Abstract
Secreted Frizzled related proteins (sFRPs) are a family of proteins that modulate Wnt signaling, which in turn regulates multiple aspects of ventral midbrain (VM) and dopamine (DA) neuron development. However, it is not known which Wnt signaling branch and what aspects of midbrain DA neuron development are regulated by sFRPs. Here, we show that sFRP1 and sFRP2 activate the Wnt/planar-cell-polarity/Rac1 pathway in DA cells. in the developing VM, sFRP1 and sFRP2 are expressed at low levels, and sFRP1−/− or sFRP2−/− mice had no detectable phenotype. However, compound sFRP1−/−;sFRP2−/− mutants revealed a Wnt/PCP phenotype similar to that previously described for Wnt5a−/− mice. This included an anteroposterior shortening of the VM, a lateral expansion of the Shh domain and DA lineage markers (Lmx1a and Th), as well as an accumulation of Nurr1+ precursors in the VM. In vitro experiments showed that, while very high concentrations of SFRP1 had a negative effect on cell survival, low/medium concentrations of sFRP1 or sFRP2 promoted the DA differentiation of progenitors derived from primary VM cultures or mouse embryonic stem cells (ESCs), mimicking the effects of Wnt5a. We thus conclude that the main function of sFRP1 and sFRP2 is to enhance Wnt/PCP signaling in DA cells and to regulate Wnt/PCP-dependent functions in midbrain development. Moreover, we suggest that low-medium concentrations of sFRPs may be used to enhance the DA differentiation of ESCs and improve their therapeutic application.
Keywords: Embryonic stem cells, Neural differentiation, Developmental biology, Parkinson’s disease
Introduction
Secreted frizzled related proteins (sFRPs) are a family of five secreted proteins (sFRP1-sFRP5) that can be separated into two subgroups based on sequence homology: sFRP1/2/5 and sFRP3/4 [1]. SFRPs are known to bind and interact with Wnts [2, 3], a large family of ligands comprising 19 members in mammals [4], many of which are expressed in the nervous system (for review see [5–7]), including the developing midbrain [8]. Early studies found that sFRP binding to Wnt prevented the activation of Wnt receptors [9–14] leading to the initial classification of sFRPs as Wnt signaling inhibitors. However, subsequent reports have indicated that, depending on the cell context, different mechanisms and different pathways can be activated by sFRPs. For instance, sFRPs can directly interact with Frizzled [15] and can activate Wnt/β-catenin signaling [3, 16, 17]. To add a further layer of complexity, the function of specific sFRPs has been found to be either redundant or distinct depending on the cell types they act upon. For example, while sFRP1 has been found to sensitize cells to apoptotic stimuli, sFRP2 (also referred to as secreted apoptosis related protein1) conferred resistance to apoptotic stimuli [16]. On the other hand, while individual sFRP1−/− or sFRP2−/− mutant mice did not show any defects in anteroposterior axis elongation and thoracic somito-genesis, double mutant mice did, suggesting that some functions are redundant [18]. Moreover, sFRPs have been found to modulate both Wnt/β-catenin and Wnt/PCP pathways, as shown by the synergy between sFRP1/2/5 triple mutants with Dkk1 or Vangl2 (looptail) loss of function mutants [19]. It has been proposed that sFRPs may also play a role in establishing, maintaining, and/or modulating Wnt protein gradients in vivo by affecting the diffusibility of Wnt ligands [20], thus regulating multiple Wnt-dependent functions. Despite the growing knowledge of the function of sFRPs, their roles in Wnt-dependent signaling events, such as development of the ventral midbrain (VM), remain unknown. We have previously reported that Wnts selectively regulate distinct aspects of VM dopamine (DA) neuron development. Within the VM, Wnt1, an activator of Wnt/β-catenin signaling, regulates the expansion of neural progenitors, the specification of DA neurons, and DA neuron survival [21–24]. Similarly, Wnt3a activates Wnt/β-catenin signaling in DA progenitors [25] and increases their proliferation [26], while Wnt5a activates Wnt/PCP signaling in DA neurons [27], promoting precursor proliferation, differentiation, and survival of DA neurons [26, 28]. Furthermore, Wnt5a−/− mice display a convergent extension (CE) defect in the development of the midbrain A9–A10 nucleus, an increase in proliferation, and a transient excess of Nurr1 + postmitotic DA neuroblasts [29]. Wnt signaling is thus known to control distinct aspects of midbrain and DA neuron development [30]; however, the functions of sFRPs remain to be examined.
This study focuses on the function of sFRPs in DA neuron development. SFRP1 and sFRP2 were found at low levels in the developing VM. Analysis of Wnt signaling in a DA neuron cell line revealed that low to high concentrations of sFRP1 and sFRP2 activated Wnt/PCP/Rac1 signaling. Subsequent analysis of primary VM cultures as well as mouse embryonic stem cells (mESCs) demonstrated differential effects of sFRPs depending on their concentration. At high concentrations, sFRP1 had deleterious effects on midbrain DA neurons, while sFRP2 had no detectable effect. Instead, low-medium concentrations of sFRP1 and sFRP2 were sufficient to promote DA neuron differentiation. Moreover, in agreement with the low levels of sFRP1 and sFRP2 expression in the VM, analysis of sFRP1−/−;sFRP2−/− mice showed a Wnt/PCP phenotype similar to that of Wnt5a−/− mice, which included morphogenesis and differentiation defects. While providing new insights into the function of sFRPs in VM DA development, our findings have important implications for the generation of DA neurons from ESCs and may thereby become useful tools to improve cell replacement strategies for Parkinson’s disease.
Materials and Methods
Rac1 Activity Assays
Activation of Rac1 in treated SN4741 cells was analyzed by pull-down of activated, GTP-bound Rac1 from cell extracts as described previously [27]. Relative levels of GTP-bound Rac1 and total Rac1 were determined by 11% polyacrylamide SDS-PAGE: sodium dodecyl sulfate polyacrylamide gel electrophoresis and subsequent Western blotting (anti-Rac1 1:1,000, Upstate). Densitometric analyses were performed with Image J software, and levels of Rac1 GTP were normalized to total Rac1 levels.
Primary Cultures
Embryonic day (E) 13.5–14.5 VMs, obtained from time-mated Sprague-Dawley rats, or E11.5 CD1 mice VMs, were dissected, mechanically dissociated in N2 media consisting of a 1:1 mixture of F12 and minimum essential media supplemented with 15 mM HEPES buffer, 1 mM glutamine, 6 mg/ml glucose, 1 mg/ml bovine serum albumin, and N2 supplement (all purchased from Invitrogen, Carlsbad, CA, http://www.invitrogen.com), and plated at a final density of 1.32 × 105 cells per centimeter square on poly-D-lysine (10 μM)-coated plates (Falcon) for rat primary cultures, or 1.5 × 105 cells per centimeter square for mouse primary cultures, as described previously [26]. Treatment with 30–300 ng/ml of Wnt5a and/or 0.01–10 μg/ml sFRP1 or sFRP2 [31] was initiated within 1 hour of plating. Cultures were incubated for 3 days in vitro, in a 37°C incubator with 5% CO2, before fixation with 4% paraformaldehyde (PFA).
mESC Cultures
Differentiation of R1 mESCs into DA neurons was performed using a slightly modified protocol [32]. Briefly, R1 mESCs were seeded at a density of 100 cells per centimeter square on mitomycin-treated PA6 stromal cells and cultured in ES-Serum Replacement Media, composed of KnockOut Dulbecco’s modified Eagle’s medium (Invitrogen), 15% KnockOut serum replacement (Invitrogen), 0.1 mM β-mercaptoethanol (Sigma), 200 mM L-glutamine (Invitrogen), 1% nonessential amino acids (Biochrom AG), and 2,000 U/ml penicillin/streptomycin (Invitrogen). After 5 days, medium was changed and supplemented with 25 ng/ml fibroblast growth factor 8 (FGF8) (R&D Systems Inc., Minneapolis, MN, http://www.rndsystems.com) and 200 ng/ml of Shh (R&D Systems). On day 6, recombinant sFRP1 or sFRP2 was added in combination with 200 ng/ml of Shh and 25 ng/ml of Fgf8. From day 8 to day 11, cells were cultured in N2 medium supplemented with 50 ng/ml FGF8, 10 ng/ml FGF2 (R&D Systems), and 200 ng/ml of Shh, with differing amounts of sFRP1 or sFRP2. From day 11 to day 14, the N2 media were replaced and supplemented with 30 ng/ml brain-derived neurotrophic factor (R&D Systems), 30 ng/ml glial-derived neurotrophic factor (R&D Systems), and 200 μM ascorbic acid (Sigma, St. Louis, MO, http://www.sigmaaldrich.com).
Results
SFRPs Are Expressed in the VM Neuroepithelium and Are Developmentally Regulated
In order to study the possible role of sFRPs in the developing VM, we first examined the expression of sFRP1–4 by quantitative polymerase chain reaction in E10.5–E14.5 rat ventral and dorsal midbrain (Supporting Information Fig. 1A–1D). All sFRPs were found to be developmentally regulated in a very dynamic manner. Moreover, each sFRP showed a region-specific temporal expression profile. Within the VM, sFRP1 and sFRP2 were downregulated at E10.5 and E15.5, while sFRP3 expression decreased from E13.5 and sFRP4 was only modestly regulated. We next examined the expression of sFRPs by in situ hybridization (ISH) in mouse E11.5 brains, at the time of birth of A9–A10 DA neurons. SFRP1 and sFRP2 were highly expressed within the lateral midbrain, in the ventricular zone (VZ) of the alar plate (AP), as assessed by digoxigenin ISH (Fig. 1A, 1B). Low levels of sFRP1 and sFRP2 were also detected in the VZ of the medial and lateral floorplate (FP), respectively, by radioactive ISH (Fig. 1A′, 1B′). While sFRP4 could not be detected, sFRP3 expression was detected only at low levels by radioactive ISH in the alar plate but not in the VM (Fig. 1C, 1C′). Based on these findings, we decided to focus our study on the role of sRFP1 and sFRP2 in VM development.
