Abstract
Objective
Our aim was to study the quality of medical care in patients with systemic lupus erythematosus (SLE) to understand gaps and to analyze the association with outcome of the disease.
Methods
Information on demographics and medical care was assessed by self-reported questionnaires among SLE patients (LuLa cohort, 2011, n = 580). In total, 21 aspects of medical care were analyzed. Univariate analysis selected 10 predictor variables for further analysis: (1) urine examination and (2) blood test in the previous year, (3) taking antimalarials, (4) taking vitamin D and calcium if the dosage of prednisolone was greater than 7.5 mg/day, counseling regarding (5) lipid metabolism, (6) vaccination, and (7) blood pressure, and treatment of the comorbidities (8) hypertension, (9) osteoporosis and (10) lipid metabolism disorder. The association of these 10 items with the outcome of the disease, assessed in 2015, was analyzed by linear regression analysis, adjusted for age, disease duration and sex.
Results
On average six of the 10 items were met (±1.7). Receiving more clinical care in 2013 was predictive for low disease activity (SLAQ, p = 0.024, β = –0.104, corr. R2 = 0.048), low progress in disease-related damage (Delta Brief Index of Lupus Questionnaire, p = 0.048, β = –0.132, corr. R2 = 0.036) and high health-related quality of life (SF-12 physical, p = 0.035, β = 0.100, corr. R2 = 0.091) in 2015.
Conclusion
Our study illustrates a link between the quality of care and the SLE outcome parameters disease activity, disease-related damage and quality of life. Consistent considerations of these care parameters, which are recommended in several management guidelines, could therefore be a good approach to improve the outcome of patients with SLE.
Keywords: Care, outcome, systemic lupus erythematosus
Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease that is associated with premature mortality and increased morbidity, both from the disease itself and associated comorbidities, such as cardiovascular diseases (CVDs).1 As a chronic disease, it requires frequent interaction with health-care providers, even in asymptomatic patients.2 In addition, the collaboration of the patient’s main medical contact and other specialists is crucial because SLE can affect numerous organs. Therefore, management of the disease is complex and heterogeneous. Recommendations have been developed to help improve the management of SLE patients (American College of Rheumatology (ACR), British Society for Rheumatology, European League Against Rheumatism (EULAR), EULAR/European Renal Association–European Dialysis and Transplant Association), but there has not been a formal evaluation of these recommendations.3–6
Although many studies have explored risk factors associated with poor outcome in SLE,7,8 only a few studies have investigated the impact of quality of health care on outcome. For other chronic diseases, such as diabetes mellitus, asthma/chronic obstructive pulmonary disease, depression and chronic heart failure, the impact of quality of care on outcome has already been described.
In addition, in musculoskeletal conditions, such as osteoarthritis, rheumatoid arthritis, chronic back pain and osteoporosis, models of care, including for example supporting access to multidisciplinary teams, resulted in improved patient outcomes.9,10
Yazdany et al. studied the effect of good clinical care on outcome parameters in a cohort of 737 SLE patients in 2014. They showed that high performance on their SLE quality indicators was protective against damage accrual. However, there was no impact on disease activity.11,12
Our aim was to study the quality of SLE medical care in Germany to understand gaps and to analyze the association of aspects of care with long-term outcome parameters. This information is critical to understanding the potential impact of clinical care and improving its management in practice.
Methods
Data source
The LuLa Study is a longitudinal nationwide survey of SLE patients. Since 2001, patients have received an annual questionnaire on a multitude of SLE-associated factors. The study organization and implementation was chosen to minimize the effect of expectancy bias such as the Rosenthal effect. In comparison with other cohort studies and in particular with reference data from the national database of the German Rheumatism Research Center, it was shown previously that data provided by the LuLa Study is reliable, comparable and can be considered as representative of SLE patients in Germany.13 In addition to demographic data, clinical parameters such as comorbidities, lupus-specific medication, disease activity, damage and health-related quality of life (HRQoL) are collected. In 2013 we additionally inquired about aspects of clinical care as a main topic.
Study organization and preparation of data acquisition is performed by the German SLE self-help community (GSHC), whereas pseudonymized data collection and scientific evaluation are guaranteed by a tertiary center. Medical care is provided independently of the study by physicians throughout Germany.
