impact of weight on all PK parameters was also investigated using an allometric model as described above in addition to a linear model: TVP =θTVP×(WTi/WTref) using a reference weight of 70 kg. This was performed by removing the 0.75 allometric scaling factor for clearances (CL and Q) and replacing it with 1. Parameters were again estimated for the time course during CPB, and during the post-CPB period. However, an additional pre-CPB period was also estimated for the final model. This was defined as the time when the subject was in the OR before the initiation of CPB, after the bolus was administered to the subject and during the infusion. Subjects except those in cohorts N1, N2, I1, and I2 underwent one PK sampling during this period. The final model was used to simulate the final dosing strategies for Css of 200, 500, 700, and 1000 pg ml−1 for both neonates and infants.
Log-likelihood profiling (LLP) was performed for each of the estimated fixed-effect parameters, in an effort to illustrate the marginal (approximate) –2 log-likelihood profile for each parameter. Assuming that the difference in –2 log-likelihood for nested models is χ2 distributed, 95% confidence intervals (CIs) were constructed by selecting fixed-effect parameter values associated with a change of 3.84 points in the MVOF, when compared with the maximum likelihood estimate. It is acknowledged that the accuracy of these methods may be compromised under conditions when an approximation to the likelihood is required, but the purpose was simply to provide a relative comparison of parameter precision. Predictive checks were performed for both the allometric and linear scaled final models.
Results
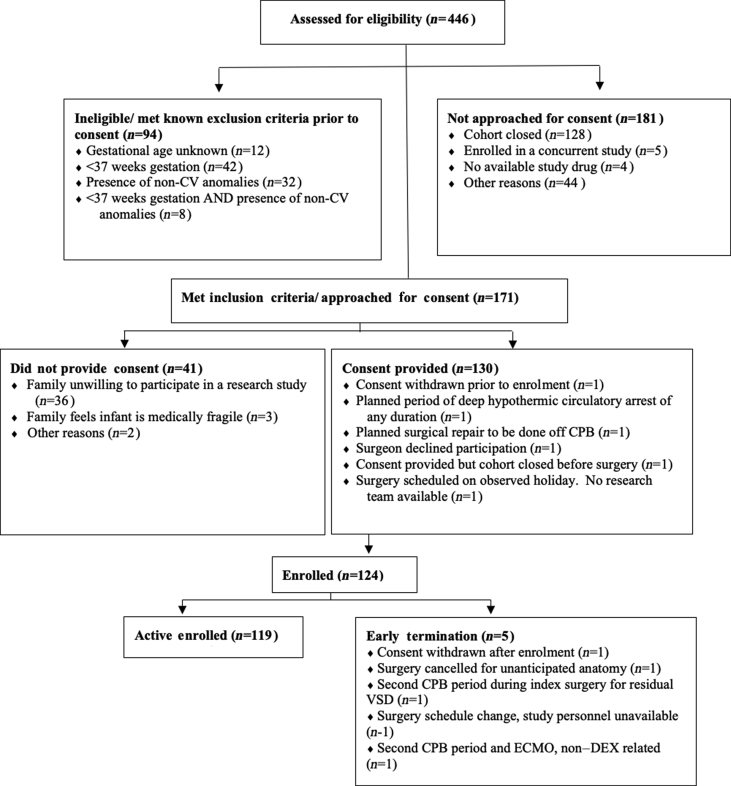
A total of 124 subjects were enrolled; there were five early withdrawals (Fig. 3). Patient characteristics and other information are reported in Table 2. The 122 subjects who entered the OR and received anaesthesia were evaluated for safety outcomes; three subjects entered the OR but did not receive all DEX dosing and sampling and did not have complete PK data; the remaining 119 subjects all had complete PK data and comprised the final PK dataset. The final number of 119 subjects, instead of the targeted 106, resulted from the addition of two additional cohorts of six subjects, and early termination of cohort I5 at six subjects, resulting in 83 Part 1 subjects (instead of the targeted 70). Part 2 had the planned 36 subjects for a total of 119; the five early withdrawal subjects were replaced, thus the total of 124 enrolled subjects.
Fig 3.
Screening/eligibility/enrolment flow diagram. CV, cardiovascular; CPB, cardiopulmonary bypass; DEX, dexmedetomidine; ECMO, extracorporeal membrane oxygenation; VSD, ventricular septal defect.
Table 2.
Subjects characteristics and medical history. *P-values from Wilcoxon test for median or Fisher exact test for frequencies, neonate vs infant groups. CPB, cardiopulmonary bypass; DEX, dexmedetomidine.
