Short abstract
Background
High doses of capsaicin are recommended for the treatment of neuropathic pain. However, low doses evoke mechanical hypersensitivity. Activation of the capsaicin chemosensor transient receptor potential vanilloid 1 (TRPV1) induces neurogenic inflammation. In addition to the release of pro-inflammatory mediators, reactive oxygen species are produced. These highly reactive molecules generate oxidised phospholipids and 4-hydroxynonenal (4-HNE) which then directly activate TRP ankyrin 1 (TRPA1). The apolipoprotein A-I mimetic peptide D-4F neutralises oxidised phospholipids. Here, we asked whether D-4F ameliorates neurogenic hypersensitivity in rodents by targeting reactive oxygen species and 4-HNE in the capsaicin-evoked pain model.
Results
Co-application of D-4F ameliorated capsaicin-induced mechanical hypersensitivity and allodynia as well as persistent heat hypersensitivity measured by Randell–Selitto, von Frey and Hargreaves test, respectively. In addition, mechanical hypersensitivity was blocked after co-injection of D-4F with the reactive oxygen species analogue H2O2 or 4-HNE. In vitro studies on dorsal root ganglion neurons and stably transfected cell lines revealed a TRPA1-dependent inhibition of the calcium influx when agonists were pre-incubated with D-4F. The capsaicin-induced calcium influx in TRPV1-expressing cell lines and dorsal root ganglion neurons sustained in the presence of D-4F.
Conclusions
D-4F is a promising compound to ameliorate TRPA1-dependent hypersensitivity during neurogenic inflammation.
Keywords: TRPA1, capsaicin, reactive oxygen species, oxidised lipids, pain, targeting
Introduction
To reduce severe side effects evoked by standard non-steroidal anti-inflammatory drugs and opioids, several novel pain killers have been tested in clinical trials. Within the last years, the transient receptor potential vanilloid type 1 (TRPV1) became one of the most extensively investigated targets. In addition to the invention of small molecule TRPV1 channel blockers, capsaicin, the hot chilli pepper extract, has been launched as a treatment option. A high-dose capsaicin-containing patch has been approved as a second-line therapy for patients with various types of neuropathic pain.1 Capsaicin, the most specific exogenous TRPV1 agonist, evokes strong heat sensation upon receptor activation. In addition, capsaicin has also been used to experimentally study pain in humans and rodents. Recently, the chemical has been discussed as a model substance for the study of ongoing neuropathic pain since low doses of capsaicin evoke long-lasting mechanical hyperalgesia due to neurogenic inflammation.2
During inflammation, endogenous mediators such as prostaglandins, bradykinin, reactive oxygen species (ROS) like hydrogen peroxide (H2O2) as well as hydroxyl radicals and downstream products are released. While bradykinin and prostaglandins indirectly modulate TRP channel responses, ROS itself and its products are direct, unselective TRP agonists that are crucial for the inflammatory response and a feedback loop promoting neurogenic inflammation.2,3 Various intermediates of ROS have strong oxidising capabilities that lead to the production of endogenous oxidation-specific epitopes, oxidised phospholipids (OxPL), and 4-hydroxynonenal (4-HNE), a smaller, more stable but highly reactive end product of lipid peroxidation.4,5 TRPA1, the ankyrin type 1 TRP homologue of TRPV1, is activated by ROS and its downstream product 4-HNE via Michael addition.6 While direct activation of TRPV1 by H2O2 is controversial, it has been proven that H2O2 directly activates TRPA1 by covalent modification of the cysteine residues located in the N-terminus of the TRPA1, crucial for the channel activation.6–8
D-4F is a peptide sequence of the apolipoprotein A-I (ApoA-I), the most important apolipoprotein of high-density lipoproteins (HDLs). Evidence on apolipoproteins being involved in pain modulation recently increased.9,10 We and others showed that apolipoprotein A-I (ApoA-I) as well as its structural mimetic peptide D-4F prevent inflammatory pain.5,11 We revealed that D-4F prevents TRPA1 activation evoked by endogenously occurring OxPL that are generated during inflammation by ROS but not by the exogenous TRPA1 agonist mustard oil (MO).5,12 Furthermore, D-4F has been used as an experimental therapeutic compound for the treatment of other diseases with inflammatory components by directly binding to OxPL, e.g. occurring in atherosclerotic plaques and pro-inflammatory reactions.13–15
In this study, the efficacy of D-4F is tested in a rodent model of neurogenic inflammation, induced by capsaicin, evoking hypersensitivity and TRP channel activity. We found that D-4F ameliorated capsaicin-induced mechanical but not TRPV1-typical thermal hypersensitivity. Therefore, we decipher effects on downstream mediators of capsaicin like H2O2 and 4-HNE that directly activate the mechanosensor TRPA1.
Material and methods
Materials
The materials used were supplied as follows: MO, H2O2, capsaicin, DMSO (Sigma-Aldrich, Taufkirchen, Germany), 4-HNE (Alpha Diagnostic International, San Antonio, TX), D-4F (custom made, Peptide Specialty Laboratories GmbH, Heidelberg, Germany), and Fura-2/AM (Life Technologies, Darmstadt, Germany).
Animals
The animal care and experimental protocols were approved by the Government of Lower Franconia (protocol number 61/11). Experimental protocols were in accordance with the international guidelines for the care and use of laboratory animals (EU Directive 2010/63/EU for animal experiments) and Animal Research: Reporting of In Vivo Experiments (ARRIVE) criteria.
To compare the data obtained in this study with our data from previous publications, we continued using rats for behavioural studies and mouse tissue for in vitro experiments.5,12 In line with the literature, no species differences regarding TRPA1 activation in rodents have been expected.16
Male Wistar rats (200–300 g; 7–9 weeks old, Janvier Labs, Le Genest-Saint-Isle, France) were accommodated on dry litter in groups of six with free access to food and water under standard conditions (12:12 h light/dark cycle, 21°C–25°C, 45%–55% humidity). Experiments were performed during daytime. In score sheets, criteria for discontinuation of the experiments and humane end points were evaluated and observed. No animals had to be euthanised beforehand.
Male C57BL/6 J mice at the age of 8 to 10 weeks weighing about 20 g were taken from the animal breeding facility of the Institute for Clinical Neurobiology, University Hospital of Würzburg, Germany, for dorsal root ganglia (DRGs) preparations. Siblings were kept in one cage on dry litter with housings, paper towels as well as access to dry SNIFF pellets and water ad libitum. The 12:12 h day–night cycle was maintained. At the end of the experiments, all animals were killed with CO2.