Participants are recruited by invitation by their rheumatologist or the GSHC itself. Inclusion criteria are a confirmed diagnosis of SLE and the returning of the completed questionnaire.
Two-pass verification was performed to reduce data entry errors for the digitization of the questionnaire in the tertiary center.
The questionnaire was sent to 636 patients by the GSHC in the year 2013, and the return rate of the completed questionnaires was 91.2% (580).
Outcome
Patient-reported disease activity, disease-related damage and HRQoL were chosen as outcome parameters. The Systemic Lupus Activity Questionnaire (SLAQ),14 based on the Systemic Lupus Activity Measure (SLAM), translated and validated in different languages, was used to assess disease activity. Disease symptoms during the previous three months are assessed by 24 items. The German version has proven to have a strong correlation with the SLAM and presented good to excellent internal consistency.15
Damage was assessed using the Brief Index of Lupus Questionnaire (BILD),16 which is based on the Systemic Lupus International Collaborating Clinics/ACR Damage index (SDI) and consists of 28 items inquiring about organ damage accumulated since the diagnosis of SLE. The questionnaire was translated and validated in different languages. The German version has proven comparable validity to the original BILD and a strong correlation with physician-reported damage (SDI).17 Once damage has occurred, it cannot recover; meaning the BILD score cannot improve. We used the delta of the BILD score (2015–2011) to analyze the progress of disease-related damage.
HRQoL was assessed by the Short Form 12 Health Survey (SF-12).18 Based on the Short Form 36 (SF-36), the SF-12 provides comparable results, with a mental (MCS) and a physical (PCS) component. Additionally, the physical functioning index of the SF-36 (SF-36-PFI) was assessed.19
Patient-oriented questionnaires for disease activity (SLAQ), disease-related damage (BILD) and HRQoL (SF-12/SF-36) have a great correlation with physician-reported questionnaires, but physician-assessed outcome parameters are warranted to validate our findings.
Clinical care parameters and statistical analysis
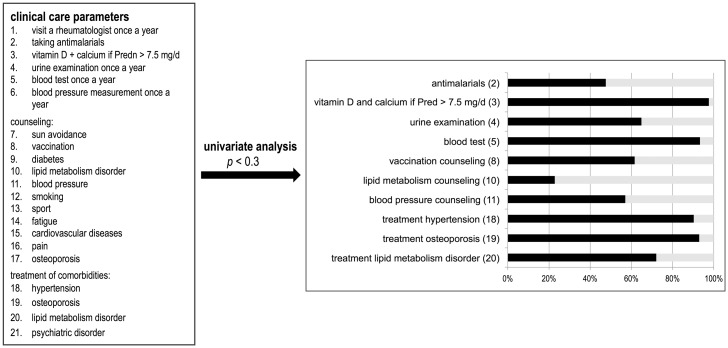
To understand the impact of clinical care in SLE, we analyzed 21 parameters predicting good clinical care, as mentioned in current management guidelines (e.g. counseling regarding sun avoidance, vaccination, diabetes, and blood pressure, visiting a rheumatologist once a year, taking antimalarials, urine and blood test once a year, and treatment of the comorbidities hypertension, osteoporosis, lipid metabolism disorder, and psychiatric disorders, as shown in Figure 1).
Figure 1.
Analysis of 21 clinical care parameters. Ten items met the univariate criteria for further analysis. The figure shows the affirmation of the individual clinical care parameters in our cohort. In 2013 urine was examined in 64.2%, and a blood test was performed in 93.3%. A total of 47.6% were on antimalarials, 97.6% took a prednisolone equivalent (Pred) less than or equal to 7.5 mg per day and/or calcium and vitamin D at a dosage greater than 7.5 mg prednisolone per day. Lipid metabolism counseling was performed in 22.8%, vaccination counseling in 61.5% and blood pressure counseling in 45.9% of patients. A total of 7.1% of participants had untreated osteoporosis. In 9.7% and 27.9% blood pressure or lipid metabolism disorder was untreated (n = 580).
