Abstract
Background.
The outcome of localized osteosarcoma has remained constant over the past 30 years. Histological response to preoperative chemotherapy is the best predictor of outcome. Strategies to alter treatment based on histological response have not resulted in increased survival.
Procedure.
Patients with localized osteosarcoma received preoperative chemotherapy with cisplatin, doxorubicin, and methotrexate. Patients whose tumors had a good histological response (≥90% necrosis) continued with the same treatment postoperatively. Patients with poor histological response (<90% necrosis) received three courses of melphalan 100 mg/m2 on day −4, cyclophosphamide 2,000 mg/m2 on days −3, and −2 followed by stem cell infusion.
Results.
Fifty-two patients were enrolled. Median age was 14 years, and 56% of patients were male. The femur was the most common site. Forty patients underwent limb salvage surgery and amputation was performed in six patients. Forty-eight percent of tumors showed good histological response. Forty patients were evaluable for outcome; 18 patients with poor histologic response received high-dose chemotherapy. The 5-year event-free survival (EFS) and overall survival (OS) for patients treated on the high-dose chemotherapy arm were 28% (95% confidence interval [CI], 10–49) and 48% (95% CI, 23–69), respectively. The 5-year EFS and OS for patients treated on the standard chemotherapy arm were 62% (95% CI, 36–80) and 74% (95% CI, 44–90), respectively. All patients who received high-dose chemotherapy developed grade 3 or higher hematological toxicity. There were no treatment-related deaths.
Conclusions.
Postoperative alkylator intensification with high-dose cyclophosphamide and melphalan in patients with localized osteosarcoma with poor histological response failed to improve survival.
Keywords: clinical trials, high-dose chemotherapy, necrosis, osteosarcoma, stem cell transplantation
INTRODUCTION
With currently available treatment regimens, approximately 60–70% of patients with nonmetastatic osteosarcoma of the extremity survive. The survival rate has remained constant during the past 30 years as shown in studies performed in Europe and North America.[1,2] The best predictor of outcome has been in vivo sensitivity, i.e., histologic response to neoadjuvant chemotherapy as determined by the pathologic examination of the resected tumor.[2,3] Approximately half of the patients with localized osteosarcoma have poor histological response defined as <90% tumor necrosis.[4–6] The 5-year survival for these patients is approximately 45%, and is almost 25% less than those with good histological response.[2,7] Efforts to improve the proportion of patients with good histologic response have not translated into better overall survival (OS).[8,9] Another approach is to intensify postoperative chemotherapy in poor responders by adding other active agents to the preoperative chemotherapy regimen consisting of high-dose methotrexate, doxorubicin, and cisplatin (MAP).
Expression of the MDR1 gene product P-glycoprotein has been shown to correlate with treatment failure in patients with osteosarcoma. In a study by Chan et al., patients whose tumors had undetectable P-glycoprotein appeared to have a better histologic response to preoperative chemotherapy, although this was not statistically significant (P = 0.057).[10] In a study by Baldini et al., both P-glycoprotein status and the extent of tumor necrosis after preoperative chemotherapy were independent predictors of clinical outcome.[11] P-glycoprotein mediates multidrug resistance to antimetabolites including doxorubicin. Alkylating agents do not share the same efflux transport mechanisms mediated by P-glycoprotein, and may be useful in patients with multidrug resistance. Alkylating agents have a steep dose–response curve. Therefore, one strategy to improve outcomes for the patients with poor histological response could be to use high doses of alkylating agents to intensify treatment. Alkylating agents such as cyclophosphamide and ifosfamide have been shown to be active in osteosarcoma both in newly diagnosed and recurrent settings.[3,12–15]
In this study, we hypothesized that postoperative dose intensification of alkylating agents in patients with localized osteosarcoma who are poor responders will improve their outcome compared to historical controls. Patients who had 90% or greater tumor necrosis were designated as good responders and received therapy similar to the preoperative therapy. Those who had less than 90% necrosis were designated as poor responders and received high-dose chemotherapy with alkylating agents with peripheral blood stem cell support.
METHODS
Patient Eligibility
Patients less than 26 years of age with newly diagnosed biopsy-proven localized high-grade osteosarcoma were eligible to participate in the study. Organ function requirements were as follows: peripheral blood absolute neutrophil count (ANC) ≥ 1,500/μl, platelet count ≥ 100,000/μl, hemoglobin ≥ 8 g/dl; total bilirubin ≤ 2 mg/dl and alanine transaminase <5× upper limit of normal for age: serum creatinine ≤1.5× upper limit of normal for age or a glomerular filtration rate of ≥70 ml/min/1.73 m2; and normal cardiac function as determined by echocardiogram. Patients with low-grade osteosarcoma, radiation-induced osteosarcoma, osteosarcoma arising from premalignant conditions, and pregnant females were excluded from the study. The research was approved by the institutional review boards of all participating institutions. Written informed consent was obtained from patients prior to enrollment. In case of minors, written informed consent was obtained from parents/legal guardians.
Treatment Plan
The first six patients on this trial received three courses of preoperative chemotherapy consisting of cisplatin (90 mg/m2/dose) administered intravenously over 6 hr, and doxorubicin (75 mg/m2/dose) administered intravenously over 15 min on day 1 of a 3-week course. Dexrazoxane (750 mg/m2) was administered prior to doxorubicin infusion. All subsequent patients received two courses of preoperative chemotherapy. Each course consisted of cisplatin (60 mg/m2/dose), doxorubicin (37.5 mg/m2/dose) administered intravenously on days 1 and 2 of week 1, and high-dose methotrexate (12 g/m2) followed by leucovorin rescue administered intravenously on day 1 of weeks 4 and 5. Dexrazoxane (375 mg/m2) was administered intravenously prior to each doxorubicin infusion. Granulocyte colony-stimulating factor (5 μg/kg) was administered subcutaneously once a day, starting 24 hr after the administration of cisplatin and doxorubicin and continued until the ANC was >5,000/μl.