| Characteristic | Enrolled (n=119) |
Neonate (n=60) |
Infant (n=59) |
P-value | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Male sex | 79 | 66 | 47 | 78 | 32 | 54 | 0.007 |
| Cardiac diagnosis | |||||||
| D-transposition of the great arteries | 53 | 45 | 51 | 85 | 2 | 3 | <0.001 |
| Tetralogy of Fallot | 37 | 31 | 8 | 13 | 29 | 49 | <0.001 |
| Ventricular septal defect |
39 |
33 |
9 |
15 |
30 |
51 |
<0.001 |
|
Median |
(Q1, Q3) |
Median |
(Q1, Q3) |
Median |
(Q1, Q3) |
||
| Gestational age (weeks) | 39 | (38, 39) | 39 | (38, 39) | 39 | (38, 40) | 0.44 |
| Age at surgery (days) | 18 | (5, 93) | 5 | (4, 6) | 93 | (70, 126) | <0.001 |
| Male | 7 | (5, 87) | 5 | (4, 6) | 97 | (61, 128) | <0.001 |
| Female | 81 | (9, 117) | 5 | (5, 8) | 93 | (80, 126) | <0.001 |
| Length at surgery (cm) | 54.0 | (50.8, 58.0) | 51.0 | (48.5, 52.1) | 58.0 | (55.5, 61.5) | <0.001 |
| Weight at surgery (kg) | 3.8 | (3.4, 5.2) | 3.4 | (3.1, 3.7) | 5.2 | (4.2, 5.7) | <0.001 |
| CPB time (min) | 92.0 | (63.0, 163.0) | 125.5 | (73.5, 206.0) | 75.0 | (42.0, 119.0) | <0.001 |
| Lowest CPB temperature (°C) | 33.1 | (28.0, 34.2) | 32.1 | (27.7, 34.0) | 33.6 | (28.0, 34.5) | 0.06 |
| Anaesthesia time (h) | 5.2 | (3.9, 6.7) | 5.7 | (4.5, 8.0) | 4.6 | (3.4, 6.0) | <0.001 |
| Duration of ICU DEX infusion (h) | 6.9 | (6.0,11.8) | 6.7 | (5.9, 10.8) | 8.1 | (6.1, 12.0) | 0.02 |
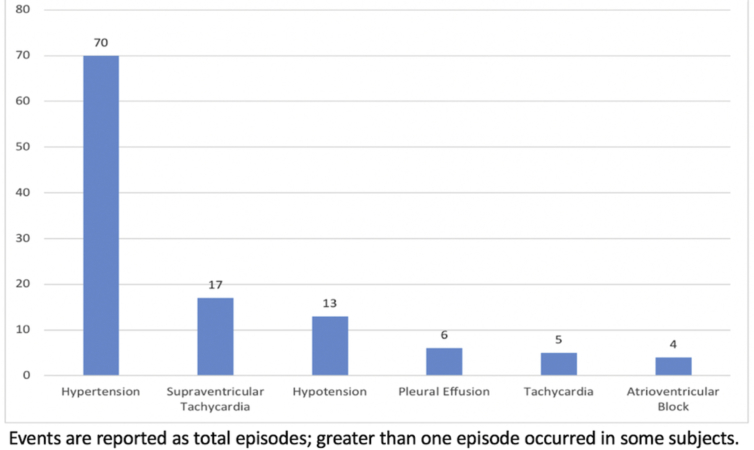
In Part 1 of the study (fixed dose escalation), the infant group I-5 (0.75 μg kg−1 h−1 prolonged infusion dose) included two adjudicated safety events, so the MTD was exceeded at that dose, and enrolment was terminated after six subjects. For the primary outcome, there were a total of five adjudicated safety events (4.1%; 95% CI, 1.8–9.2%). The events are described in detail in Table 3. An additional lower dose cohort I-4A was added (Table 5). Because the neonatal group N-5 dosing did not meet the MTD, an additional higher dose cohort N-6 was added, per protocol. The MTD was neither met nor exceeded in neonates (maximum prolonged infusion dose, 0.7 μg kg−1 h−1). There were two episodes of junctional rhythm with heart rate slowing to 65–109 beats min−1, two episodes of second-/third-degree atrioventricular block with HR 85–95 beats min−1. None of these episodes was associated with hypotension; all four required temporary atrial or atrioventricular pacing for 30 min to 48 h. Three of four were receiving drugs with known effects on the conduction system: digoxin, amiodarone, or β-adrenergic receptor blocking drugs. In each of these events, DEX was deemed possibly related, but not the primary cause of the event. There was one episode of hypotension with multifactorial aetiology; DEX was deemed possibly related. All adjudicated safety outcomes occurred in the Infant age group; there were none in the Neonatal age group. Of the five safety outcomes, two were deemed serious AEs, and three non-serious AEs. There were no instances of oversedation, cardiac arrest, or ECMO cannulation related to DEX administration. There were no unexpected AEs. The most common secondary safety events are displayed in Supplementary Figure S1, and complete AE data are reported in Supplementary Appendix 2.
Table 3.
Adjudicated safety events. I, infant cohort; VSD, ventricular septal defect; TOF, tetralogy of Fallot; DEX, dexmedetomidine; pg, picograms; AV, atrioventricular; DDD, dual-chamber atrioventricular; MAP, mean arterial pressure; CPB, cardiopulmonary bypass.
| Cohort | Cardiac diagnosis | Age at time of event (days) | Weight (kg) | Event | DEX level during start of event (pg ml−1) | Description | Additional drugs | Outcome |
|---|---|---|---|---|---|---|---|---|
| I-2 | VSD | 175 | 5.73 | Junctional rhythm rate 65–85 beats min−1; sinus bradycardia/junctional rhythm rate 85–95 beats min−1 | 126 | Junctional rhythm for 60 min after CPB requiring atrial pacing at 112 beats min−1. Postoperative intermittent sinus bradycardia and junctional rhythm requiring atrial pacing for 12 h. | Preoperative digoxin | Resolved |
| I-5 | TOF | 98 | 5.30 | Second-degree AV block 90–95 beats min−1 | 738–977 | DDD paced 48 h. | Esmolol, amiodarone | Resolved |
| I-5 | VSD | 173 | 6.22 | Junctional rhythm rate 103–109 beats min−1 | 455–677 | Junctional rhythm 75 min after termination of DEX infusion requiring atrial pacing for 2 h. | Propranolol | Resolved |
| I-4a | TOF | 42 | 3.50 | Third-degree AV block rate 85–95 beats min−1 | 159–289 | Third-degree AV block requiring DDD pacing after CPB, resolved within 30 min. | Resolved | |
| I-High | TOF | 32 | 3.60 | Hypotension | 233 | 32 min after DEX infusion stopped MAP was <40 mm Hg, agitation and hypovolaemia. | Lorazepam, midazolam, morphine, fentanyl | Resolved |
Table 5.
Final dexmedetomidine dosing scheme for Part 1 of the DEX study: loading dose plus infusion cohorts. DEX, dexmedetomidine; CPB, cardiopulmonary bypass.
| Cohort | Age (days), number of subjects | Loading dose to subject (μg kg−1) | Infusion to subject (μg kg−1 h−1) | Loading dose to CPB prime volume (μg ml−1) |
|---|---|---|---|---|
| N-3 | 0–21, n=7 | 0.2 | 0.1 | 0.0004 |
| N-4 | 0–21, n=7 | 0.4 | 0.2 | 0.0008 |
| N-5 | 0–21, n=7 | 0.6 | 0.3 | 0.0012 |
| N-6 | 0–21, n=7 | 0.8 | 0.4 | 0.0016 |
| I-3 | 22–180, n=7 | 0.35 | 0.25 | 0.0004 |
| I-4 | 22–180, n=7 | 0.7 | 0.5 | 0.0008 |
| I-4a | 22–180, n=7 | 0.85 | 0.625 | 0.0010 |
| I-5 | 22–180, n=6 | 1 | 0.75 | 0.0012 |
The final dosing schema, after addition of cohorts N-6, and I-4A, are displayed in Table 4, Table 5. In the infant age group, two of the three additional safety events occurred at 0.6 and 0.625 μg kg−1 h−1, and the final safety event occurred with DEX dosing only to the CPB circuit. Thus, four of five safety outcomes occurred at higher DEX dosing. DEX plasma concentrations at the time of the five adjudicated safety events ranged from 126 to 977 pg ml−1, and thus were not consistently associated with high DEX concentrations.