In a first univariate analysis, we studied the association of these variables to selected outcome parameters (Kruskal-Wallis Test). A p value of <0.3 was chosen as criteria for further multivariable analysis. Univariate analysis is a common way to select predictor variables for multivariable analysis, reducing problems of overfitting and stepwise selection.20
Ten items met the univariate criteria (p < 0.3), including (1) urine examination and (2) blood test in the previous year, (3) taking antimalarials, (4) taking vitamin D and calcium if the dosage of prednisolone was greater than 7.5 mg per day, counseling regarding (5) lipid metabolism, (6) vaccination, and (7) blood pressure, and treatment of the comorbidities (8) hypertension, (9) osteoporosis and (10) lipid metabolism disorder.
In a next step the association of these care parameters to disease outcome was studied by linear regression, adjusted for age, sex and disease duration.
Data were analyzed with the statistical software program IBM SPSS Statistic 23 (IBM Corp, Armonk, NY, USA). A p value <0.05 was considered significant.
The LuLa cohort is approved by the Heinrich-Heine-University Düsseldorf Institutional Review Board (study numbers 2260 and 3708) and is registered in the German World Health Organization primary registry German Clinical Trial Register, www.germanctr.de (ID: DRKS00011053). The study did not require additional approval.
Results
Characterization of the cohort
In total, 580 patients were included in our analysis. Participants were mostly female (93.8%) with a mean age of 54 years and mean disease duration of 20 years. Forty-seven percent of the participants were treated with antimalarials and 50% with prednisolone at a dosage less than 7.5 mg per day in 2013.
In 66% a rheumatologist was reported as the main contact for SLE. For 15% the general practitioner (GP) was the main contact, and another 18% selected another specialist (e.g. nephrologist, Table 1). Patients with a rheumatologist as their main contact were comparatively younger (53.0 vs 56.9 years, Kruskal-Wallis Test Multiple comparisons, p < 0.03) and the proportion of patients taking 7.5 mg or less of a prednisolone equivalent per day was higher in this group (chi-square test of independence, p = 0.003). We found no significant differences in the number of concomitant diseases, disease activity, disease damage and HRQoL in the groups.
Table 1.
Characteristics of the study cohort in the baseline year, 2013
| n (%) | Mean | SD | |
|---|---|---|---|
| Age, y | 54.8 | 13.2 | |
| Female | 544 (93.8) | ||
| Disease duration, y | 20.0 | 9.0 | |
| Physical functioning (SF-36 PFI) | 67.8 | 28.5 | |
| Physical HRQoL (SF-12) | 40.7 | 11.8 | |
| Mental HRQoL (SF-12) | 46.3 | 11.7 | |
| Disease activity (SLAQ) | 13 | 7 | |
| No. of comorbidities | 1.0 | 1.6 | |
| Prednisolone ≤7.5 mg | 293 (50.5) | ||
| Prednisolone >7.5 mg | 61 (10.5) | ||
| Immunosuppression | 278 (48.0) | ||
| No immunosuppression | 301 (52.0) | ||
| Antimalarials | 276 (47.6) | ||
| No. of visits | |||
| GP per year | 7 | 6 | |
| Rheumatologist per year | 3 | 3 | |
| Main contact for lupus | |||
| GP | 89 (15.3) | ||
| Rheumatologist | 381 (65.7) | ||
| Other specialist | 102 (17.6) |
A total of 580 patients participated. Immunosuppression includes azathioprine, methotrexate, leflunomide, ciclosporine A, mycophenolic acid, cyclophosphamide, rituximab and belimumab.
GP: general practitioner; HRQoL: health-related quality of life; PFI: physical functioning index; SF-12: Short Form 12 Health Survey; SLAQ: Systemic Lupus Activity Questionnaire.
The number of physician visits during the year was dependent on the disease activity. The distance to the physician’s office had no influence on the frequency of visits (Kruskal-Wallis Test Multiple comparisons, p < 0.01). On average, the distance was 5 km to the GP and 36.8 km to the rheumatologist. Eighty-one percent of the participants visited a rheumatologist at least once during the year.
Disease outcome
Disease outcome was assessed by three patient-reported outcome parameters: disease activity (SLAQ), disease-related damage (BILD) and HRQoL (SF-12).
At baseline (2013) mean disease activity (SLAQ) was 13.1 (±7.5) compared to 12.7 ± 7.2 in 2015. Twenty percent of participants had a significant increase in SLAQ of four or more points in 2015.