Experiments were grouped by agonist. All rats needed for one trial were ordered on the same day at the age of six weeks and 180 g. Before handling, rats were numbered by chance. Numbers were randomly allocated to group ‘D-4F’ and group ‘vehicle’. The order of injections as well as the order of threshold measurements remained constant throughout the experiment. The experimenter was blinded to treatments but performed all intraplantar injections of pronociceptive mediators and measurement of pain behaviour.
Capsaicin (245 nmol; 1% DMSO), H2O2 (19.2 µmol), 4-HNE (6 µmol) and D-4F (2.5 mg/kg) were diluted in saline. The chosen doses matched with our earlier studies and were used by others as well.5,12,13,17–21 Previously, we demonstrated that the injection of isotonic saline does not elicit hyperalgesia, infiltration with leukocytes or increased production of proalgesic mediators like prostaglandins or cytokines.22
Behavioural testing
Nociceptive withdrawals are fast reflexive retractions of the hind paw in response to nociceptive sensory input that are provoked by either mechanical or thermal stimuli often referring to a prolonged hypersensitivity. The taxonomy suggested by the International Association for the Study of Pain was used.
Nociceptive reflexive measurements: Pungent reagents were injected at time point 0 under isoflurane anaesthesia. D-4F or saline were applied after a 5-min interval. The total volume of each injection was 100 µL. A paw pressure algesiometer (modified Randall-Selitto test; Ugo Basile, Comerio, Italy) and von Frey filaments to test mechanical reflexive nociceptive thresholds as well as a Hargreaves’ apparatus (IITC Life Science Inc., Woodland Hills, CA) to measure thermal reflexive nociceptive thresholds were used. Mechanical and thermal thresholds were measured before injection (time point 0 (= baseline)) as well as 1, 3 and 6 h after the injection.
Calcium imaging and DRG preparation
Fura-2-based calcium imaging on primary adult DRG neuron cultures from C57BL/6 male mice and HEK-293 cells stably transfected with either recombinant human TRPA1 or rat TRPV1 were conducted as reported before.5 In brief, cells were loaded with fura-2/AM before time-lapse image series were acquired at intervals of 2 s, with an excitation of 340/380 nm and emission at 512 nm. To maintain the comparability of our studies, the concentrations of capsaicin (1 µM), H2O2 (10 µM), 4-HNE (30 µM), MO (10 µM), KCL (45 mM) as well as D-4F (1 and 10 mg/ml) used here matched those from our earlier studies.5,12 As before D-4F, dissolved in calcium imaging buffer containing 134 mM NaCl, 6 mM KCl, 2 mM MgCl2, 1 mM CaCl2, 10 mM Hepes and 5 mM glucose (pH 7.4), was either pre-incubated for 30 min with the cells at 37°C, 5% CO2 in the incubator or with the agonists at room temperature. Calcium imaging data were processed before the statistical analysis of the number of reacting cells and the area under curve (AUC). The mean of basal fluorescence in each experiment was determined. By defining ‘reacting cells’ as cells with a 1.5-fold increase in mean basal fluorescent intensity after stimulation, the number of reacting cells (%) was calculated. The AUC was calculated of the mean for DRGs from 270 to 420 s and 740 to 860 s, as indicated in the figure legend, starting 150 s after the application time point of the stimulus of interest to exclude onset differences, and in HEK-293 cell experiments from 120 to 390 s. As lack of responsiveness could not be differentiated from lack of transfection non-responding HEK-293 cells were not excluded from the results. All data are presented as individual traces of each cell plus mean indicated by grey and black lines, respectively.
Statistical analysis
The data and statistical analysis obey with the recommendations on experimental design and analysis in pharmacology.23
Prism 7 for Mac OS X (GraphPad Software, San Diego, CA) or SigmaPlot 14 (Systat Software GmbH, Erkrath, Germany) were used for the analysis of the in vitro and in vivo data, respectively. Regardless the outcome of the normality test, according to Shapiro–Wilk, repeated measurement ANOVA was performed as recommended before.24–26 The minimum number of the animals to reach statistical significance was used corresponding to a priori power analysis performed with G*power based on the means and standard deviations of our previous studies including either the agonist or D-4F.27 Data were taken from maximum effects regularly reached after 3 h. Overall, power analysis revealed that often N about 3 would be sufficient. To perform statistical analyses, N numbers were normally increased to N = 6. The effect of D-4F on capsaicin was already significant with low animal numbers, and solvent controls did not differ from our previous data.12 Therefore, an artificial increase in animal numbers to justify the results was not regarded to be necessary and in accordance with the aim to reduce animal numbers.
In behavioural experiments, a two-way repeated measurement ANOVA followed by Holm–Sidak post hoc multiple comparison was used. Values given in the results section are the P-values for interaction. Stars in the figures indicate a significant difference (P > 0.05) between the treatment groups at indicated time points. Data are shown as mean ± S.E.M. Even though only four rats were used in the capsaicin control group, data differ significantly from the treatment group. In our previous study, more vehicle control data are published.12 Including these data, the statistics provided would strengthen the results. However, we omit to re-use our published data since the results presented here differ significantly already.
In vitro duplicated experiments of 100 cells per coverslip (pseudo-replications) were counted as a single experiment. The means were considered as genuine replications and were evaluated by the Mann–Whitney U test or a one-way ANOVA, post hoc Holm–Sidak, respectively. Differences were considered as significant when P < 0.05. The Holm–Sidak post hoc comparison was chosen because of the higher power in opposite to the Bonferroni’s test. Analyses of in vitro data are shown as scatter plots for the AUC, whereas the number of responding cells is presented as median with interquartile range.
