Abstract
The advent of sodium-glucose cotransporter 2 (SGLT2) inhibitors represents a major advance for people with type 2 diabetes (T2DM) and chronic kidney disease (CKD). The results of the Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) trial have clearly demonstrated that canagliflozin prevents kidney failure and cardiovascular events. The results from three other large-scale randomized trials, collectively enrolling >30 000 participants, have provided further evidence that the effects of SGLT2 inhibition on major kidney outcomes in people with T2DM may be present across the class, although this will only be known for certain when Dapagliflozin and Renal Outcomes and Cardiovascular Mortality in Patients with CKD (DAPA-CKD) (NCT03036150) and The Study of Heart and Kidney Protection with Empagliflozin (EMPA-KIDNEY) (NCT03594110) are reported over coming years. Importantly, the benefits of SGLT2 inhibition have been achieved in addition to the current standard of care. This review summarizes evidence for SGLT2 inhibition in people with T2DM and CKD, evaluates key patient characteristics and concomitant drug use that may influence the use of these drugs in people with CKD, discusses current guideline recommendations and explores how these drugs may be used in people with CKD in the future, including in combination with other treatments.
Keywords: chronic kidney disease, clinical outcomes, SGLT2 inhibitors, type 2 diabetes
INTRODUCTION
Since 2008, the US Food and Drug Administration (FDA) and other regulators have mandated that all new glucose-lowering agents undergo long-term cardiovascular outcome trials to demonstrate safety, primarily in response to concerns that drugs (notably rosiglitazone) that were effective in improving glycaemic control could increase cardiovascular risk [1]. Just over a decade later, almost 20 large-scale cardiovascular or kidney outcome trials have been completed, resulting in an explosion of evidence that has transformed diabetes care. Of the newer classes of glucose-lowering agents, sodium-glucose cotransporter 2 (SGLT2) inhibitors have been shown to consistently reduce the risk of clinically important, patient-level cardiovascular outcomes, including atherosclerotic cardiovascular events and hospitalization for heart failure (HF) [2]. The advent of SGLT2 inhibitors thus represents a major advance for individuals with type 2 diabetes mellitus (T2DM) and those who care for them.
The emergence of evidence for the SGLT2 inhibitor class has been watched with great anticipation in the nephrology community. It has been almost two decades since the benefits of renin–angiotensin system (RAS) blockade were confirmed in Irbesartan Diabetic Nephropathy Trial (IDNT) [3] and Reduction of Endpoints in Non-Insulin-Dependent Diabetes Mellitus with Angiotensin 2 Antagonist Losartan (RENAAL) [4]; however, despite this, growth in the global burden of diabetic kidney disease (DKD) has continued unabated [5, 6]. The capacity of SGLT2 inhibitors to reduce albuminuria created hope for a clinically meaningful kidney benefit. This optimism was further reinforced by secondary analyses of the SGLT2 cardiovascular outcome trials, which demonstrated that these drugs consistently reduced the risk of serum creatinine-based kidney outcomes [7–10]. However, most participants in these trials were at low risk of end-stage kidney disease (ESKD) and thus the effect of SGLT2 inhibitors on the most important patient-level kidney outcome—namely the need for dialysis or transplantation—was uncertain.
The Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) trial was designed to specifically address this evidence gap [11]. The trial demonstrated that an SGLT2 inhibitor, canagliflozin, substantially reduced the progression of DKD (doubling of serum creatinine, ESKD or cardiovascular or kidney-related death) in participants with T2DM and severely increased albuminuria who were already receiving RAS blockade. Indeed, CREDENCE demonstrated, for the first time ever, that a single intervention reduced the need for dialysis, transplantation or death due to kidney disease in its own right. A 2019 meta-analysis [12] synthesizing the accumulated trial evidence provided further strong support for the role of SGLT2 inhibition for kidney protection in people with T2DM and that the combination of RAS blockade and SGLT2 inhibition should be routinely offered to patients with T2DM who have, or are at high risk of, progressive kidney disease, with the strongest evidence for canagliflozin.
In the face of these clear and substantial benefits for cardiovascular and kidney outcomes, a number of other important questions remain. Who might benefit most from treatment? How should this evidence be applied in routine practice to maximize benefits and ensure potential harms are minimized? Are there other patient groups, aside from those with T2DM, who may benefit from SGLT2 inhibitors? Finally, how should these agents be used in combination with other currently available and future treatments?
Some of these issues, including SGLT2 inhibition in people with type 1 diabetes and in people with non-DKD, have been examined in this special issue of NDT. In this analysis we explore how select patient characteristics and how concomitant drug use might influence the use of SGLT2 inhibitors in people with T2DM and chronic kidney disease (CKD), discuss current guidelines recommendations and explore future research priorities in this area.
