Abstract
Background:
Intensive care unit–acquired weakness (ICU-AW) is a common complication of critical illness and is associated with increased mortality, longer mechanical ventilation and longer hospital stay. Little is known about the causes of mortality in patients with ICU-AW. In this study, we aimed to give an overview of the causes of death in a population diagnosed with ICU-AW during hospital admission.
Methods:
Data from a prospective cohort study in the mixed medical–surgical ICU of the Academic Medical Center in Amsterdam were used. Patients were included when mechanically ventilated for more than 48 hours. Intensive care unit–acquired weakness was defined as a mean medical research council score <4. Baseline data and data on the time of death were collected.
Results:
Fifty-three patients were included. Irreversible shock with multiple organ failure (MOF) was the most common cause of death (28/53 of patients; 26 patients with septic shock and 2 patients with hypovolemic shock). Most common site of sepsis was abdominal (38.5%) and pulmonary (19.2%). On admission to the ICU, 53% had a do-not-resuscitate code. In 74% of the patients, further treatment limitations were implemented during their ICU stay.
Conclusion:
In this cohort of patients with ICU-AW, most patients died of irreversible shock with MOF, caused by sepsis.
Keywords: ICU-acquired weakness, mortality, weakness
Introduction
Intensive care unit–acquired weakness (ICU-AW) is a common complication of critical illness with far-reaching consequences. The incidence of ICU-AW on the intensive care unit (ICU) is approximately 50%.1 Intensive care unit–acquired weakness is associated with longer duration of mechanical ventilation and longer ICU and hospital stay. Recovery is often slow and incomplete. Six months after hospital discharge, physical functioning is still impaired in patients with ICU-AW.2
Besides increased morbidity, ICU-AW is also associated with increased mortality.1 In several analyses, ICU-AW was found to be an independent predictor of ICU mortality and in-hospital mortality.2–4 Increased mortality is also associated with the severity of ICU-AW; mortality increases when weakness is more severe as indicated by a lower medical research council (MRC) score.3
Little is known about the causes of death in patients with ICU-AW. In this study, we studied the causes of death while admitted in the hospital in a population diagnosed with ICU-AW.
Methods
Patients
Data from a prospective cohort study including patients, who were mechanically ventilated for at least 48 hours, in the mixed medical–surgical ICU of the Academic Medical Center in Amsterdam were used.2 Patients admitted because of stroke, traumatic brain or spinal injury, a neuromuscular disorder, central nervous system infection, or cardiac arrest were excluded. Furthermore, patients with a poor prehospital functional status (modified Rankin scale >3) were excluded.5 For the current study, data from patients who were diagnosed with ICU-AW and who died during the ICU or hospital admission were used.
Diagnosis of ICU-AW
Intensive care unit–acquired weakness was prospectively diagnosed as soon as patients were awake and responsive. Trained physiotherapists used the MRC score, the experts’ proposed method, to diagnose ICU-AW.6 Six muscle groups were tested bilaterally: shoulder abductors, elbow flexors, wrist flexors, hip flexors, knee extensors, and ankle dorsiflexors. Intensive care unit–acquired weakness was defined as a mean MRC score <4. No additional neurophysiological tests to further differentiate between neuropathy and myopathy were performed.
Data Collection
Baseline data (including age, sex, medical vs surgical admission, planned vs emergency admission, and highest Acute Physiology and Chronic Health Evaluation IV [APACHE-IV] and Sequential Organ Failure Assessment [SOFA] score) were collected.7–8 In case of readmission, the highest score overall was collected. Data on the clinical parameters at the time of death or within 12 hours prior to death were retrospectively collected from the medical charts. These included data on duration of mechanical ventilation, presence of tracheostomy, presence of sepsis, use of continuous veno-venous hemofiltration, and noradrenaline use (>0.1 μg/kg/min for >1 hour). Sepsis was defined by 2 systemic inflammatory response syndrome criteria plus the administration of antibiotics for a suspected infection, as was the standard definition at that time.9 If patients were readmitted to the ICU during the same hospital admission, the length of stay and days on mechanical ventilation were added to the previous admission. Decisions on limitation or withdrawal of supportive care were also recorded. The location of death was recorded as ICU or hospital ward. Two independent observers (E.W. and J.H.) determined causes of death by reviewing the medical charts and discharge letters. The causes of death were divided into 7 categories: cardiac arrest, cardiac failure, fatal surgical complication, irreversible shock, respiratory failure, irreversible neurological damage and other. Interobserver disagreements were discussed, and a final cause of death was determined. In patients diagnosed with sepsis as cause of death, the focus of sepsis was determined.