Definitive surgery was planned 2 weeks after the end of preoperative chemotherapy. Pathology was centrally reviewed to determine the percentage of necrosis. Patients with ≥90% tumor necrosis went on to receive four more courses of chemotherapy identical to preoperative chemotherapy. Cisplatin was not administered during the last two courses. Patients with <90% tumor necrosis underwent collection of stem cells by bone marrow harvest. They received three courses of high-dose chemotherapy consisting of melphalan (100 mg/m2) on day −4 and cyclophosphamide (2,000 mg/m2) on days −3 and −2, and followed by stem cell infusion on day 0. G-CSF (5 μg/kg) was administered intravenously once a day, starting 24 hr after the stem cell infusion until count recovery. Toxicities were graded using common toxicology criteria v2.0. Patients who developed progressive disease or toxicity requiring a change in treatment plan were removed from protocol therapy. Patients who died or those who withdrew consent were considered off study.
Following an interim analysis, the study was amended and the high-dose chemotherapy arm was closed after 40 patients were enrolled. All subsequent patients were enrolled onto the standard chemotherapy arm irrespective of tumor necrosis.
Follow-up
After the end of treatment, each patient was followed using physical examination, complete blood count, computed tomography scan of the chest, nuclear medicine bone scintigraphy, and radiographs of the primary site every 3 months for the first 2 years, then every 6 months for 2 years, and yearly thereafter.
Statistical Analysis
The study was descriptive with planned comparison to historical data. Event-free survival (EFS) was defined as the interval between the date of diagnosis and the date of progressive disease/relapse or date of death. OS was defined as the interval between the date of diagnosis and date of death or last follow up. Survival analysis was performed using Kaplan–Meier method. All analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
Between March 1999 and October 2006, 52 patients with localized osteosarcoma were enrolled at the four participating institutions (Fig. 1). Six patients were removed from protocol therapy prior to surgery: three due to progressive disease and three due to treatment-related toxicity. Six patients withdrew from study after surgery: four due to patient/physician preference against high-dose chemotherapy, one transferred to other institution, and another one was removed due to noncompliance with the treatment regimen. The characteristics of enrolled patients are described in Table I.
Fig. 1.
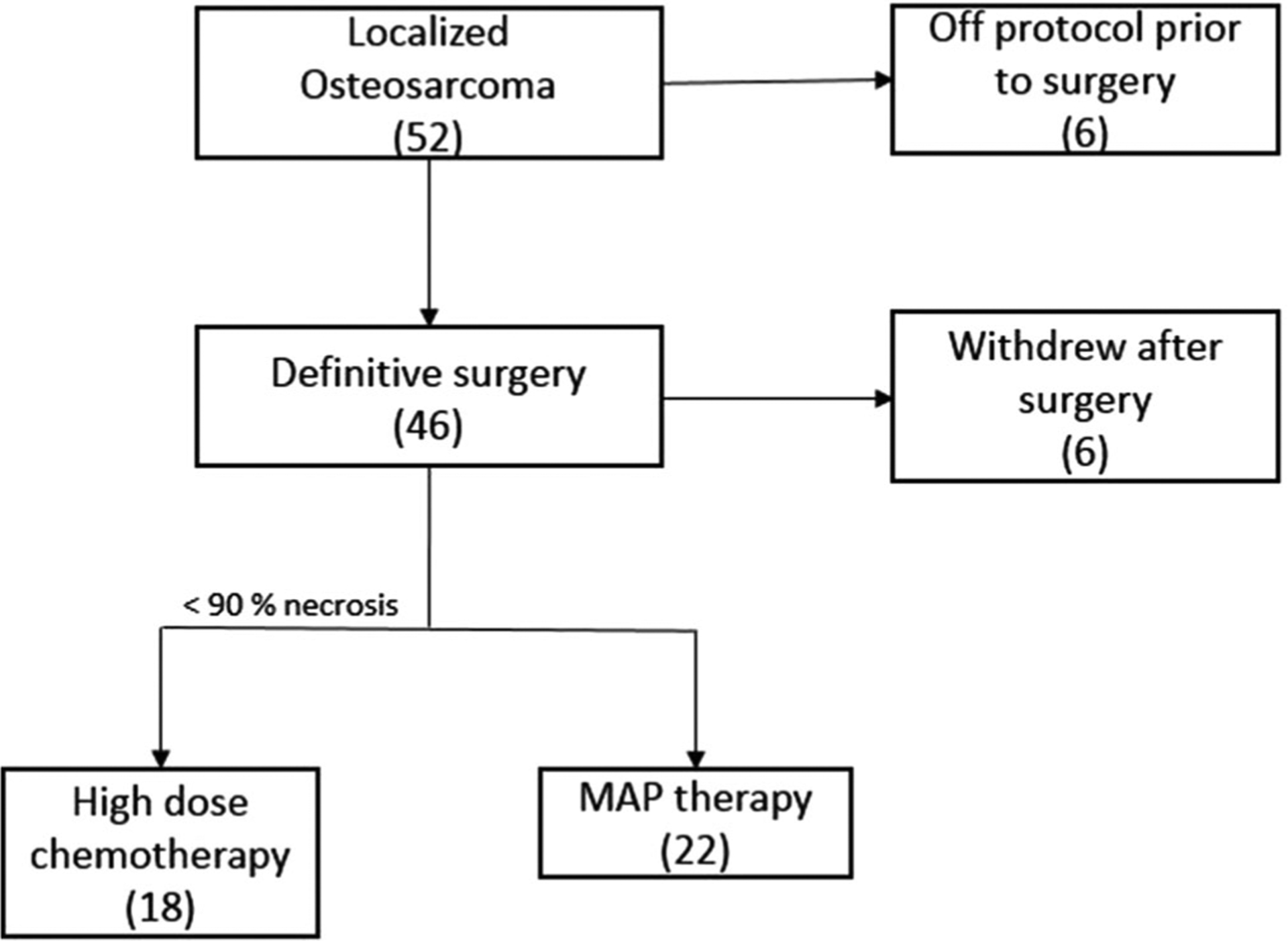
Patient flow.