Figure 1.
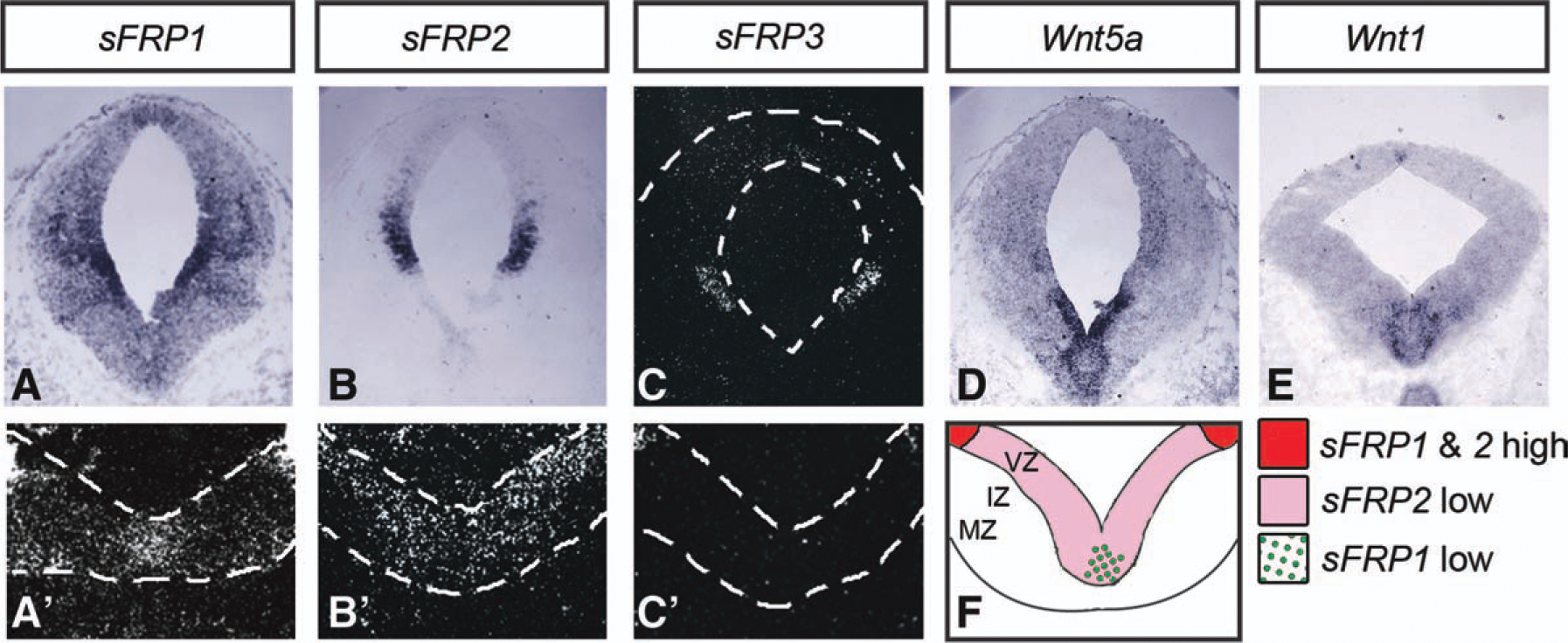
Spatial pattern of expression of sFRP1, sFRP2, and sFRP3 in the developing midbrain at E11.5. (A–C, A′–C′): In situ hybridization for sFRP1, sFRP2, and sFRP3 mRNA on coronal sections of mouse midbrain at E11.5 revealed high expression in the midbrain AP. Higher magnification of radioactive in situ hybridization detected expression of sFRP1 in the midline and a low expression of sFRP2 in the whole ventral midbrain (VM). In contrast, low levels of sFRP3 were only detected laterally, in the midbrain AP but not in the VM. (D, E): Wnt5a and Wntl expression was also detected in the VM. (F): Scheme summarizing the overlap and expression levels of sFRP1 and sFRP2 in the VM. Abbreviations: sFRP, secreted Frizzled related protein; IZ, intermediate zone; MZ, marginal zone; VZ, ventricular zone.
Given the known function of sFRPs as Wnt modulators, we compared the expression pattern of sFRP1 and sFRP2 with that of two Wnts known to regulate DA neuron development, Wntl and Wnt5a [23, 26, 29, 33]. Wnt5a was expressed throughout the VZ of the VM (Fig. 1D), thereby overlapping with the VM expression of both sFRP1 and sFRP2. Wntl, however, was expressed in two stripes flanking the VM midline (Fig. 1E), following a pattern that overlapped with sFRP2 expression. Thus, our results suggest that while the medial FP expresses Wnt5a and low levels of sFRP1 and sFRP2, the lateral FP expresses Wnt5a, Wntl, and low levels of sFRP2 (Fig. 1F).
SFRP1 and SFRP2 Enhance Wnt/PCP/Racl Signaling in DA Cells
Initially, we assessed the function of sFRPs using SN4741 cells, a DA cell line that has been extensively used to study Wnt signaling [25, 34, 35]. Treatment of SN cells with sFRP1 or sFRP2 alone (up to 10 μg/ml) had no effect on Dvl or β-catenin accumulation (data not shown). Since sFRPs have been described as modulators of Wnt signaling [36], we therefore examined their effects on Wnt signaling (Fig. 2). We previously reported that Wnt3a activates β-catenin and induces hyperphosphorylation of Dvl in DA cells, which is manifested by a mobility shift [25, 34]. Instead, Wnt5a does not activate β-catenin but induces Dvl hyperphosphorylation [25, 34] and activates the small GTPase Rac1 in DA cells [27, 29]. However, we previously found that a greater activation of Wnt/PCP/Rac1 pathway decreases Dvl phosphorylation [27].
Figure 2.
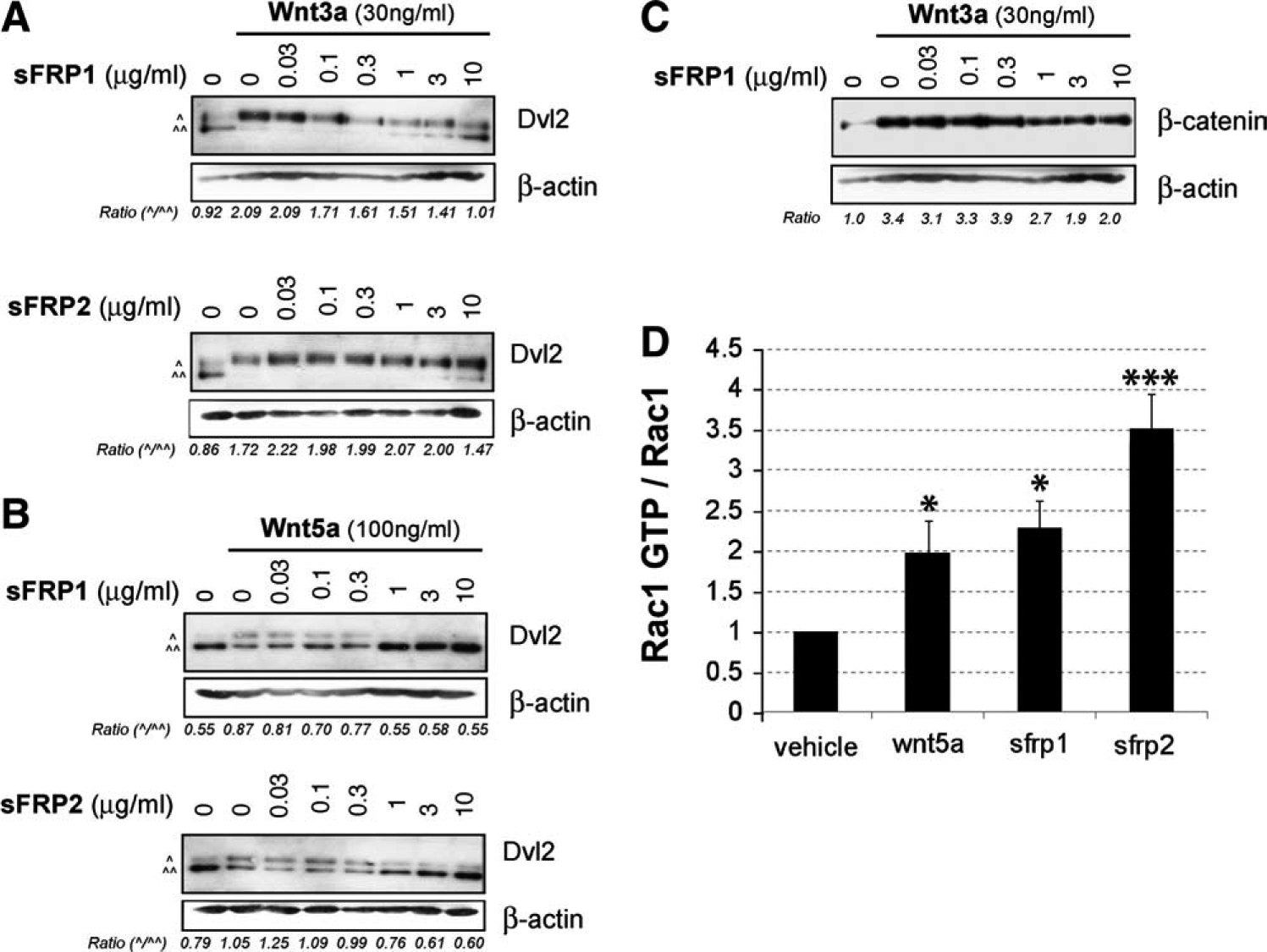
SFRP1 and sFRP2 inhibit the Wnt3a- or Wnt5a-induced Dvl shift and activate Rac1 in a dopaminergic neuron cell line. SN4741 cells were treated with 30 ng/ml Wnt3a (A) or 100 ng/ml Wnt5a (B) and increasing concentrations (0, 0.03, 0.1, 0.3, 1, 3, and 10 μg/ml) of sFRP1or sFRP2. (A): The Wnt3a-induced Dvl2 phosphorylation mobility shift was reduced by a lower concentration of sFRP1 than sFRP2. (B): The Wnt5a-induced Dvl2 phosphorylation mobility shift was efficiently reduced by both sFRP1 and sFRP2. The ratio between the hyperphosphory-lated form (upper band ^) and the nonshifted lower band (^^) is given for representative gels (n ≥ 3). (C): SFRP1 also reduced the increase in active β-catenin induced by Wnt3a. The induction of active β-catenin was expressed as the ratio between the densitometric quantification of active β-catenin and the loading control actin (ratio: β-catenin/actin). (D): Both sFRP1 and sFRP2 (2 μg/ml) increased Rac1 activity by twofold and 3.5-fold, respectively, compared with vehicle. ***, p < .001 and *, p < .05 by one-way analysis of variance, with Turkey’s post hoc test for multiple comparisons (n = 3). Abbreviation: sFRP, secreted Frizzled related protein.