Baseline damage (BILD) averaged 2.5 (±2.3). In 23.4% BILD increased by two or more points in 2015 (mean 2.7 ± 2.4).
In 2013 the mean mental HRQoL (SF-12 mental) was 46.3 ± 11.8 and physical HRQoL (SF-12 physical) was 40.7 ± 11.8. Additionally the physical functioning index (SF-36 PFI) was assessed 67.7 ± 23.5.
Quality of care predicts disease outcome
In total we were able to measure 21 clinical care parameters because they are recommended in current management guidelines (Figure 1).
Univariate analysis was used to evaluate predictor variables for further multivariable analysis. Ten parameters met the criteria of a p value less than 0.3.
On average six of the 10 parameters were affirmed (±1.7). The affirmation of the individual medical care items are shown in Figure 1 and varied between 22.8% (lipid metabolism counseling) and 97.6% (prednisolone equivalent ≤7.5 mg per day or osteoporosis protection at a dosage >7.5 mg).
Subsequent linear regression analysis was used to analyze the association to the outcome of the disease.
Receiving more clinical care, measured by the 10 clinical care parameters in 2013, was predictive for low progress in disease-related damage (delta-BILD (2015-2011), p = 0.048, β = –0.132, corr. R2 = 0.036) and low disease activity in 2015 (SLAQ p = 0.024, β = –0.104, corrected R2 = 0.039).
In addition, receiving more clinical care was associated with a high physical HRQoL (SF-12 physical 2015 p = 0.035, β = 0.100, corr. R2 = 0.091 and SF-36 PFI 2015 p = 0.005, β = 0.124, corr. R2 = 0.138). The impact on the mental component of HRQoL (SF-12) was not significant (p = 0.290). Detailed results are given in Table 2.
Table 2.
Good clinical care (GCC) predicts outcome
| Dependent variable | adjusted R2 | βstand. (GCC) | SD | p |
|---|---|---|---|---|
| Disease-related damage | ||||
| Delta BILDa | 0.036 | −0.132 | −2.752 | 0.048 |
| Disease activity | ||||
| SLAQ 2015a | 0.039 | −0.104 | 0.192 | 0.024 |
| HRQoL | ||||
| SF-12 mental 2015 | 0.008 | 0.052 | 0.317 | 0.290 |
| SF-12 physical 2015a | 0.091 | 0.100 | 0.318 | 0.035 |
| Physical functioning index | ||||
| SF-36 PFI 2015b | 0.138 | 0.124 | 0.714 | 0.005 |
Linear regression adjusted for sex, age and disease duration. Independent variable: GCC with 10 items. Dependent variable: outcome parameters (delta BILD, SLAQ, SF-12 mental/physical and SF-36-PFI. β regression coefficient, HRQoL).
BILD: Brief Index of Lupus Questionnaire; HRQoL: health-related quality of life; PFI: physical functioning index; SF-12: Short Form 12 Health Survey; SLAQ: Systemic Lupus Activity Questionnaire.
p < 0.05. bp < 0.01.
For better visual presentation we chose three groups of patients, depending on the number of clinical care parameters affirmed. Significant differences between the three groups are shown in Figure 2.
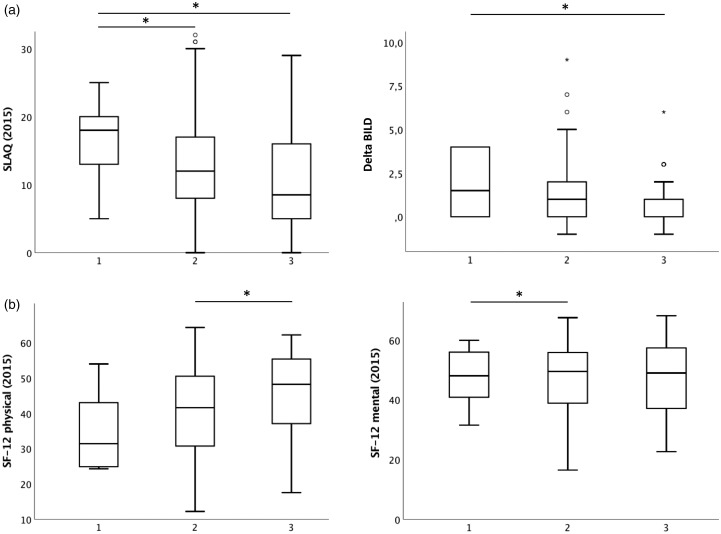
Figure 2.