TABLE I.
Patient Characteristics
| Total (n = 52) | |
|---|---|
| Age at DX (years) | 14 (7, 22) |
| Gender, n (%) | |
| Male | 29 (56) |
| Female | 23 (44) |
| Primary site (%) | |
| Femur | 27 (52) |
| Tibia | 15 (29) |
| Humerus | 6 (11) |
| Pelvis | 1 (2) |
| Other | 3 (6) |
| Surgery | |
| Limb salvage | 40 |
| Amputation | 6 |
| Not performed | 6 |
| Necrosis grade, n (%) | |
| Grade 1 (<50%) | 6 (13) |
| Grade 2 (50–89%) | 17 (38) |
| Grade 3 (90–99%) | 18 (40) |
| Grade 4 (100%) | 4 (9) |
| Follow-up time (months) | 38.5 (23.6, 71.2) |
| Median time interval between neoadjuvant chemotherapy and surgery (days) | 20 (6, 45) |
| Median time interval between surgery and adjuvant chemotherapy (days) | 38 (26.5, 43) |
Surgery and Tumor Response
Of the 46 patients who underwent definitive surgery, 40 patients underwent tumor resection with limb salvage while six patients underwent amputation. Tumor necrosis information was available on 45 patients; four tumors had 100% necrosis, 18 tumors had 90–99% necrosis, 17 tumors had 50–89% necrosis, and six had <50% necrosis. The first six patients on this trial received preoperative chemotherapy without methotrexate. All of their tumors had <90% necrosis. Subsequently, the study was amended to include methotrexate. The median time interval between the last dose of preoperative chemotherapy and definitive surgery was 20 days (range 6–45 days).
Adjuvant Chemotherapy
Twenty-two patients received standard chemotherapy (including four with <90% necrosis enrolled after interim analysis), whereas 18 patients with a poor histological response received high-dose chemotherapy followed by stem cell rescue. Six patients in the high-dose chemotherapy arm did not complete all three intended courses: two developed progressive disease during treatment, two refused further therapy after two courses, one patient did not have adequate stem cells for a third course, and one patient developed renal cell carcinoma. The median interval between definitive surgery and initiation of adjuvant chemotherapy was 27.5 days (range: 16–71 days) in the standard chemotherapy arm and 40.5 days (range: 29–64 days) in the high-dose chemotherapy arm.
Toxicity
The toxicities observed during preoperative chemotherapy and standard chemotherapy arm were as expected and comparable to those described in previous publications. Three patients were removed from protocol therapy due to toxicity during preoperative chemotherapy: two patients due to prolonged methotrexate clearance with kidney injury and mucositis one patient due to central nervous system ischemia. All 18 patients who received high-dose chemotherapy developed grade 4 neutropenia, grade 3/4 thrombocytopenia, and grade 3 anemia. In addition, six patients developed grade 3 infection, four patients had grade 3/4 mucositis, and one patient had grade 4 hemorrhagic cystitis. No treatment-related deaths were observed.
Outcome
An interim analysis performed following the treatment of the first 18 patients with less than 90% tumor necrosis showed a 2-year EFS of 41%. The objective was to show a 2-year EFS of 75% compared to historical data of 55% by treating 48 patients with nonmetastatic extremity disease who showed less than 90% necrosis following preoperative chemotherapy. Based on statistical analysis, it was extremely unlikely that 31 additional patients could have a high enough 2-year EFS to achieve the study objective. Therefore, all subsequent patients with poor histological response were enrolled on the standard arm.
Of the 40 evaluable patients, after a median follow up of 39 months, 19 patients experienced progressive disease or relapse. In six patients, the disease relapsed locally, nine patients had isolated lung relapse, two patients developed distant bone relapse, and two patients developed combined lung and bone relapse. In two patients, the first event was a second malignancy; renal cell carcinoma, colorectal adenocarcinoma. All patient deaths were attributable to osteosarcoma progression except in the patient with adenocarcinoma. The 5-year EFS and OS for all 40 evaluable patients was 46% and 62%, respectively. The 5-year EFS and OS for patients treated on the standard chemotherapy arm were 62% (95% confidence interval [CI], 36–80) and 74% (95% CI, 44–90), respectively (Figs. 2 and 3). The 5-year EFS and OS for patients treated on the high-dose chemotherapy arm were 28% (95% CI, 10–49) and 48% (95% CI, 23–69), respectively.
Fig. 2.