SGLT2 inhibition across different levels of estimated glomerular filtration rate (eGFR)
The completed trials included participants with varying levels of baseline eGFR and albuminuria. In the three cardiovascular outcome trials, the proportion of participants with baseline eGFR <60 mL/min/1.73 m2 ranged from 7.4 to 25.9% [8, 13, 14]. In contrast, ∼60% of participants in CREDENCE had a baseline eGFR <60 mL/min/1.73 m2. Most participants in the cardiovascular outcome trials had levels of albuminuria within the normal range at baseline [9, 15, 16], whereas those in the CREDENCE trial were required to have a urinary albumin:creatinine ratio of at least 300 mg/g at enrolment. While protection against kidney failure with SGLT2 inhibitors other than canagliflozin remains to be demonstrated in the ongoing kidney outcome trials, the accumulated trial evidence has allowed for a robust assessment of the effects of SGLT2 inhibition in patients with T2DM across varying levels of eGFR and albuminuria.
The glucose-lowering effect of SGLT2 inhibitors is directly proportional to glomerular filtration, and thus glycaemic efficacy decreases substantially as kidney function declines [17]. Because regulatory approvals for these drugs have been primarily based on their ability to reduce glycated haemoglobin (HbA1c), SGLT2 inhibitors have largely not been approved for use in people with an eGFR <45 mL/min1.73 m2 [18]. However, in the CREDENCE trial, the effect of canagliflozin on doubling of serum creatinine, ESKD or death due to kidney disease {hazard ratio [HR] 0.66 [95% confidence interval (CI) 0.53–0.81]} was consistent across all levels of kidney function down to an eGFR of 30 mL/min/1.73 m2. The effect of canagliflozin on cardiovascular death, non-fatal myocardial infarction or non-fatal stroke [HR 0.80 (95% CI 0.67–0.95)] was also not modified by baseline kidney function. While largely powered by the effects of canagliflozin observed in the CREDENCE trial, a recent meta-analysis [12] of the four major trials showed that SGLT2 inhibition reduces the risk of progression of kidney disease across all levels of kidney function studied to date, including ∼30% proportional risk reduction in people with an eGFR of 30–45 mL/min/1.73 m2, in whom the glucose-lowering effect is almost completely abrogated.
The disconnect between glucose lowering and effects on kidney and cardiovascular outcomes is a characteristic feature of SGLT2 inhibition. This is further supported by head-to-head data showing that canagliflozin slows the loss of kidney function compared with glimepiride [19] and that kidney benefits are independent of HbA1c before and during therapy, and by the degree of HbA1c lowering [20].
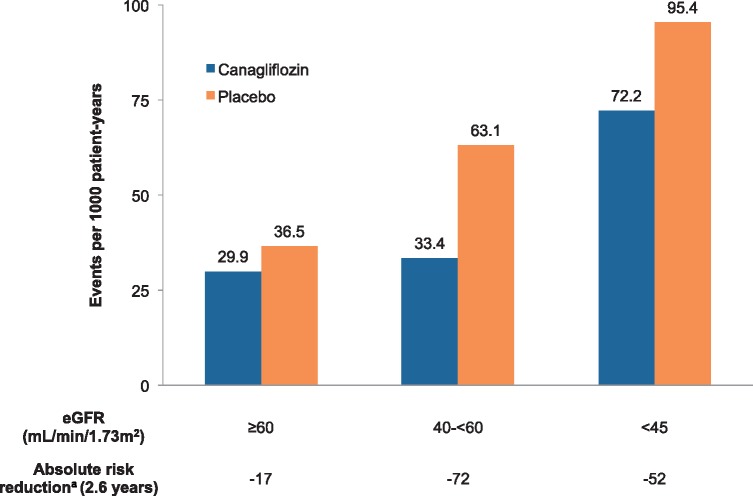
Because the risk of ESKD and cardiovascular mortality increases substantially as eGFR declines [21, 22], the absolute effects of treatment with SGLT2 inhibition are likely to be at least as large, if not greater, in patients with an eGFR <60 mL/min/1.73 m2 compared with those with preserved kidney function. In the CREDENCE trial, the estimated number of primary events prevented per 1000 patients treated over 2.6 years is substantially greater in participants with a starting eGFR <60 mL/min/1.73 m2 (Figure 1).
FIGURE 1.
Estimated number of primary events (doubling of serum creatinine, ESKD or cardiovascular or kidney-related death) prevented per 1000 patients treated over 2.6 years in the CREDENCE trial by baseline eGFR. aAbsolute risk reductions estimated as the number of events prevented per 1000 patients treated over 2.6 years.
The accumulated trial evidence provides strong evidence that SGLT2 inhibition should be prioritized in people with T2DM and CKD, including those with a starting eGFR of 30–45 mL/min/1.73 m2. Importantly, in the CREDENCE trial, participants whose eGFR fell to <30 mL/min/1.73 m2 continued on randomized treatment until dialysis or transplantation. As a result, the FDA now permits the continued use of canagliflozin with an eGFR <30 mL/min/1.73 m2 until dialysis or transplantation in people already initiated on therapy [23].