Table 4.
Final dexmedetomidine dosing scheme for Part 1 of the DEX study: CPB only cohorts. DEX, dexmedetomidine; CPB, cardiopulmonary bypass.
| Cohort | Age (days), number of subjects | DEX dose for CPB circuit volume (μg ml−1) | DEX dose for subject weight (μg kg−1) to CPB circuit |
|---|---|---|---|
| N-1 | 0–21, n = 7 | 0.0004 | 0.2 |
| N-2 | 0–21, n =7 | 0.0008 | 0.4 |
| I-1 | 22–180, n =7 | 0.0004 | 0.35 |
| I-2 | 22–180, n =7 | 0.0008 | 0.70 |
No subjects died during the study period or the 30 day follow-up period. Of the secondary safety outcomes, adrenal insufficiency was suspected in five subjects, with three exhibiting catecholamine-resistant hypotension. ACTH stimulation test was performed in two of these subjects, with one exhibiting test results diagnostic of adrenal insufficiency (basal serum cortisol level ≤20 μg dl−1, with an increase of ≤9 μg dl−1 after ACTH).28 Four of five subjects received supplemental corticosteroid treatment. Hypertension was the most common secondary outcome; 70 episodes were recorded in 35 subjects.
PK modelling
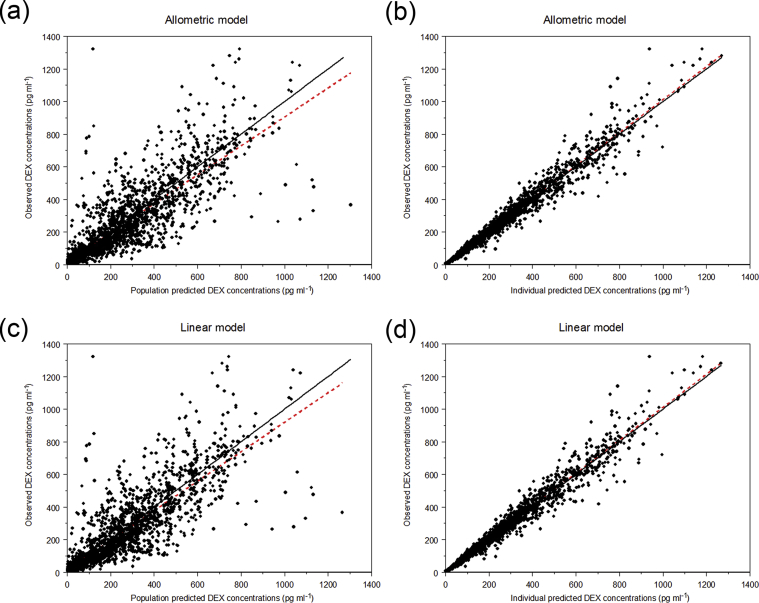
The final model included 1967 plasma concentrations from 119 subjects. There were 168 samples below the level of quantification (8.5%). The model progression from the development of Model 1 at the end of Part 1, through Part 2, with dosing strategies, model evolution, and success of external validations, are displayed in Table 6. The external validation was successful for all subjects for all targeted DEX concentrations with 96.1% of 597 measured DEX concentrations falling within the 5th–95th percentile prediction intervals in Part 2 (Supplementary Appendix 3). The PK parameters for both the allometrically and linearly weight scaled models and the final model are displayed in Table 7. The linear scaled model demonstrated a 10 point reduction in the MVOF and Akaike information criterion (AIC). DEX clearance after CPB was 623 (allometric) or 1240 (linear) ml min−1 70 kg−1 and decreased by about 95% during CPB in both models when compared with pre-CPB values. Ultrafiltration (whether conventional during CPB or modified after CPB) and duration of CPB had minimal effect on DEX PK parameters, and were not included in the final PK model. Despite the exclusion of subjects who underwent deep hypothermic cardiac arrest, temperature did have an effect on V1 during CPB, in which there was an increase in V1 during CPB for temperatures lower than 37°C (Temp/37)−1.6 in both models). Neonates achieved 90% maximum clearance at approximately 18 days (100% maximum clearance=180-day-old infant for this study). The impact of age and temperature in the model is demonstrated in Table 8 through a backwards reduction, for both the allometrically and linearly scaled models. Predictive checks demonstrated that the allometrically scaled model had 7.6% of observations outside of the 90th prediction interval, whereas the linearly scaled model had 5.8% of observations outside of the 90th prediction interval. The observed vs population predicted, and observed vs individual predicted, DEX plasma concentrations for allometrically and linearly scaled models are displayed in Figure 4. The final mathematical model is displayed in Supplementary Appendix 4, and the NONMEM® control files are contained in Supplementary Appendix 5. The individual log likelihood profile (LLP) curves are displayed in Supplementary Appendix 6a for the allometric model and in Supplementary Appendix 6b for the linear model.
Table 6.