Statistical Analysis
Normally distributed data are presented as mean and standard deviation, and nonnormally distributed data are presented as median with interquartile range (IQR). Proportions are presented with percentages and total numbers.
Results
Patient Demographics
A total of 53 patients was included. Table 1 shows the baseline characteristics. The average age of the patients was 63 years (± 16), and 53% was male. The median number of days on mechanical ventilation was 13 (IQR: 8-27), with a length of stay on the ICU of 17 days (IQR: 9-33). The highest SOFA score was 14 (± 5), and the APACHE-IV score was 93 (± 25). Characteristics at the moment of death are presented in Table 2.
Table 1.
Patient Demographics.
| Age, mean (SD), years | 63 (16) |
| Male, n (%) | 28 (53) |
| Type of patient n (%) | |
| Surgical planned | 9 (17) |
| Surgical nonplanned | 11 (21) |
| Medical | 33 (62) |
| Patients with ICU readmissions, n (%) | 8 (15) |
| Highest SOFA score, mean (SD) | 14 (5) |
| Highest APACHE IV score, mean (SD) | 93 (25) |
| Length of ICU stay, median (IQR), days | 17 (9-33) |
| Mechanical ventilation, median (IQR), days | 13 (8-27) |
| MRC score, mean (SD) | 2.0 (1.4) |
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; ICU-AW, intensive care unit–acquired weakness; IQR, interquartile range; MRC, medical resource council; SOFA, Sequential Organ Failure Assessment; SD, standard deviation.
Table 2.
Characteristics at the Time of Death (or Within 12 Hours Prior to Death).a
| Mechanical ventilation, n (%) | 45 (85) |
| Tracheostomy, n (%) | 22 (41) |
| CVVH, n (%) | 21 (40) |
| Sepsis, n (%) | 43 (81) |
| Received vasopression, n (%) | 27 (51) |
| Location of death (%) | |
| Intensive care | 75 |
| Hospital ward | 25 |
Abbreviations: CVVH, continuous veno-venous hemofiltration.
aPresence of sepsis as defined by 2 systemic inflammatory response syndrome criteria plus the administration of antibiotics for a suspected infection.
Cause of Death
The most common cause of death (53%) was irreversible shock with multiple organ failure (MOF; Table 3). Shock was septic shock in 26 patients and hypovolemic shock in only 2 patients. Most common sites of sepsis were abdominal (38.5%) and pulmonary (19.2%). In 4 patients, no infectious focus causing the sepsis could be identified. Twelve of the patients who died of sepsis were admitted with the same infectious diagnosis. In 14, another ICU-acquired infection was diagnosed. Respiratory and cardiac failure were 2 other major causes of death in patients with ICU-AW (16.9% and 11.3%, respectively).
Table 3.
Cause of Death.
| MOF/irreversible shock, n (%) | 28 (53) |
| Septic shock, n (%) | 26 (49) |
| Hypovolemic shock, n (%) | 2 (4) |
| Respiratory failure, n (%) | 9 (17) |
| Cardiac failure, n (%) | 6 (11) |
| Cardiac arrest, n (%) | 4 (7) |
| Fatal surgical complication, n (%) | 1 (2) |
| Irreversible neurological damage, n (%) | 1 (2) |
| Other, n (%) | 4 (8) |
Abbreviations: ICU-AW, intensive care unit–acquired weakness; MOF, multiple organ failure.