Impact of albuminuria
SGLT2 inhibition ameliorates albuminuria by approximately a third in patients with moderate or severely increased albuminuria, with lesser effects in those with normal albuminuria [15, 24]. If these drugs protect the kidney solely by lowering albuminuria as hypothesized, then people with higher levels of albuminuria should benefit more. However, a meta-analysis of the major SGLT2 inhibitor trials, which included CREDENCE, demonstrated that the proportional kidney benefits are consistent irrespective of baseline albuminuria [12]. Secondary analyses of the cardiovascular outcome trials also suggest that cardiovascular benefits might be similar across different levels of albuminuria [15, 16].
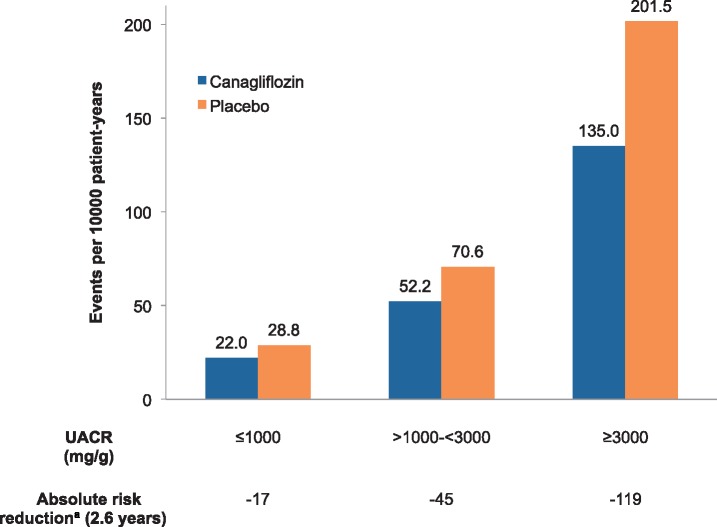
The consistent evidence of kidney protection across the spectrum of albuminuria has important implications because the clinical phenotype and presentation of DKD have changed in recent decades [25]. Normoalbuminuric DKD is increasingly common; it is estimated that ∼40% of people with T2DM develop reduced kidney function without having albuminuria documented [26, 27]. While the risk of ESKD for these individuals is low, progressive loss of kidney function can still occur [28], with a heterogeneous range of structural changes on kidney biopsy [25], suggesting that there are probably a number of different mechanisms of disease progression aside from classically progressive albuminuria. SGLT2 inhibition clearly reduces cardiovascular events and may reduce kidney events even in people with normal albuminuria and thus represents a promising therapeutic option for this patient population. Furthermore, T2DM with normal albuminuria is much more common than classically progressive DKD, therefore SGLT2 inhibition may be an important strategy for kidney (and cardiovascular) risk reduction at a population level. At the same time, because patients with substantially increased albuminuria are at much higher risk of ESKD, treatment with SGLT2 inhibitors in these individuals should be prioritized because the absolute benefits are greater (Figures 2 and 3; note the scale in Figure 2 is twice as wide as in Figure 1) [15].
FIGURE 2.
Estimated number of primary events (doubling of serum creatinine, ESKD or cardiovascular or kidney-related death) prevented per 1000 patients treated over 2.6 years in the CREDENCE trial by baseline UACR. Absolute risk reductions estimated as the number of events prevented per 1000 patients treated over 2.6 years. UACR: urinary albumin:creatinine ratio.
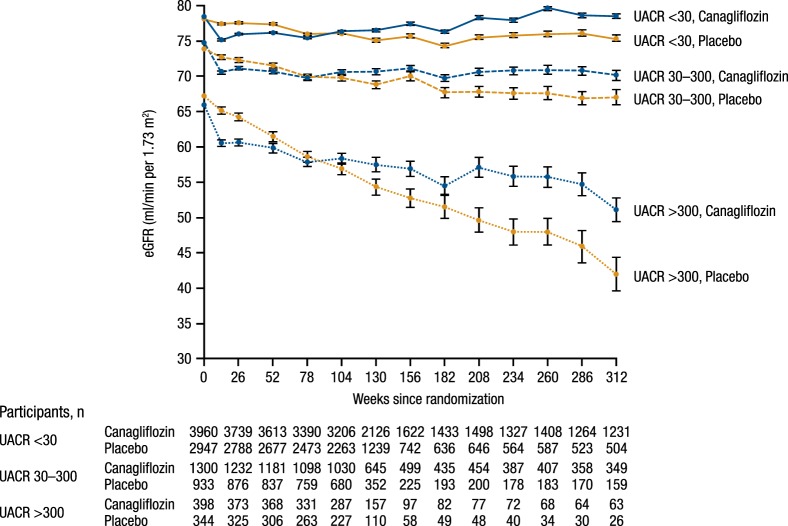
FIGURE 3.
Effect of canagliflozin on eGFR over time by baseline UACR in the Canagliflozin Cardiovascular Asssessment Study (CANVAS) Program. P-interaction <0.0001 for differences between UACR subgroups. UACR: urinary albumin:creatinine ratio. Reproduced from Neuen et al. [15].