Results
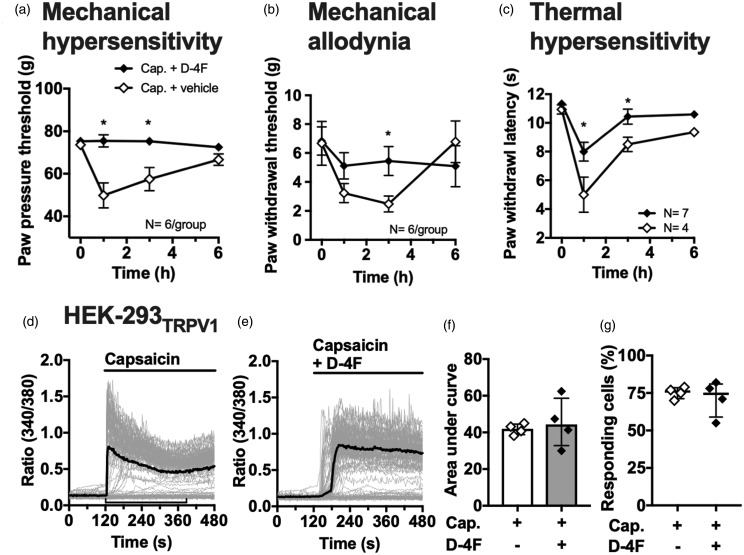
Subcutaneous injection of capsaicin induced transient mechanical and thermal nocifensive behaviour in rats for up to 6 h (Figure 1(a) to (c)). Additional local injection of D-4F, after a 5-min interval, significantly prevented capsaicin-induced mechanical allodynia as well as mechanical and thermal hypersensitivity at indicated time points (Figure 1(a): F(3, 15) = 9.1, P(treatment × time) = 0.001; (b): F(3, 15) = 8.413, P(treatment × time) = 0.0016; (c): F(3, 27) = 2.3; P(treatment × time) = 0.099). While the onset of mechanical hypersensitivity upon local capsaicin injection was prompt, maximum mechanical allodynia was only measured 3 h after capsaicin application indicating that sensitisation of other mechanisms rather than direct TRPV1 activation is involved. D-4F ameliorated capsaicin-evoked thermal hypersensitivity. To decipher a direct inhibition of TRPV1 by D-4F upon capsaicin stimulation, Fura-2-based in vitro calcium imaging experiments on HEK-293 cells stably transfected with TRPV1 were performed (Figure 1(d) to (g)). The robust calcium influx via TRPV1 upon capsaicin stimulation remained unaffected by co-applicated D-4F as well as the TRPV1 desensitisation. However, analyses reveal an increased variability of the AUC when cells were co-stimulated with capsaicin and D-4F. Statistical analysis of the AUC as well as the number of responding cells revealed equal numbers (Figure 1(f): N = 4, P(AUC) = 0.69, (g): N = 4, P(RC) > 0.99).
Figure 1.
D-4F inhibits capsaicin-induced mechanical and thermal hyperalgesia independent of TRPV1 activation. (a–c) Measurements of mechanical paw pressure threshold by Randall–Selitto and paw withdrawal thresholds by von Frey test as well as thermal paw withdraw latency by Hargreaves test before (0) and 1, 3, and 6 h after intraplantar injection of capsaicin (245 nM) plus subsequent application of D-4F (2.5 mg/kg i.p.) or vehicle in male Wistar rats. Filled symbols indicate D-4F; open symbols for vehicle treatment groups. N as indicated in the graphs. Two-way repeated-measurement ANOVA, post hoc Holm–Sidak; *P < 0.05 highlighting significant differences between treatments at indicated time points. (d and e) Representative traces of calcium transients in Fura-2-based calcium imaging experiments on HEK-293TRPV1 cells upon stimulation with capsaicin alone (1 µM) or a pre-incubated mixture with D-4F (100 μg/ml; 30 min). Grey lines: individual cells, black line: mean; indicator line in (d) highlights interval chosen for AUC calculations. (f and g) Statistical analysis of the area under curve (interval: 120–390 s) and the number of responding cells upon stimulation. Scatter dot plots including median with interquartile ranges. Filled symbols indicate D-4F application; open symbols for vehicle treatment groups. N = 4 independent experiments of averaged duplicates. Mann–Whitney U test. *P < 0.05. 4-HNE: 4-hydroxynonenal; H2O2: hydrogen peroxide; D-4F: D-amino-4-phenylalanine mimetic peptide of apolipoprotein A-I.
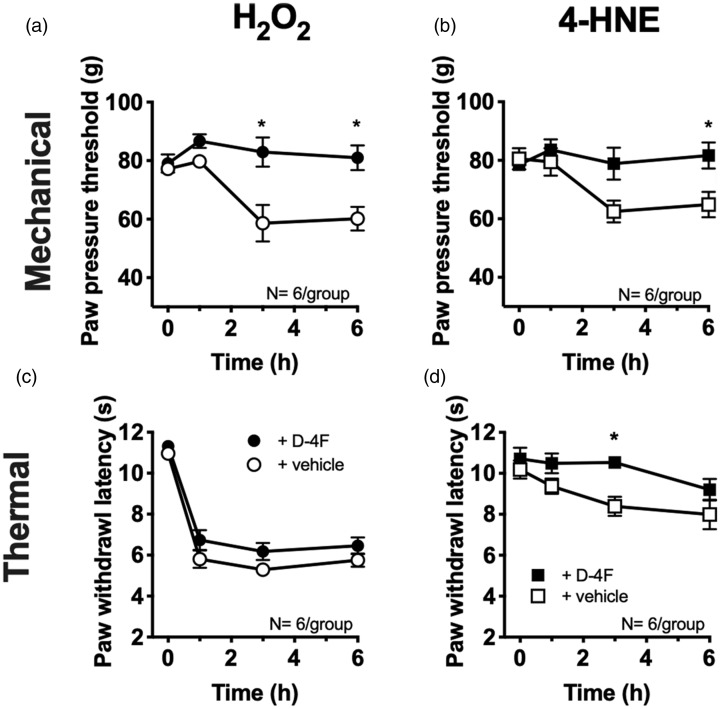
To dissect the mode of action of D-4F in capsaicin-induced hypersensitivity, the effect of D-4F on mechanical and thermal hypersensitivity after local injection of the ROS source H2O2 and the downstream lipid peroxidation product 4-HNE, both non-selective TRP channel agonists, were measured (Figure 2). After application of either of them, mechanical hypersensitivity was completely abolished by D-4F as revealed by statistical analysis comparing effects over time with baseline (0 h). In H2O2-treated rats, D-4F significantly reduced mechanical hypersensitivity (Figure 2(a) (H2O2): F(3, 15) = 5.21; p = 0.01). Despite an overall significant interaction in 4-HNE-injected animals, a significant difference between treatment groups was only obtained after 6 h (Figure 2(b) (4-HNE): F(3, 15) = 4.2; p = 0.02). While H2O2-evoked thermal hypersensitivity remained unaffected, 4-HNE-induced thermal hypersensitivity, although not pronounced, was prevented by D-4F for about 3 h (Figure 2(c) (H2O2): F(3, 30) = 0.52; p = 0.67, (d) (4-HNE): F(3, 15) = 1.48; p = 0.26).
Figure 2.
D-4F abolishes mechanical hypersensitivity evoked by a ROS source and 4-HNE. (a–d) Measurement of mechanical and thermal hypersensitivity at indicated time points in rats after local injection of the ROS equivalent H2O2 (a and b; 19.2 µM) or 4-HNE (c and d; 6 µM) plus D-4F (2.5 mg/kg i.p.) or saline. Filled symbols indicate D-4F treatment; open symbols for vehicle groups. N = 6/group. Two-way repeated-measurement ANOVA, post hoc Holm–Sidak; *P < 0.05 highlighting significant differences between treatments at indicated time points. 4-HNE: 4-hydroxynonenal; H2O2: hydrogen peroxide; D-4F: D-amino-4-phenylalanine mimetic peptide of apolipoprotein A-I.
