Abstract
Diabetic kidney disease (DKD) is a common complication of type 1 diabetes (T1D) and a major risk factor for premature death from cardiovascular disease (CVD). Current treatments, such as control of hyperglycaemia and hypertension, are beneficial, but only partially protect against DKD. Finding new, safe and effective therapies to halt nephropathy progression has proven to be challenging. Sodium-glucose cotransporter 2 (SGLT2) inhibitors have demonstrated, in addition to glycaemic lowering, impressive protection against DKD and CVD progression in people with type 2 diabetes. Although these beneficial cardiorenal effects may also apply to people with T1D, supporting data are lacking. Furthermore, the increased rates of euglycaemic diabetic ketoacidosis may limit the use of this class in people with T1D. In this review we highlight the pathophysiology of DKD in T1D and the unmet need that exists. We further detail the beneficial and adverse effects of SGLT2 inhibitors based on their mechanism of action. Finally, we balance the effects in people with T1D and indicate future lines of research.
Keywords: diabetic ketoacidosis, diabetic kidney disease, natriuresis, SGLT2 inhibitors, type 1 diabetes
DIABETIC KIDNEY DISEASE IN TYPE 1 DIABETES
Over 30 million people suffer from type 1 diabetes (T1D), increasing risk for early death mainly from cardiorenal disease [1, 2]. Despite advances in glycaemic and blood pressure control, a child diagnosed with T1D is expected to live up to 17 years less than non-diabetic peers [3–6]. The strongest risk factor for cardiovascular disease (CVD) and mortality in T1D is diabetic kidney disease (DKD) [7, 8]. DKD remains a common complication of T1D. Historically up to 40% of people with T1D had onset of elevated urinary albumin excretion and the majority of these progressed to end-stage kidney disease (ESKD) within 10–15 years [9]. Although the cumulative incidence of DKD has been reduced with the achievement of intensive glycaemic control and renin–angiotensin–aldosterone system (RAAS) blockade, it remains a major morbid complication. In the Diabetes Control and Complications Trial (DCCT) and its observational follow-up study, the Epidemiology of Diabetes Interventions and Complication (EDIC) study, 25% of participants assigned to intensive therapy still developed elevated urinary albumin excretion during follow-up [10]. Current treatment of cardiorenal risk factors, such as control of hyperglycaemia and hypertension, is beneficial, but only partially protect against DKD. Additionally, while intensive glycaemic control is crucial throughout the disease duration, RAAS blockade may be more important in individuals with some degree of elevated urinary albumin excretion. Clinical trials in DKD in T1D have yielded disappointing results [11–18], potentially due to the lack of interventions at early stages of disease when the benefit is most likely. The recently published results from the Adolescent Type 1 Cardiorenal Intervention Trial demonstrated that the use of angiotensin-converting enzyme inhibitor and statin failed to change urinary albumin excretion over time in youth with T1D [19]. To effectively mitigate DKD risk in T1D, therapeutic strategies may have to target pathophysiology specific to T1D, and caution should be exercised when extrapolating trial data from people with type 2 diabetes (T2D). Thus identifying therapies to impede DKD in T1D remain a public health priority.
For people with T2D, sodium-glucose cotransporter 2 (SGLT2) inhibitors have been introduced to improve hyperglycaemia. The glycaemic-lowering effects of SGLT2 inhibition are based on the fact that the kidneys approximately filter and completely reabsorb 180 g of glucose daily, an amount that is augmented in people with hyperglycaemia. The process of reabsorption is carried out by two transporters located in the proximal tubule: the high-capacity, low-affinity SGLT2 and the low-capacity, high-affinity SGLT1, the functions of which are upregulated to facilitate increased glucose fluxes in the state of chronic hyperglycaemia. As non-specific SGLT inhibition by phlorizin provoked intolerable gastrointestinal side effects, more specific inhibitors were developed. These more specific SGLT2 inhibitors, initially brought to the market for glucose-lowering in people with T2D, now include canagliflozin, dapagliflozin, empagliflozin (EMPA), luseogliflozin and ertugliflozin. Sotagliflozin is different from other SGLT2 inhibitors, as it also inhibits intestinal SGLT1 activity and was specifically developed for people with T1D [20]. The glucose-lowering effects of SGLT2 inhibitors in people with T2D have been discussed in detail elsewhere [20], but it is important to realize that it is largely dependent on the filtered glycaemic load, which is determined by prevailing glucose concentrations (level of glycaemic control) and glomerular filtration rate (GFR). In T2D, SGLT2 inhibitors have also been shown to protect pancreatic β cells against glucose toxicity and preserve insulin secretory capacity, and murine models suggest that this may also hold true for T1D [21]. The SGLT2 inhibitors have received attention not due to their glucose-lowering efficacy, but through their remarkable cardiorenal effects. Indeed, SGLT2 inhibitors were shown to reduce cardiovascular risk (in people with previous CVD) and progression of DKD in several large trials, including Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME), CANagliflozin cardioVascular Assessment Study (CANVAS), Multicenter Trial to Evaluate the Effect of Dapagliflozin on the Incidence of Cardiovascular Events (DECLARE-TIMI58) (NCT01730534), Evaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants With Diabetic Nephropathy (CREDENCE) (NCT02065791) as detailed below. Several mechanisms of DKD progression overlap in people with T2D and T1D, and SGLT2 is a promising nephroprotective agent in people with T1D, supported by some recent data from the inTandem 1 and 2 trials (NCT02384941 and NCT02421510, respectively) [22]. However, there are also important differences that may limit generalization of T2D cardiovascular outcome trials (CVOTs) data to people living with T1D, as discussed below.
PATHOPHYSIOLOGY OF DKD IN T1D
The natural history of the pathophysiology of DKD
The natural history of DKD in T1D is characterized by progressive pathological changes that develop over a long silent period without clinical evidence of kidney dysfunction [23]. In fact, kidney biopsy data have established that structural defects precede functional impairment [24–26]. The International Diabetic Nephropathy Study (IDNS) demonstrated that the principal morphometric abnormalities of early DKD included increased glomerular basement membrane width and fractional volume of mesangium and mesangial matrix [23]. Furthermore, IDNS found these abnormalities as early as 2 years after T1D onset and structural defects advanced once increased urinary albumin excretion becomes detectable [23]. Strong and robust relationships between glomerular structure and function have also been demonstrated in people with T1D [27, 28]. Notably, the histological features of DKD may differ in T1D and T2D. Compared with T1D, there appears to be greater structural lesion heterogeneity in people with T2D, for which reasons data should not be directly extrapolated [27–30]. The vast majority of studies have focused on the morphometry of the glomerulus, yet the proximal tubule may be equally if not more important in the initial onset of DKD [31]. In addition, there have been substantial advances and maturation of transcriptomic technologies that offer a platform to identify key genes and pathways involved in DKD in T1D. However, crucial components of the future success of these endeavours are deep phenotyping and access to renal tissue.