Stratification of risk based on atherosclerotic cardiovascular disease
The presence or absence of atherosclerotic cardiovascular disease has typically been used to stratify absolute cardiovascular risk and identify which patients should be treated with cardioprotective therapies (i.e. primary versus secondary prevention). While this may be a useful approach for the prevention of atherosclerotic vascular events in patients without CKD, this distinction is probably of limited use in patients with CKD. A 2018 meta-analysis of the cardiovascular outcome trials showed that benefits for HF and kidney disease progression are consistent irrespective of a history of atherosclerotic cardiovascular disease [2]. Because individuals with T2DM and CKD are at very high risk of both outcomes, treatment with SGLT2 inhibitors should be offered regardless, as suggested in the most recent clinical practice guidelines (discussed below). In CREDENCE, consistent protection against cardiovascular and kidney outcomes was observed for primary and secondary prevention cohorts [29], further emphasizing that stratifying absolute risk based on the history of atherosclerotic cardiovascular disease alone is short-sighted, particularly in patients with T2DM and severely increased albuminuria, for whom the absolute risk of adverse outcomes is particularly high.
SGLT2 inhibition with and without RAS blockade
The CREDENCE trial provided strong evidence that kidney and cardiovascular protection with canagliflozin is achieved in addition to RAS blockade, as the use of RAS blockade was mandated for entry into the trial. In comparison, there is less data on whether SGLT2 inhibitor monotherapy is effective at slowing the progression of kidney disease, although subgroup data from the cardiovascular outcome trials appear promising. A meta-analysis of these trials found that kidney protection was consistent regardless of baseline use of RAS blockade, a finding limited somewhat by the fact all but ∼20% of participants were receiving RAS blockade [12]. Nevertheless, these data provide some justification for the use of SGLT2 inhibitors without background RAS blockade in certain patients, for example, those with normoalbuminuric DKD, for whom there is somewhat less data on kidney protection at lower levels of albuminuria [30], or those experiencing hyperkalaemia, which occurs more frequently as kidney function declines [31]. Reassuringly, no increased risk of acute kidney injury, volume depletion or hyperkalaemia were observed in the CREDENCE trial, suggesting that the combined use of SGLT2 inhibition and RAS blockade should be well tolerated from a haemodynamic perspective, if used appropriately.
Concomitant diuretics: safe and effective?
The efficacy and safety of SGLT2 inhibitors in combination with diuretics are of particular clinical relevance because the use of diuretics becomes increasingly common as kidney function declines and because hypertension and HF are highly prevalent in patients with DKD [32, 33]. Concerns have been expressed about the potential for volume depletion with broad untargeted use of SGLT2 inhibitors, particularly, when used in conjunction with loop diuretics [34]. To minimize this risk, SGLT2 inhibitors should not be initiated in people with unstable volume status or those with hypovolaemia. Furthermore, loop diuretic dose should be adjusted appropriately (SGLT2 inhibitors are loop diuretic sparing). Subgroup analyses from the cardiovascular outcome trials suggest that the effects of SGLT2 inhibition on kidney and cardiovascular outcomes are mostly consistent in patients receiving and not receiving diuretics, and data from the EMPA-REG OUTCOME trial (NCT01131676) showed that diuretic use did not alter the risk of kidney or volume-related adverse events [7, 9, 35, 36]. However, the overall risk of adverse kidney outcomes in these trials was low, and corresponding data in a high-risk population, such as CREDENCE, will provide further important information.
Metformin and the question of first-line treatment
In almost all clinical practice guidelines, metformin remains the preferred first-line pharmacotherapy for T2DM, in large part due to its low cost, tolerability and safety. However, evidence for cardiovascular and kidney benefits with new glucose-lowering agents has led to some debate about metformin’s place as first-line treatment for all patients [37], especially since its effects on cardiovascular outcomes are not clear [38], with little direct evidence for kidney outcomes. Most recently, joint guidelines from the European Society of Cardiology (ESC) and European Association for the Study of Diabetes (EASD) suggest SGLT2 inhibitors and glucagon-like peptide-1 (GLP-1) agonists should be used in patients with T2DM who have or are at high risk of cardiovascular disease, whether they are treatment naïve or already receiving metformin [39]. In the EMPA-REG OUTCOME trial, compared with participants receiving metformin, the effects on cardiovascular death and on hospitalization for HF were at least as large in participants not on metformin [36, 40], but otherwise there are currently scant data on the effects of SGLT2 inhibitors with and without metformin. Absolute benefits, possible adverse effects, reimbursement and out-of-pocket costs would all be important to consider if SGLT2 inhibitors are to be used as first-line oral therapy.
Latest guideline recommendations on the use of SGLT2 inhibitors
Several major clinical practice guidelines have been updated in the past 18 months to reflect the evolving evidence for cardiovascular and kidney protection from SGLT2 trials. Major updates include a joint consensus report from the American Diabetes Association (ADA) and the EASD, and the ADA Standards of Care [41, 42]. These have been accompanied by a number of statements from other organizations including the American College of Cardiology, ESC, American Association of Clinical Endocrinologists, Diabetes Canada and European Renal Association-European Dialysis and Transplant Association [39, 43–46].