Pharmacokinetic model progression during Part 2 of the study. *Infusion dose 1 to subject before and during CPB, to be given in the operating room (OR) through 2 h 13 min CPB. †Infusion dose 1 to subject pre and 1 h during CPB. ‡Infusion dose 2 to subject restarted or decreased (if less than 2 h 13 min CPB) in the OR after CPB before ICU admission. ¶Infusion dose 2 to subject after infusion dose 1 completed during CPB through hour 4 (omitted if CPB 1 h or less). §Infusion dose 2 to subject after infusion dose 1 completed during CPB (omitted if CPB 1 h or less). ||Postoperative infusion dose 3 to subject 2 h after CPB. #Postoperative infusion dose 3 to subject 1 h after CPB. I, infant; N, Neonatal; DEX, dexmedetomidine; CPB, cardiopulmonary bypass; CL, clearance; CPB, cardiopulmonary bypass; POST OP, after operation; V1, volume of distribution to compartment 1; Q, intercompartmental clearance; V2, volume of distribution to compartment 2; CPBt, cardiopulmonary bypass temperature; Css, steady state plasma concentration. NA, not available.
| Cohort contributions | I-Low | I-Med | I-High | N-Low | N-High |
|---|---|---|---|---|---|
| N1 | n=7 | n=7 | n=7 | n=7 | n=7 |
| N2 | n=7 | n=7 | n=7 | n=7 | n=7 |
| N3 | n=7 | n=7 | n=7 | n=7 | n=7 |
| N4 | n=7 | n=7 | n=7 | n=7 | n=7 |
| N5 | NA | n=7 | n=7 | n=7 | n=7 |
| N6 | NA | NA | n=7 | n=7 | n=7 |
| I1 | n=7 | n=7 | n=7 | n=7 | n=7 |
| I2 | n=7 | n=7 | n=7 | n=7 | n=7 |
| I3 | n=7 | n=7 | n=7 | n=7 | n=7 |
| I4 | n=7 | n=7 | n=7 | n=7 | n=7 |
| I4a | n=7 | n=7 | n=7 | n=7 | n=7 |
| I5 | n=6 | n=6 | n=6 | n=6 | n=6 |
| I low | NA | n=6 | n=6 | n=6 | n=6 |
| I med | NA | NA | n=6 | n=6 | n=6 |
| I high | NA | NA | NA | NA | n=6 |
| N low | NA | NA | NA | NA | n=6 |
| N med | NA | NA | NA | NA | n=6 |
| N high | NA | NA | NA | NA | NA |
| CL POST OP (ml min−1 70 kg−1) | 771 | 727 | 686 | 686 | 756 |
| CL CPB (ml min−1 70 kg−1) | 0.276 | 0.153 | 0.845 | 0.845 | 0.047 |
| V1 POST OP (L 70 kg−1) | 167 | 149 | 157 | 157 | 245 |
| V1 CPB (L 70 kg−1) | 36.4 | 118 | 68.7 | 68.7 | 35.1 |
| Q POST OP (ml min−1 70 kg−1) | 189 | 100 | 228 | 228 | 388 |
| Q CPB (ml min−1 70 kg−1) | 7930 | 4270 | 9610 | 9610 | 4590 |
| V2 POST OP (L 70 kg−1) | 64.4 | 70.5 | 93.5 | 93.5 | 79.2 |
| CPB V2 (L 70 kg−1) | 370 | 386 | 362 | 362 | 547 |
| AGE on CL POST OP (days) | 0.795 | 2.1 | 0.935 | 0.935 | 1.02 |
| CPBt on V1 CPB | 1.4 | 0.268 | 0.625 | 0.625 | NA |
| Model name | Model 1 | Model 2 | Model 3 | Model 3 | Model 4 |
| Target Css (pg ml−1) in simulations | 200–300 | 400–500 | 600–700 | 200–300 | 600–700 |
| Loading dose to subject (μg kg−1) | 0.5 | 0.9 | 1.1 | 0.4 | 1 |
| Loading dose to CPB (μg ml−1) | 0.0006 | 0.0024 | 0.014 | 0.006 | 0.016 |
| Infusion to Subject 1 (μg kg−1 h−1) | 0.4* | 0.8† | 1.4† | 0.3† | 0.8† |
| Infusion to Subject 2 (μg kg−1 h−1) | 0.25‡ | 0.2¶ | 0.15§ | 0. § | 0.25§ |
| Infusion to Subject 3 (μg kg−1 h−1) | NA | 0.5|| | 0.6# | 0.25# | 0.7# |
| n (%) within 90th percentile prediction interval | 95 (90.5) | 106 (94.6) | 86 (100) | 116 (96.7) | 77 (96) |
Table 7.
Dexmedetomidine pharmacokinetic parameters for final models. 95% CI are derived from log likelihood profiles (LLP—individual LLP curves are presented in Supplementary Appendix 6) for fixed effect parameter estimates are indicated in parentheses. se% is the standard error percent derived from the NONMEM asymptotic standard errors. Inter-individual variability and proportional residual variability point estimates are presented as percent coefficient of variation (square root of variance)×100. σ2 additive point estimate is expressed as a standard deviation. AIC, Akaike information criterion; CI, confidence interval; CL, clearance; Q, intercompartmental clearance; V1, central volume of distribution; V2, peripheral volume of distribution; CPB, cardiopulmonary bypass; post, post-CPB values; cpb, values during CPB; ω2, between-subject variability.