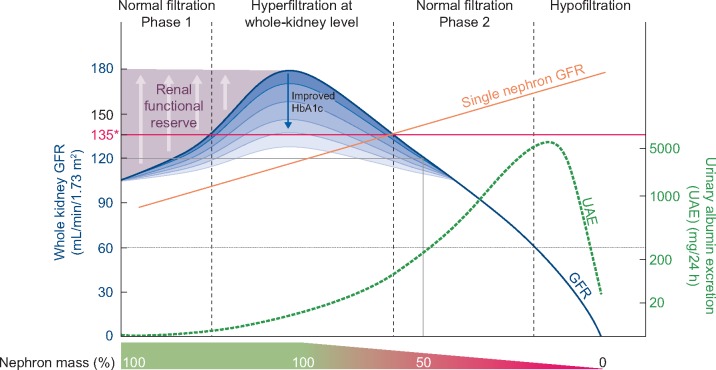
The sequence of progression of DKD in T1D has been proposed to start with hyperfiltration, resulting in glomerular injury followed by elevated urinary albumin excretion and progressive estimated GFR (eGFR) decline, eventually resulting in chronic kidney disease (CKD) and ESKD [32] (Figure 1). The data on hyperfiltration, however, are conflicting. For example, recent analysis of DCCT/EDIC data did not support hyperfiltration as an independent risk factor for the development of CKD and ESKD [33] and contradicts prior data from other groups [34, 35]. Although animal research and experimental models strongly support single-nephron hyperfiltration as an important early phenotype of kidney disease, whole-kidney GFR is measured in clinical research rather than single-nephron GFR. This is potentially problematic, as whole-kidney GFR is a product of the number of nephrons and the individual single-nephron GFR. Accordingly, to accurately diagnose single-nephron hyperfiltration from whole-kidney GFR, one relies on preserved nephron mass. However, nephron mass starts to progressively decline at ∼25–30 years of age, and the decrease is faster in people with risk factors such as diabetes and hypertension [36]. Therefore there is likely a substantial portion of people with single-nephron hyperfiltration who are misclassified as being normofilterers based on their apparently normal ‘whole-kidney GFR’ in the setting of a reduced nephron mass. This is a recognized problem in nephrology, for which reason there are substantial efforts being made to non-invasively quantify nephron mass (e.g. cationic-enhanced magnetic resonance imaging) and thereby estimate single-nephron GFR [37].
FIGURE 1.
Progression of DKD in T1D. * whole-kidney hyperfiltration (>= 135 ml/min/1.73m2)
This is also the reason it is preferred to study hyperfiltration as defined by ‘whole-kidney GFR’ in young people prior to progressive nephron loss, as there may be less heterogeneity in nephron numbers and therefore less misclassification bias. There is also no consensus on what rate of annual GFR loss constitutes rapid GFR decline. Annual declines >3–5 mL/min/1.73 m2 or 3.3% have been proposed to predict DKD progression and mortality by different groups [34, 35, 38–40]. The implication of moderately increased albuminuria, previously known as microalbuminuria, has also been questioned over the past few years after the demonstration that it does not necessarily imply progressive nephropathy and regresses to normoalbuminuria in a significant proportion of people without therapy [41, 42]. However, in those with persistent moderately increased albuminuria in the DCCT/EDIC study, the 15-year cumulative incidences of increased albuminuria (previously known as macroalbuminuria), impaired GFR and ESKD were 39, 19 and 7%, respectively [43]. Accordingly, persistent moderately increased albuminuria remains an important early phenotype of DKD. Further longitudinal research is needed to better understand the relationships between single-nephron hyperfiltration, rapid GFR decline and progressive nephropathy in people with T1D.
An energetic role of SGLT2 inhibition in DKD pathogenesis in people with T1D
The salutatory effects of SGLT2 inhibition in impeding DKD progression are incompletely accounted for by the modest improvements in HbA1c, weight and blood pressure. Metabolic and non-metabolic effects of SGLT2 inhibition have been proposed to explain the impressive cardiorenal benefits. The kidneys are highly metabolically active and are second only to the heart with respect to oxygen (O2) consumption per tissue mass. To sustain this activity, the kidneys rely on various substrates to generate adenosine triphosphate (ATP), including citrate, glutamine, glucose and free fatty acids [44]. Early DKD is associated with an environment that exacerbates renal O2 consumption in experimental models due to (i) elevated GFR and increased filtered sodium [34, 45, 46], (ii) increased activity of the Na+/K+ ATPase pump due to high tubular glucose and Na+ reabsorption and (iii) neurohormonal changes including increased vasopressinergic and RAAS activity [47–50]. In fact, animal models suggest that renal O2 consumption is increased by 40% in all cortical segments and by 160% each in the S3 segment and medullary collecting duct in the setting of renal hypertrophy and hyperfiltration [51–55]. Furthermore, emerging animal data suggest that in diabetes the kidneys are unable to sufficiently compensate for the increased O2 consumption due to the effects of insulin resistance and mitochondrial dysfunction on energy utilization [44, 56–58]. Data on renal O2 consumption in DKD are currently limited to animal models. SGLT2 inhibition attenuates whole-kidney hyperfiltration in adults with T1D [59] and single-nephron hyperfiltration in animal models [60] and offers renal protection in adults with T2D and CKD [61]. In addition, adult data suggest that SGLT2 inhibition can improve insulin sensitivity [62]. Finally, animal data suggest that SGLT2 inhibition improves renal oxygenation and ameliorates renal hypoxia [63]. It is unclear whether the improved renal oxygenation in response to SGLT2 inhibition relates to natriuresis, as sodium excretion is not expected to be altered with prolonged treatment [64, 65], likely through compensatory sodium reabsorption at more distal tubular segments. However, alterations in the location of sodium reabsorption may affect the reuptake of other molecules, such as uric acid, that may also play a role in ATP consumption and generation [20, 66]. The consequences of SGLT2 inhibitor-induced alterations in sodium handling are not limited to renal energetics and include important non-metabolic changes in interstitial fluid volume, systemic haemodynamics and vascular function, which are all likely to contribute to the observed cardiorenal benefits [67, 68].