Here we show that while sFRP1 had no effect on the Wnt3a- or Wnt5a-induced Dvl shift at low concentrations, it decreased Dvl phosphorylation shift at 0.3–1 μg/ml (Fig. 2A, 2B) and increased the levels of activated GTP-bound Rac1 at 2 μg/ml (Fig. 2D), indicating that sFRP1 enhances Wnt/PCP/Rac1 signaling. Surprisingly, high concentrations of sFRP1 (3 μg/ml) reduced the Wnt3a-induced increase in active β-catenin (Fig. 2C) but did not affect the Wnt/β-catenin reporter, TOPFLASH (not shown), indicating that sFRP1 mainly works as an activator of Wnt/PCP signaling.
We also found that while low concentrations of sFRP2 (30 ng/ml) enhanced the effects of Wnt3a or Wnt5a on Dvl phosphorylation, higher concentrations inhibited such effects (Fig. 2A, 2B). Unlike sFRP1, sFRP2 (2 μg/ml) was not able to modulate the Wnt3a-induced increase in active β-catenin (not shown) but efficiently increased Rac1 activity (Fig. 2D). The effects of Wnt5a and sFRPs on Rac1 activation were not additive (data not show), indicating that sFRP2 and Wnt5a work by a similar mechanism. These results show that sFRP2 selectively and progressively activates Wnt/PCP/Rac1 signaling from low to high concentrations.
Combined, our results indicate that despite the minor effect of sFRP1 on the levels of active β-catenin both sFRP1 and sFRP2 work as activators of the Wnt/PCP/Rac1 pathway. Importantly, sFRP2 began to activate Wnt/PCP at low concentrations (increased Dvl shift), and at higher concentrations, both sFRP1 and sFRP2 further activated Wnt/PCP (decreased Dvl shift and activation of Rac1). The similarities between sFRPs and Wnt5a prompted us to examine the function of sFRPs on midbrain DA neurons.
SFRP1 and SFRP2 Regulate DA Neuron Differentiation and Neuritogenesis
We have previously shown that Wnts regulate the proliferation and differentiation of DA neurons in VM precursor cultures [26]; however, the effects of sFRPs are unknown. We thus treated VM precursor cultures with increasing concentrations of sFRP1 or sFRP2 for 3 days and examined their differentiation into Tuj1+ or tyrosine hydroxylase (TH+) neurons. While sFRP2 increased the proportion of TH+ neurons at low concentrations (10–100 ng/ml), sFRP1 worked at medium-high concentrations (100 ng to 1 μg/ml, Fig. 3A, 3B). Higher concentrations of sFRP1 dramatically decreased the survival of TuJ1+ and TH+ neurons in culture (Supporting Information Fig. 2A, 2B) but had no effect on SN cells, indicating that different cells respond at different sFRP concentrations. The cell loss observed in cultures with very high concentrations of sFRP1 resembled the loss of DA neurons in the Wntl−/− mice [23]. However, neither sFRP1 nor sFRP2 had an effect on proliferation, as assessed by BrdU: (5-bromo-2′-deoxyuridine) incorporation in the cultures (BrdU/Hoechst, data not shown) and by the analysis of Ki67+ cells in sFRP1−/−;sFRP2−/− mice, which showed no change (Supporting Information Fig. 3). Finally, higher concentrations of sFRP2 (5 μg/ml) neither had any effect on TH+ or TuJ1 + cells nor modified the effects of Wnt5a (Supporting Information Fig. 2C, 2D). Combined, our results indicate that sFRPs work in a concentration-dependent manner to regulate DA neuron differentiation.
Figure 3.
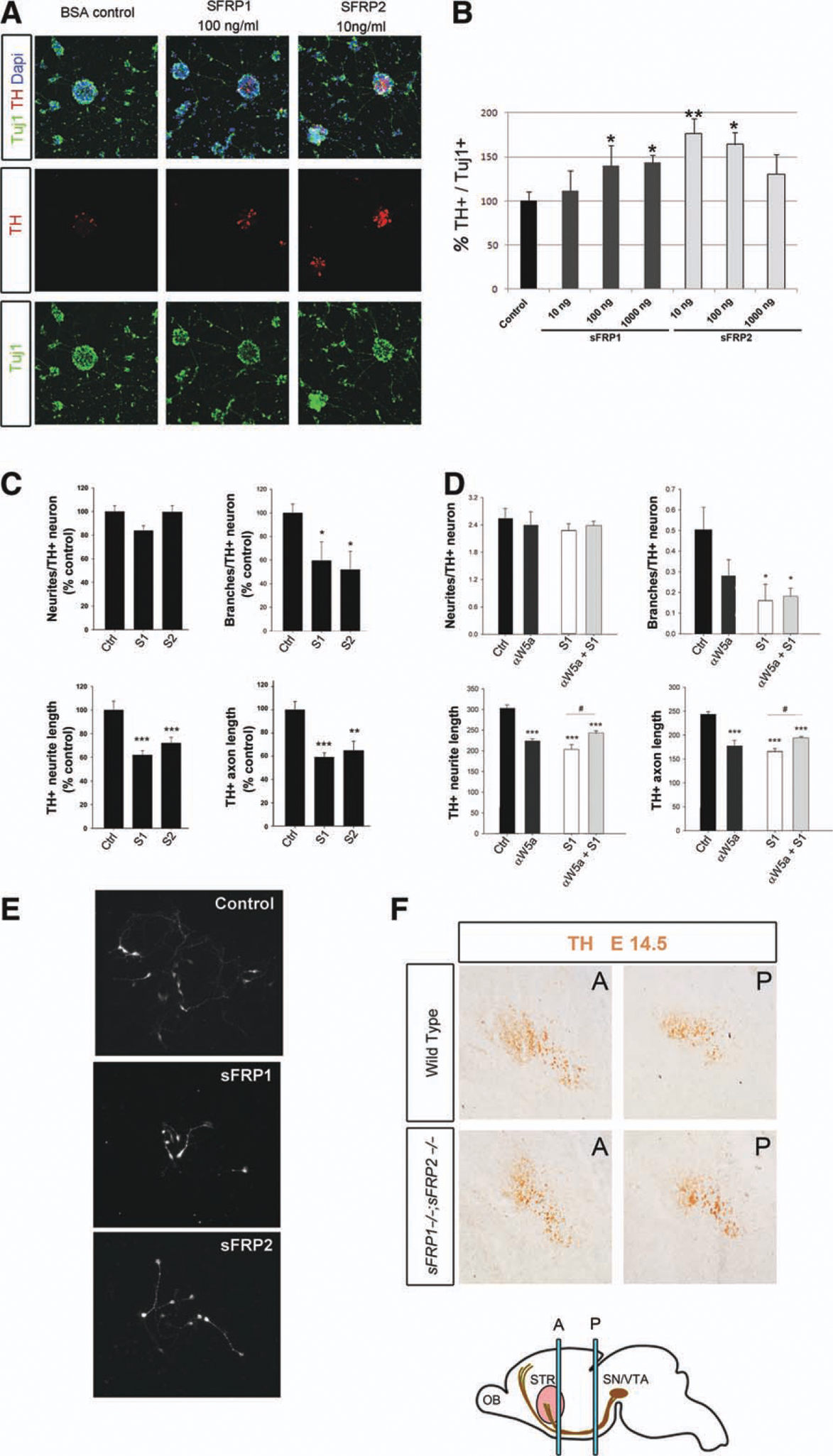
Differential effects of sFRP1 and sFRP2 on TH+ cell numbers and neuritogenesis. Mouse E11.5 ventral midbrain cultures were treated for 3 days with vehicle (BSA Ctrl) or different concentrations of sFRP1 or sFRP2 (10, 100, or 1,000 ng/ml) and analyzed for expression of beta III-tubulin (TuJ1, green) and TH (red), using DAPI (blue) as counterstaining. (A): SFRP1 (100 ng/ml) and sFRP2 (10 ng/ml) increased the percentage of Th+/Tuj1 + cells in primary cultures. (B): The percentage of TH+/TuJ1+ neurons increased in a dose-dependent manner in sFRP1- and sFRP2-treated primary cultures. Significant effects were detected at 100–1,000 ng/ml of sFRP1 and 10–100 ng/ml of sFRP2 as assessed by one-way analysis of variance with Tukey’s post hoc test for multiple comparisons (n = 3). p < .001; **, p < .01; *, p < .05. (C): The effects of sFRP1 or sFRP2 on neuritogenesis were evaluated in rat E13.5 primary precursor cells at concentrations capable of activating Rac1 (2.5 μg/ml, S1 or S2). DA neurite length was expressed in four different ways, as neurites/TH+ neuron, branches/TH+ neuron, length of the dominant neurite (axon), and average length per neurite. (D): Wnt5a effect was analyzed by adding a Wnt5a blocking antibody (αWnt5a) to the cultures, in combination with sFRP1 (S1). αWnt5a alone reduced the number of branches, TH+ neurite length, and TH+ axon length. Moreover, aWnt5a partially prevented the effect of sFRP1. ***, p < .001; **, p < .01; *, p < .05 by one-way analysis of varaiance, with Tukeys’s post hoc test for multiple comparisons (n = 3). (E): TH immunocytochemistry showing the reduction of neurite branching, length, and axonal length in SFRP1- and sFRP2-treated cultures. (F): TH im-munostaining showing DA axons at anterior and posterior levels of the medial forebrain bundle at E14.5 (A, P, STR [pink], SN/VTA [brown], and OB). Abbreviations: A, anterior level; bovine serum albumin, BSA; 4′,6-diamidino-2-, DAP; OB, olfactory bulb; P, posterior level; STR, striatum; SN/VTA, substantia nigra and ventral tegmental area; sFRP, secreted Frizzled related protein; TH, tyrosine hydroxylase.