Treatment Limitations
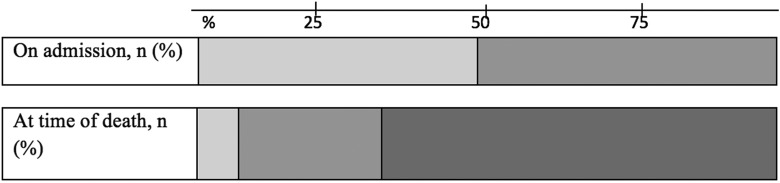
On admission to the ICU, 47% of patients had no treatment limitations and 53% had a do-not-resuscitate (DNR) code. In 74% of the patients in this study, further treatment limitations were implemented during their ICU stay. This usually involved no renal replacement therapy or no mechanical ventilation. These treatment limitations were initiated at a median of 8 days (IQR: 2-21 days) after admission. In the majority of patients (66%), supportive care was in the end withdrawn (Figure 1). Of the 4 patients with no limitations in care, 3 died of cardiac arrest and 1 of irreversible shock. Once supportive ICU care was withdrawn and palliative care was initiated, patients died on average within 1 day.
Figure 1.
Care limitations on time during the intensive care unit (ICU) stay, n (%). Lightest gray indicates no care limitation, on admission 25 (47) and at the time of death 4 (8). Medium gray indicates do not resuscitate, on admission 28 (53) and at the time of death 14 (26). Darkest gray indicates supportive care withheld, on admission 0 (0) and at the time of death 35 (66).
Discussion
In this single-center retrospective case series, we found that most patients with ICU-AW died of irreversible septic shock with MOF, mostly from an abdominal or pulmonary focus. Other common causes of death were respiratory and cardiac failure. In a large proportion of these patients, supportive treatment was withdrawn.
Intensive care unit–acquired weakness was first described by Bolton et al in 1983.10 Known in-hospital risk factors for ICU-AW are sepsis, MOF, or prolonged mechanical ventilation.2,11 Although we can identify patients at risk and we know its association with a higher morbidity, mortality, and length of stay, little is known about the cause of death of patients with ICU-AW. Hermans et al performed a study to identify the impact of weakness on mortality and short-term outcome.12 In this study, the cause of death of all patients who died in the hospital, both on ICU and on the ward, was registered. The majority of ICU-AW patients died because of MOF or irreversible shock, which is similar to our data. Hermans et al found no difference in the cause of death between patients with and without ICU-AW, but patients with ICU-AW were more often DNR coded (98% vs 83%) on time of death and more often care was withheld (52% vs 30%). A large proportion of our patients were also DNR coded. We deliberately did not make a comparison between patients with and patients without ICU-AW, as this was not the aim of our study. We did not study if the presence of ICU-AW was taken into consideration in the decision to limit or withdraw care in ICU patients. If so, this may be a contributable factor in the high mortality rates in ICU-AW patients. The majority of patients in our study died because of sepsis with MOF and shock. Fourteen of 26 patients who died of sepsis were diagnosed with a new infection during their admission. These ICU-acquired infections are known to be attributable to mortality.13 No data are available on the relation between ICU-AW and ICU-acquired infections.
Sometimes the exact cause of death was difficult to determine, as multiple problems were found in 1 patient. This was usually a combination of infection, organ failure leading to respiratory insufficiency, and treatment limitations. As described in the Methods section, we discussed these patients and decided on the most important factor leading to death.
Care limitations are known to influence outcome. Hemphill et al described a multivariate analysis on outcome after intracerebral hemorrhage, in which higher DNR rate per hospital was associated with higher mortality.14 This study suggests that the use of DNR order in the first 24 hours of care has a high impact on overall treatment and outcome. Do-not-resuscitate orders were also found to be an independent risk factor for mortality in sepsis and septic shock.15 Walkey et al described a wide variation between hospitals in invasive and organ supportive interventions.16 Patients with an early DNR code in a hospital with a high DNR rate were less likely to receive organ support and more likely to receive palliative care. Burns et al found that there is a disparity on DNR order rates by patient characteristics such as sex, race, and current illness.17 This is mainly due to the patients’ expectation of prognosis and functional status.
A DNR code is guided by the preference of the patients, but also the likelihood of success and the prognosis of survival estimated by the treating clinician. In our study, 53% of patients were DNR coded on ICU admission. The presence of such a high DNR rate at admission might be due to comorbidities or life expectancy of these patients prior to ICU admission. The mean SOFA and APACHE IV scores were high in our population, indicating that we studied a group of severely ill patients.
Some particular topics of our study should be stressed. Only patients who regained consciousness during ICU admission were included since being awake and responsive is a requirement for a reliable MRC score. Whether our population is representative for the ICU patients in general is uncertain, as this study was performed in a high-level university ICU with many complex patients. Finally, it has to be noted that cause of death was not confirmed by postmortem autopsy.