DIFFERENCES IN INTRARENAL HAEMODYNAMIC EFFECTS OF SGLT2 INHIBITORS IN PEOPLE WITH T1D AND T2D
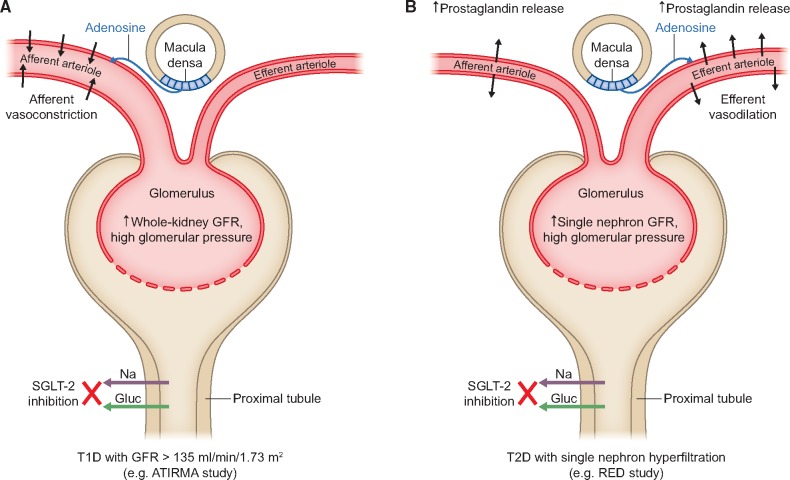
Above, we described that several similarities exist between DKD in people with T1D and T2D. However, important differences should also be noted. In young adults with T1D, 8 weeks of SGLT2 inhibition resulted in afferent arteriolar vasoconstriction and a decrease in GFR in participants with baseline hyperfiltration [59]. The afferent arteriolar vasoconstriction is proposed to be mediated by increased distal sodium delivery and tubuloglomerular feedback. In contrast, recent data in older adults with T2D suggest that SGLT2 inhibition confers efferent arteriolar vasodilation with attenuated renal vascular resistance, possibly due to increased prostaglandin release [69]. The mechanisms underlying the different effects on intrarenal haemodynamic function remain incompletely understood but may relate to differences in factors governing arteriolar tone, including RAAS blockade and prostaglandin activity (Figure 2). For example, RAAS activation in DKD in T1D is associated with greater afferent arteriolar than efferent arteriolar vasoconstriction [70]. People with T1D are also known to have elevated circulating plasma cyclic guanosine monophosphate, which has been linked to greater efferent arteriolar tone and may explain the lack of efferent vasodilation in response to SGLT2 inhibition in people with T1D [71]. Furthermore, the role of renal vasoactive factors such as adenosine may be markedly different in the presence or absence of concurrent RAAS blockade, a group of drugs that is more commonly prescribed in adults with T2D. It also remains poorly understood whether these differences in intrarenal haemodynamic function relate to T1D versus T2D or rather are a function of age and diabetes duration. Future research should interrogate the mechanisms underlying the differences observed in people with T1D and T2D to better understand the role of SGLT2 inhibition in people with T1D. To better understand these differences, trials should enrol people with both T1D and T2D in adequate numbers to allow meaningful comparative analyses.
FIGURE 2.
Intrarenal haemodynamic function changes in mechanistic trials with SGLT2 inhibitors in T1D versus T2D. The figure illustrates potential mechanisms explaining the differences observed in two mechanistic trials with SGLT2 inhibitor in young adults (∼24 years of age) with T1D [ATIRMA study (NCT01392560)] and older adults (∼63 years of age) with T2D [RED (NCT02682563)].
UNMET MEDICAL NEED IN T1D TREATMENT
Due to the deleterious effects of hyperglycaemia on microvascular and macrovascular outcomes, intensive insulin therapy, either by multiple insulin injections or via continuous subcutaneous insulin infusion, is employed in people with T1D to achieve optimal glucose control (HbA1c <7.0% or 53 mmol/mol). Based on the data from the DCCT/EDIC, optimal glycaemic control was shown to impede the onset of vascular complications [72, 73]. In terms of DKD, in the DCCT/EDIC study, the development of macroalbuminuria was reduced by 54% (range 19–74) by strict glycaemic control, indicating the importance of glycaemic control for the kidney [73]. However, rigorous lowering of glycaemia by intensive insulin therapy, due to the absence of a feedback system, comes at the price of (severe) hypoglycaemia, particularly in the face of impaired awareness of hypoglycaemia in people with long-standing disease. Another undesired consequence of intensive insulin therapy is clinically significant weight gain, particularly in patients with frequent hypoglycaemia, and potentially insulin resistance, which has been causally linked to vascular complications [39, 74, 75]. Thus it is not surprising that many people with T1D fail to reach glycaemic targets, which represents a clear unmet medical need. Several efforts have been implemented to address these needs. First, pancreatic transplantation to restore islet cell function is sometimes combined with kidney transplant. Although pancreatic transplant can induce sustained diabetes remission, the surgery is associated with high mortality rates. Therefore ongoing research is focused on transplanting functional human pancreatic islets to people with T1D. In addition, current research aims to halt β-cell destruction through immunomodulatory therapies. Another approach is directed at improving insulin delivery, e.g. with closed-loop systems that allow glucose feedback on insulin delivery by reducing the risk for both hypo- and hyperglycaemia. Finally, strategies have been pursued whereby additional (oral) pharmacotherapies are initiated to complement insulin therapy. Focusing on the latter, this has proven to be a challenging area. Studies with metformin [REMOVAL trial (NCT01483560)], the glucagon-like peptide-1 receptor agonist liraglutide [ADJUNCT ONE (NCT01836523)], the dipeptidyl peptidase-4 inhibitor sitagliptin and pramlintide showed overall modest benefits in adults with T1D when added to insulin therapy and could even increase adverse effects such as hypoglycaemia [76]. On the other hand, metformin therapy compared with placebo in adolescents with T1D was found to improve markers of CVD [77, 78]. The rationale for adjunct therapies in T1D is clearly evident based on the need for both glycaemic control and cardiorenal protection. However, the evidence to support the use of the above-mentioned therapies remains limited and future studies are needed.