| Allometric weight normalised model |
Linear weight normalised model |
|||
|---|---|---|---|---|
| AIC=16 328 | AIC=16 318 | |||
|
Parameter |
Point estimate (NONMEM SE%) |
95% CI from LLP |
Point estimate (NONMEM SE%) |
95% CI from LLP |
| CLpre (ml min−1 70 kg−1) | 1240 (14) | 1030, 1470 | 2580 (14) | 1950, 3400 |
| CLcpb (ml min−1 70 kg−1) | 74.1 (42.1) | 59, 126 | 142 (53.5) | 130, 300 |
| CLpost (ml min−1 70 kg−1) | 623 (7.9) | 560, 670 | 1240 (8.39) | 1020, 1400 |
| V1pre(L 70 kg−1) | 132 (26.4) | 109, 152 | 139 (25.8) | 94.6, 202 |
| V1cpb (L 70 kg−1) | 115 (14.7) | 106, 136 | 116 (14.9) | 103, 146 |
| V1post (L 70 kg−1) | 155 (7.61) | 141, 167 | 159 (7.92) | 129, 185 |
| Qpre (ml min−1/70 kg−1) | 2300 (96.1) | 50, 6800 | 4120 (107) | 100, 400 000 |
| Qcpb (ml min−1 70 kg) | 2980 (18.7) | 2410, 3710 | 6160 (16.9) | 4300, 8400 |
| Qpost (ml min 70 kg−1) | 209 (18.6) | 161, 270 | 422 (20.3) | 280, 700 |
| V2pre(L 70 kg−1) | 78.9 (36) | 19.5, 154 | 69.6 (43) | 5, 90 |
| V2cpb (L 70 kg−1) | 144 (12.4) | 135, 162 | 147 (12.4) | 101, 149 |
| V2post (L 70 kg−1) | 105 (9.4) | 92.3, 113 | 97 (10.6) | 78.6, 130 |
| Age CLpost 50% mature (days) | 1.77 (25.4) | 1.11, 2.28 | 1.29 (33.9) | 0.4, 2 |
| Temp effect V1cpb |
–1.6 (6.6) |
–1.69, –1.41 |
–1.57 (6.43) |
–1.73, –1.21 |
|
Inter-individual variability |
Covariance |
se% |
Covariance |
se% |
| ω2CLpre | 75.5% | 30.4 | 73.3% | 31.1 |
| ω2CLCPB | 192.6% | 46.1 | 195.4% | 50.8 |
| ω2CLpost | 48.0% | 13 | 47.6% | 13.1 |
| ω2V1pre | 65.4% | 54 | 63.0% | 55.7 |
| ω2V1CPB | 70.3% | 16.8 | 70.6% | 16.8 |
| ω2V1post | 49.5% | 13.7 | 48.9% | 12.8 |
| ω2V2CPB | 63.9% | 24.5 | 71.6% | 24.5 |
| ω2V2post | 71.1% | 28.5 | 66.4% | 30.7 |
| ω2QCPB | 55.7% | 57.4 | 49.2% | 86 |
| ω2Qpost |
137.1% |
25.7 |
134.2% |
27.1 |
|
Inter-individual covariance |
Point estimate (correlation) |
se% |
Point estimate (correlation) |
se% |
| CLpost, CLcpb | 0.23 | 72.2 | 0.23 | 71.6 |
| CLpost, V1post | 0.81 | 129 | 0.84 | 12.5 |
| CLcpb, V1post |
0.54 |
42.8 |
0.51 |
45.3 |
|
Residual variability |
Point estimate |
se% |
Point estimate |
se% |
| σ2 proportional | 0.0199 | 4.3 | 0.0198 | 4.11 |
| σ2 additive | 9.99 | 18.5 | 11.0 | 17.5 |
Table 8.
Results of stepwise reductions. MVOF, minimum value of the objective function; CL, clearance; V1CPB, volume of distribution in compartment 1 during cardiopulmonary bypass.
| Description | MVOF | Change in MVOF |
|---|---|---|
| Allometric weight normalised model | ||
| Final allometric model | 16 265.6 | |
| Remove age on CL from full model | 16 292.9 | 27.3 |
| Remove temperature on V1CPB from full model | 16 458.9 | 193.3 |
| Remove both age and temperature from full model | 16 481.9 | 216.3 |
| Linear weight normalised model | ||
| Final linear model | 16 256.1 | |
| Remove age on CL from full model | 16 268.6 | 12.5 |
| Remove temperature on V1CPB from full model | 16 449.3 | 193.2 |
| Remove both age and temperature from full model | 16 456.8 | 200.7 |
Fig 4.
Observed vs predicted plasma dexmedetomidine concentrations. Black solid lines are lines of identity, whereas red lines are slope of observed vs population predicted concentrations. (a) Observed vs population predicted dexmedetomidine (DEX) plasma concentrations for the allometrically scaled covariate model. (b) Observed vs individual predicted dexmedetomidine plasma concentrations for the allometrically scaled covariate model. (c) Observed vs population predicted dexmedetomidine plasma concentrations for the linearly scaled covariate model. (d) Observed vs individual predicted dexmedetomidine plasma concentrations for the linearly scaled covariate model.
Recommendations for DEX dosing to achieve and maintain steady-state plasma concentrations of 200, 500, 700, and 1000 pg ml−1 by age group are displayed in Table 9. These dosing recommendations are based on simulations using the subjects enrolled in the study, including weights, ages, and temperatures, a minimum CPB temperature of 32°C. Simulated concentrations based on this dosing guidance are demonstrated in Supplementary Appendix 7.
Table 9.
Dosing recommendations for dexmedetomidine steady-state concentrations. Css, steady-state concentration; CPB, cardiopulmonary bypass.
| Age group (days) | Target Css (pg ml−1) | Initial loading dose (μg kg−1) | Infusion 1: pre-CPB, first 60 min of CPB (μg kg−1 h−1) | Loading dose to CPB prime volume (μg ml−1) | Infusion 2: after 60 min of CPB until end of CPB (μg kg−1 h−1) | Infusion 3: 60 min after CPB (μg kg−1 h−1) |
|---|---|---|---|---|---|---|
| Neonatal (0–21) | 200 | 0.24 | 0.22 | 0.004 | 0.04 | 0.14 |
| Neonatal (0–21) | 500 | 0.6 | 0.55 | 0.01 | 0.1 | 0.35 |
| Neonatal (0–21) | 700 | 0.84 | 0.77 | 0.014 | 0.14 | 0.49 |
| Neonatal (0–21) | 1000 | 1.2 | 1.1 | 0.02 | 0.2 | 0.7 |
| Infant (22–180) | 200 | 0.29 | 0.26 | 0.005 | 0.05 | 0.17 |
| Infant (22–180) | 500 | 0.72 | 0.66 | 0.012 | 0.12 | 0.42 |
| Infant (22–180) | 700 | 1.01 | 0.92 | 0.017 | 0.17 | 0.59 |
| Infant (22–180) | 1000 | 1.44 | 1.32 | 0.024 | 0.24 | 0.84 |
Discussion
Safety
Despite concern about bradyarrhythmias and hypotension, in the current study these events were of low incidence and severity, demonstrating that DEX can be a feasible addition to the anaesthetic regimen for infant cardiac surgery. In a previously published study by Su and colleagues21 in an infant cardiac surgery population aged 0–36 months receiving postoperative DEX infusion, five subjects experienced bradyarrhythmias, hypotension, or oversedation. This incidence of five AEs in 59 subjects (8.5%) is within the range reported in the current study; thus DEX infusion before and during CPB does not appear to increase the incidence of AEs compared with the study of Su and colleagues,21 who assessed AEs with DEX administered only after CPB.