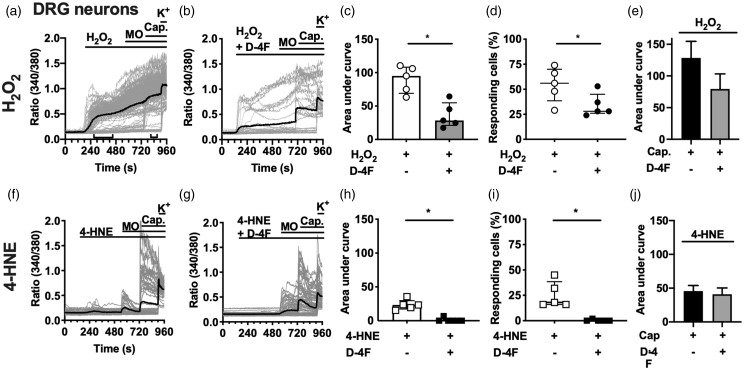
Calcium imaging experiments on primary cultured DRG neurons from adult C57BL/6 mice revealed a significant reduction in calcium influx upon stimulation of the cells with a mixture of D-4F and H2O2 compared to H2O2 alone (Figure 3(a) to (d)). Mustard oil (MO), capsaicin, and potassium served as positive controls to indicate neuronal populations. The subsequent application of MO, the main exogenous TRPA1 agonist, failed to potentiate the H2O2-evoked calcium influx, indicating the involvement of TRPA1 on DRG neurons. Recently, we showed that TRPA1 activation by MO remained statistically unchanged after adding D-4F.5 Statistical analyses of the AUC and the number of responding cells upon stimulation with H2O2 and D-4F were significantly different, whereas capsaicin failed to alter the AUC (Figure 3(c): P(AUC) = 0.03 and (d): P(RC) = 0.032; (e): P(AUC(Cap.) = 0.56). The non-significantly decreased responsiveness of DRG neurons to capsaicin after H2O2 stimulation might be explained by the calcium kinetics revealed before capsaicin application. The calcium influx in DRG neurons evoked by the endogenous TRPA1 agonist 4-HNE was also prevented by the application of a mixture with D-4F (Figure 3(e) to (h); (g): P(AUC) = 0.004 and (h): P(RC) = 0.008). Since 4-HNE-evoked transients, due to its potency, were only temporarily, lasting for about 4.5 min, further stimulation of TRPA1-positive cells by MO was possible. The capsaicin-elicited additional calcium influx remained unchanged after D-4F or vehicle treatment (Figure 3(j): P(AUC(4-HNE) = 0.76).
Figure 3.
D-4F reduces calcium influx elicited by ROS or 4-HNE in DRG neurons. (a, b, f, and g) Representative measurements of calcium imaging experiments on cultured DRG neurons upon stimulation with H2O2 (a; 10 µM) or 4-HNE (e; 30 µM) alone or mixed with D-4F (100 µg/ml; b and f). Mustard oil (10 µM) and capsaicin (1 µM) served as internal control for corresponding TRP channel activation; potassium to verify neuron activation. Grey lines: individual cells, black line: mean; indicator lines in (a) highlight interval chosen for AUC calculations. (c–e, h–j) Statistical analysis of the area under curve and the number of responding cells (interval 4-HNE or H2O2: 120–540 s and capsaicin: 740–860 s). Filled symbols and grey bars indicate D-4F experiments; open symbols and black bars for agonists only. N = 5 independent experiments of averaged duplicates. Mann–Whitney U test. *P < 0.05. 4-HNE: 4-hydroxynonenal; H2O2: hydrogen peroxide; D-4F: D-amino-4-phenylalanine mimetic peptide of apolipoprotein A-I; DRG: dorsal root ganglion; MO: mustard oil.
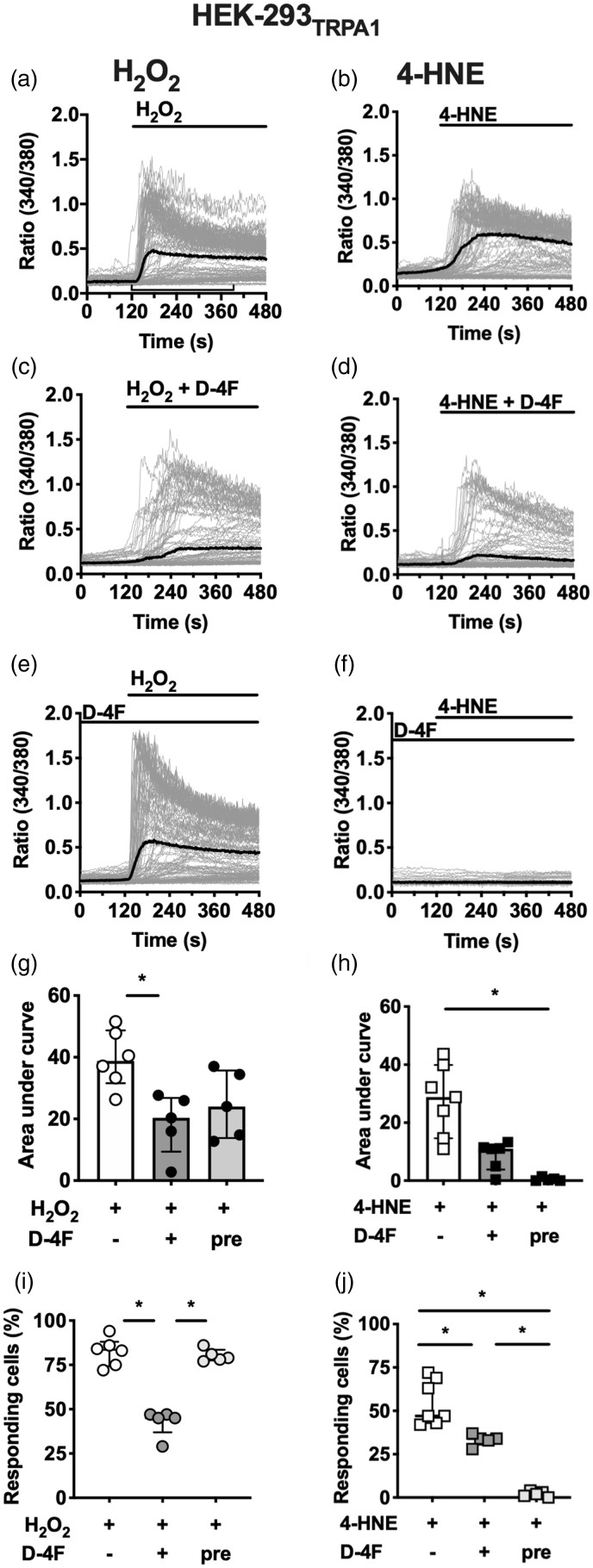
In DRG neurons, multiple co-expressed ion channels such as TRPA1 and TRPV are sensitised upon neurogenic inflammation. To further investigate the TRPA1 dependency of D-4F-mediated inhibitory effects, the compound was tested in in vitro calcium imaging experiments on TRPA1 stably expressed in HEK-293. D-4F does not prevent MO-evoked TRPA1 activation.5 Interestingly, the inhibitory potential of D-4F upon stimulation of TRPA1 with H2O2 and 4-HNE was dependent on the time-point of application, revealing different modes of action. H2O2 and 4-HNE evoked a robust TRPA1-mediated calcium influx after application of the drugs to the bath solution (Figure 4(a) and (b)). The application of a mixture of D-4F with either substance, H2O2 or 4-HNE, diminished the TRPA1 channel activation (Figure 4(c), (d), (g) to (j)). Even though the AUC was not substantially reduced after co-stimulation with 4-HNE, the number of responding cells differed significantly. When the HEK-293TRPA1 cells themselves were pre-incubated with D-4F, the AUC was non-significantly reduced (Figure 4(g): F(2, 13) = 6.35; p = 0.01) and the number of responding cells remained unchanged in comparison to the control (Figure 4(i): F(2, 15) = 17.8; P = 0.0001). On the contrary, pre-treatment of the cells with D-4F completely abolished the 4-HNE-evoked TRPA1 activation (Figure 4(f)). None of the TRPA1 channels were activated (Figure 4(i): F (2, 13) = 56.2, P < 0.0001, (j): F(2, 14) = 54.4; P < 0.0001).
Figure 4.