GLYCAEMIC EFFECTS OF SGLT2 INHIBITION IN T1D
Recent studies have defined the effects of different SGLT2 inhibitors and dual SGLT1 and 2 inhibitors in people with T1D, adjunctive to standard of care insulin therapy (Table 1). In the DEPICT 1 trial (NCT02268214), dapagliflozin 5 and 10 mg once daily, compared with placebo, reduced HbA1c by 0.42 and 0.45%, respectively, at 24 weeks of treatment [79]; at Week 52, the differences remained −0.33% and −0.36% [80]. In the Empagliflozin as Adjunctive to InSulin thErapy trials, similar reductions were observed for 10 and 25 mg dosages (currently approved in T2D): −0.53% and −0.54%, respectively. The dosage of 2.5 mg yielded a placebo-corrected HbA1c reduction of −0.28% [81]. Finally, sotagliflozin reduced HbA1c by 0.36% and 0.41% in the inTandem 1 and inTandem 2 studies [82, 83]. Most patients in the trial programmes had reasonable glycaemic control prior to drug initiation due to insulin optimization during run-in and had preserved kidney function. It is important to emphasize that the achieved reduction in HbA1c was not accompanied by increased occurrence of (severe) hypoglycaemic episodes. Insulin reductions were seen across the trials and were mostly on the order of 10–15%. In glucose management of T1D, much attention has shifted from average glucose values as determined by HbA1c (an HbA1c value on target may include many hypo- and hyperglycaemic events that average out) to more complex measurements done by devices, such as flash glucose monitoring or continuous glucose monitoring (CGM). As such, the glycaemic parameter time in range (TIR), usually defined as glucose levels between 3.9 and 10.0 mmol/L, has received much attention. In the Dapagliflozin Evaluation in Patients With Inadequately Controlled Type 1 Diabetes trials, in 1591 participants with CGM analyses, dapagliflozin dosages increased TIR by 10–11% versus placebo [84, 85]. Furthermore, reductions in mean amplitude of glucose excursion (MAGE) were observed as well as 24-h mean glucose values, while values <3.9 mM were not increased. For sotagliflozin, a placebo-adjusted increase in TIR occurred in 5.4% for the 200 mg dose and 11.2% for the 400 mg dose [85]. Finally, EMPA 25 mg also showed increases of 10% TIR, while reducing glucose variability (glucose interquartile range and MAGE) [86]. The TIR percentages indicated above corresponded to ∼3 h/day extra at the highest dosages of each drug, without an increase in time for hypoglycaemia. It should be mentioned that the association between TIR and the development of microvascular complication is less well established as compared with the risk of high HbA1c levels and microvascular complications. However, two recent publications in people with T1D and T2D [87, 88] linked lower TIR to progression of retinopathy and albuminuria. With the increased use of sensor technology, more information will likely be available in the near future.
Table 1.
Studies with high-dose SGLT2 and dual SGLT1 and SGLT2 inhibitors in people with T1D
| Clinical Trials in People with Type 1 Diabetes |
||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| DEPICT 1 | DEPICT 2 | inTandem 1 | inTandem 2 | inTandem 3 | EASE 1 | EASE 2 | EASE 3 | |
| Number of patients,an | 556 | 442 | 530 | 521 | 1402 | 37 | 487 | 486 |
| Study drug | DAPA | DAPA | SOTA | SOTA | SOTA | EMPA | EMPA | EMPA |
| Drug high dose (mg) | 10 | 10 | 400 | 400 | 400 | 25 | 25 | 25 |
| Study duration (weeks) | 52 | 24 | 52 | 52 | 24 | 4 | 52 | 26 |
| Baseline HbA1c (%) | 8.5 | 8.4 | 7.5 | 7.8 | 8.2 | 8.2 | 8.1 | 8.2 |
| Change in HbA1cb (%) | −0.36 | −0.42 | −0.31 | −0.32 | −0.46 | −0.49 | −0.45 | −0.52 |
| Change in insulin TDDb (%) | ∼−10 | −11.1 | −12.6 | −8.2 | −9.7 | −13 | −12.9 | −12.6 |
| Change in body weightb (kg) | −3.6 | −3.0 | −4.3 | −2.9 | −3.0 | −1.9 | −3.6 | −3.4 |
| Change in time in rangeb,c (%) | +10.7 | +10.4 | +13.4 | NA | +10.9 | +12.5 | +7.4 | |
| Ketoacidosis incidence rates,d,en | 13 versus 3 | 3 versus 0 | 11 versus 1 | 9 versus 0 | 21 versus 4 | 0 versus 0 | 16 versus 6 | |
High-dose treatment with SGLT1 or SGLT2 inhibitor: dapagliflozin 10 mg, EMPA 25 mg or sotagliflozin 400 mg.
Including placebo and high-dose SGLT1 or SGLT2 inhibitor treatment group.
Placebo-adjusted.
CGM data for DEPICT-1 and DEPICT-2 were pooled for analyses.
Incidence rates for SGLT1/2 inhibitor versus placebo.
Ketoacidosis incidence rates were pooled for EASE 2 and EASE 3 studies.
DAPA: dapagliflozin; SOTA, sotagliflozin; TDD, total daily dosage; NA, not available.
EXTRAGLYCAEMIC BENEFITS OF SGLT2 INHIBITION IN PEOPLE WITH T1D
The reason that SGLT2 inhibitors have received wide attention recently is not due to their glucose-lowering potential, as described above, but rather their impressive cardiorenal benefits. As mandated by the Food and Drug Administration (FDA), all trials with SGLT2 inhibitors have included placebo-controlled cardiovascular safety trials in the past couple of years. Unexpectedly, this drug class showed reductions in cardiovascular endpoints (i.e. the composite 3-MACE endpoint) in people with prior CVD, as reviewed extensively elsewhere [89, 90]. This improvement was driven by unprecedented reductions in hospitalization for heart failure [hazard ratios (HRs) ranging from 0.66 to 0.83]. Additionally, the composite endpoint of progression of nephropathy was improved (HRs ranging from 0.54 to 0.60), a finding that was confirmed in a study with DKD patients [61, 91, 92]. Of great importance, hard renal outcomes such as progression to ESKD were also reduced. The mechanisms behind the observed cardiorenal benefit continue to be incompletely understood. Although SGLT2 inhibitors modestly improve cardiovascular and renal risk factors such as blood pressure (∼2–3 mmHg), body weight (∼3 kg) and uric acid, it is unlikely that these factors completely mediate the observed benefits. Indeed, in a mediation analysis of the EMPA-REG OUTCOME trial, the largest predictor of favourable outcome was haematocrit, a marker of plasma volume. By inhibition of SGLT2, sodium and glucose reabsorption are concomitantly blocked. This in turn leads to temporary natriuresis, until sodium balance is restored by upregulation of other sodium transporters [64, 65]. However, the natriuresis-induced volume contraction is sustained over time. Thus alterations in sodium homeostasis and extracellular volume are thought to drive the observed cardiovascular benefit. At the kidney level, alterations in sodium handling may also drive the observed renohaemodynamic actions, where more distal sodium uptake could drive beneficial amelioration of hyperfiltration as detailed above.