Wnts are known to regulate both neuritogenesis and axo-nogenesis [5, 7]. Moreover, we recently reported that Wnt5a can modulate DA axon morphogenesis by a mechanism involving Rac1 activation [33]. We thus examined the effects of sFRP1 and sFRP2 on DA neuritogenesis and axon morphogenesis in rat E13.5 cultures at concentrations capable of activating Rac1 (2.5 μg/ml). SFRP1 or sFRP2 treatment reduced Th+ neurite branches in a similar manner to Wnt5a in mouse E11.5 cultures [33] and decreased the length of TH+ neurites and axons (Fig. 3C, 3E), as previously described for Wnt5a-treated mouse E14.5 cultures [33]. Surprisingly, cotreatment with sFRP1 and a Wnt5a blocking antibody partially prevented the effect of sFRP1 on TH+ neurite length and axon length (Fig. 3D), suggesting that, to some extent, sFRPs require Wnt5a for their effects. Finally, as analysis of Wnt5a−/− mice revealed that Wnt5a regulates the fasciculation of DA axons in vivo [33], we then analyzed the medial forebrain bundle of sFRP1−/−;sFRP2−/− mice at E14.5. Surprisingly, no significant difference in the number of TH+ axons was detected at posterior or anterior levels of the medial forebrain bundle, including near the striatum (Fig. 3F). Thus, our results suggest that sFRP1 and sFRP2 may regulate aspects of neuritogenesis and axon morphogenesis that are not required for the development of the medial forebrain bundle.
Deletion of SFRP1 and SFRP2 Alters the Positioning of DA Cells and VM Morphogenesis
We next studied the number of TH+ cells in A9–A10 areas at the beginning (E10.5) and end (E14.5) of the DA neurogenic period in the VM. While TH counts remained unchanged across all genotypes (wild type [WT], sFRP1−/−, sFRP2−/−, and sFRP1−/−;sFRP2−/−) (Supporting Information Fig. 3A), the area occupied by TH+ neurons in coronal sections was notably greater in sFRP1−/−;sFRP2−/− embryos (Fig. 4A, 4B and not shown). Interestingly, we have previously shown that deletion of Wnt5a results in a medial-lateral (ML) widening and anterior-posterior (AP) shortening of the domain occupied by VM DA neurons [29]. Moreover, our finding that sFRP1 and sFRP2 regulated Wnt/PCP signaling in DA cells similarly to Wnt5a (Fig. 2) prompted us to examine the distribution of TH+ cells in more detail. Neither sFRP1−/− nor sFRP2−/− showed a significant difference in the ML or AP distribution of DA neurons compared with WT mice (Fig. 4C). However, sFRP1−/−;sFRP2−/− double-knockout mice showed a clear ML widening (Fig. 4A) as well as a significant AP shortening of the VM at E11.5 (Fig. 4C). While the AP defect was rescued by E14.5 (Fig. 4D), TH+ cells remained aberrantly distributed in the ML and dorsal-ventral axis of the VM (Fig. 4B). This finding indicated that sFRP1−/−;sFRP2−/− mice exhibit a CE phenotype similar to that described for Wnt5a [29]. To investigate this in greater detail, we next examined the expression of other DA genes that define the VM progenitor domain and examined whether their position was also altered.
Figure 4.
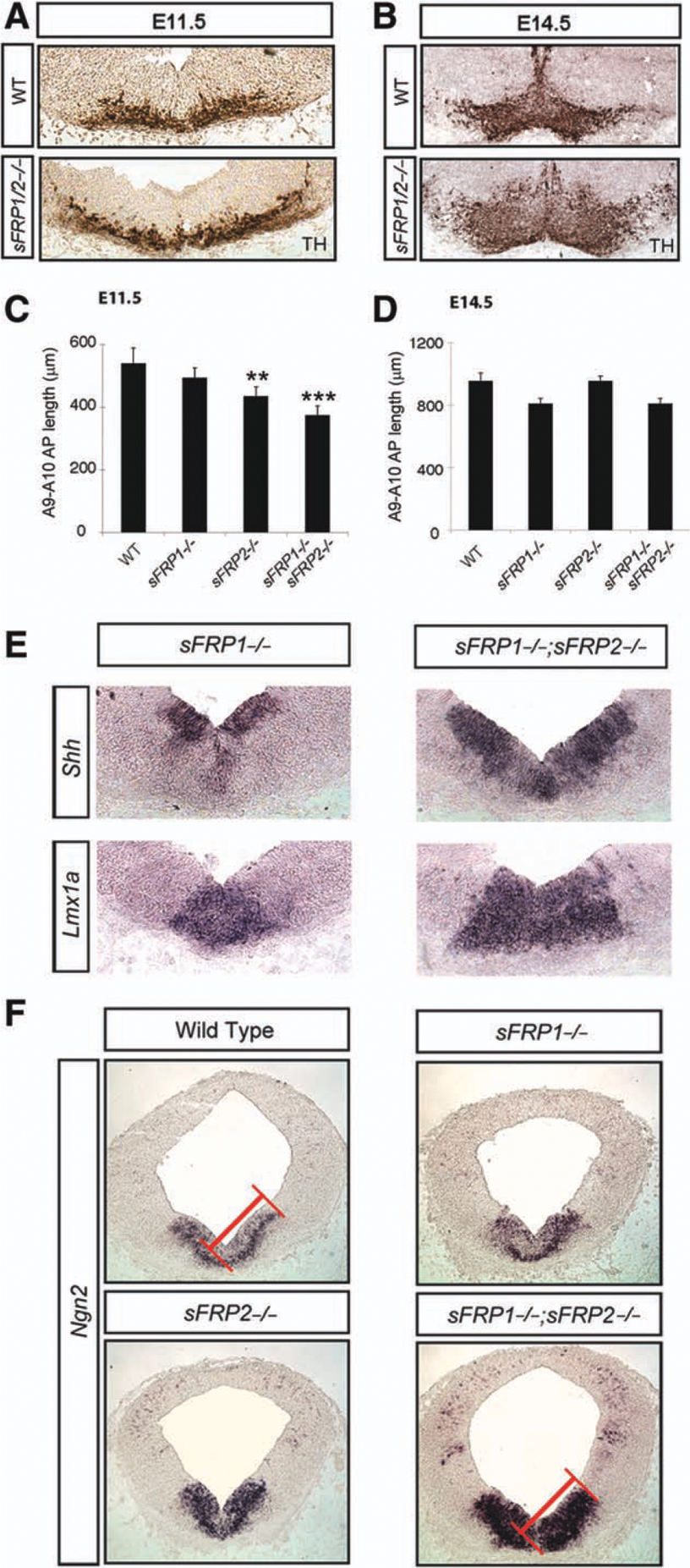
Convergent extension defects in the ventral midbrain of sFRP1−/−;sFRP2−/− mice. While the number of TH+ cells in the ventral midbrain (VM) was not altered in sFRP1−/−;sFRP2−/− mice, their medial-lateral distribution at E11.5 (A) and their medial-lateral and dorsal-ventral distribution at E14.5 (B) were increased. This was accompanied by a reduction in the rostral-caudal distribution of TH+ cells at E11.5 (C) but not at E14.5 (D). *** p < .001; **, p < .01 by one-way analyses of variance, with Bonferroni’s post hoc test for multiple comparisons (n = 4 for E11.5 and n = 3 for E14.5). In addition to the increased lateral distribution of TH, other markers such as Shh (floorplate [FP] and basal plate [BP]) and Lmxla (FP) were also laterally expanded in sFRP1−/−;sFRP2−/− mice at E11.5 (E). The expression of Ngn2 in the VM expanded dorsoven-trally, but not laterally, in sFRP1−/−;sFRP2−/− mice at E11.5 (F). Abbreviations: Ngn2, neurogenin 2; sFRP, secreted Frizzled related protein; TH, tyrosine hydroxylase; WT, wild type. Shh, Sonic hedgehog homolog; Lmx1a, LIM homeobox transcription factor 1 alpha.
First, we examined the expression of Shh and Lmx1a, two genes required for the specification of VM progenitors. We found a lateral expansion of the expression domain of Shh and Lmxla in sFRP1−/−;sFRP2−/− mice, compared with WT, sFRP2−/−, or sFRP1−/− mice at E11.5 (Fig. 4E and data not shown). This domain expansion was accompanied by a characteristic change in the morphology of the ventricle, normally forming a V- or Y-shaped invagination, yet displaying a U-shape in sFRP1−/−;sFRP2−/− mice (Fig. 5A). These results show that sFRP1 and sFRP2 together regulate VM morphogenesis and show phenotypes similar to other Wnt-related mutants (Scribble, Vangl2, and Wnt5a) [29, 38–40].
Figure 5.