Most noticeably, guidelines now recommended the selection of agents based on end-organ protection and patient co-morbidities. Most guidelines recommend either SGLT2 inhibitors or GLP-1 receptor agonists as second-line treatment (after metformin) in people with T2DM and a history of atherosclerotic cardiovascular disease (i.e. for the secondary prevention of cardiovascular events). The ESC–EASD guidelines recommend either an SGLT2 inhibitor or GLP-1 receptor agonist in people with T2DM at high or very high risk of cardiovascular disease, irrespective of whether they are treatment naïve or already on metformin.
Given the consistent evidence that SGLT2 inhibition can reduce the risk of hospitalization for HF and kidney disease progression, the ADA–EASD consensus report also recommends the use of SGLT2 inhibitors in people with T2DM and HF or CKD (irrespective of a history of atherosclerotic cardiovascular disease). Based on the results of the CREDENCE trial, the updated 2019 ADA Standards of Care specifically endorse the use of SGLT2 inhibitors for the prevention of kidney failure, cardiovascular events or both in patients with an eGFR >30 mL/min/1.73 m2, particularly in those with severely increased albuminuria (Grade A recommendation) [47].
Implementation of evidence into clinical practice
There is strong evidence from completed trials that patients with T2DM and CKD are among those who are likely to benefit most from SGLT2 inhibition, with larger absolute risk reductions for those with reduced kidney function and higher levels of albuminuria (Figures 1 and 2). The strongest evidence for kidney protection is with canagliflozin, and while data from cardiovascular outcome trials of other SGLT2 inhibitors are promising, a class effect on patient-level kidney outcomes (i.e. dialysis or transplantation) remains to be demonstrated in ongoing kidney outcome trials. Nevertheless, there is currently limited evidence that key patient characteristics or use of concomitant drugs modifies the efficacy or safety of SGLT2 inhibition (Table 1), supporting that these drugs should be routinely offered to most patients with T2DM and CKD.
Table 1.
Key patient characteristics and concomitant drug use influencing the decision to use SGLT2 inhibitors
| Key patient characteristics/ concomitant drug use | Overall conclusion | Level of evidence | Limitations and other considerations |
|---|---|---|---|
| eGFR | Kidney protection achieved across all levels of starting eGFR >30 mL/min/1.73 m2 | Meta-analysis of subgroup data from the major SGLT2 trials |
|
| Albuminuria | Kidney protection consistent across different levels of albuminuria | Meta-analysis of subgroup data from the major SGLT2 trials |
|
| ASCVD | Consistent protection against progression of kidney disease and HF irrespective of prior ASCVD | Meta-analysis of CVOTs and pre-specified secondary analysis of CREDENCE | Risk stratification based on ASCVD alone likely to be of limited value in people with CKD who are already at elevated risk of cardiovascular events |
| RAS blockade | Effect on kidney outcomes probably similar in participants receiving and not receiving RAS blockade | Meta-analysis of subgroup data from major SGLT2 trials |
|
| Diuretics | Benefits probably unaltered by concomitant diuretics and safe if used appropriately | Subgroup data from CVOTs (efficacy) and EMPA-REG OUTCOME (safety) | Participants in the CVOTs were generally at low risk of kidney-related adverse events |
| Metformin | Possibly similar irrespective of metformin use (cardiovascular outcomes) | Subgroup data from the EMPA-REG OUTCOME trial |
|
| GLP-1 receptor agonists | Potential for additive protection by different mechanisms | Theoretical benefits | No randomized evidence for patient-level cardiovascular or kidney outcomes |
ASCVD, atherosclerotic cardiovascular disease; CVOT, cardiovascular outcome trial.
Collaborative efforts from the nephrology community and other stakeholders are now required to ensure that findings from randomized trials are translated into routine clinical practice. In this respect, initiatives such as the Diabetic Kidney Disease Collaborative (DKD-C) are welcome. The DKD-C was recently launched by the American Society of Nephrology to increase coordination between primary care, nephrologists and other specialties to deliver optimal care to people with diabetes and CKD. Current data suggest that a substantial proportion of patients with CKD who would benefit from treatment with RAS blockade still do not receive it [48]. As such, implementation research should be a priority to speed the incorporation of SGLT2 inhibitors and other proven therapies into clinical practice for the benefit of patients.
Looking to the future
With the growing number of therapeutic options for people with T2DM, there is a need to better understand the optimal combination of treatments. There is strong evidence that GLP-1 receptor agonists also reduce the risk of a range of cardiovascular events [49]. The types of events prevented with GLP-1 receptor agonists, and time frames over which benefits accrue, highlight that the mechanism of cardioprotection with these agents is likely to be distinct from SGLT2 inhibitors and mediated mainly through anti-atherothrombotic effects. In contrast, SGLT2 inhibitors have greater benefits on HF and kidney outcomes, underscoring their unique haemodynamic effects. Additionally, GLP-1 receptor agonists are permitted for use down to an eGFR of 15 mL/min/1.73 m2 in some regions, presenting an important treatment option for patients with advanced CKD. The contrasting mechanisms and benefits of these two classes of drugs raise the possibility that combination therapy with GLP-1 receptor agonists and SGLT2 inhibitors could provide additive cardiovascular and kidney benefits. Short-term trials of semaglutide and dulaglutide suggest that combination treatment reduces HbA1c and body weight to a greater extent than SGLT2 inhibition alone, without additional safety concerns [50, 51]. However, randomized evidence on patient-level cardiovascular and kidney outcomes is currently lacking and a trial designed to test these agents in combination would be very valuable. In lieu of such trials, the uptake of SGLT2 inhibitors in routine clinical practice will mean that future randomized studies, including of GLP-1 receptor agonists, may well include substantial numbers of participants receiving SGLT2 inhibitor therapy, and thus provide indirect evidence on the effects of these drug classes used in combination.