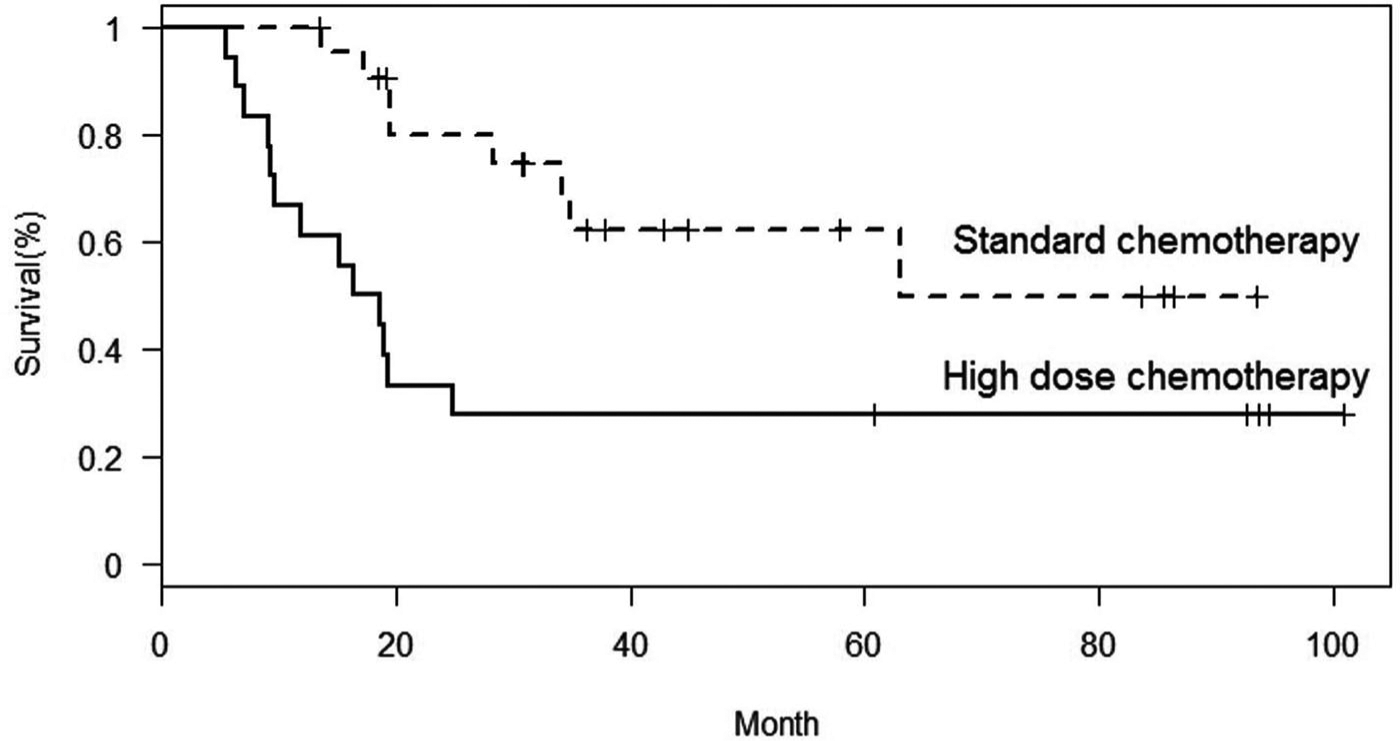
Event-free survival by treatment regimen.
Fig. 3.
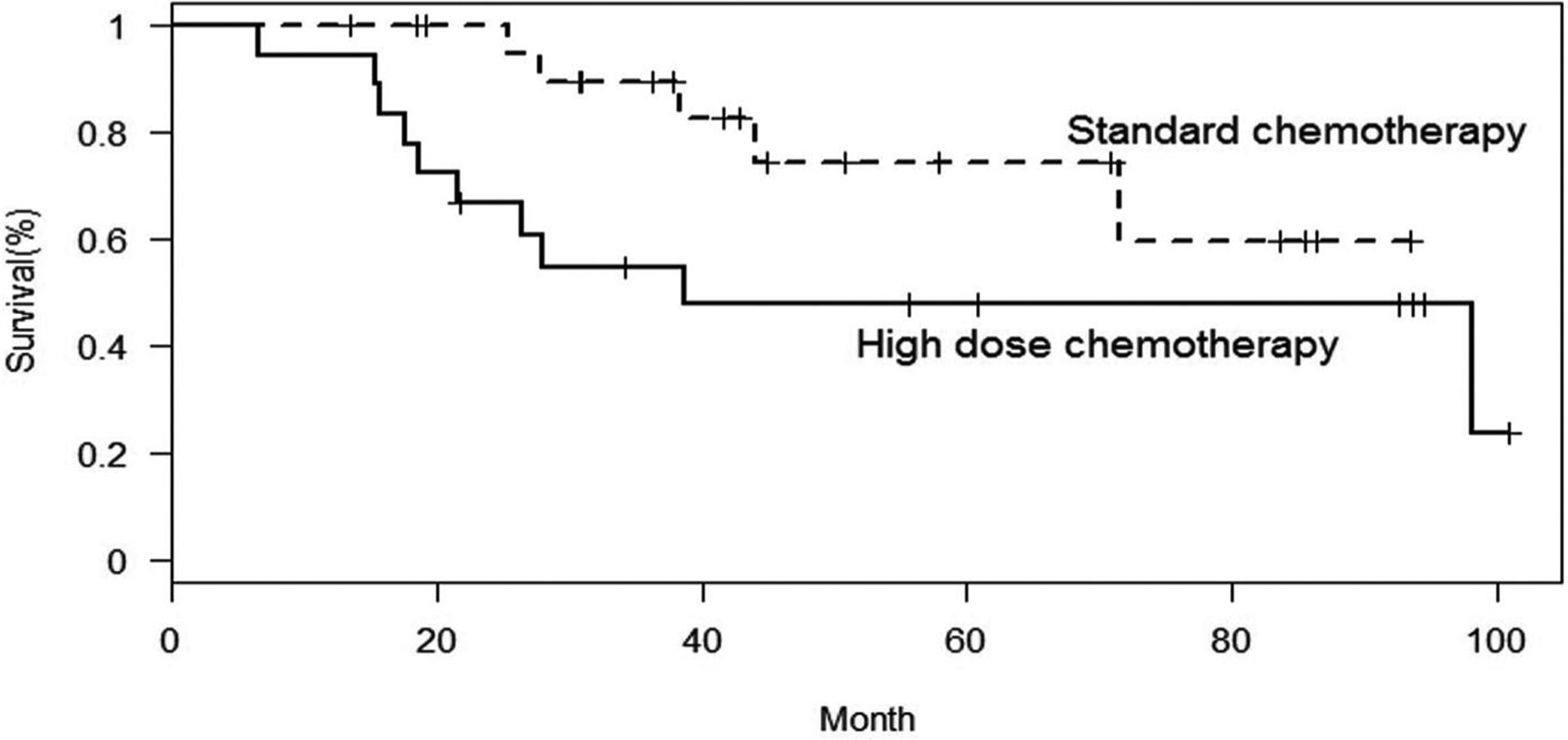
Overall survival by treatment regimen.