D-4F modulates TRPA1-mediated calcium influx in HEK-293 cells evoked by ROS and 4-HNE. (a–f) Representative calcium traces from HEK-293TRPA1 in Fura-2 experiments after stimulation with H2O2 (a) or 4-HNE (b) alone, each pre-incubated with D-4F (c and d), or pre-incubated cells in D-4F (e and f; 10 µg/ml) before application of H2O2 and 4-HNE, respectively. Grey lines: individual cells, black line: mean; indicator lines in (a) highlight interval chosen for AUC calculations. (g–j), Statistical analysis of the area under curve and the number of responding cells during a 4-min interval (120–390 s). N = 5 (H2O2) or 7 (4-HNE) independent experiments of averaged duplicates. One-way ANOVA, post hoc Holm–Sidak. *P < 0.05. 4-HNE: 4-hydroxynonenal; H2O2: hydrogen peroxide; D-4F: D-amino-4-phenylalanine mimetic peptide of apolipoprotein A-I.
Discussion
In this study, we provide evidence that D-4F prevents reflexive nocifensive behaviour in a model of neurogenic inflammation. Besides its well-known function as a very potent acute TRPV1 agonist, capsaicin leads to ROS generation.28 ROS such as H2O2 directly stimulate different TRP receptors by disulphide bond formation, e.g. via direct binding to cysteine and lysin residues of TRPA1.29 Furthermore, ROS, a source of highly reactive hydroxyl radicals, induces the formation of OxPL in vivo.12,30,31 Here, we show that D-4F averted H2O2-elicited nociceptive behaviour upon reflexive mechanical testing. On DRG neurons, only a partial blockade of the H2O2-evoked calcium influx has been conceived. It is well known that DRG neurons express a variety of TRP channels such as the co-expressed TRPV1 and TRPA1 but also TRPC5 and TRPM2. All are known as redox sensors.29 Pre-incubation of D-4F with H2O2 prior to HEK-293TRPA1 stimulation prevented calcium influx suggesting a direct interaction of the two compounds, most likely via binding to lysine residues of D-4F. In contrast, pre-incubation of the HEK-293TRPA1 with D-4F before H2O2 application failed to significantly inhibit the calcium influx. Due to its highly reactive amino acid residues, it is most likely that H2O2 has a higher affinity to TRPA1 than to D-4F. Hence, the results suggest that the inhibitory effect of D-4F cannot only be explained by a scavenging mechanism.
During ROS exposure, lipids of the plasma membrane are oxidised resulting in an increase of OxPL species. D-4F scavenges OxPL by binding to these species with distinctive affinities.15 Previously, we have shown that components of OxPAPC such as PGPC and POVPC also activate TRPV1; however, higher concentrations are needed than for the stimulation of TRPA1.5 Even though D-4F blocks TRPV1-mediated thermal hypersensitivity, different affinities and dose effects of OxPL on proteins like TRP channels and D-4F peptide indicate an incomplete, more selective scavenging mechanism.
One of the downstream products of lipid peroxidation is 4-HNE that itself induces the generation of E06-reactive OxPLs after local paw injections.12 The pre-incubation of D-4F with 4-HNE diminished the TRPA1 activation in accordance to the results obtained after H2O2 stimulation. Similar to H2O2, 4-HNE is also known to interact with histidine, lysine and cysteine residues. 4-HNE covalently binds via Michael addition or Schiff base formation. Studies with the whole ApoA-I molecule revealed a direct interaction of 4-HNE with histidine residues.32 Since the ApoA-I mimetic peptide D-4F lacks histidine residues, 4-HNE could possibly bind to the lysine residues in D-4F and consecutively being inactivated.33 Our data show convincingly a D-4F-mediated inhibition of 4-HNE-evoked nocifensive behaviour. In line, 4-HNE production was decreased after D-4F treatment in murine asthma model.34 Both extracellular and intracellular cysteine residues of TRPA1 as well as its histidine residues are likely to be subject to the modification of direct 4-HNE-evoked TRPA1 activation and enhancement of TRPV1 stimulation.8,35–37 We hypothesise that D-4F might directly interfere with the abovementioned amino acid residues and therefore competitively block the channel opening. On the contrary, D-4F failed to inhibit the activation of TRPA1 by its main exogenous agonist MO; thus, different binding sites of TRPA1 agonists and antagonists must be considered.5 Furthermore, pre-incubation of HEK-293TRPA1 completely prevented the 4-HNE-elicited activation of TRPA1. Taken together, the data presented here highlight the presence of a supplementary, different mechanism.