PK model
After bypass, DEX clearance and other PK parameters for the overall study population were similar to previously published single-centre data in neonates and infants up to 24 months of age.21 We determined postoperative CL to be 623 ml min−1 70 kg−1 in an allometrically scaled model and 1240 ml min−1 70 kg−1 in the linear model. Pre-CPB CL was precisely estimated at 1240 and 2580 ml min−1 70 kg−1 in the linear model. The model determined that CL in the immediate postoperative period was reduced by approximately 50% and CL on CPB was reduced by approximately 95% when compared with pre-CPB values. Weight and scaling adjustments should be performed when comparing values from the allometric model to other individuals or populations. For example, 623 ml min−1 70 kg−1 translates to 243 ml min−1 for a 20 kg child, 86 ml min−1 for a 5 kg child, and can be calculated using the equation: CL (ml min−1)=623×(wt/70)0.75. Both the allometric and linearly scaled models performed well, although the linear model was statistically superior to the allometric model. Clearance can also be calculated using a simpler approach with the linear scaled model. For example, a 20 kg child would have a postoperative clearance of 354 ml min−1, calculated using the equation CL (ml min−1)=1240×(wt/70). However, clearance during CPB diminished by ∼95%, a novel and very important finding for the appropriate use of DEX during CPB. Explanations for this profound decrease in clearance are likely secondary to reduced hepatic blood flow with CPB, and reduced DEX metabolism with hypothermia; a similar effect has been demonstrated with fentanyl.38, 39
Because of this reduced clearance, we recommend that the DEX infusion dose be reduced significantly after 1 h of bypass according to the dosing recommendations in Table 9. Hepatic metabolism of DEX increased rapidly in the first 3 weeks of life, concurring with published studies of DEX and other drugs, with 50% of maximum clearance at 6 months reached by 2 days of life, and 90% by 18 days.21 This finding supports the age grouping in the current study by designating neonates as 0–21 days for PK purposes. The very narrow SE percentages for the PK parameters, the inter-individual and residual variability, the inter-individual covariance, and the model plots of actual vs expected DEX plasma concentrations, for the entire population and for individuals, indicate a robust model that should achieve excellent generalisability for this patient population. Zimmerman and colleagues20 reported a single-centre DEX PK model during CPB in 18 infants and children; DEX infusion was started without loading dose at 0.5 μg kg−1 h−1; clearance after CPB was estimated at 701 ml min−1 70 kg−1, similar to the value in this study. Clearance on CPB decreased by 68%.19 However, 21% of DEX concentrations were below the lower concentration of quantification of the assay or of insufficient quantity for analysis, and ses for the PK parameters were much larger because of the significantly smaller subject population. Therapeutic DEX concentrations, as predicted, would require 4–5 half-lives, or 8–10 h, with this common approach to DEX administration, and therefore will have little effect for adding to anaesthetic and sedation depth in the OR period.20
Our study is the first to report DEX PK model creation and then model validation in a separate phase of the study in infants and children. Only a small number of published PK studies, in both children and adults, report model validation.35, 36, 37 In the validation experiment, all cohorts had >90% of measured DEX concentrations within the 5th–95th percentile prediction interval, indicating successful model validation for all dose ranges and age cohorts. The performance of this study in four centres with significantly different CPB durations should make this model more generalisable to the actual clinical population.
In preclinical studies, DEX is reported to have neuroprotective properties, ameliorating the histopathologic injuries induced by GABA and NMDA binding agents, and hypoxic–ischaemic and inflammatory injuries.10, 11, 12 In addition, DEX does not cause neurodegeneration in animal models, including non-human primates, except at doses that are significantly supratherapeutic.40 Therefore, DEX is an attractive candidate to study for neuroprotection in neonatal and infant cardiac surgery. Although exact plasma concentrations for neuroprotection in preclinical studies have not been reported, the robust PK model described in this study allows a range of plasma concentrations to be targeted, with low risk of haemodynamic AEs and oversedation.
Conclusions
When used with a careful dosing strategy, DEX results in low incidence and severity of safety events in infants undergoing cardiac surgery with CPB. The robust, validated PK model derived from this study should assist clinicians in selecting appropriate DEX dosing as a component of a balanced anaesthetic regimen starting before surgical incision that maintains steady-state concentrations throughout the entire perioperative procedure. The results of this phase 1 trial provide preliminary data for dosing for a phase 3 trial of DEX neuroprotection in this population.
Authors' contributions
Principal investigator: DBA
Study conception and design: DBA, AFZ, NSW, SCN
Data acquisition: DBA, AFZ, NSW, SCN, EAG, JI, TS
Analysis and interpretation of data: DBA, AFZ, NSW, SCN, EAG, JI, TS, KMB, MS, FT, BN
Pharmacokinetic design and modelling: DBA, AFZ
All authors participated in writing the first draft of the manuscript
Acknowledgements
The authors thank Janice Prodell for coordinating procedures for collecting, processing, handling, and shipping plasma samples, and producing detailed worksheets for each dosing cohort. The authors also acknowledge Ganesh Moorthy for performing the DEX assays. The authors acknowledge the cooperation of the anaesthesiologists, surgeons, intensivists, cardiologists, and bedside nurses at Texas Children's Hospital, the Children's Hospital of Philadelphia, C.S. Mott Children's Hospital, and Boston Children's Hospital for their support of the study.
Handling editor: A.R. Absalom
Editorial decision: 19 June 2019
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2019.06.026.
Declarations of interest
The views expressed in this manuscript are those of the authors and do not reflect official positions of the National Heart, Lung, and Blood Institute or the National Institutes of Health. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the manuscript, and its final contents. The authors declare no other conflicts of interest.
Funding
Pediatric Heart Network of the US National Heart, Lung, and Blood Institute of the US National Institutes of Health (grant numbers HL109818, HL109737, HL109741, HL068270). Pfizer, Inc. (New York, NY, USA; formerly Hospira, Inc.) provided DEX for the first 100 subjects enrolled in the study.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Fig. S1.