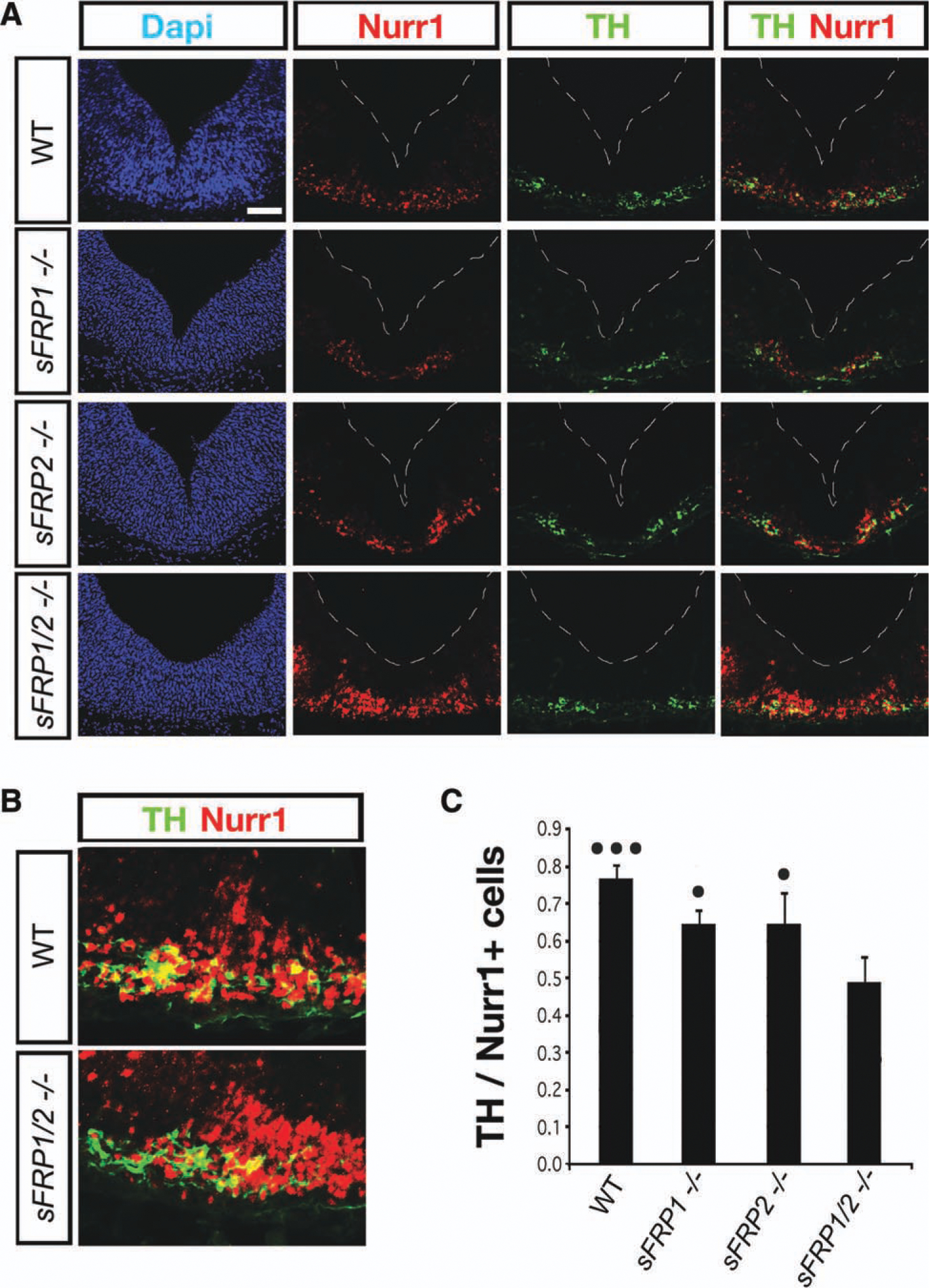
Deletion of sFRP1, sFRP2, and sFRP1/2 reduced the differentiation of Nurr1+/TH− DA precursors into Nurr1+/TH+ DA neurons. Immunohistochemistry for Nurr1 revealed an increase in the medial-lateral distribution of Nurr1+ cells in sFRP1−/−;sFRP2−/− compared with WT (A). This increase in Nurr1+ cells did not result in an increase in the number of Nurr1+/TH+ neurons in sFRP1−/−;sFRP2−/− but rather in an accumulation of Nurr1+/TH− DA precursors compared with WT (B). The proportion of TH+/Nurr1+ cells decreased modestly in sFRP1−/− or sFRP2−/− mice (n = 4 and 5, respectively). sFRP1−/−;sFRP2−/− mice (n = 5) showed a greater reduction in the proportion of TH+/Nurr1+ cells (C), which was significantly different from single mutants (•p < .05) and WT (•••p < .001), as assessed by analysis of variance with Tukey’s post hoc test. Our results thus suggest that the effects of endogenous low levels of sFRP1 and sFRP2 are additive. Abbreviations: DAPI, 4′,6-diami-dino-2-phenylindole; sFRP, secreted Frizzled related protein; TH, tyrosine hydroxylase; WT, wild type. Nurr1, nuclear receptor related 1.
Impaired DA Differentiation of Nurr1 Precursors in the VM of sFRP1−/−;sFRP2−/− Mice
The increased ML expression of Shh and Lmx1a in the VM of sFRP1−/−;sFRP2−/− mice suggested the possibility of increased proliferation, neurogenesis, and postmitotic cell accumulation, as described in the Wnt5a−/− mice [29]. However, staining for the cell cycle marker Ki67 and the pan-neuronal marker TuJ1 did not reveal any differences in the distribution or total number of proliferating or postmitotic cells between WT and sFRP1−/−, sFRP2−/−, or sFRP1−/−;sFRP2−/− (Supporting Information Fig. 3B, 3C and data not shown), suggesting that sFRPs may work on smaller subpopulation of cells. We next explored whether neurogenesis was affected by examining the expression of Neurogenin 2 (Ngn2), a gene required for the differentiation of Sox2+ VZ progenitors into Nurr1+ postmitotic DA neuroblasts in the intermediate zone (IZ), and for their subsequent differentiation into DA neurons in the marginal zone (MZ) [41]. Ngn2 expression was unchanged in sFRP1−/− or sFRP2−/− mice. However, a striking upregulation of Ngn2 expression was observed in sFRP1−/−;sFRP2−/− mice, which did not extend laterally, but occupied all layers of cells in the VM, from VZ to MZ (Fig. 4F). These findings suggested an increase in neurogenesis and a possible increase in postmitotic Nurr1+/TH– migratory neuroblasts in the IZ. In agreement, sFRP1−/−;sFRP2−/− mice revealed a significant increase in the number of Nurr1+ cells in the VM (Fig. 5A, 5B). As Nurr1 is expressed both by DA precursors and neurons, we performed double TH and Nurr1 immunohistochemistry, to distinguish DA neuroblasts/precursors (Nurr1+/TH−) from DA neurons (Nurr1+/TH+). While the number of TH+ cells did not change in sFRP1−/−;sFRP2−/− mice, the number of Nurr1+ cells increased compared with WT (Fig. 5A, 5B). Moreover, we found that the proportion of Nurr1+ cells that had acquired TH expression significantly decreased in single and double sFRP mutants at E11.5 (Fig. 5C). While deletion of SFRP1 or sFRP2 induced only a modest but significant decrease in the TH/Nurr1+ cell ratio, deletion of both sFRP1 and sFRP2 decreased this ratio by a 30%. Combined, our results show that sFRP1−/−;sFRP2−/− mice phenocopy several aspects of the Wnt5a−/− mice and indicate that endogenous/low levels of sFRP1 and sFRP2 are required for VM morphogenesis and the generation of postmitotic DA neuroblasts.
SFRP1 and SFRP2 Regulate Midbrain DA Neuron Development in mESC Preparations
In light of these findings, we next examined the effect of sFRP1 and sFRP2 on the generation of DA neurons from mESCs. Under our differentiation conditions (Fig. 6A), the majority of mESC-derived TH+ neurons expressed typical midbrain DA markers, including Lmx1a, Foxa2, Nurr1, and Pitx3 [42–44] (Fig. 6C and data not shown). Administration of either sFRP1 or sFRP2 from day 6 to day 11 was found to regulate the number of TH+ cells derived from mESCs in a dose-dependent manner (Fig. 6B). Interestingly, the effects of sFRP1 did not follow a sigmoidal curve but rather a bell-shaped curve. At relatively low concentrations (10 ng/ml), sFRP1 and sFRP2 promoted the generation of TH+ cells, which acquired phenotypic features of midbrain DA neurons, such as the expression of Nurr1 and Lmx1a (Fig. 6C). However, the effects of higher concentrations of sFRPs clearly differed for sFRP1 and sFRP2. While high concentrations of sFRP1 induced a drastic decrease in the number of TH+ cells, no such decrease was observed in sFRP2-treated cultures. Importantly, these results are in agreement with our in vitro (Fig. 3B and Supporting Information Fig. 2) and in vivo results (Figs. 4 and 5), whereby only high concentrations of sFRP1 negatively affected the number of DA neurons and medium-low/endogenous concentrations of sFRP1 and sFRP2 promote/were required for DA neuron development.
Figure 6.