Synergies of TRPA1 and TRPV1 have been well demonstrated before. Capsaicin-induced TRPV1 activation modulates TRPA1 sensitivity and vice versa, partially by the formation of heterodimers.38–41 Stimulation of either of the TRP channels enables neurogenic inflammation-typical neurotransmitter release of calcitonin gene-related peptide (CGRP) and substance P. The close connection of TRPA1 and TRPV1 complicates to decipher whether D-4F-mediated analgesia underlies attenuation of neurogenic inflammation or neuronal hyperexcitability since either perpetuates the other. We suggest that neurogenic inflammation, inducing an increase of products such as ROS and OxPL, is necessary for the efficacy of D-4F in evoked mechanical hyperalgesia and allodynia. Our study lacks of evidence that D-4F directly modulates TRPV1. A decreased TRPV1-mediated CRGP release from DRG neurons upon capsaicin stimulation in the presence of D-4F is unexpected. Previously, we showed that oxidised lipid-evoked CRGP release from mice hind paw skin is not completely abolished in TRPA1 ko, TRPV1 ko or double-ko mice.5 This strengthens the idea that neurogenic inflammation is a complex process not only facilitated by TRPV1 and blocking only one component would not be sufficient to completely interrupt the CGRP release and subsequent cascades. In addition, considering the abovementioned mechanisms of D-4F interfering with ROS and its products, it is less likely that D-4F-mediated TRPA1 inhibition is due to TRPV1-evoked desensitisation rather than D-4F-mediated scavenging of TRPA1 agonists.
The in vivo mode of action of D-4F must be more complex. ROS induce an upregulation of pro-inflammatory cytokines that lead to a sensitisation of TRP channels.42 D-4F reduces the release of pro-inflammatory cytokines and is known to alter cholesterol levels of cellular membranes by the formation of new HDL molecules.13,15 It also changes lipid rafts, another suggested mode of action. While depletion of cholesterol from membranes facilitates hypersensitivity, changes of the lipid composition of cellular membranes also modulate TRP channel activation.43,44 As demonstrated in this study and before, D-4F predominantly inhibits mechanical hyperalgesia.5 It is likely that other mechanoreceptors might be involved in the efficacy of D-4F.45,46
In addition, an effect of D-4F on other target cells is possible. The release of CGRP induces degranulation of mast cells.47 Also, direct capsaicin-evoked activation of TRPV1 on mast cells is possible emphasising mast cells as a key cell of the mode of action of D-4F.48 These local immune cells but also TRPV1-positive DRG neurons express the ubiquitous toll-like receptor 4 (TLR-4).49,50 So far, it was thought that 4-HNE supresses TLR-4 receptor activation, but recently, it has been shown that 4-HNE also activates TLR-4.46 TLR-4 activation sensitises TRPV1.51 Furthermore, the neurotransmitter substance P and CGRP modulate TLR expression and signalling pathways.52,53 There is evidence that ApoA-I supresses the TLR-4 signalling cascade by alternating the TLR-4 membrane integrity into lipid rafts.54,55 This is in line with the data that TLR-4 interacts with ApoA-I binding protein, recently shown in a pain model.11
Oral D-4F is safe and well tolerated by high-risk cardiovascular patients. The daily application of single doses of D-4F reduced their HDL-inflammatory index.14 In this study as well as previously, a single dose of D-4F injected locally lasted 6 h to inhibit inflammatory and capsaicin-induced pain.5 For clinical use, a longer duration of action would be necessary. Enhanced bioavailability and stability of D-4F could improve the pharmacokinetics on the road to a promising pain killer.
In summary, mechanical hyperalgesia, evoked by low-dose capsaicin, could be explained by the generation of local ROS and downstream components mainly activating the mechanosensor TRPA1. Co-application of D-4F might be a complimentary approach to ameliorate pain evoked by neurogenic inflammation while using the capsaicin patch for pain treatment.
Acknowledgments
The authors thank the IZKF for funding (N-261 to BO and HLR; Z2/CSP-2 to BO). The authors gratefully thank Prof. Michael Sendtner, MD, and Robert Blum, PhD, Institute of Clinical Neurobiology, University Hospital of Würzburg, Germany, for the availability of the cell culture and the calcium imaging equipment.
Author Contributions
BO conceptualised the in vitro part of the project, analysed the data and wrote the manuscript. Together with JK, calcium imaging experiments were conducted. MM and ABK performed the behavioural tests and analysed the data. HLR designed the project and perused the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Department of Anaesthesiology, University Hospital of Würzburg, by the Interdisciplinary Centre for Clinical Research (IZKF) of the Medical Faculty of the University of Würzburg, Germany (N-261 to BO and HR; Z2/CSP-2 to BO) and by the Open Access Publication Fund of the University of Würzburg.