Most recently, the results of the first dedicated HF outcome trial of an SGLT2 inhibitor, DAPA-HF (dapagliflozin; NCT03036124), were presented at the 2019 ESC Congress. DAPA-HF is notable because it is the first SGLT2 inhibitor trial enrolling participants with and without T2DM. In this trial, the effect on the primary outcome of urgent HF visit, hospitalization for HF or cardiovascular death [HR 0.74 (95% CI 0.65–0.85)] was similar in participants with and without T2DM [52], further strengthening the hypothesis that these drugs may also benefit individuals with non-diabetic CKD. Likewise, DAPA-CKD and EMPA-KIDNEY are also enrolling participants without T2DM, and thus are expected to provide important evidence on the effects of SGLT2 inhibition on kidney outcomes in people without diabetes [53, 54]. These trials also include participants with a starting eGFR as low as 20 mL/min/1.73 m2 and thus may potentially provide some important information in people with advanced CKD.
CONCLUSION
SGLT2 inhibitors are undoubtedly a practice-changing development for patients with T2DM and CKD. Evidence from completed trials strongly supports the role of SGLT2 inhibition to prevent kidney and cardiovascular events in patients with T2DM, and those with lower levels of eGFR and higher levels of albuminuria are among those who stand to gain the greatest absolute benefits.
ACKNOWLEDGEMENTS
This work was not specifically funded. B.L.N. is supported by an Australian National Health and Medical Research Council Postgraduate Scholarship; the John Chalmers PhD Scholarship from the George Institute for Global Health; a University Postgraduate Award from the University of New South Wales Sydney; the Graduate Research Fund at Lincoln College, University of Oxford; and an Oxford Australia Clarendon Scholarship from the University of Oxford. M.J.J. is supported by a Medical Research Future Fund Next-Generation Clinical Researchers Programme Career Development Fellowship; V.P. reports receiving research support from the Australian National Health and Medical Research Council (Senior Research Fellowship and Programme Grant).
CONFLICT OF INTEREST STATEMENT
He has received travel support from Janssen. Serves on steering committees for Janssen and CSL; is responsible for research projects that have received unrestricted funding from Gambro, Baxter, CSL, Amgen, Eli Lilly and Merck; has served on advisory boards sponsored by Akebia, Baxter and Boehringer Ingelheim; and has spoken at scientific meetings sponsored by Janssen, Amgen and Roche, with any consultancy, honoraria or travel support paid to her institution. Serving on steering committees for AbbVie, Boehringer Ingelheim, Gilead, GlaxoSmithKline, Janssen, Novo Nordisk and Pfizer; serving on advisory boards and/or speaking at scientific meetings for AbbVie, Astellas, AstraZeneca, Bayer, Baxter, Bristol-Myers Squibb, Boehringer Ingelheim, Durect, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Merck, Novartis, Novo Nordisk, Pfizer, Pharmalink, Relypsa, Roche, Sanofi, Servier and Vitae; and receiving personal fees for consulting and scientific presentations related to canagliflozin from Mitsubishi Tanabe and Mundipharma.