DISCUSSION
Outcomes for patients with localized osteosarcoma have remained constant over the past 30 years. Identifying new and more effective chemotherapeutic agents may improve outcomes. However, no new agents have been recently identified and the only active agents available are high-dose methotrexate, cisplatin, doxorubicin, and alkylating agents (ifosfamide and cyclophosphamide). Another avenue for further therapeutic advance is to tailor therapy based on prognostic factors. Histological response to preoperative chemotherapy is consistently shown to predict outcome across multiple clinical studies.[2,3] Most clinical trials report a good histological response rate of 45–50%.[4,6] The 5-year survival for patients with good histological response is approximately 75–80% compared to 45%–50% for those with poor histological response.[2,6]
Various groups have attempted to improve outcomes for patients with osteosarcoma by either intensifying preoperative chemotherapy to increase good histological response or by intensifying postoperative chemotherapy in patients with a poor histological response. An increase in the proportion of patients achieving a good histological response does not correlate with improved outcome. The Memorial Sloan-Kettering T12 protocol intensified preoperative chemotherapy by administering high-dose methotrexate, cisplatin, doxorubicin, bleomycin, cyclophosphamide, and dactinomycin.[8] The proportion of patients with good histological response was higher in the experimental arm (44% vs. 37%), but there was no significant difference in the EFS. The European Osteosarcoma Intergroup conducted a randomized phase III trial comparing dose-dense cisplatin and doxorubicin administered every 2 weeks with the same chemotherapy agents administered every 3 weeks. While the proportion of those with good histological response was higher in the dose-intensified group (50% vs. 36%), there was no difference in progression-free survival or OS.[9] Approximately half of the patients in the current study had ≥90% tumor necrosis. This is similar to the data reported from the EURAMOS-1 international trial.[4] The first six patients in this study were treated without high-dose methotrexate, and all of them had <90% tumor necrosis. This may have resulted from the omission of methotrexate. A meta-analysis by Anninga et al. showed that better outcomes were achieved with clinical trials using three active agents when compared to those using two agents.[16]
Intensification of postoperative chemotherapy for patients with poor histological response has generally involved the addition of alkylating agent to the preoperative MAP regimen. Cyclophosphamide was part of the earliest chemotherapy regimens used for osteosarcoma.[3,17,18] It was used in combination with bleomycin and dactinomycin (BCD regimen). In combination with etoposide, cyclophosphamide produced an 88% response rate in newly diagnosed osteosarcoma.[12] The activity of ifosfamide in osteosarcoma was reported initially in a recurrent setting.[14] Subsequently, the combination of high-dose ifosfamide and etoposide showed 60% response rate in patients with newly diagnosed metastatic osteosarcoma.[13] A regimen containing carboplatin, ifosfamide, and doxorubicin produced similar outcomes to the MAP regimen in newly diagnosed patients with localized osteosarcoma.[15]
Since ifosfamide and cyclophosphamide are considered equivalent in their clinical spectrum and activity, and high-dose ifosfamide is associated with significant renal injury and ototoxicity in patients previously exposed to cisplatin, we used cyclophosphamide in this study. Melphalan, at lower doses (0.3 mg/kg), was part of initial chemotherapy regimens for osteosarcoma.[19] The response rate to single-agent melphalan at lower dose was 15%. Melphalan at doses greater than 65 mg/m2 with bone marrow transplant support produced higher response rates in solid tumors including sarcomas and neuroblastoma when compared to melphalan at lower doses.[20] The combination of high–dose cyclophosphamide and melphalan followed by autologous stem cell rescue was well tolerated in children with refractory central nervous system malignancies.[21] This formed the basis of combining these agents. To reduce interference with the dose intensity of the alkylating agents, other agents were not used in the adjuvant setting.