Good clinical care is predictive for low disease activity (Systemic Lupus Activity Questionnaire, SLAQ), (a) low progress in disease-related damage and (b) high health-related quality of life. Boxplots present three groups of patients: 1) patients who have one or two clinical care parameters (CCPs) affirmed (n = 37), 2) patients who have three to eight CCPs (n = 501) and 3) patients who have nine or 10 CCPs affirmed (n = 45). The Kruskal-Wallis test was performed for comparison of the different groups, p < 0.05. BILD: Brief Index of Lupus Questionnaire; SF-12: Short Form 12 Health Survey.
Regarding the impact of the individual clinical care item on the outcome, taking antimalarials and osteoporosis protection had the greatest impact on damage (BILD), whereas blood pressure counseling and osteoporosis protection had the greatest impact on lowering disease activity (SLAQ). In addition, blood pressure counseling was important for the improvement of the mental and the physical component of HRQoL (SF-12). Table 3 shows the standardized regression coefficients (β) of individual clinical care items.
Table 3.
The importance of the individual clinical care parameter for the outcome
| Delta BILD | SLAQ | SF-12 physical | SF-12 mental | |
|---|---|---|---|---|
| Urine examination | –0.037 (5) | –0.009 (5) | 0.012 (6) | 0.014 (6) |
| Blood test | 0.005 (7) | –0.005 (6) | –0.034 (9) | –0.033 (7) |
| Antimalarials | –0.116 (2) | 0.109 (10) | –0.024 (8) | –0.049 (8) |
| Vitamin D and calcium if prednisolone >7.5 mg/d | –0.129 (1) | –0.240 (1) | 0.308 (1) | 0.031 (5) |
| Lipid metabolism counseling | 0.057 (10) | 0.105 (9) | –0.064 (10) | –0.130 (10) |
| Vaccination counseling | 0.007 (8) | –0.057 (4) | 0.038 (3) | 0.098 (3) |
| Blood pressure counseling | –0.049 (3) | –0.141 (2) | 0.091 (2) | 0.154 (1) |
| Treatment osteoporosis | –0.043 (4) | 0.026 (7) | 0.016 (5) | 0.031 (4) |
| Treatment blood pressure | –0.022 (6) | –0.093 (3) | 0.020 (4) | 0.105 (2) |
| Treatment lipid metabolism disorder | 0.008 (9) | 0.039 (8) | 0.002 (7) | –0.098 (9) |
Standardized regression coefficients (β) of linear regression analysis (2015). Dependent variables: outcome parameters (delta BILD, SLAQ and the mental and physical component of the SF-12). The number in parentheses represents the relevance (rank) of the individual clinical care parameter for the outcome. Number 1 means the parameter has the greatest impact on the outcome parameter.
BILD: Brief Index of Lupus Questionnaire; HRQoL: health-related quality of life; PFI: physical functioning index; SF-12: Short Form 12 Health Survey; SLAQ: Systemic Lupus Activity Questionnaire.
In addition, it is noteworthy that some of the parameters of clinical care were primarily met by the GP and others by specialists (Figure 3).
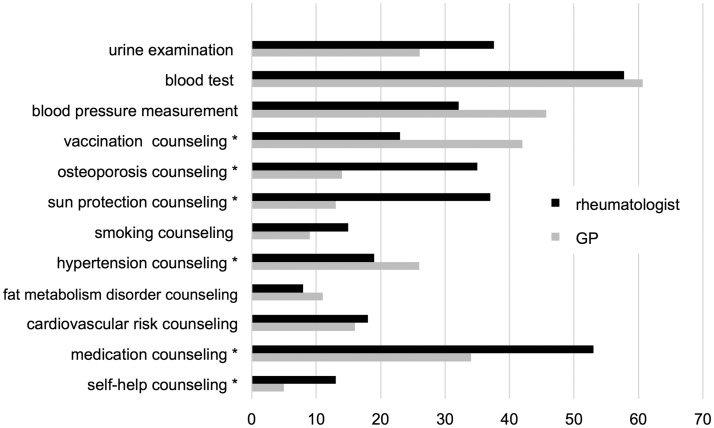
Figure 3.