REFERENCES
- 1. Cefalu WT, Kaul S, Gerstein HC. et al. Cardiovascular outcomes trials in type 2 diabetes: where do we go from here? Reflections from a diabetes care editors’ expert forum. Diabetes Care 2018; 41: 14–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zelniker TA, Wiviott SD, Raz I. et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019; 393: 31–39 [DOI] [PubMed] [Google Scholar]
- 3. Parving HH, Lehnert H, Bröchner-Mortensen J. et al. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med 2001; 345: 870–878 [DOI] [PubMed] [Google Scholar]
- 4. Brenner BM, Cooper ME, De Zeeuw D. et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345: 861–869 [DOI] [PubMed] [Google Scholar]
- 5. Neuen BL, Chadban SJ, Demaio AR. et al. Chronic kidney disease and the global NCDs agenda. BMJ Glob Health 2017; 2: e000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xie Y, Bowe B, Mokdad AH. et al. Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int 2018; 94: 567–581 [DOI] [PubMed] [Google Scholar]
- 7. Perkovic V, de Zeeuw D, Mahaffey KW. et al. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS Program randomised clinical trials. Lancet Diabetes Endocrinol 2018; 6: 691–704 [DOI] [PubMed] [Google Scholar]
- 8. Wanner C, Inzucchi SE, Lachin JM. et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016; 375: 323–334 [DOI] [PubMed] [Google Scholar]
- 9. Mosenzon O, Wiviott SD, Cahn A. et al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE–TIMI 58 randomised trial. Lancet Diabetes Endocrinol 2019; 7: 606–617. [DOI] [PubMed] [Google Scholar]
- 10. Neal B, Perkovic V, Mahaffey KW. et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377: 644–657 [DOI] [PubMed] [Google Scholar]
- 11. Perkovic V, Jardine MJ, Neal B. et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019; 380: 2295–2306 [DOI] [PubMed] [Google Scholar]
- 12. Neuen BL, Young T, Heerspink HJ. et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 2019; 7: 845–854 [DOI] [PubMed] [Google Scholar]
- 13. Neuen BL, Ohkuma T, Neal B. et al. Cardiovascular and renal outcomes with canagliflozin according to baseline kidney function: data from the CANVAS Program. Circulation 2018; 138: 1537–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wiviott SD, Raz I, Bonaca MP. et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019; 380: 347–357 [DOI] [PubMed] [Google Scholar]
- 15. Neuen BL, Ohkuma T, Neal B. et al. Effect of canagliflozin on renal and cardiovascular outcomes across different levels of albuminuria: data from the CANVAS Program. J Am Soc Nephrol 2019; 30: 2229–2242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wanner C, Lachin JM, Inzucchi SE. et al. Empagliflozin and clinical outcomes in patients with type 2 diabetes mellitus, established cardiovascular disease, and chronic kidney disease. Circulation 2018; 137: 119–129 [DOI] [PubMed] [Google Scholar]
- 17. Heerspink HJ, Perkins BA, Fitchett DH. et al. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation 2016; 134: 752–772 [DOI] [PubMed] [Google Scholar]
- 18. Toyama T, Neuen BL, Jun M. et al. Effect of SGLT2 inhibitors on cardiovascular, renal and safety outcomes in patients with type 2 diabetes mellitus and chronic kidney disease: a systematic review and meta‐analysis. Diabetes Obes Metab 2019; 21: 1237–1250 [DOI] [PubMed] [Google Scholar]
- 19. Heerspink HJ, Desai M, Jardine M. et al. Canagliflozin slows progression of renal function decline independently of glycemic effects. J Am Soc Nephrol 2017; 28: 368–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cooper ME, Inzucchi SE, Zinman B. et al. Glucose control and the effect of empagliflozin on kidney outcomes in type 2 diabetes: an analysis from the EMPA-REG OUTCOME trial. Am J Kidney Dis 2019; 74: 713. [DOI] [PubMed] [Google Scholar]
- 21. Gansevoort RT, Matsushita K, van der Velde M. et al. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int 2011; 80: 93–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Matsushita K, Coresh J, Sang Y. et al. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol 2015; 3: 514–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Highlights of prescribing information. Invokana (canagliflozin) tablets. http://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/INVOKANA-pi.pdf (15 November 2019, date last accessed)
- 24. Cherney DZ, Zinman B, Inzucchi SE. et al. Effects of empagliflozin on the urinary albumin-to-creatinine ratio in patients with type 2 diabetes and established cardiovascular disease: an exploratory analysis from the EMPA-REG OUTCOME randomised, placebo-controlled trial. Lancet Diabetes Endocrinol 2017; 5: 610–621 [DOI] [PubMed] [Google Scholar]
- 25. Alicic RZ, Rooney MT, Tuttle KR.. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol 2017; 12: 2032–2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. MacIsaac RJ, Tsalamandris C, Panagiotopoulos S. et al. Nonalbuminuric renal insufficiency in type 2 diabetes. Diabetes Care 2004; 27: 195–200 [DOI] [PubMed] [Google Scholar]
- 27. Retnakaran R, Cull CA, Thorne KI. et al. Risk factors for renal dysfunction in type 2 diabetes: UK Prospective Diabetes Study 74. Diabetes 2006; 55: 1832–1839 [DOI] [PubMed] [Google Scholar]
- 28. Vistisen D, Andersen GS, Hulman A. et al. Progressive decline in estimated glomerular filtration rate in patients with diabetes after moderate loss in kidney function—even without albuminuria. Diabetes Care 2019; 42:1886–1894. [DOI] [PubMed] [Google Scholar]