Though controversial, this approach of changing postoperative chemotherapy regimen in poor responders has been utilized in the past in COSS-82 study.[22] Patients with poor histological response who received high-dose methotrexate and BCD as preoperative therapy received doxorubicin and cisplatin postoperatively. The 4-year EFS was 41% in this arm. Patients with poor histological response who received MAP regimen as a part of initial therapy received BCD and cisplatin/ifosfamide postoperatively. The 4-year EFS was 52%. The lower outcome in preoperative BCD arm may be due to the development of resistance in cells exposed to a lower intensity regimen. In contrast, in the current study, we used standard agents at standard doses in the preoperative phase and in the postoperative phase used alkylating agents at high dose intensity.
Other groups have investigated the addition of alkylators for poor responders to improve outcome. Ferrari et al. added ifosfamide to standard MAP regimen in poor responders.[23] The 5-year EFS of 50% was less than the EFS of 64% achieved in a previous trial with MAP regimen conducted by the same group. The authors concluded that the reduction in methotrexate dose or dose intensity, due to the addition of ifosfamide, was responsible for the worse outcome. The Intergroup Study 0133 prospectively compared the addition of ifosfamide to MAP regimen in the postoperative setting.[1] The 6-year EFS for patients treated with or without ifosfamide was 64% and 63%, respectively, and the difference was not statistically significant. The ISG/OS-1 study compared the addition of ifosfamide to the standard MAP chemotherapy in 246 patients in the neoadjuvant setting.[5] The addition of ifosfamide failed to increase the good response rate and resulted in increased hematological toxicity. In the recently concluded EURAMOS-1 trial, the addition of ifosfamide and etoposide to MAP regimen in patients with poor histological response failed to improve outcome.[24] This study provides additional evidence for lack of improvement in outcome with the addition of an alkylator in tumors with a poor histological response.
High-dose chemotherapy has been investigated in both preoperative and adjuvant settings. High-dose ifosfamide with stem cell rescue was added to cisplatin and doxorubicin preoperatively in 22 patients with localized osteosarcoma.[25] Eighty-two percent of patients had at least 90% necrosis and 3-year EFS and OS were 70% and 83%, respectively. Investigators from Italian and Scandinavian sarcoma groups added two courses of high-dose chemotherapy with carboplatin and etoposide followed by stem cell rescue in patients with metastatic osteosarcoma or localized pelvic primary.[26] This was associated with significant toxicity with no improvement in survival when compared to standard three drug regimens. The current study failed to demonstrate improvement in survival with high-dose chemotherapy.
The EFS for poor responders in this study is lower than other studies in a similar setting.[27] This may have resulted from several factors. There was a significant delay in starting postoperative chemotherapy. Patients had to wait for histological response data prior to treatment allotment. In the high-dose chemotherapy arm, there was an additional delay caused by procurement of stem cells prior to postoperative chemotherapy. This caused delays in instituting postoperative chemotherapy for an extended period of time, which may have affected the outcome. In addition, patients with poor histological response did not receive postoperative MAP regimen, which has been shown to achieve approximately 50% EFS in these patients. Although these outcomes are inferior to the outcomes achieved in patients with good histological response, they are superior to the outcomes achieved without adjuvant chemotherapy.
Our strategy was to intensify alkylating agent therapy. Toward our effort, we substituted a preoperative regimen with high-dose cyclophosphamide and melphalan with autologous stem cell support in the postoperative phase. Higher doses of melphalan with stem cell support had shown improved response in other sarcomas and we extrapolated these results to osteosarcoma. The outcome of the poor responders in this study was based on 18 patients, but this strategy clearly resulted in a lower EFS for patients with poor histological response when compared to all previous studies. We conclude that postoperative intensification with alkylating agents failed to improve outcomes for patients with localized osteosarcoma with poor histological response to neoadjuvant chemotherapy.
Footnotes
Conflict of interest: Nothing to declare.
REFERENCES
- 1.Meyers PA, Schwartz CL, Krailo MD, Healey JH, Bernstein ML, Betcher D, Ferguson WS, Gebhardt MC, Goorin AM, Harris M, Kleinerman E, Link MP, Nadel H, Nieder M, Siegal GP, Weiner MA, Wells RJ, Womer RB, Grier HE. Osteosarcoma: The addition of muramyl tripeptide to chemotherapy improves overall survival—A report from the Children’s Oncology Group. J Clin Oncol 2008;26:633–638. [DOI] [PubMed] [Google Scholar]