Differences in counseling and performance of examinations by the general practitioner (GP) and the rheumatologist (percentages). *Significant differences, chi square test, p < 0.05.
The GP for example more frequently informed patients about vaccination (31.9%, n = 185) and blood pressure (18.4%, n = 107), whereas urine examination was more often carried out by the rheumatologist (31.1%, n = 218).
Discussion
To identify addressable deficiencies in SLE health care, the measurement of performance on quality indicators in SLE is crucial. Whereas many large observational studies have monitored clinical and biological variables, little is known about the health-care quality in these cohorts even though significant gaps in care of patients with SLE have been revealed, including low vaccination rates and a low proportion of treated osteoporosis and other comorbidities.21–23
Our aim was to evaluate the quality of care in SLE and its association with patient outcome.
The importance of clinical care in SLE
Our data show a beneficial effect of 10 aspects of clinical care on the outcome of the disease including disease activity, disease-related damage and HRQoL in SLE.
Different statements on the relevance of the individual aspects of clinical care in SLE exist. A positive effect on survival and disease activity in SLE has been described previously only for the intake of antimalarials.24,25
In patients with SLE an increased incidence of CVD and premature atherosclerosis is described that is partially explained by an increased prevalence of hypertension and dyslipidemia (11.5% to 75%).26–28 Present agreement exists on the need for monitoring traditional CVD risk factors and treating modifiable risk factors according to existing guidelines.29 The impact on patients’ survival is undisputed, whereas an influence on SLE disease activity and damage has not been described. Counseling regarding blood pressure and lipid metabolism as well as the treatment of these as comorbidities were included as four individual items in our linear regression analysis, selected by univariate analysis.
In addition, osteoporosis protection was included in our analysis. Risk factors for osteoporosis in SLE include the treatment with glucocorticosteroids and vitamin D insufficiency, often related to the avoidance of sun exposure.
No data are available to suggest an optimal frequency of clinical and laboratory assessment in patients with SLE. Urine and blood test examination is recommended every six to 12 months for patients with inactive disease to monitor organ involvement, which may occur without symptoms.4 We included urine examination and blood tests, performed in the previous year, in our analysis.
Our data reveal significant gaps in clinical care of patients with SLE because on average only 61% of the quality care parameters endorsed in current management recommendations were met in our cohort. These data are consistent with the observations of Yazdany et al., who showed that individual patients with SLE received approximately only 65% of services recommended in the SLE process.12
Quality of care predicts disease outcome
Within our analysis, 10 parameters of clinical care predicted disease outcome. Receiving more clinical care was associated with low progress in disease-related damage, a low disease activity and a high HRQoL (physical component) in 2015.
There was no significant impact on the mental component of HRQoL in 2015, which could be explained by the fact that our 10 parameters mainly affect the clinical care of patients and disregarded the psychosocial care.
It remains to be discussed that not all possible aspects of good care were surveyed. Aspects, which potentially have an influence on our outcome parameters, like the adherence to medication, for example, may not have been considered. Additionally, there is a possibility of unmeasured confounding factors, which might have an impact both on receiving higher quality of care and better outcome parameters.
Furthermore, the 10 clinical care items were considered unweighted in the linear regression analysis compared to the work of Yazdany et al.11 The impact of the individual clinical care item on the outcome varied depending on the considered outcome parameter shown. For instance, taking antimalarials had the greatest impact on damage, and blood pressure counseling was important for the improvement of the mental and the physical component of HRQoL.
It remains to be discussed that the time interval between reporting the quality of care (2013) and measuring the outcome of the disease (2015) is only two years. But the quality of care may not change significantly over the years, and we asked about general counseling, for example, of vaccination, which covers a longer period of time.
Study design
In the LuLa cohort, patients with SLE answer annual questionnaires on a multitude of SLE-associated factors. The study organization and implementation was chosen to minimize the effect of expectancy bias such as the Rosenthal effect. In comparison with other cohort studies and in particular with reference data from the national database of the German Rheumatism Research Center, it was shown previously that data provided by the LuLa Study are reliable, comparable and can be considered representative of SLE patients in Germany.13 Patient-oriented questionnaires for disease activity (SLAQ), disease-related damage (BILD) and HRQoL (SF-12/SF-36) have a great correlation with physician-reported questionnaires, but physician-assessed outcome parameters are warranted to validate our findings.