The key question is whether the renal and cardiovascular benefits of SGLT2 inhibitors observed in people with T2D also apply to individuals with T1D. However, no such trials have been conducted, and it remains uncertain whether large-scale CVOTs will be performed in this population. At present, we have to carefully extrapolate T2D data and rely on biomarkers of cardiorenal health. In this regard, post hoc analyses from the EASE, DEPICT and inTandem trials may provide important insights. In a pooled analysis of the inTandem 1 and 2 studies, we found that sotagliflozin increased haematocrit by ∼2%, as well as serum albumin, confirming volume contraction [22]. This may indicate similar cardiovascular mechanisms as in T1D adults. Salutary effects on blood pressure, body weight and uric acid were also reported. Given the role of elevated blood pressure, overweight and hyperuricaemia on hyperfiltration, reduction of these parameters could contribute to improved renal outcomes in adults with T1D. In addition, an early drop in eGFR similar to what has been shown in T2D was observed, and in those with albuminuria at baseline, a 40–60% attenuation of urinary albumin excretion was demonstrated [22]. These findings may implicate a reduction in glomerular pressure as a potential mediator of the nephroprotective effects consistent with the study by Cherney et al. [59]. Thus, while SGLT2 inhibitors have shown impressive beneficial effects on the cardiorenal axis in people with T2D, biomarkers suggest that these effects may also present in people with T1D. Given the burden of renal disease in people afflicted by T1D, this provides impetus for dedicated large-scale trials and cohort studies.
DIABETIC KETOACIDOSIS RISK IN T1D MECHANISMS: PREVALENCE AND IMPLICATIONS
SGLT2 and dual SGLT1 and 2 inhibitors are associated with several side effects. Most of the adverse reactions relate to their mode of action and are seen across the class. SGLT2 inhibitors induce glucosuria that makes the urine an attractive culture medium for bacteria, resulting in a slight increase in genitourinary infections. Most commonly observed infections are fungal infections of the genital skin (5–10% of treated women). However, for people with T1D, the most critical potential adverse effect concerns euglycaemic diabetic ketoacidosis (DKA). SGLT2 inhibitors increase ketonaemia, also in people with T2D. This is caused by reductions in plasma insulin concentrations or a reduction in insulin dosage and concomitant increments in glucagon concentrations. While ketone bodies have been hypothesized to explain beneficial cardiorenal effects of SGLT2 inhibitors, in people with T1D they increase the risk for acidosis. Due to apparent normoglycaemia secondary to increased glucosuria, misdiagnosis of euglycaemic DKA continues to be a concern that could lead to delayed management. Risk factors for DKA in people with T1D have been identified and include large reductions in basal insulin therapy, insulin pump failure, reduced carbohydrate intake, use of alcohol, acute illness, vomiting and volume depletion/dehydration [93]. The percentage of DKA in the conducted trials in people with T1D was reported as 3.5% (4076 individuals treated with dual SGLT1 and 2 inhibitors) versus 0.6% (among 2362 placebo-treated individuals), which yields a 5.8-fold relative risk increase. As these numbers are derived from trials with motivated patients and expert physicians using careful surveillance and monitoring of ketonaemia (illustrated by very low DKA events in the placebo groups), it is plausible that the relative risk may be higher in clinical practice. An exception to these data concerns the novel low dose of EMPA (2.5 mg; currently not available), which demonstrated no increase in DKA rates, albeit at the expense of attenuated glucose-lowering actions. It should be mentioned that this low dose of EMPA is not yet available for clinical use.
A recently written consensus report written by international experts highlights the need for appropriate patient selection for SGLT2 inhibition and crucial knowledge available at the medical team. Finally, patients are required to measure ketones in addition to glucose levels and be trained on how to act upon increments, which is uncommon in clinical practice in most countries at present. Despite the usage of ketone metres, DKA rates were significant, which suggests that ketone body measurements are insufficient to mitigate DKA risk in people with T1D.
WEIGHING THE RISKS AND BENEFITS OF SGLT2 INHIBITORS IN T1D
The risk–benefit assessment of SGLT1 and 2 inhibitors in people with T1D remains challenging for health authorities and medical providers. In the USA, the FDA decided not to approve sotagliflozin as adjunctive therapy in people with T1D. In Europe, the European Committee for Medical Products for Humans Use recommended the use of sotagliflozin and dapagliflozin for people with T1D, however, it was restricted to individuals with BMI >27 kg/m2, based on post hoc analyses that showed these individuals might have a reduced risk to develop DKA. Based on preliminary data and biomarker analysis, low-dose SGLT2 inhibition may hold promise as an adjunctive therapy in people with T1D, especially those at high risk of DKD and CVD. However, CVOTs in people with T1D are needed to better understand the risk–benefit assessment, as data from people with T2D may not be generalizable to people with T1D. In fact, mechanistic studies suggest different effects of SGLT2 inhibition on intrarenal haemodynamic function in people with T1D versus T2D. T1D continues to be an exclusion criterion in the vast majority of pharma-sponsored clinical trials. However, the ongoing EMPA-Kidney includes a subset of people with T1D and DKD and may shed some important light. The medical community will likely remain sceptical until CVOTs in people with T1D data are available. Accordingly, future efforts should focus on designing a pragmatic CVOTs in people with T1D who are at high risk of DKD and CVD. Strategic partnerships between academia, pharma, organizations (e.g. Juvenile Diabetes Research Foundation, American Diabetes Association and European Foundation for the Study of Diabetes) and federal and state sponsors are needed to facilitate the development of pragmatic CVOTs in people with T1D. Real-world data from carefully designed studies could also help in this regard. This particularly holds true for the adverse effects. While efficacy can be accurately determined in randomized clinical trials, real-world evidence regarding ketoacidosis rates will provide crucial information on the risk of this drug class in people with T1D and will determine their future use.