References
- 1.Mahmoud M., Mason K.P. Dexmedetomidine: review, update, and future considerations of paediatric perioperative and periprocedural applications and limitations. Br J Anaesth. 2015;115:171–182. doi: 10.1093/bja/aev226. [DOI] [PubMed] [Google Scholar]
- 2.Mukhtar A.M., Obayah E.M., Hassona A.M. The use of dexmedetomidine in pediatric cardiac surgery. Anesth Analg. 2006;103:52–56. doi: 10.1213/01.ane.0000217204.92904.76. [DOI] [PubMed] [Google Scholar]
- 3.Chrysostomou C., Sanchez De Toledo J., Avolio T. Dexmedetomidine use in a pediatric cardiac intensive care unit: can we use it in infants after cardiac surgery? Pediatr Crit Care Med. 2009;10:654–660. doi: 10.1097/PCC.0b013e3181a00b7a. [DOI] [PubMed] [Google Scholar]
- 4.Su F., Nicolson S.C., Zuppa A.F. A dose–response study of dexmedetomidine administered as the primary sedative in infants following open heart surgery. Pediatr Crit Care Med. 2013;14:499–507. doi: 10.1097/PCC.0b013e31828a8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Achuff B.J., Nicolson S.C., Elci O.U., Zuppa A.F. Intraoperative dexmedetomidine reduces postoperative mechanical ventilation in infants after open heart surgery. Pediatr Crit Care Med. 2015;16:440–447. doi: 10.1097/PCC.0000000000000382. [DOI] [PubMed] [Google Scholar]
- 6.Chrysostomou C., Sanchez-de-Toledo J., Wearden P. Perioperative use of dexmedetomidine is associated with decreased incidence of ventricular and supraventricular tachyarrhythmias after congenital cardiac operations. Ann Thorac Surg. 2011;92:964–972. doi: 10.1016/j.athoracsur.2011.04.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leriger M., Naguib A., Galantowicz M., Tobias J.D. Dexmedetomidine controls junctional ectopic tachycardia during tetralogy of Fallot repair in an infant. Ann Card Anaesth. 2012;15:224–228. doi: 10.4103/0971-9784.97978. [DOI] [PubMed] [Google Scholar]
- 8.Tobias J.D., Chrysostomou C. Dexmedetomidine: antiarrhythmic effects in the pediatric cardiac patient. Pediatr Cardiol. 2013;34:779–785. doi: 10.1007/s00246-013-0659-7. [DOI] [PubMed] [Google Scholar]
- 9.Wong J., Steil G.M., Curtis M. Cardiovascular effects of dexmedetomidine sedation in children. Anesth Analg. 2012;114:193–199. doi: 10.1213/ANE.0b013e3182326d5a. [DOI] [PubMed] [Google Scholar]
- 10.Sanders R.D., Xu J., Shu Y. Dexmedetomidine attenuates isoflurane-induced neurocognitive impairment in neonatal rats. Anesthesiology. 2009;110:1077–1085. doi: 10.1097/ALN.0b013e31819daedd. [DOI] [PubMed] [Google Scholar]
- 11.Olutoye O.A., Lazar D.A., Akinkuotu A.C., Adesina A., Olutoye O.O. Potential of the ovine brain as a model for anaesthesia-induced neuroapoptosis. Pediatr Surg Int. 2015;31:865–869. doi: 10.1007/s00383-015-3751-7. [DOI] [PubMed] [Google Scholar]
- 12.Koo E., Oshodi T., Meschter C., Ebrahimnejad A., Dong G. Neurotoxic effects of dexmedetomidine in foetal cynomolgus monkey brains. J Toxicol Sci. 2014;39:251–262. doi: 10.2131/jts.39.251. [DOI] [PubMed] [Google Scholar]
- 13.Istaphanous G.K., Ward C.G., Loepke A.W. The impact of the perioperative period on neurocognitive development, with a focus on pharmacological concerns. Best Pract Res Clin Anaesthesiol. 2010;24:433–449. doi: 10.1016/j.bpa.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Sanders R.D., Sun P., Patel S. Dexmedetomidine provides cortical neuroprotection: impact on anaesthetic-induced neuroapoptosis in the rat developing brain. Acta Anaesthesiol Scand. 2010;54:710–716. doi: 10.1111/j.1399-6576.2009.02177.x. [DOI] [PubMed] [Google Scholar]
- 15.Rajakumaraswamy N., Ma D., Hossain M. Neuroprotective interaction produced by xenon and dexmedetomidine on in vitro and in vivo neuronal injury models. Neurosci Lett. 2006;409:128–133. doi: 10.1016/j.neulet.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 16.Sifringer M., von Haefen C., Krain M. Neuroprotective effect of dexmedetomidine on hyperoxia-induced toxicity in the neonatal rat brain. Oxid Med Cell Longev. 2015;2015:530371. doi: 10.1155/2015/530371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz L.I., Twite M., Gulack B., Hill K., Kim S., Vener D.F. The perioperative use of dexmedetomidine in pediatric patients with congenital heart disease: an analysis from the congenital cardiac anesthesia society–society of thoracic surgeons congenital heart disease database. Anesth Analg. 2016;123:715–721. doi: 10.1213/ANE.0000000000001314. [DOI] [PubMed] [Google Scholar]
- 18.Garisto C., Ricci Z., Tofani L., Benegni S., Pezzella C., Cogo P. Use of low dose dexmedetomidine in combination with opioids and midazolam in paediatric cardiac surgical patients: randomized controlled trial. Minerva Anestesiol. 2018;84:1053–1062. doi: 10.23736/S0375-9393.18.12213-9. [DOI] [PubMed] [Google Scholar]
- 19.Pan W., Wang Y., Lin L., Zhou G., Hua X., Mo L. Outcomes of dexmedetomidine treatment in pediatric patients undergoing congenital heart disease surgery: a meta-analysis. Paediatr Anaesth. 2016;26:239–248. doi: 10.1111/pan.12820. [DOI] [PubMed] [Google Scholar]