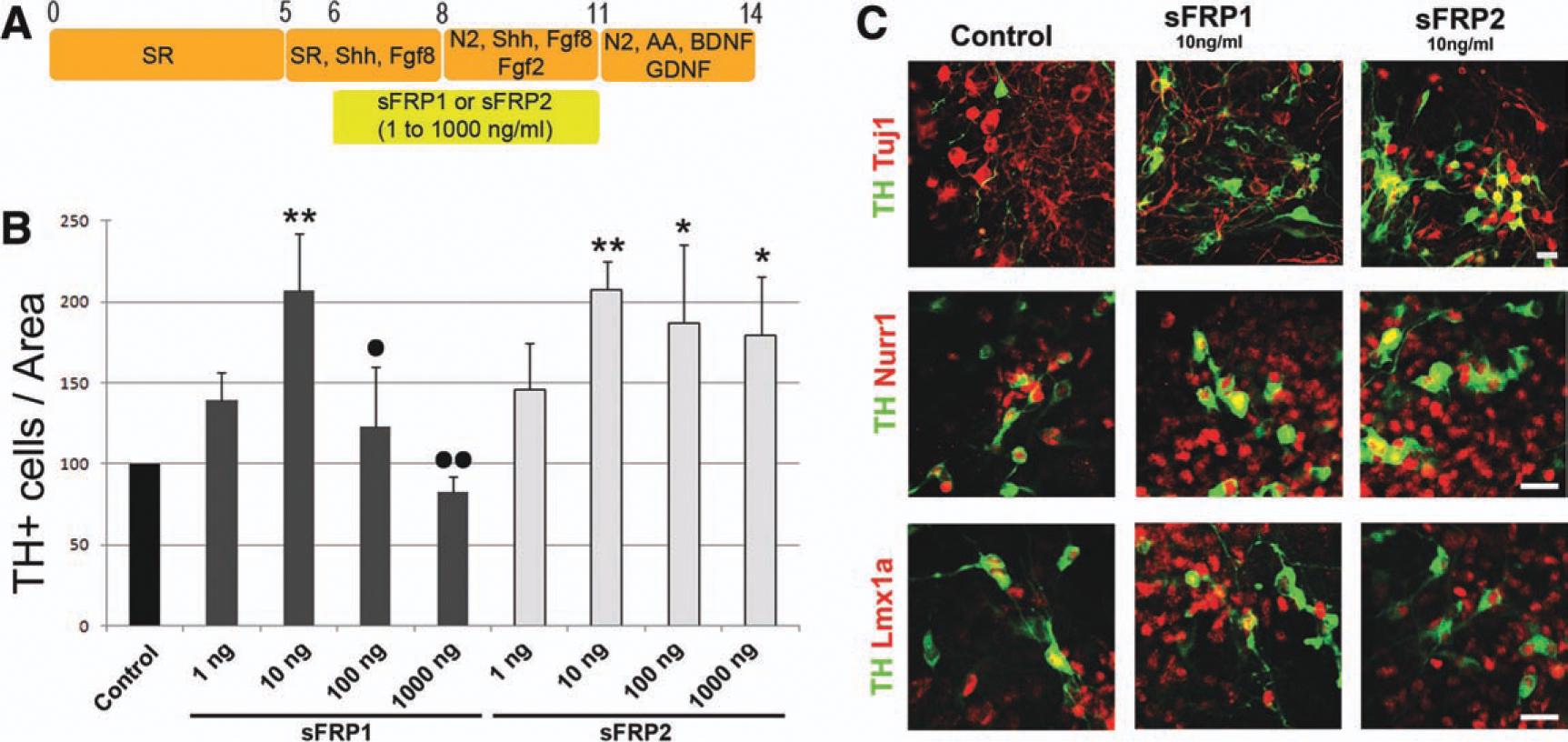
SFRP1 and sFRP2 promote the generation of dopamine (DA) neurons from mouse embryonic stem cells (mESCs). (A): Schematic diagram illustrating the culture conditions used to generate DA neurons from mESCs. mESCs were cultured in the presence of serum replacement medium (SRM), followed by basal conditions, including SRM with FGF8 (for 3 days), SRM with FGF2 and FGF8 (for 3 days), and finally N2 supplement with AA, GDNF, and BDNF (for 3 days). Modified treatment conditions include the addition of sFRP1 or sFRP2 from day 6 to day 11 (from 1 to 1,000 ng/ml). (B): SFRP1 and sFRP2 increased the numbers of TH+ cells per Tuj1+ area up to a concentration of 10 ng/ml. While higher concentrations of sFRP1 decreased the numbers of TH, the effects of sFRP2 were sustained. *, p < .05; **, p < .001 compared with control; •, p < .05; ••, p < .01 compared with 10 ng/ml sFRP1, by one-way analysis of variance, with Tukey’s post hoc test for multiple comparisons. (C): SFRP1 or sFRP2 (10 ng/ml) did not affect Tuj1 expression but increased the expression of Nurr1 and Lmx1a in TH+ cells. The data show representative results from five independent differentiation experiments. Abbreviations: AA, ascorbic acid; BDNF, brain-derived neurotrophic factor; FGF, fibroblast growth factor; GDNF, glial-derived neurotrophic factor; sFRP, secreted Frizzled related protein; TH, tyrosine hydroxylase.
Discussion
Our findings indicate that sFRP1 and sFRP2 are both sufficient and required for the appropriate development of midbrain DA neurons. We found that sFRP1 and sFRP2 regulate diverse functions in the developing midbrain such as morphogenesis, the generation of DA neuroblasts, as well as DA differentiation and survival. The effects of endogenous low levels of sFRP1 and sFRP2 were mainly redundant, as assessed by the deletion of these two genes, which resulted in a phenotype that resembled that of the Wnt5a−/− mice [29]: broadening of the DA domain in the VM, an accumulation of Nurr1+,TH− postmitotic cells due to increased neurogenesis, and normal Nurr1+,TH+ cell numbers due to decreased differentiation of Nurr1+,TH− cells. Surprisingly, we also found that the effects of high concentrations of sFRP1 and sFRP2 differed to some extent. While sFRP1 impaired DA neuron survival in primary cultures and mESC preparations, sFRP2 did not. These activities correlated well with the modulatory effects of sFRPs and with their dual regulation of Wnt signaling in DA neuron (Fig. 2) and eye development [45]. Indeed, we found that sFRP1 and sFRP2 activated the small GTPase Rac1 and reduced Wnt5a-induced Dvl phosphorylation, two activities consistent with a role of sFRP1 and sFRP2 in promoting Wnt/PCP/Rac1 signaling [27]. SFRP1 and sFRP2 also inhibited Wnt3a-induced Dvl phosphorylation; however, only sFRP1 inhibited the Wnt3a-induced stabilization of active β-catenin. Interestingly, in accordance with these results, high concentrations of sFRP1, but not sFRP2, impaired DA neuron survival. Moreover, medium-low levels of sFRP1 and sFRP2 enhanced Wnt/PCP signaling and endogenous levels regulated Wnt/PCP related functions in a coordinated manner, as shown by the finding that deletion of both sFRP1/2 partially phenocopied the Wnt5a−/− mice. Combined, our findings indicate that sFRPs regulate Wnt signaling and diverse aspects of midbrain DA neuron development in a dose-dependent manner.
SFRP1 and SFRP2 Differentially Regulate Cell Survival
sFRPs have been traditionally classified as Wnt inhibitors. However, we hereby report that sFRP1 and sFRP2 activate Wnt/PCP signaling in DA neurons, a function very different of that initially expected. In fact, in recent years, distinct sFRPs have been found to regulate several different functions [20, 36, 46], some of which are Wnt-related, but also Wnt-unrelated, and can even antagonize each other’s effects. Thus, sFRPs should not be considered as mere Wnt inhibitors.
In our study, we found that high concentrations of sFRP1, but not sFRP2, induced cell death. In agreement with our finding, it has previously been reported that sFRP1 increases, while sFRP2 decreases, the vulnerability of breast tumor cells to apoptotic stimuli [16]. Similar results have been reported in other cell types [47, 48], including neural cells in the developing hindbrain [49]. Our results also indicate that sFRP1, but not sFRP2, decreases the Wnt3a-induced levels of active β-catenin, a finding that underlines that the differences between sFRP1 and sFRP2 are not limited to biological activity but also to the signaling pathways they regulate in DA cells. It remains to be determined whether a reduction of active β-catenin, but not TOPFLASH, by sFRP1 in DA cells could impact on DA neuron development as deletion of β-catenin in the midbrain FP [50], or deletion of Wntl [21, 24], which result in dramatic impairment of midbrain DA development. Moreover, deletion of Wntl results in a loss of Pitx3 expression, a transcription factor required for DA neuron survival [51–53] and in the loss of DA neurons [23]. However, since the expression levels of sFRPs in the VM were low and the in vitro effects of sFRP1 were in response to relatively high concentrations, the physiological relevance of our in vitro findings, in the context of VM development, is unclear. Indeed, analysis of sFRP1−/−, sFRP2−/−, and sFRP1−/−;sFRP2−/− mice showed no alteration in the number of midbrain DA neurons, indicating that the endogenous low levels of sFRP1 are not sufficient to negatively regulate Wnt/β-catenin signaling and DA neuron survival.
SFRP1 and SFRP2 Are Required for Wnt/PCP-Dependent Functions in the Developing VM
While sFRP1−/−;sFRP2−/− mice had no defect on the number of DA neurons, their position in the developing midbrain was abnormal, with TH+ cells found in more lateral and dorsal regions of the VM while occupying a shorter anteroposterior domain. These results suggested that the loss of sFRP1 and sFRP2 results in a Wnt/PCP/Rac1 phenotype similar to that previously described for Wnt5a−/− mice [29]. In agreement with this finding, additional alterations in VM morphogenesis were also detected. A flattening of the ventricular cavity and a broadening of the Shh, Lmxla, and Nurrl expression domains were detected in sFRP1−/−;sFRP2−/− mice. However, the Ngn2 expression domain did not extend laterally, as seen in the Wnt5a−/− mice but instead extended from the VZ to the MZ. In addition, the expression levels of Ngn2 increased, resulting in an increase in neurogenesis, that is, an increase in the number of Nurr1+,TH− neuroblasts or precursor cells in the IZ, which are the first postmitotic cells in the DA lineage. Moreover, as described for the Wnt5a−/− mice, the differentiation of these cells into midbrain DA neurons decreased in sFRP1−/−;sFRP2−/− mice. Conversely, administration of sFRP1 or sFRP2 at low-intermediate concentrations (10–100 ng/ml) promoted DA differentiation of primary cells and ESC cultures, similar to Wnt5a [54]. Thus, our data show that sFRP1 and sFRP2 are redundantly required to regulate most Wnt5a-dependent functions in the developing VM. Interestingly, sFRP1−/−;sFRP2−/− mutant mice also exhibited other previously described Wnt/PCP phenotypes such as a shortening of the AP axis as the Wnt5a−/− mice [18] and abnormalities in embryonic male sexual development similar to those exhibited by the Looptail (Vangl2) and Wnt5a mutants [55, 56]. Moreover, severe neural tube defects have been detected in sFRP1;sFRP2;looptail triple mutants, indicating that sFRP1 and sFRP2 genetically interact with Vangl2, and that sFRP1 and sFRP2 regulate the Wnt/PCP pathway [19]. Importantly, our analyses of Wnt signaling in midbrain DA cells also support a role for sFRP1 and sFRP2 in the activation of the Wnt/PCP/Rac1 pathway, as previously reported for Wnt5a [27, 29]. Indeed, we found that sFRPs decreased the Dvl phosphorylation shift and effectively increased Rac1 activity in DA cells. Moreover, while Wnt5a and sFRP1 increased Rac1 activity to the same extent, sFRP2 was more efficient at inducing Rac1 activity, but their effects were not additive, indicating that they both activate this common critical signaling event. In summay, our results show that sFRP1 and sFRP2 share with Wnt5a their capacity to activate the Wnt/PCP/Rac1 pathway and regulate Wnt/PCP-related functions.