CONCLUSION
Trials have established that SGLT2 inhibitors impede DKD progression in people with T2D. Although the mechanisms of nephroprotection remain uncertain, it may relate to improvements in renal haemodynamics, including reduced glomerular pressure, as well as improvements in renal risk factors such as blood pressure, hyperglycaemia, body weight and uric acid. However, DKD pathogenesis may be different in people with T1D compared with T2D. DKD in T1D may be characterized by distinct metabolic and renal haemodynamic perturbations, and data suggest that renal lesions from research biopsies also differ in people with T1D and T2D. Thus the question remains whether the renal benefits of SGLT2 inhibitors are also present in T1D. At present, we are unable to address this question due to the lack of dedicated trials in T1D, although certain renal biomarkers in non-dedicated renal studies as well as a mechanistic study focusing on renal haemodynamics suggest that SGLT2 inhibition may also confer nephroprotection in people with T1D. Renal trials investigating the effects of SGLT2 inhibitors in adults with T1D are now urgently needed. A major focus when designing these trials should be safety, as SGLT2 inhibitors, despite extensive surveillance measures, increase DKA risk. Without dedicated renal trials in people with T1D, the benefit–risk ratio cannot be meaningfully balanced for individual patients.
FUNDING
D.H.v.R. is supported by a research fellowship from the Dutch Diabetes Foundation and European Union Marie Curie program. P.B. receives salary and research support from the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (K23 DK116720-01), in addition to research support from the Juvenile Diabetes Research Foundation (2-SRA-2018-627-M-B, 2-SRA-2019-845-S-B), NIDDK/DiaComp, Thrasher Research Fund, International Society of Pediatric and Adolescent Diabetes, Colorado Clinical & Translational Sciences Institute and Center for Women’s Health Research at the University of Colorado.
AUTHORS’ CONTRIBUTIONS
The authors are fully responsible for all content and editorial decisions and were involved at all stages of manuscript development and have approved the final version.
CONFLICT OF INTEREST STATEMENT
P.B. has acted as a consultant for Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Sanofi, Novo Nordisk and Horizon Pharma. P.B. also serves on the advisory board of XORTX. D.H.v.R. has acted as a consultant and received honoraria from Boehringer Ingelheim, Eli Lilly, Merck, Novo Nordisk and Sanofi and has received research operating funds from the Boehringer Ingelheim–Eli Lilly Diabetes Alliance, MSD, AstraZeneca and Novo Nordisk.
REFERENCES
- 1. Rewers M, Zimmet P.. The rising tide of childhood type 1 diabetes—what is the elusive environmental trigger? Lancet 2004; 364: 1645–1647 [DOI] [PubMed] [Google Scholar]
- 2. Vehik K, Hamman RF, Lezotte D. et al. Increasing incidence of type 1 diabetes in 0- to 17-year-old Colorado youth. Diabetes Care 2007; 30: 503–509 [DOI] [PubMed] [Google Scholar]
- 3. Narayan KM, Boyle JP, Thompson TJ. et al. Lifetime risk for diabetes mellitus in the United States. JAMA 2003; 290: 1884–1890 [DOI] [PubMed] [Google Scholar]
- 4. Miller RG, Mahajan HD, Costacou T. et al. A contemporary estimate of total mortality and cardiovascular disease risk in young adults with type 1 diabetes: the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care 2016; 39: 2296–2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Harding JL, Shaw JE, Peeters A. et al. Age-specific trends from 2000–2011 in all-cause and cause-specific mortality in type 1 and type 2 diabetes: a cohort study of more than one million people. Diabetes Care 2016; 39: 1018–1026 [DOI] [PubMed] [Google Scholar]
- 6. Lind M, Svensson AM, Kosiborod M. et al. Glycemic control and excess mortality in type 1 diabetes. N Engl J Med 2014; 371: 1972–1982 [DOI] [PubMed] [Google Scholar]
- 7. Groop PH, Thomas MC, Moran JL. et al. The presence and severity of chronic kidney disease predicts all-cause mortality in type 1 diabetes. Diabetes 2009; 58: 1651–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Orchard TJ, Secrest AM, Miller RG. et al. In the absence of renal disease, 20 year mortality risk in type 1 diabetes is comparable to that of the general population: a report from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetologia 2010; 53: 2312–2319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Krolewski AS, Warram JH, Christlieb AR. et al. The changing natural history of nephropathy in type I diabetes. Am J Med 1985; 78: 785–794 [DOI] [PubMed] [Google Scholar]
- 10.DCCT/EDIC Research Group. Effect of intensive diabetes treatment on albuminuria in type 1 diabetes: long-term follow-up of the Diabetes Control and Complications Trial and Epidemiology of Diabetes Interventions and Complications study. Lancet Diabetes Endocrinol 2014; 2: 793–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Zeeuw D, Akizawa T, Audhya P. et al. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med 2013; 369: 2492–2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lewis EJ, Greene T, Spitalewiz S. et al. Pyridorin in type 2 diabetic nephropathy. J Am Soc Nephrol 2012; 23: 131–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mauer M, Zinman B, Gardiner R. et al. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med 2009; 361: 40–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mann JF, Anderson C, Gao P. et al. Dual inhibition of the renin-angiotensin system in high-risk diabetes and risk for stroke and other outcomes: results of the ONTARGET trial. J Hypertens 2013; 31: 414–421 [DOI] [PubMed] [Google Scholar]
- 15. Cherney DZ, Konvalinka A, Zinman B. et al. Effect of protein kinase Cβ inhibition on renal hemodynamic function and urinary biomarkers in humans with type 1 diabetes: a pilot study. Diab Care 2009; 32: 91–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tuttle KR, McGill JB, Haney DJ. et al. Kidney outcomes in long-term studies of ruboxistaurin for diabetic eye disease. Clin J Am Soc Nephrol 2007; 2: 631–636 [DOI] [PubMed] [Google Scholar]
- 17. Bjornstad P, Maahs DM.. Diabetes complications in childhood diabetes—new biomarkers and technologies. Curr Pediatr Rep 2015; 3: 177–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bjornstad P, Cherney D, Maahs DM.. Early diabetic nephropathy in type 1 diabetes: new insights. Curr Opin Endocrinol Diabetes Obes 2014; 21: 279–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marcovecchio ML, Chiesa ST, Bond S. et al. ACE inhibitors and statins in adolescents with type 1 diabetes. N Engl J Med 2017; 377: 1733–1745 [DOI] [PubMed] [Google Scholar]