- 2.Whelan JS, Jinks RC, McTiernan A, Sydes MR, Hook JM, Trani L, Uscinska B, Bramwell V, Lewis IJ, Nooij MA, van Glabbeke M, Grimer RJ, Hogendoorn PCW, Taminiau AHM, Gelderblom H. Survival from high-grade localised extremity osteosarcoma: Combined results and prognostic factors from three European Osteosarcoma Intergroup randomised controlled trials. Ann Oncol 2012;23:1607–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Provisor AJ, Ettinger LJ, Nachman JB, Krailo MD, Makley JT, Yunis EJ, Huvos AG, Betcher DL, Baum ES, Kisker CT, Miser JS. Treatment of nonmetastatic osteosarcoma of the extremity with preoperative and postoperative chemotherapy: A report from the Children’s Cancer Group. J Clin Oncol 1997;15:76–84. [DOI] [PubMed] [Google Scholar]
- 4.Whelan JS, Bielack SS, Marina N, Smeland S, Jovic G, Hook JM, Krailo M, Anninga J, Butterfass-Bahloul T, Böhling T, Calaminus G, Capra M, Deffenbaugh C, Dhooge C, Eriksson M, Flanagan AM, Gelderblom H, Goorin A, Gorlick R, Gosheger G, Grimer RJ, Hall KS, Helmke K, Hogendoorn PCW, Jundt G, Kager L, Kuehne T, Lau CC, Letson GD, Meyer J, Meyers PA, Morris C, Mottl H, Nadel H, Nagarajan R, Randall RL, Schomberg P, Schwarz R, Teot LA, Sydes MR, Bernstein M; EURAMOS collaborators. EURAMOS-1, an international randomised study for osteosarcoma: Results from pre-randomisation treatment. Ann Oncol 2015;26:407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrari S, Ruggieri P, Cefalo G, Tamburini A, Capanna R, Fagioli F, Comandone A, Bertulli R, Bisogno G, Palmerini E, Alberghini M, Parafioriti A, Linari A, Picci P, Bacci G. Neoadjuvant chemotherapy with methotrexate, cisplatin, and doxorubicin with or without ifosfamide in nonmetastatic osteosarcoma of the extremity: An Italian sarcoma group trial ISG/OS-1. J Clin Oncol 2012;30:2112–2118. [DOI] [PubMed] [Google Scholar]
- 6.Meyers PA, Schwartz CL, Krailo M, Kleinerman ES, Betcher D, Bernstein ML, Conrad E, Ferguson W, Gebhardt M, Goorin AM, Harris MB, Healey J, Huvos A, Link M, Montebello J, Nadel H, Nieder M, Sato J, Siegal G, Weiner M, Wells R, Wold L, Womer R, Grier H. Osteosarcoma: A randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J Clin Oncol 2005;23:2004–2011. [DOI] [PubMed] [Google Scholar]
- 7.Bielack SS, Smeland S, Whelan JS, Marina N, Jovic G, Hook JM, Krailo MD, Gebhardt M, Pápai Z, Meyer J, Nadel H, Randall RL, Deffenbaugh C, Nagarajan R, Brennan B, Letson GD, Teot LA, Goorin A, Baumhoer D, Kager L, Werner M, Lau CC, Hall KS, Gelderblom H, Meyers P, Gorlick R, Windhager R, Helmke K, Eriksson M, Hoogerbrugge PM, Schomberg P, Tunn P-U, Kühne T, Jürgens H, van den Berg H, Böhling T, Picton S, Renard M, Reichardt P, Gerss J, Butterfass-Bahloul T, Morris C, Hogendoorn PCW, Seddon B, Calaminus G, Michelagnoli M, Dhooge C, Sydes MR, Bernstein M. Methotrexate, doxorubicin, and cisplatin (MAP) plus maintenance pegylated interferon Alfa-2b versus MAP alone in patients with resectable high-grade osteosarcoma and good histologic response to preoperative MAP: First results of the EURAMOS-1 good response randomized controlled trial. J Clin Oncol 2015;33:2279–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyers PA, Gorlick R, Heller G, Casper E, Lane J, Huvos AG, Healey JH. Intensification of preoperative chemotherapy for osteogenic sarcoma: Results of the memorial Sloan-Kettering (T12) protocol. J Clin Oncol 1998;16:2452–2458. [DOI] [PubMed] [Google Scholar]
- 9.Lewis IJ, Nooij MA, Whelan J, Sydes MR, Grimer R, Hogendoorn PCW, Memon MA, Weeden S, Uscinska BM, van Glabbeke M, Kirkpatrick A, Hauben EI, Craft AW, Taminiau AHM; MRC BO06 and EORTC 80931 collaborators, European Osteosarcoma Intergroup. Improvement in histologic response but not survival in osteosarcoma patients treated with intensified chemotherapy: A randomized phase III trial of the European Osteosarcoma Intergroup. J Natl Cancer Inst 2007;99:112–128. [DOI] [PubMed] [Google Scholar]
- 10.Chan HS, Grogan TM, Haddad G, DeBoer G, Ling V. P-glycoprotein expression: Critical determinant in the response to osteosarcoma chemotherapy. J Natl Cancer Inst 1997;89:1706–1715. [DOI] [PubMed] [Google Scholar]
- 11.Baldini N, Scotlandi K, Barbanti-Bròdano G, Manara MC, Maurici D, Bacci G, Bertoni F, Picci P, Sottili S, Campanacci M. Expression of P-glycoprotein in high-grade osteosarcomas in relation to clinical outcome. N Engl J Med 1995;333:1380–1385. [DOI] [PubMed] [Google Scholar]
- 12.Cassano WF, Graham-Pole J, Dickson N. Etoposide, cyclophosphamide, cisplatin, and doxorubicin as neoadjuvant chemotherapy for osteosarcoma. Cancer 1991;68:1899–1902. [DOI] [PubMed] [Google Scholar]