Is the Function of Wnt5a on Midbrain DA Neurons Completely Phenocopied by sFRP1 and sFRP2?
While sFRP1−/−;sFRP2−/− mice show many similarities to the Wnt5a−/− mice, they also show distinct phenotypic differences. In Wnt5a−/− mice, the number of Ki67+ proliferating progenitors increased by 45% [29] and TH+ axons in the medial forebrain bundle showed abnormal fasciculation [33], yet these parameters remained unchanged in sFPRl/sFRP2 mutant mice. Additionally, while the Ngn2 domain was laterally expanded in the Wnt5a−/− mutant, a dorsoventral (but not a lateral) expansion was observed in sFRP1−/−;sFRP2−/− midbrains. These results indicate that sFRP1−/−;sFRP2−/− mutants only partially phenocopy the Wnt/PCP phenotype of Wnt5a−/− mice. Similarly, gain-of-function experiments have shown only partial similarities between the effects of sFRPs and Wnt5a. For instance, primary VM cultures showed that high concentrations of sFRP1 protein differed from the effects of Wnt5a, in that it did not promote DA precursor differentiation [26] but rather induced cell death. Similarly, while Wnt5a promoted DA neurite and axonal elongation in VM primary cultures [33], sFRP1 and sFRP2 reduced their growth. However, we also found that both sFRP1 and sFRP2 mimicked the effects of Wnt5a and promoted DA differentiation of primary progenitors and mESC at intermediate concentrations. Combined, these results suggest that sFRP1 and sFRP2 control several but not all of the events regulated by Wnt5a and indicate that the function of sFRPs are highly context, cell type- and dose-dependent.
Implementation of sFRP1 and sFRP2 to the Generation of Midbrain DA Neurons from Stem Cells
The VM phenotype observed in the sFRP1−/−;sFRP2−/− mutant mice suggested that administration of relatively low concentrations of sFRP to ESCs could improve the generation of midbrain DA neurons. Indeed, we found that 10–100 ng/ml sFRP2 was able to increase the yield of TH+ DA neurons in primary and ESC cultures. Importantly, higher concentrations of sFRP1 became ineffective, but no adverse effects were detected. Instead, the effects of sFRP1 were achieved at varying concentrations, depending on the cells. While sFRP1 promoted DA neuron differentiation of ESCs at 10 ng/ml, it required 0.1–1 μg/ml to promote differentiation in primary cells. Moreover, high concentrations (1 μg/ml) had no effect on ESCs and 5 μg/ml impaired the survival or primary neurons. We thus suggest that sFRP2, because of its positive effects on DA differentiation at low concentrations and the lack of deleterious effects at high concentrations, may be a useful tool to promote the DA differentiation of ESCs in vitro. In addition, the negative effect of sFRP2 on neuritogenesis may be an additional advantage when preparing DA neurons for cell transplantation and cell replacement therapy for Parkinson’s disease, as cells with long processes are easily axotomized during cell preparation for transplantation and survive very poorly in vivo. We thus think that sFRP2 may be a particularly well-suited tool to promote the DA differentiation without the disadvantage of inducing excessive neuritogenesis, which could impair their survival during grafting. Future experiments will determine whether sFRP2 represents an advantage with respect to Wnt5a in terms of improving functional engraftment of stem cells in animal models of Parkinson’s disease.
Conclusion
In summary, our study shows that sFRP1 and sFRP2 activate Wnt/PCP/Rac1 signaling in midbrain DA neurons. We suggest that the low expression level of sFRP1, restricted to the VM midline domain, and the broad expression of sFRP2 are important for appropriate VM development. Deletion of sFRP1 and sFRP2 indicated that endogenous levels of sFRPs indicated that they are redundantly required for the regulation of several Wnt/PCP-dependent functions in the VM such as morphogenesis and DA differentiation. Moreover, low-medium levels of either sFRP1 or sFRP2 promoted the generation of DA neurons from DA progenitors as well as mESCs. Thus, our findings show that the coordinated expression and the fine tuning of sFRP1/2 levels is important for VM development and suggest that sFRP2, because of its stable effects, may find an application in the preparation of ESC-derived DA neurons for transplantation in Parkinson’s disease.
Supplementary Material
Acknowledgments
We thank Carmen Ramírez-Castillejo, Helena Mira, and Carmen Saltó, for support and assistance; Alessandra Nanni for secretarial help; Johan Glad for help with statistical analysis; Johnny Soderlund and Lottie Jansson-Sjöstrand for additional assistance. This work was supported by grants from the Swedish Research Council (DBRM, VR2008:2811 and 3287), European Union (Neurostemcell and Eurostemcell), Swedish Foundation for Strategic Research (INGVAR and DBRM), Norwegian Research Council, and Karolinska Institute, National Health and Medical Research Council (NHMRC), Australia. J.K. was supported by the Swedish National Neuroscience Network. J.C.V. was supported by a FEBS Long-Term Fellowship. V.B. was supported by EMBO Installation Grant and Ministry of Education, Youth and Sports of the Czech Republic (MSM0021622430). C.L.P was supported by an NHMRC Career development Fellowship, Australia. J.K. is currently affiliated with Ludwig Institute for Cancer Research Ltd., Stockholm, Sweden; E.R.A. is currently affiliated with Department of Cellular and Molecular Biology, Karolinska Institute, Stockholm, Sweden; S.B. is currently affiliated with Instituto de Biomedicina de Sevilla, Hospital Virgen del Rocio, Sevilla, Spain; V.B. is currently affiliated with Institute of Experimental Biology, Faculty of Science, Masaryk University, Brno, Czech Republic, and Institute of Biophysics, Brno, Czech Republic.
Footnotes
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Rattner A, Hsieh JC, Smallwood PM et al. A family of secreted proteins contains homology to the cysteine-rich ligand-binding domain of frizzled receptors. Proc Natl Acad Sci USA 1997;94: 2859–2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lopez-Rios J, Esteve P, Ruiz JM et al. The Netrin-related domain of Sfrp1 interacts with Wnt ligands and antagonizes their activity in the anterior neural plate. Neural Dev 2008;3:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uren A, Reichsman F, Anest V et al. Secreted frizzled-related protein-1 binds directly to Wingless and is a biphasic modulator of Wnt signaling. J Biol Chem 2000;275:4374–4382. [DOI] [PubMed] [Google Scholar]
- 4.van Amerongen R, Nusse R. Towards an integrated view of Wnt signaling in development. Development 2009;136:3205–3214. [DOI] [PubMed] [Google Scholar]
- 5.Ciani L, Salinas PC. WNTs in the vertebrate nervous system: From patterning to neuronal connectivity. Nat Rev Neurosci 2005;6: 351–362. [DOI] [PubMed] [Google Scholar]
- 6.Malaterre J, Ramsay RG, Mantamadiotis T. Wnt-Frizzled signalling and the many paths to neural development and adult brain homeostasis. Front Biosci 2007;12:492–506. [DOI] [PubMed] [Google Scholar]
- 7.Salinas PC, Zou Y. Wnt signaling in neural circuit assembly. Annu Rev Neurosci 2008;31:339–358. [DOI] [PubMed] [Google Scholar]
- 8.Rawal N, Castelo-Branco G, Sousa KM et al. Dynamic temporal and cell type-specific expression of Wnt signaling components in the developing midbrain. Exp Cell Res 2006;312:1626–1636. [DOI] [PubMed] [Google Scholar]
- 9.Dennis S, Aikawa M, Szeto W et al. A secreted frizzled related protein, FrzA, selectively associates with wnt-1 protein and regulates wnt-1 signaling. J Cell Sci 1999;112(pt 21):3815–3820. [DOI] [PubMed] [Google Scholar]
- 10.Leyns L, Bouwmeester T, Kim SH et al. Frzb-1 is a secreted antagonist of Wnt signaling expressed in the Spemann organizer. Cell 1997; 88:747–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin K, Wang S, Julius MA et al. The cysteine-rich frizzled domain of Frzb-1 is required and sufficient for modulation of Wnt signaling. Proc Natl Acad Sci USA 1997;94:11196–11200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang S, Krinks M, Lin K et al. Frzb, a secreted protein expressed in the Spemann organizer, binds and inhibits Wnt-8. Cell 1997;88: 757–766. [DOI] [PubMed] [Google Scholar]
- 13.Wang S, Krinks M, Moos M, Jr. Frzb-1, an antagonist of Wnt-1 and Wnt-8, does not block signaling by Wnts −3A, −5A, or −11. Biochem Biophys Res Commun 1997;236:502–504. [DOI] [PubMed] [Google Scholar]
- 14.Xu Q, D’Amore PA, Sokol SY. Functional and biochemical interactions of Wnts with FrzA, a secreted Wnt antagonist. Development 1998;125:4767–4776. [DOI] [PubMed] [Google Scholar]
- 15.Bafico A, Gazit A, Pramila T et al. Interaction of frizzled related protein (FRP) with Wnt ligands and the frizzled receptor suggests alternative mechanisms for FRP inhibition of Wnt signaling. J Biol Chem 1999;274:16180–16187. [DOI] [PubMed] [Google Scholar]