- 20. van Bommel EJ, Muskiet MH, Tonneijck L. et al. SGLT2 inhibition in the diabetic kidney-from mechanisms to clinical outcome. Clin J Am Soc Nephrol 2017; 12: 700–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cheng ST, Chen L, Li SY. et al. Effects of empagliflozin, an SGLT2 inhibitor, on pancreatic β-cell mass and glucose homeostasis in type 1 diabetes. PLoS One 2016; 11: e0147391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Van Raalte D, Bjornstad P, Persson F. et al. The impact of sotagliflozin on renal function, albuminuria, blood pressure, and hematocrit in adults with type 1 diabetes. Diabetes Care 2019; 42: 1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Drummond K, Mauer M.. The early natural history of nephropathy in type 1 diabetes: II. Early renal structural changes in type 1 diabetes. Diabetes 2002; 51: 1580–1587 [DOI] [PubMed] [Google Scholar]
- 24. Fioretto P, Mauer M.. Histopathology of diabetic nephropathy. Semin Nephrol 2007; 27: 195–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fioretto P, Steffes MW, Mauer M.. Glomerular structure in nonproteinuric IDDM patients with various levels of albuminuria. Diabetes 1994; 43: 1358–1364 [DOI] [PubMed] [Google Scholar]
- 26. Bilous RW, Mauer SM, Sutherland DE. et al. Mean glomerular volume and rate of development of diabetic nephropathy. Diabetes 1989; 38: 1142–1147 [DOI] [PubMed] [Google Scholar]
- 27. Mauer M, Caramori ML, Fioretto P. et al. Glomerular structural-functional relationship models of diabetic nephropathy are robust in type 1 diabetic patients. Nephrol Dial Transplant 2015; 30: 918–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Najafian B, Mauer M.. Morphologic features of declining renal function in type 1 diabetes. Semin Nephrol 2012; 32: 415–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ruggenenti P, Gambara V, Perna A. et al. The nephropathy of non-insulin-dependent diabetes: predictors of outcome relative to diverse patterns of renal injury. J Am Soc Nephrol 1998; 9: 2336–2343 [DOI] [PubMed] [Google Scholar]
- 30. Fufaa GD, Weil EJ, Lemley KV. et al. Structural predictors of loss of renal function in American Indians with type 2 diabetes. Clin J Am Soc Nephrol 2016; 11: 254–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gilbert RE. Proximal tubulopathy: prime mover and key therapeutic target in diabetic kidney disease. Diabetes 2017; 66: 791–800 [DOI] [PubMed] [Google Scholar]
- 32. Mogensen CE, Christensen CK, Vittinghus E.. The stages in diabetic renal disease. With emphasis on the stage of incipient diabetic nephropathy. Diabetes 1983; 32(Suppl 2): 64–78 [DOI] [PubMed] [Google Scholar]
- 33. Molitch ME, Gao X, Bebu I. et al. Early glomerular hyperfiltration and long-term kidney outcomes in type 1 diabetes: the DCCT/EDIC experience. Clin J Am Soc Nephrol 2019; 14: 854–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bjornstad P, Cherney DZ, Snell-Bergeon JK. et al. Rapid GFR decline is associated with renal hyperfiltration and impaired GFR in adults with type 1 diabetes. Nephrol Dial Transplant 2015; 30: 1706–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bjornstad P, Costacou T, Miller RG. et al. Predictors of early renal function decline in adults with type 1 diabetes: the Coronary Artery Calcification in Type 1 Diabetes and the Pittsburgh Epidemiology of Diabetes Complications studies. Diabetes Med 2017; 34: 1532–1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Denic A, Mathew J, Lerman LO. et al. Single-nephron glomerular filtration rate in healthy adults. N Engl J Med 2017; 376: 2349–2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xie L, Bennett KM, Liu C. et al. MRI tools for assessment of microstructure and nephron function of the kidney. Am J Physiol Renal Physiol 2016; 311: F1109–F1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Krolewski AS, Niewczas MA, Skupien J. et al. Early progressive renal decline precedes the onset of microalbuminuria and its progression to macroalbuminuria. Diabetes Care 2014; 37: 226–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bjornstad P, Snell-Bergeon JK, Rewers M. et al. Early diabetic nephropathy: a complication of reduced insulin sensitivity in type 1 diabetes. Diabetes Care 2013; 36: 3678–3683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Thomson HJ, Ekinci EI, Radcliffe NJ. et al. Elevated baseline glomerular filtration rate (GFR) is independently associated with a more rapid decline in renal function of patients with type 1 diabetes. J Diabetes Complicat 2016; 30: 256–261 [DOI] [PubMed] [Google Scholar]
- 41. Gross JL, de Azevedo MJ, Silveiro SP. et al. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care 2005; 28: 164–176 [DOI] [PubMed] [Google Scholar]
- 42. Perkins BA, Ficociello LH, Silva KH. et al. Regression of microalbuminuria in type 1 diabetes. N Engl J Med 2003; 348: 2285–2293 [DOI] [PubMed] [Google Scholar]
- 43. de Boer IH, Rue TC, Cleary PA. et al. Long-term renal outcomes of patients with type 1 diabetes mellitus and microalbuminuria: an analysis of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications cohort. Arch Intern Med 2011; 171: 412–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mudaliar S, Alloju S, Henry RR.. Can a shift in fuel energetics explain the beneficial cardiorenal outcomes in the EMPA-REG OUTCOME study? A unifying hypothesis. Diabetes Care 2016; 39: 1115–1122 [DOI] [PubMed] [Google Scholar]
- 45. Cherney DZ, Miller JA, Scholey JW. et al. Renal hyperfiltration is a determinant of endothelial function responses to cyclooxygenase 2 inhibition in type 1 diabetes. Diabetes Care 2010; 33: 1344–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lovshin JA, Skrtic M, Bjornstad P. et al. Hyperfiltration, urinary albumin excretion, and ambulatory blood pressure in adolescents with type 1 diabetes mellitus. Am J Physiol Renal Physiol 2018; 314: F667–F674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ricksten SE, Bragadottir G, Redfors B.. Renal oxygenation in clinical acute kidney injury. Crit Care 2013; 17: 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bragadottir G, Redfors B, Nygren A. et al. Low-dose vasopressin increases glomerular filtration rate, but impairs renal oxygenation in post-cardiac surgery patients. Acta Anaesthesiol Scand 2009; 53: 1052–1059 [DOI] [PubMed] [Google Scholar]