- 29. Mahaffey K, Jardine M, Bompoint S. et al. Canagliflozin and cardiovascular and renal outcomes in type 2 diabetes and chronic kidney disease in primary and secondary cardiovascular prevention groups: results from the randomized CREDENCE trial. Circulation 2019; 140: 739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ruilope LM, Solini A.. RAS blockade for every diabetic patient: pro and con. Diabetes Care 2011; 34(Suppl 2): S320–S324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lazich I, Bakris GL.. Prediction and management of hyperkalemia across the spectrum of chronic kidney disease. Semin Nephrol 2014; 34: 333–339 [DOI] [PubMed] [Google Scholar]
- 32. House AA, Wanner C, Sarnak MJ. et al. Heart failure in chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 2019; 95: 1304–1317 [DOI] [PubMed] [Google Scholar]
- 33. Horowitz B, Miskulin D, Zager P.. Epidemiology of hypertension in CKD. Adv Chronic Kidney Dis 2015; 22: 88–95 [DOI] [PubMed] [Google Scholar]
- 34. Cherney DZ, Udell JA.. Use of sodium glucose cotransporter 2 inhibitors in the hands of cardiologists: with great power comes great responsibility. Circulation 2016; 134: 1915–1917 [DOI] [PubMed] [Google Scholar]
- 35. Mayer GJ, Wanner C, Weir MR. et al. Analysis from the EMPA-REG OUTCOME® trial indicates empagliflozin may assist in preventing the progression of chronic kidney disease in patients with type 2 diabetes irrespective of medications that alter intrarenal hemodynamics. Kidney Int 2019; 96: 489–504 [DOI] [PubMed] [Google Scholar]
- 36. Zinman B, Wanner C, Lachin JM. et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128 [DOI] [PubMed] [Google Scholar]
- 37. Khunti K, Seidu S, Davies MJ.. Should sodium glucose co-transporter 2 inhibitors be considered as first line oral therapy for people with type 2 diabetes? Diabetes Obes Metab 2018; 21: 207–209 [DOI] [PubMed] [Google Scholar]
- 38. Griffin SJ, Leaver JK, Irving GJ.. Impact of metformin on cardiovascular disease: a meta-analysis of randomised trials among people with type 2 diabetes. Diabetologia 2017; 60: 1620–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cosentino F, Grant PJ, Aboyans V. et al. 2019 ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASDThe task force for diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and the European Association for the Study of Diabetes (EASD). Eur Heart J 2013; 34: 3035–3087 [DOI] [PubMed] [Google Scholar]
- 40. Fitchett D, Zinman B, Wanner C. et al. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME® trial. Eur Heart J 2016; 37: 1526–1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Davies MJ, D’Alessio DA, Fradkin J. et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2018; 61: 2461–2498 [DOI] [PubMed] [Google Scholar]
- 42. American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2019. Diabetes Care 2019; 42(Suppl 1): S90–S102 [DOI] [PubMed] [Google Scholar]
- 43. Das SR, Everett BM, Birtcher KK. et al. 2018 ACC expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes and atherosclerotic cardiovascular disease: a report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol 2018; 72: 3200–3223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Garber AJ, Abrahamson MJ, Barzilay JI. et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm–2018 executive summary. Endocr Pract 2018; 24: 91–120 [DOI] [PubMed] [Google Scholar]
- 45. Lipscombe L, Booth G, Butalia S. et al. Pharmacologic glycemic management of type 2 diabetes in adults. Can J Diabetes 2018; 42(Suppl 1): S88–S103 [DOI] [PubMed] [Google Scholar]
- 46. Sarafidis P, Ferro CJ, Morales E. et al. SGLT-2 inhibitors and GLP-1 receptor agonists for nephroprotection and cardioprotection in patients with diabetes mellitus and chronic kidney disease. A consensus statement by the EURECA-m and the DIABESITY working groups of the ERA-EDTA. Nephrol Dial Transplant 2019; 34: 208–230 [DOI] [PubMed] [Google Scholar]
- 47. American Diabetes Association. 11. Microvascular complications and foot care: standards of medical care in diabetes—2019. Diabetes Care 2019; 42(Suppl 1): S124–S138 [DOI] [PubMed] [Google Scholar]
- 48. Murphy DP, Drawz PE, Foley RN.. Trends in angiotensin-converting enzyme inhibitor and angiotensin II receptor blocker use among those with impaired kidney function in the United States. J Am Soc Nephrol 2019; 30: 1314–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kristensen SL, Rørth R, Jhund PS. et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol 2019; 7: 776–785 [DOI] [PubMed] [Google Scholar]
- 50. Zinman B, Bhosekar V, Busch R. et al. Semaglutide once weekly as add-on to SGLT-2 inhibitor therapy in type 2 diabetes (SUSTAIN 9): a randomised, placebo-controlled trial. Lancet Diabetes Endocrinol 2019; 7: 356–367 [DOI] [PubMed] [Google Scholar]
- 51. Ludvik B, Frías JP, Tinahones FJ. et al. Dulaglutide as add-on therapy to SGLT2 inhibitors in patients with inadequately controlled type 2 diabetes (AWARD-10): a 24-week, randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 2018; 6: 370–381 [DOI] [PubMed] [Google Scholar]
- 52. McMurray JJ, Solomon SD, Inzucchi SE. et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019; 381: 1995–2008 [DOI] [PubMed] [Google Scholar]
- 53. Herrington WG, Preiss D, Haynes R. et al. The potential for improving cardio-renal outcomes by sodium-glucose co-transporter-2 inhibition in people with chronic kidney disease: a rationale for the EMPA-KIDNEY study. Clin Kidney J 2018; 11: 749–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pecoits-Filho R, Perkovic V.. Are SGLT2 inhibitors ready for prime time for CKD? Clin J Am Soc Nephrol 2018; 13: 318–320 [DOI] [PMC free article] [PubMed] [Google Scholar]