ORCID iD
Beatrice Oehler https://orcid.org/0000-0003-3150-2102
References
- 1.Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, Gilron I, Haanpää M, Hansson P, Jensen TS, Kamerman PR, Lund K, Moore A, Raja SN, Rice ASC, Rowbotham M, Sena E, Siddall P, Smith BH, Wallace M. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 2015; 14: 162–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gouin O, L’Herondelle K, Lebonvallet N, Le Gall-Ianotto C, Sakka M, Buhé V, Plée-Gautier E, Carré J-L, Lefeuvre L, Misery L, Le Garrec R. TRPV1 and TRPA1 in cutaneous neurogenic and chronic inflammation: pro-inflammatory response induced by their activation and their sensitization. Protein Cell 2017; 8: 644–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Binder CJ, Papac-Milicevic N, Witztum JL. Innate sensing of oxidation-specific epitopes in health and disease. Nat Rev Immunol 2016; 16: 485–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sisignano M, Angioni C, Ferreiros N, Schuh C-D, Suo J, Schreiber Y, Dawes JM, Antunes-Martins A, Bennett DLH, McMahon SB, Geisslinger G, Scholich K. Synthesis of lipid mediators during UVB-induced inflammatory hyperalgesia in rats and mice. PLoS One 2013; 8: e81228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oehler B, Kistner K, Martin C, Schiller J, Mayer R, Mohammadi M, Sauer R-S, Filipovic MR, Nieto FR, Kloka J, Pflücke D, Hill K, Schaefer M, Malcangio M, Reeh PW, Brack A, Blum R, Rittner HL. Inflammatory pain control by blocking oxidized phospholipid-mediated TRP channel activation. Sci Rep 2017; 7: 5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andersson DA, Gentry C, Moss S, Bevan S. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J Neurosci 2008; 28: 2485–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trevisan G, Benemei S, Materazzi S, De Logu F, De Siena G, Fusi C, Fortes Rossato M, Coppi E, Marone IM, Ferreira J, Geppetti P, Nassini R. TRPA1 mediates trigeminal neuropathic pain in mice downstream of monocytes/macrophages and oxidative stress. Brain 2016; 139: 1361–1377. [DOI] [PubMed] [Google Scholar]
- 8.Trevisani M, Siemens J, Materazzi S, Bautista DM, Nassini R, Campi B, Imamachi N, Andrè E, Patacchini R, Cottrell GS, Gatti R, Basbaum AI, Bunnett NW, Julius D, Geppetti P. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc Natl Acad Sci USA 2007; 104: 13519–13524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khoonsari PE, Ossipova E, Lengqvist J, Svensson CI, Kosek E, Kadetoff D, Jakobsson P-J, Kultima K, Lampa J. The human CSF pain proteome. J Proteomics 2019; 190: 67–76. [DOI] [PubMed] [Google Scholar]
- 10.Dhillon H, Singh S. Role of apolipoprotein E in the tangled mystery of pain. Med Hypotheses 2018; 114: 58–64. [DOI] [PubMed] [Google Scholar]
- 11.Woller SA, Choi S-H, An EJ, Low H, Schneider DA, Ramachandran R, Kim J, Bae YS, Sviridov D, Corr M, Yaksh TL, Miller YI. Inhibition of neuroinflammation by AIBP: spinal effects upon facilitated pain states. Cell Rep 2018; 23: 2667–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohammadi M, Oehler B, Kloka J, Martin C, Brack A, Blum R, Rittner HL. Antinociception by the anti-oxidized phospholipid antibody E06. Br J Pharmacol 2018; 175: 2940–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charles-Schoeman C, Banquerigo ML, Hama S, Navab M, Park GS, Van Lenten BJ, Wagner AC, Fogelman AM, Brahn E. Treatment with an apolipoprotein A-1 mimetic peptide in combination with pravastatin inhibits collagen-induced arthritis. Clin Immunol 2008; 127: 234–244. [DOI] [PubMed] [Google Scholar]
- 14.Dunbar RL, Movva R, Bloedon LT, Duffy D, Norris RB, Navab M, Fogelman AM, Rader DJ. Oral apolipoprotein A-I mimetic D-4F lowers HDL-inflammatory index in high-risk patients: a first-in-human multiple-dose, randomized controlled trial. Clin Transl Sci 2017; 10: 455–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Lenten BJ, Wagner AC, Jung C-L, Ruchala P, Waring AJ, Lehrer RI, Watson AD, Hama S, Navab M, Anantharamaiah GM, Fogelman AM. Anti-inflammatory apoA-I-mimetic peptides bind oxidized lipids with much higher affinity than human apoA-I. J Lipid Res 2008; 49: 2302–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bianchi BR, Zhang X-F, Reilly RM, Kym PR, Yao BB, Chen J. Species comparison and pharmacological characterization of human, monkey, rat, and mouse TRPA1 channels. J Pharmacol Exp Ther 2012; 341: 360–368. [DOI] [PubMed] [Google Scholar]
- 17.Kruger AL, Peterson S, Turkseven S, Kaminski PM, Zhang FF, Quan S, Wolin MS, Abraham NG. D-4F induces heme oxygenase-1 and extracellular superoxide dismutase, decreases endothelial cell sloughing, and improves vascular reactivity in rat model of diabetes. Circulation 2005; 111: 3126–3134. [DOI] [PubMed] [Google Scholar]
- 18.Ning R, Venkat P, Chopp M, Zacharek A, Yan T, Cui X, Seyfried D, Chen J. D-4F increases microRNA-124a and reduces neuroinflammation in diabetic stroke rats. Oncotarget 2017; 8: 95481–95494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song X, Fischer P, Chen X, Burton C, Wang J. An apoA-I mimetic peptide facilitates off-loading cholesterol from HDL to liver cells through scavenger receptor BI. Int J Biol Sci 2009; 5: 637–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song X, Shi Y, You J, Wang Z, Xie L, Zhang C, Xiong J. D-4F, an apolipoprotein A-I mimetic, suppresses IL-4 induced macrophage alternative activation and pro-fibrotic TGF-beta1 expression. Pharm Biol 2019; 57: 470–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tian H, Yao S-T, Yang N-N, Ren J, Jiao P, Zhang X, Li D-X, Zhang G-A, Xia Z-F, Qin S-C. D4F alleviates macrophage-derived foam cell apoptosis by inhibiting the NF-kappaB-dependent Fas/FasL pathway. Sci Rep 2017; 7: 7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rittner HL, Mousa SA, Labuz D, Beschmann K, Schäfer M, Stein C, Brack A. Selective local PMN recruitment by CXCL1 or CXCL2/3 injection does not cause inflammatory pain. J Leukoc Biol 2006; 79: 1022–1032. [DOI] [PubMed] [Google Scholar]
- 23.Curtis MJ, Alexander S, Cirino G, Docherty JR, George CH, Giembycz MA, Hoyer D, Insel PA, Izzo AA, Ji Y, MacEwan DJ, Sobey CG, Stanford SC, Teixeira MM, Wonnacott S, Ahluwalia A. Experimental design and analysis and their reporting II: updated and simplified guidance for authors and peer reviewers. Br J Pharmacol 2018; 175: 987–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pagano RR. Understanding statistics in the behavioral sciences. 10th ed. Belmont: Wadsworth/Cengage Learning, 2013. [Google Scholar]
- 25.Salkind NJ. Encyclopedia of research design. Thousand Oaks: SAGE, 2010. [Google Scholar]
- 26.Wilcox RR. Introduction to robust estimation and hypothesis testing. 3rd ed. Amsterdam: Academic Press, 2012. [Google Scholar]
- 27.Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007; 39: 175–191. [DOI] [PubMed] [Google Scholar]