- 20.Zimmerman K.O., Wu H., Laughon M. Dexmedetomidine pharmacokinetics and a new dosing paradigm in infants supported with cardiopulmonary bypass. Anesth Analg Adv. 2018 doi: 10.1213/ANE.0000000000003700. Access published on August 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Su F., Gastonguay M.R., Nicolson S.C., DiLiberto M., Ocampo-Pelland A., Zuppa A.F. Dexmedetomidine pharmacology in neonates and infants after open heart surgery. Anesth Analg. 2016;122:1556–1566. doi: 10.1213/ANE.0000000000000869. [DOI] [PubMed] [Google Scholar]
- 22.Wagner D., Pasko D., Phillips K., Waldvogel J., Annich G. In vitro clearance of dexmedetomidine in extracorporeal membrane oxygenation. Perfusion. 2013;28:40–46. doi: 10.1177/0267659112456894. [DOI] [PubMed] [Google Scholar]
- 23.Su F., Nicolson S.C., Gastonguay M.R. Population pharmacokinetics of dexmedetomidine in infants after open heart surgery. Anesth Analg. 2010;110:1383–1392. doi: 10.1213/ANE.0b013e3181d783c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Versmold H.T., Kitterman J.A., Phibbs R.H., Gregory G.A., Tooley W.H. Aortic blood pressure during the first 12 hours of life in infants with birth weight 610 to 4,220 grams. Pediatrics. 1981;67:607–613. [PubMed] [Google Scholar]
- 25.Pejovic B., Peco-Antic A., Marinkovic-Eric J. Blood pressure in non-critically ill preterm and full-term neonates. Pediatr Nephrol. 2007;22:249–257. doi: 10.1007/s00467-006-0311-3. [DOI] [PubMed] [Google Scholar]
- 26.Kent A.L., Kecskes Z., Shadbolt B., Falk M.C. Normative blood pressure data in the early neonatal period. Pediatr Nephrol. 2007;22:1335–1341. doi: 10.1007/s00467-007-0480-8. [DOI] [PubMed] [Google Scholar]
- 27.Kent A.L., Kecskes Z., Shadbolt B., Falk M.C. Blood pressure in the first year of life in healthy infants born at term. Pediatr Nephrol. 2007;22:1743–1749. doi: 10.1007/s00467-007-0561-8. [DOI] [PubMed] [Google Scholar]
- 28.Garcia X., Bhutta A.T., Dyamenahalli U., Imamura M., Jaquiss R.D., Prodhan P. Adrenal insufficiency in hemodynamically unstable neonates after open-heart surgery. Congenit Heart Dis. 2010;5:422–429. doi: 10.1111/j.1747-0803.2010.00447.x. [DOI] [PubMed] [Google Scholar]
- 29.Lee J.I., Su F., Shi H., Zuppa A.F. Sensitive and specific liquid chromatography-tandem mass spectrometric method for the quantitation of dexmedetomidine in pediatric plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;852:195–201. doi: 10.1016/j.jchromb.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 30.Diaz S.M., Rodarte A., Foley J., Capparelli E.V. Pharmacokinetics of dexmedetomidine in postsurgical pediatric intensive care unit patients: preliminary study. Pediatr Crit Care Med. 2007;8:419–424. doi: 10.1097/01.PCC.0000282046.66773.39. [DOI] [PubMed] [Google Scholar]
- 31.Petroz G.C., Sikich N., James M. A phase I, two-center study of the pharmacokinetics and pharmacodynamics of dexmedetomidine in children. Anesthesiology. 2006;105:1098–1110. doi: 10.1097/00000542-200612000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Potts A.L., Warman G.R., Anderson B.J. Dexmedetomidine disposition in children: a population analysis. Paediatr Anaesth. 2008;18:722–730. doi: 10.1111/j.1460-9592.2008.02653.x. [DOI] [PubMed] [Google Scholar]
- 33.Anderson B.J., McKee A.D., Holford N.H. Size, myths and the clinical pharmacokinetics of analgesia in paediatric patients. Clin Pharmacokinet. 1997;33:313–327. doi: 10.2165/00003088-199733050-00001. [DOI] [PubMed] [Google Scholar]
- 34.Potts A.L., Anderson B.J., Warman G.R., Lerman J., Diaz S.M., Vilo S. Dexmedetomidine pharmacokinetics in pediatric intensive care—a pooled analysis. Paediatr Anaesth. 2009;19:1119–1129. doi: 10.1111/j.1460-9592.2009.03133.x. [DOI] [PubMed] [Google Scholar]
- 35.Brendel K., Dartois C., Comets E. Are population pharmacokinetic and/or pharmacodynamic models adequately evaluated? A survey of the literature from 2002 to 2004. Clin Pharmacokinet. 2007;46:221–234. doi: 10.2165/00003088-200746030-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holford N., Ma S.C., Ploeger B.A. Clinical trial simulation: a review. Clin Pharmacol Ther. 2010;88:166–182. doi: 10.1038/clpt.2010.114. [DOI] [PubMed] [Google Scholar]
- 37.Boessen R., Knol M.J., Groenwold R.H., Roes K.C. Validation and predictive performance assessment of clinical trial simulation models. Clin Pharmacol Ther. 2011;89:487–488. doi: 10.1038/clpt.2010.277. [DOI] [PubMed] [Google Scholar]
- 38.Mathie R.T., Ohri S.K., Batten J.J., Peters A.M., Keogh B.E. Hepatic blood flow during cardiopulmonary bypass operations: the effect of temperature and pulsatility. J Thorac Cardiovasc Surg. 1997;114:292–293. doi: 10.1016/S0022-5223(97)70162-4. [DOI] [PubMed] [Google Scholar]
- 39.Koren G., Barker C., Goresky G. The influence of hypothermia on the disposition of fentanyl—human and animal studies. Eur J Clin Pharmacol. 1987;32:373–376. doi: 10.1007/BF00543972. [DOI] [PubMed] [Google Scholar]
- 40.Liu J.R., Yuki K., Baek C., Han X.H., Soriano S.G. Dexmedetomidine-induced neuroapoptosis is dependent on its cumulative dose. Anesth Analg. 2016;123:1008–1017. doi: 10.1213/ANE.0000000000001527. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.