Our study illustrates a strong link between quality of care and important SLE outcome parameters including quality of life, disease-related damage and disease activity, assessed by self-reported questionnaires. Improvement of health care provided on an individual level could therefore be a good approach to improve the outcome of patients with lupus erythematosus. The 10 parameters identified in our analysis should be of particular importance in the care of patients with lupus erythematosus.
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: A.K., M.S., G.C., R.F.-B. and J.G.R. received unrestricted grants from GlaxoSmithKline and UCB Pharma for performing the LuLa Study. The other authors have nothing to declare.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The LuLa Study is supported by unrestricted grants from GlaxoSmithKline and UCB Pharma.
ORCID iD
A Kernder https://orcid.org/0000-0002-7742-7526
References
- 1.Borchers AT, Keen CL, Shoenfeld Y, Gershwin ME. Surviving the butterfly and the wolf: Mortality trends in systemic lupus erythematosus. Autoimmun Rev 2004; 3: 423–453. [DOI] [PubMed] [Google Scholar]
- 2.Gladman DD, Ibañez D, Ruiz I, Urowitz MB. Recommendations for frequency of visits to monitor systemic lupus erythematosus in asymptomatic patients: Data from an observational cohort study. J Rheumatol 2013; 40: 630–633. [DOI] [PubMed] [Google Scholar]
- 3.Bertsias G, Ioannidis JPA, Boletis J, et al. Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics. EULAR recommendations for the management of systemic lupus erythematosus. Report of a Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics. Ann Rheum Dis 2008; 67: 195–205. [DOI] [PubMed] [Google Scholar]
- 4.Mosca M, Tani C, Aringer M, et al. European League Against Rheumatism recommendations for monitoring patients with systemic lupus erythematosus in clinical practice and in observational studies. Ann Rheum Dis 2010; 69: 1269–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guidelines for referral and management of systemic lupus erythematosus in adults. American College of Rheumatology Ad Hoc Committee on Systemic Lupus Erythematosus Guidelines. Arthritis Rheum 1999; 42: 1785–1796. [DOI] [PubMed] [Google Scholar]
- 6.Gordon C, Amissah-Arthur MB, Gayed M, et al. The British Society for Rheumatology guideline for the management of systemic lupus erythematosus in adults. Rheumatology (Oxford) 2018; 57: e1–e45. [DOI] [PubMed] [Google Scholar]
- 7.Karlson EW, Daltroy LH, Lew RA, et al. The relationship of socioeconomic status, race, and modifiable risk factors to outcomes in patients with systemic lupus erythematosus. Arthritis Rheum 1997; 40: 47–56. [DOI] [PubMed] [Google Scholar]
- 8.Sutcliffe N, Clarke AE, Gordon C, Farewell V, Isenberg DA. The association of socio-economic status, race, psychosocial factors and outcome in patients with systemic lupus erythematosus. Rheumatology (Oxford) 1999; 38: 1130–1137. [DOI] [PubMed] [Google Scholar]
- 9.Speerin R, Slater H, Li L, et al. Moving from evidence to practice: Models of care for the prevention and management of musculoskeletal conditions. Best Pract Res Clin Rheumatol 2014; 28: 479–515. [DOI] [PubMed] [Google Scholar]
- 10.Coleman K, Austin BT, Brach C, Wagner EH. Evidence on the Chronic Care Model in the new millennium. Health Aff (Millwood) 2009; 28: 75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yazdany J, Trupin L, Schmajuk G, Katz PP, Yelin EH. Quality of care in systemic lupus erythematosus: The association between process and outcome measures in the Lupus Outcomes Study. BMJ Qual Saf 2014; 23: 659–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yazdany J, Trupin L, Tonner C, et al. Quality of Care in systemic lupus erythematosus: Application of quality measures to understand gaps in care. J Gen Intern Med 2012; 27: 1326–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischer-Betz R, Wessel E, Richter J, Winkler-Rohlfing B, Willers R, Schneider M. Lupus in Germany: Analysis within the German Lupus Self-Help Organization (LULA) [article in German]. Z Rheumatol 2005; 64: 111–122. [DOI] [PubMed] [Google Scholar]