- 28.Westlund KN, Kochukov MY, Lu Y, McNearney TA. Impact of central and peripheral TRPV1 and ROS levels on proinflammatory mediators and nociceptive behavior. Mol Pain 2010; 6: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogawa N, Kurokawa T, Mori Y. Sensing of redox status by TRP channels. Cell Calcium 2016; 60: 115–122. [DOI] [PubMed] [Google Scholar]
- 30.Zhang X, Li L, McNaughton PA. Proinflammatory mediators modulate the heat-activated ion channel TRPV1 via the scaffolding protein AKAP79/150. Neuron 2008; 59: 450–461. [DOI] [PubMed] [Google Scholar]
- 31.Patwardhan AM, Scotland PE, Akopian AN, Hargreaves KM. Activation of TRPV1 in the spinal cord by oxidized linoleic acid metabolites contributes to inflammatory hyperalgesia. Proc Natl Acad Sci USA 2009; 106: 18820–18824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shao B. Site-specific oxidation of apolipoprotein A-I impairs cholesterol export by ABCA1, a key cardioprotective function of HDL. Biochim Biophys Acta 2012; 1821: 490–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Requena JR, Fu MX, Ahmed MU, Jenkins AJ, Lyons TJ, Baynes JW, Thorpe SR. Quantification of malondialdehyde and 4-hydroxynonenal adducts to lysine residues in native and oxidized human low-density lipoprotein. Biochem J 1997; 322: 317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nandedkar SD, Weihrauch D, Xu H, Shi Y, Feroah T, Hutchins W, Rickaby DA, Duzgunes N, Hillery CA, Konduri KS, Pritchard KA. D-4F, an apoA-1 mimetic, decreases airway hyperresponsiveness, inflammation, and oxidative stress in a murine model of asthma. J Lipid Res 2011; 52: 499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu H, Bandell M, Petrus MJ, Zhu MX, Patapoutian A. Zinc activates damage-sensing TRPA1 ion channels. Nat Chem Biol 2009; 5: 183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kishimoto E, Naito Y, Handa O, Okada H, Mizushima K, Hirai Y, Nakabe N, Uchiyama K, Ishikawa T, Takagi T, Yagi N, Kokura S, Yoshida N, Yoshikawa T. Oxidative stress-induced posttranslational modification of TRPV1 expressed in esophageal epithelial cells. Am J Physiol Gastrointest Liver Physiol 2011; 301: G230–G238. [DOI] [PubMed] [Google Scholar]
- 37.DelloStritto DJ, Connell PJ, Dick GM, Fancher IS, Klarich B, Fahmy JN, Kang PT, Chen Y-R, Damron DS, Thodeti CK, Bratz IN. Differential regulation of TRPV1 channels by H2O2: implications for diabetic microvascular dysfunction. Basic Res Cardiol 2016; 111: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spahn V, Stein C, Zollner C. Modulation of transient receptor vanilloid 1 activity by transient receptor potential ankyrin 1. Mol Pharmacol 2014; 85: 335–344. [DOI] [PubMed] [Google Scholar]
- 39.Akopian AN, Ruparel NB, Jeske NA, Hargreaves KM. Transient receptor potential TRPA1 channel desensitization in sensory neurons is agonist dependent and regulated by TRPV1-directed internalization. J Physiol (Lond) 2007; 583: 175–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patil MJ, Jeske NA, Akopian AN. Transient receptor potential V1 regulates activation and modulation of transient receptor potential A1 by Ca2+. Neuroscience 2010; 171: 1109–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Staruschenko A, Jeske NA, Akopian AN. Contribution of TRPV1-TRPA1 interaction to the single channel properties of the TRPA1 channel. J Biol Chem 2010; 285: 15167–15177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Obata K, Katsura H, Mizushima T, Yamanaka H, Kobayashi K, Dai Y, Fukuoka T, Tokunaga A, Tominaga M, Noguchi K. TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury. J Clin Invest 2005; 115: 2393–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amsalem M, Poilbout C, Ferracci G, Delmas P, Padilla F. Membrane cholesterol depletion as a trigger of Nav1.9 channel-mediated inflammatory pain. Embo J 2018; 37: e97349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Startek JB, Boonen B, Talavera K, Meseguer V. TRP channels as sensors of chemically-induced changes in cell membrane mechanical properties. Int J Mol Sci 2019; 20: 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murthy SE, Dubin AE, Whitwam T, Jojoa-Cruz S, Cahalan SM, Mousavi SAR, Ward AB, Patapoutian A. OSCA/TMEM63 are an evolutionarily conserved family of mechanically activated ion channels. Elife 2018; 7: e41844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang M, Wang Y, Geng J, Zhou S, Xiao B. Mechanically activated piezo channels mediate touch and suppress acute mechanical pain response in mice. Cell Rep 2019; 26: 1419–1431.e1414. [DOI] [PubMed] [Google Scholar]
- 47.Bunker CB, Cerio R, Bull HA, Evans J, Dowd PM, Foreman JC. The effect of capsaicin application on mast cells in normal human skin. Agents Actions 1991; 33: 195–196. [DOI] [PubMed] [Google Scholar]
- 48.Ständer S, Moormann C, Schumacher M, Buddenkotte J, Artuc M, Shpacovitch V, Brzoska T, Lippert U, Henz BM, Luger TA, Metze D, Steinhoff M. Expression of vanilloid receptor subtype 1 in cutaneous sensory nerve fibers, mast cells, and epithelial cells of appendage structures. Exp Dermatol 2004; 13: 129–139. [DOI] [PubMed] [Google Scholar]
- 49.Sandig H, Bulfone-Paus S. TLR signaling in mast cells: common and unique features. Front Immunol 2012; 3: 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wadachi R, Hargreaves KM. Trigeminal nociceptors express TLR-4 and CD14: a mechanism for pain due to infection. J Dent Res 2006; 85: 49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Diogenes A, Ferraz CCR, Akopian AN, Henry MA, Hargreaves KM. LPS sensitizes TRPV1 via activation of TLR4 in trigeminal sensory neurons. J Dent Res 2011; 90: 759–764. [DOI] [PubMed] [Google Scholar]
- 52.Tancowny BP, Karpov V, Schleimer RP, Kulka M. Substance P primes lipoteichoic acid- and Pam3CysSerLys4-mediated activation of human mast cells by up-regulating Toll-like receptor 2. Immunology 2010; 131: 220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baliu-Pique M, Jusek G, Holzmann B. Neuroimmunological communication via CGRP promotes the development of a regulatory phenotype in TLR4-stimulated macrophages. Eur J Immunol 2014; 44: 3708–3716. [DOI] [PubMed] [Google Scholar]
- 54.Cheng AM, Handa P, Tateya S, Schwartz J, Tang C, Mitra P, Oram JF, Chait A, Kim F. Apolipoprotein A-I attenuates palmitate-mediated NF-kappaB activation by reducing Toll-like receptor-4 recruitment into lipid rafts. PLoS One 2012; 7: e33917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van Linthout S, Spillmann F, Graiani G, Miteva K, Peng J, Van Craeyveld E, Meloni M, Tölle M, Escher F, Subasigüller A, Doehner W, Quaini F, De Geest B, Schultheiss H-P, Tschöpe C. Down-regulation of endothelial TLR4 signalling after apo A-I gene transfer contributes to improved survival in an experimental model of lipopolysaccharide-induced inflammation. J Mol Med 2011; 89: 151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]