- 13.Goorin AM, Harris MB, Bernstein M, Ferguson W, Devidas M, Siegal GP, Gebhardt MC, Schwartz CL, Link M, Grier HE. Phase II/III trial of etoposide and high-dose ifosfamide in newly diagnosed metastatic osteosarcoma: A pediatric oncology group trial. J Clin Oncol 2002;20:426–433. [DOI] [PubMed] [Google Scholar]
- 14.Kung FH, Pratt CB, Vega RA, Jaffe N, Strother D, Schwenn M, Nitschke R, Homans AC, Holbrook CT, Golembe B. Ifosfamide/etoposide combination in the treatment of recurrent malignant solid tumors of childhood. A Pediatric Oncology Group Phase II study. Cancer 1993;71:1898–1903. [DOI] [PubMed] [Google Scholar]
- 15.Daw NC, Neel MD, Rao BN, Billups CA, Wu J, Jenkins JJ, Quintana J, Luchtman-Jones L, Villarroel M, Santana VM. Frontline treatment of localized osteosarcoma without methotrexate: Results of the St. Jude Children’s Research Hospital OS99 trial. Cancer 2011;117:2770–2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anninga JK, Gelderblom H, Fiocco M, Kroep JR, Taminiau AHM, Hogendoorn PCW, Egeler RM. Chemotherapeutic adjuvant treatment for osteosarcoma: Where do we stand? Eur J Cancer 2011;47:2431–2445. [DOI] [PubMed] [Google Scholar]
- 17.Rosen G, Caparros B, Huvos AG, Kosloff C, Nirenberg A, Cacavio A, Marcove RC, Lane JM, Mehta B, Urban C. Preoperative chemotherapy for osteogenic sarcoma: Selection of postoperative adjuvant chemotherapy based on the response of the primary tumor to preoperative chemotherapy. Cancer 1982;49:1221–1230. [DOI] [PubMed] [Google Scholar]
- 18.Goorin AM, Schwartzentruber DJ, Devidas M, Gebhardt MC, Ayala AG, Harris MB, Helman LJ, Grier HE, Link MP, Pediatric Oncology Group. Presurgical chemotherapy compared with immediate surgery and adjuvant chemotherapy for nonmetastatic osteosarcoma: Pediatric Oncology Group Study POG-8651. J Clin Oncol 2003;21:1574–1580. [DOI] [PubMed] [Google Scholar]
- 19.Sutow WW, Sullivan MP, Fernbach DJ, Cangir A, George SL. Adjuvant chemotherapy in primary treatment of osteogenic sarcoma. A Southwest Oncology Group study. Cancer 1975;36:1598–1602. [DOI] [PubMed] [Google Scholar]
- 20.Sarosy G, Leyland-Jones B, Soochan P, Cheson BD. The systemic administration of intravenous melphalan. J Clin Oncol 1988;6:1768–1782. [DOI] [PubMed] [Google Scholar]
- 21.Graham ML, Herndon JE, Casey JR, Chaffee S, Ciocci GH, Krischer JP, Kurtzberg J, Laughlin MJ, Longee DC, Olson JF, Paleologus N, Pennington CN, Friedman HS. High-dose chemotherapy with autologous stem-cell rescue in patients with recurrent and high-risk pediatric brain tumors. J Clin Oncol 1997;15:1814–1823. [DOI] [PubMed] [Google Scholar]
- 22.Winkler K, Beron G, Delling G, Heise U, Kabisch H, Purfürst C, Berger J, Ritter J, Jürgens H, Gerein V. Neoadjuvant chemotherapy of osteosarcoma: Results of a randomized cooperative trial (COSS-82) with salvage chemotherapy based on histological tumor response. J Clin Oncol 1988;6:329–337. [DOI] [PubMed] [Google Scholar]
- 23.Ferrari S, Meazza C, Palmerini E, Tamburini A, Fagioli F, Cozza R, Ferraresi V, Bisogno G, Mascarin M, Cefalo G, Manfrini M, Capanna R, Biagini R, Donati D, Picci P. Nonmetastatic osteosarcoma of the extremity. Neoadjuvant chemotherapy with methotrexate, cisplatin, doxorubicin and ifosfamide. An Italian Sarcoma Group study (ISG/OS-Oss). Tumori 2014;100:612–619. [DOI] [PubMed] [Google Scholar]
- 24.Marina N, Smeland S, Bielack SS, Bernstein M, Jovic G, Hook JM, Krailo M, Butterfass-Bahloul T, Kuhne T, Eriksson M, Teot LA, Gelderblom H, Kager L, Hall KS, Gorlick R, Randall RL, Hogendoorn P, Calaminus G, Sydes MR, Whelan J. MAPIE vs MAP as postoperative chemotherapy in patients with a poor response to preoperative chemotherapy for newly-diagnosed osteosarcoma: Results from EURAMOS-1, 2014 presented at Connective Tissue Oncology Society meeting, Berlin, October 2014. [Google Scholar]
- 25.Arpaci F, Ataergin S, Ozet A, Erler K, Basbozkurt M, Ozcan A, Komurcu S, Ozturk B, Celasun B, Kilic S, Kuzhan O. The feasibility of neoadjuvant high-dose chemotherapy and autologous peripheral blood stem cell transplantation in patients with nonmetastatic high grade localized osteosarcoma: Results of a phase II study. Cancer 2005;104:1058–1065. [DOI] [PubMed] [Google Scholar]
- 26.Boye K, Del Prever AB, Eriksson M, Saeter G, Tienghi A, Lindholm P, Fagioli F, Skjeldal S, Ferrari S, Hall KS. High-dose chemotherapy with stem cell rescue in the primary treatment of metastatic and pelvic osteosarcoma: Final results of the ISG/SSG II study. Pediatr Blood Cancer 2014;61:840–845. [DOI] [PubMed] [Google Scholar]
- 27.Andreou D, Bielack SS, Carrle D, Kevric M, Kotz R, Winkelmann W, Jundt G, Werner M, Fehlberg S, Kager L, Kühne T, Lang S, Dominkus M, Exner GU, Hardes J, Hillmann A, Ewerbeck V, Heise U, Reichardt P, Tunn P-U. The influence of tumor- and treatment-related factors on the development of local recurrence in osteosarcoma after adequate surgery. An analysis of 1355 patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. Ann Oncol 2011;22:1228–1235. [DOI] [PubMed] [Google Scholar]