- 16.Melkonyan HS, Chang WC, Shapiro JP et al. SARPs: A family of secreted apoptosis-related proteins. Proc Natl Acad Sci USA 1997;94: 13636–13641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yokota T, Oritani K, Garrett KP et al. Soluble frizzled-related protein 1 is estrogen inducible in bone marrow stromal cells and suppresses the earliest events in lymphopoiesis. J Immunol 2008;181:6061–6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Satoh W, Gotoh T, Tsunematsu Y et al. Sfrp1 and Sfrp2 regulate anteroposterior axis elongation and somite segmentation during mouse embryogenesis. Development 2006;133:989–999. [DOI] [PubMed] [Google Scholar]
- 19.Satoh W, Matsuyama M, Takemura H et al. Sfrp1, Sfrp2, and Sfrp5 regulate the Wnt/beta-catenin and the planar cell polarity pathways during early trunk formation in mouse. Genesis 2008;46:92–103. [DOI] [PubMed] [Google Scholar]
- 20.Mii Y, Taira M. Secreted Frizzled-related proteins enhance the diffusion of Wnt ligands and expand their signalling range. Development 2009;136:4083–4088. [DOI] [PubMed] [Google Scholar]
- 21.McMahon AP, Bradley A. The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell 1990;62:1073–1085. [DOI] [PubMed] [Google Scholar]
- 22.Panhuysen M, Vogt Weisenhorn DM, Blanquet V et al. Effects of Wnt1 signaling on proliferation in the developing mid-/hindbrain region. Mol Cell Neurosci 2004;26:101–111. [DOI] [PubMed] [Google Scholar]
- 23.Prakash N, Brodski C, Naserke T et al. A Wnt1-regulated genetic network controls the identity and fate of midbrain-dopaminergic progenitors in vivo. Development 2006;133:89–98. [DOI] [PubMed] [Google Scholar]
- 24.Thomas KR, Capecchi MR. Targeted disruption of the murine int-1 proto-oncogene resulting in severe abnormalities in midbrain and cerebellar development. Nature 1990;346:847–850. [DOI] [PubMed] [Google Scholar]
- 25.Bryja V, Schulte G, Rawal N et al. Wnt-5a induces Dishevelled phosphorylation and dopaminergic differentiation via a CK1-dependent mechanism. J Cell Sci 2007;120:586–595. [DOI] [PubMed] [Google Scholar]
- 26.Castelo-Branco G, Wagner J, Rodriguez FJ et al. Differential regulation of midbrain dopaminergic neuron development by Wnt-1, Wnt-3a, and Wnt-5a. Proc Natl Acad Sci USA 2003;100:12747–12752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bryja V, Schambony A, Cajanek L et al. Beta-arrestin and casein kinase 1/2 define distinct branches of non-canonical WNT signalling pathways. EMBO Rep 2008;9:1244–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parish CL, Castelo-Branco G, Rawal N et al. Wnt5a-treated midbrain neural stem cells improve dopamine cell replacement therapy in parkinsonian mice. J Clin Invest 2008;118:149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andersson ER, Prakash N, Cajanek L et al. Wnt5a regulates ventral midbrain morphogenesis and the development of A9–A10 dopaminergic cells in vivo. PLoS One 2008;3:e3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inestrosa NC, Arenas E. Emerging roles of Wnts in the adult nervous system. Nat Rev Neurosci 2010;11:77–86. [DOI] [PubMed] [Google Scholar]
- 31.Wolf V, Endo Y, Rubin JS. Purification and Wnt-inhibitory activities of secreted frizzled-related proteins. Methods Mol Biol 2008;468: 31–4. [DOI] [PubMed] [Google Scholar]
- 32.Barberi T, Klivenyi P, Calingasan NY et al. Neural subtype specification of fertilization and nuclear transfer embryonic stem cells and application in parkinsonian mice. Nat Biotechnol 2003;21:1200–1207. [DOI] [PubMed] [Google Scholar]
- 33.Blakely BD, Bye CR, Fernando CV et al. Wnt5a regulates midbrain dopaminergic axon growth and guidance. PLoS One 2011;6:e18373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schulte G, Bryja V, Rawal N et al. Purified Wnt-5a increases differentiation of midbrain dopaminergic cells and dishevelled phosphorylation. J Neurochem 2005;92:1550–1553. [DOI] [PubMed] [Google Scholar]
- 35.Castelo-Branco G, Rawal N, Arenas E. GSK-3beta inhibition/beta-catenin stabilization in ventral midbrain precursors increases differentiation into dopamine neurons. J Cell Sci 2004;117:5731–5737. [DOI] [PubMed] [Google Scholar]
- 36.Bovolenta P, Esteve P, Ruiz JM et al. Beyond Wnt inhibition: New functions of secreted Frizzled-related proteins in development and disease. J Cell Sci 2008;121:737–746. [DOI] [PubMed] [Google Scholar]
- 37.Fenstermaker AG, Prasad AA, Bechara A et al. Wnt/planar cell polarity signaling controls the anterior-posterior organization of monoaminergic axons in the brainstem. J Neurosci 2010;30:16053–16064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kibar Z, Vogan KJ, Groulx N et al. Ltap, a mammalian homolog of Drosophila Strabismus/Van Gogh, is altered in the mouse neural tube mutant Loop-tail. Nat Genet 2001;28:251–255. [DOI] [PubMed] [Google Scholar]
- 39.Murdoch B, Chadwick K, Martin M et al. Wnt-5A augments repopulating capacity and primitive hematopoietic development of human blood stem cells in vivo. Proc Natl Acad Sci USA 2003;100:3422–3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murdoch JN, Henderson DJ, Doudney K et al. Disruption of scribble (Scrb1) causes severe neural tube defects in the circletail mouse. Hum Mol Genet 2003;12:87–98. [DOI] [PubMed] [Google Scholar]
- 41.Kele J, Simplicio N, Ferri AL et al. Neurogenin 2 is required for the development of ventral midbrain dopaminergic neurons. Development 2006;133:495–505. [DOI] [PubMed] [Google Scholar]
- 42.Hedlund E, Pruszak J, Lardaro T et al. Embryonic stem cell-derived Pitx3-enhanced green fluorescent protein midbrain dopamine neurons survive enrichment by fluorescence-activated cell sorting and function in an animal model of Parkinson’s disease. Stem Cells 2008;26:1526–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodriguez-Gomez JA, Lu JQ, Velasco I et al. Persistent dopamine functions of neurons derived from embryonic stem cells in a rodent model of Parkinson disease. Stem Cells 2007;25:918–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang M, Villaescusa JC, Luo SX et al. Interactions of Wnt/beta-cate-nin signaling and sonic hedgehog regulate the neurogenesis of ventral midbrain dopamine neurons. J Neurosci 2010;30:9280–9291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Esteve P, Bovolenta P. Secreted inducers in vertebrate eye development: More functions for old morphogens. Curr Opin Neurobiol 2006; 16:13–19. [DOI] [PubMed] [Google Scholar]
- 46.Esteve P, Sandonis A, Cardozo M et al. SFRPs act as negative modulators of ADAM10 to regulate retinal neurogenesis. Nat Neurosci 2011;14:562–569. [DOI] [PubMed] [Google Scholar]
- 47.Han X, Amar S. Secreted frizzled-related protein 1 (SFRP1) protects fibroblasts from ceramide-induced apoptosis. J Biol Chem 2004;279: 2832–2840. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Z, Deb A, Pachori A et al. Secreted frizzled related protein 2 protects cells from apoptosis by blocking the effect of canonical Wnt3a. J Mol Cell Cardiol 2009;46:370–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ellies DL, Church V, Francis-West P et al. The WNT antagonist cSFRP2 modulates programmed cell death in the developing hindbrain. Development 2000;127:5285–5295. [DOI] [PubMed] [Google Scholar]
- 50.Joksimovic M, Yun BA, Kittappa R et al. Wnt antagonism of Shh facilitates midbrain floor plate neurogenesis. Nat Neurosci 2009;12: 125–131. [DOI] [PubMed] [Google Scholar]
- 51.Chung S, Hedlund E, Hwang M et al. The homeodomain transcription factor Pitx3 facilitates differentiation of mouse embryonic stem cells into AHD2-expressing dopaminergic neurons. Mol Cell Neurosci 2005;28:241–252. [DOI] [PubMed] [Google Scholar]
- 52.Nunes I, Tovmasian LT, Silva RM et al. Pitx3 is required for development of substantia nigra dopaminergic neurons. Proc Natl Acad Sci USA 2003;100:4245–4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van den Munckhof P, Luk KC, Ste-Marie L et al. Pitx3 is required for motor activity and for survival of a subset of midbrain dopaminergic neurons. Development 2003;130:2535–2542. [DOI] [PubMed] [Google Scholar]
- 54.Lonardo E, Parish CL, Ponticelli S et al. A small synthetic cripto blocking peptide improves neural induction, dopaminergic differentiation, and functional integration of mouse embryonic stem cells in a rat model of Parkinson’s disease. Stem Cells 2010;28:1326–1337. [DOI] [PubMed] [Google Scholar]
- 55.Heikkila M, Peltoketo H, Vainio S. Wnts and the female reproductive system. J Exp Zool 2001;290:616–623. [DOI] [PubMed] [Google Scholar]
- 56.Warr N, Siggers P, Bogani D et al. Sfrp1 and Sfrp2 are required for normal male sexual development in mice. Dev Biol 2009;326: 273–284. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