In conclusion, in this cohort of patients with ICU-AW, most patients died of irreversible shock with MOF, caused by sepsis.
Footnotes
Authors’ Note: Linda van Wagenberg and Esther Witteveen contributed equally to the article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Stevens RD, Dowdy DW, Michaels RK, Mendez-Tellez PA, Pronovost PJ, Needham DM. Neuromuscular dysfunction acquired in critical illness: a systematic review. Intensive Care Med. 2007;33(11):1876–1891. [DOI] [PubMed] [Google Scholar]
- 2. Wieske L, Dettling-Ihnenfeldt DS, Verhamme C, et al. Impact of ICU-acquired weakness on post-ICU physical functioning: a follow-up study. Crit Care. 2015;19:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sharshar T, Bastuji-Garin S, Stevens RD, et al. Presence and severity of intensive care unit-acquired paresis at time of awakening are associated with increased intensive care unit and hospital mortality. Crit Care Med. 2009;37(12):3047–3053. [DOI] [PubMed] [Google Scholar]
- 4. Garnacho-Montero J, Madrazo-Osuna J, García-Garmendia JL, et al. Critical illness polyneuropathy: risk factors and clinical consequences. A cohort study in septic patients. Intensive Care Med. 2001;27(8):1288–1296. [DOI] [PubMed] [Google Scholar]
- 5. van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19(5):604–607. [DOI] [PubMed] [Google Scholar]
- 6. Fan E, Cheek F, Chlan L, et al. An official American Thoracic Society clinical practice guideline: the diagnosis of intensive care unit-acquired weakness in adults. Am J Respir Crit Care Med. 2014;190(12):1437–1446. [DOI] [PubMed] [Google Scholar]
- 7. Dahhan T, Jamil M, Al-Tarifi A, Abouchala N, Kherallah M. Validation of the APACHE IV scoring system in patients with severe sepsis and comparison with the APACHE II system. Crit Care. 2009;13(suppl 1):P511. [Google Scholar]
- 8. Ferreira FL, Bota DP, Bross A, Mélot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286(14):1754–1758. [DOI] [PubMed] [Google Scholar]
- 9. Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101(6):1644–1655 [DOI] [PubMed] [Google Scholar]
- 10. Bolton CF, Brown JD, Sibbald WJ. The electrophysiologic investigation of respiratory paralysis in critically ill patients. Neurology. 1983;33:186. [Google Scholar]
- 11. Jolley SE, Bunnell AE, Hough CL. ICU-acquired weakness. Chest. 2016;150(5):1129–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hermans G, Van Mechelen H, Clerckx B, et al. Acute outcomes and 1-year mortality of intensive care unit-acquired weakness. A cohort study and propensity-matched analysis. Am J Respir Crit Care Med. 2014;190(4):410–420. [DOI] [PubMed] [Google Scholar]
- 13. van Vught LA, Klein Klouwenberg PM, Spitoni C, et al. Incidence, risk factors and attributable mortality of secondary infections in the intensive care unit after admission for sepsis. JAMA. 2016;315(14):1469–1479. [DOI] [PubMed] [Google Scholar]
- 14. Hemphill JC, Newman J, Zhao S, Johnston SC. JC. Hemphill Hospital usage of early do-not-resuscitate orders and outcome after intracerebral hemorrhage. Stroke. 2004;35(5):1130–1134. [DOI] [PubMed] [Google Scholar]
- 15. Huang CT, Chuang YC, Tsai YJ, Ko WJ, Yu CJ. High mortality in severe sepsis and septic shock patients with do-not-resuscitate orders in East Asia. PLos One. 2016;11(7):e0159501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Walkey AJ, Weinberg J, Wiener RS, Cooke CR, Lindenauer PK. Hospital variation in utilization of life-sustaining treatments among patients with do not resuscitate orders. Health Serv Res. 2017. doi: 10.1111/1475-6773.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Burns JP, Edwards J, Johnson J, Cassem NH, Truog RD. Do-not-resuscitate order after 25 years. Crit Care Med. 2003;31(5):1543–1550. [DOI] [PubMed] [Google Scholar]