- 14.Karlson EW, Daltroy LH, Rivest C, et al. Validation of a Systemic Lupus Activity Questionnaire (SLAQ) for population studies. Lupus 2003; 12: 280–286. [DOI] [PubMed] [Google Scholar]
- 15.Chehab G, Richter J, Sander O, et al. Validation and evaluation of the German version of the Systemic Lupus Activity Questionnaire (SLAQ). Clin Exp Rheumatol 2015; 33: 354–359. [PubMed] [Google Scholar]
- 16.Yazdany J, Trupin L, Gansky SA, et al. Brief index of lupus damage: a patient-reported measure of damage in systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2011; 63: 1170–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chehab G, Sander O, Richter J, et al. Validation and evaluation of the German Brief Index of Lupus Damage (BILD)—a self-reported instrument to record damage in systemic lupus erythematosus. Lupus 2013; 22: 1050–1055. [DOI] [PubMed] [Google Scholar]
- 18.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: Construction of scales and preliminary tests of reliability and validity. Med Care 1996; 34: 220–233. [DOI] [PubMed] [Google Scholar]
- 19.Ware JE. SF-36 Health Survey: Manual and interpretation guide, Boston: Health Institute, New England Medical Center, 1993. [Google Scholar]
- 20.Steyerberg EW. Clinical prediction models: A practical approach to development, validation, and updating, NY: Springer Science+Business Media LLC, 2009. [Google Scholar]
- 21.Chehab G, Richter JG, Brinks R, Fischer-Betz R, Winkler-Rohlfing B, Schneider M. Vaccination coverage in systemic lupus erythematosus—a cross-sectional analysis of the German long-term study (LuLa cohort). Rheumatology (Oxford) 2018; 57: 1439–1447. [DOI] [PubMed] [Google Scholar]
- 22.Demas KL, Keenan BT, Solomon DH, Yazdany J, Costenbader KH. Osteoporosis and cardiovascular disease care in SLE according to new quality indicators. Semin Arthritis Rheum 2010; 40: 193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmajuk G, Yelin E, Chakravarty E, Nelson LM, Panopolis P, Yazdany J. Osteoporosis screening, prevention, and treatment in systemic lupus erythematosus: Application of the systemic lupus erythematosus quality indicators. Arthritis Care Res 2010; 62: 993–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sisó A, Ramos-Casals M, Bové A, et al. Previous antimalarial therapy in patients diagnosed with lupus nephritis: Influence on outcomes and survival. Lupus 2008; 17: 281–288. [DOI] [PubMed] [Google Scholar]
- 25.Wang F, Zhang W, Wang S, et al. AB0447 Antimalarials improve survival of systemic lupus erythematosus on cholesterol: Results of a fifteen-year Chinese multicenter retrospective study in Jiangsu Province. Ann Rheum Dis 2017; 76: 1206–1207. [Google Scholar]
- 26.Calvo-Alén J, Toloza SM, Fernández M, et al. Systemic lupus erythematosus in a multiethnic US cohort (LUMINA). XXV. Smoking, older age, disease activity, lupus anticoagulant, and glucocorticoid dose as risk factors for the occurrence of venous thrombosis in lupus patients. Arthritis Rheum 2005; 52: 2060–2068. [DOI] [PubMed] [Google Scholar]
- 27.Asanuma Y, Oeser A, Shintani AK, et al. Premature coronary-artery atherosclerosis in systemic lupus erythematosus. N Engl J Med 2003; 349: 2407–2415. [DOI] [PubMed] [Google Scholar]
- 28.Esdaile JM, Abrahamowicz M, Grodzicky T, et al. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum 2001; 44: 2331–2337. [DOI] [PubMed] [Google Scholar]
- 29.Wajed J, Ahmad Y, Durrington PN, Bruce IN. Prevention of cardiovascular disease in systemic lupus erythematosus—proposed guidelines for risk factor management. Rheumatology (Oxford) 2004; 43: 7–12. [DOI] [PubMed] [Google Scholar]