- 49. Bjornstad P, Johnson RJ, Snell-Bergeon JK. et al. Albuminuria is associated with greater copeptin concentrations in men with type 1 diabetes: a brief report from the T1D exchange Biobank. J Diabetes Complicat 2017; 31: 387–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bjornstad P, Maahs DM, Jensen T. et al. Elevated copeptin is associated with atherosclerosis and diabetic kidney disease in adults with type 1 diabetes. J Diabetes Complicat 2016; 30: 1093–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Korner A, Eklof AC, Celsi G. et al. Increased renal metabolism in diabetes. Mechanism and functional implications. Diabetes 1994; 43: 629–633 [DOI] [PubMed] [Google Scholar]
- 52. Layton AT, Laghmani K, Vallon V. et al. Solute transport and oxygen consumption along the nephrons: effects of Na+ transport inhibitors. Am J Physiol Renal Physiol 2016; 311: F1217–F1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Layton AT, Vallon V, Edwards A.. A computational model for simulating solute transport and oxygen consumption along the nephrons. Am J Physiol Renal Physiol 2016; 311: F1378–F1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Layton AT, Vallon V, Edwards A.. Predicted consequences of diabetes and SGLT inhibition on transport and oxygen consumption along a rat nephron. Am J Physiol Renal Physiol 2016; 310: F1269–F1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Palm F, Cederberg J, Hansell P. et al. Reactive oxygen species cause diabetes-induced decrease in renal oxygen tension. Diabetologia 2003; 46: 1153–1160 [DOI] [PubMed] [Google Scholar]
- 56. Nangaku M. Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J Am Soc Nephrol 2006; 17: 17–25 [DOI] [PubMed] [Google Scholar]
- 57. Haase VH. The VHL/HIF oxygen-sensing pathway and its relevance to kidney disease. Kidney Int 2006; 69: 1302–1307 [DOI] [PubMed] [Google Scholar]
- 58. Singh DK, Winocour P, Farrington K.. Mechanisms of disease: the hypoxic tubular hypothesis of diabetic nephropathy. Nat Rev Nephrol 2008; 4: 216–226 [DOI] [PubMed] [Google Scholar]
- 59. Cherney DZ, Perkins BA, Soleymanlou N. et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 2014; 129: 587–597 [DOI] [PubMed] [Google Scholar]
- 60. Kidokoro K, Cherney DZI, Bozovic A. et al. Evaluation of glomerular hemodynamic function by empagliflozin in diabetic mice using in vivo imaging. Circulation 2019; 140: 303. [DOI] [PubMed] [Google Scholar]
- 61. Wanner C, Inzucchi SE, Lachin JM. et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016; 375: 323–334 [DOI] [PubMed] [Google Scholar]
- 62. Merovci A, Solis-Herrera C, Daniele G. et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest 2014; 124: 509–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bessho R, Takiyama Y, Ota T.. Luseogliflozin inhibits HIF-1α expression in renal proximal tubular epithelial cells. ADA 2018 (Oral Presentation, 90-or) 2018 [Google Scholar]
- 64. Komoroski B, Vachharajani N, Feng Y. et al. Dapagliflozin, a novel, selective SGLT2 inhibitor, improved glycemic control over 2 weeks in patients with type 2 diabetes mellitus. Clin Pharmacol Ther 2009; 85: 513–519 [DOI] [PubMed] [Google Scholar]
- 65. Heise T, Jordan J, Wanner C. et al. Pharmacodynamic effects of single and multiple doses of empagliflozin in patients with type 2 diabetes. Clin Ther 2016; 38: 2265–2276 [DOI] [PubMed] [Google Scholar]
- 66. Bobulescu IA, Moe OW.. Renal transport of uric acid: evolving concepts and uncertainties. Adv Chronic Kidney Dis 2012; 19: 358–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wilcox CS, Shen W, Boulton DW. et al. Interaction between the sodium-glucose-linked transporter 2 inhibitor dapagliflozin and the loop diuretic bumetanide in normal human subjects. J Am Heart Assoc 2018; 7: e007046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hallow KM, Greasley PJ, Helmlinger G. et al. Evaluation of renal and cardiovascular protection mechanisms of SGLT2 inhibitors: model-based analysis of clinical data. Am J Physiol Renal Physiol 2018; 315: F1295–F1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Van Bommel E, Muskiet MH, Van Baar MJB. et al. ADA Presidents' select abstract: dapagliflozin reduces measured GFR by reducing renal efferent arteriolar resistance in type 2 diabetes. Am Diabetes Assoc Sci Sessions 2019; 2019https://www.kidney-international.org/article/S0085-2538(19)30991-3/abstract [Google Scholar]
- 70. Lovshin JA, Boulet G, Lytvyn Y. et al. Renin-angiotensin-aldosterone system activation in long-standing type 1 diabetes. JCI Insight 2018; 3: e96968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bjornstad P, Lovshin JA, Lytvyn Y. et al. Elevated plasma cyclic guanosine monophosphate may explain greater efferent arteriolar tone in adults with longstanding type 1 diabetes: a brief report. J Diabetes Complicat 2019; 33: 547–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Nathan DM, Cleary PA, Backlund JY. et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353: 2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Nathan DM, Genuth S, Lachin J. et al. Diabetes Control and Complications Trial Research Group, Nathan DM, Genuth S et al The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329: 977–986 [DOI] [PubMed] [Google Scholar]
- 74. Corbin KD, Driscoll KA, Pratley RE. et al. Obesity in type 1 diabetes: pathophysiology, clinical impact, and mechanisms. Endocr Rev 2018; 39: 629–663 [DOI] [PubMed] [Google Scholar]
- 75. Bjornstad P, Maahs DM, Johnson RJ. et al. Estimated insulin sensitivity predicts regression of albuminuria in type 1 diabetes. Diabet Med 2015; 32: 257–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. McCrimmon RJ, Henry RR.. SGLT inhibitor adjunct therapy in type 1 diabetes. Diabetologia 2018; 61: 2126–2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Bjornstad P, Schafer M, Truong U. et al. Metformin improves insulin sensitivity and vascular health in youth with type 1 diabetes mellitus. Circulation 2018; 138: 2895–2907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Anderson JJA, Couper JJ, Giles LC. et al. Effect of metformin on vascular function in children with type 1 diabetes: a 12-month randomized controlled trial. J Clin Endocrinol Metab 2017; 102: 4448–4456 [DOI] [PubMed] [Google Scholar]
- 79. Dandona P, Mathieu C, Phillip M. et al. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (DEPICT-1): 24 week results from a multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol 2017; 5: 864–876 [DOI] [PubMed] [Google Scholar]
- 80. Dandona P, Mathieu C, Phillip M. et al. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes: the DEPICT-1 52-week study. Diabetes Care 2018; 41: 2552–2559 [DOI] [PubMed] [Google Scholar]
- 81. Rosenstock J, Marquard J, Laffel LM. et al. Empagliflozin as adjunctive to insulin therapy in type 1 diabetes: the EASE trials. Diabetes Care 2018; 41: 2560–2569 [DOI] [PubMed] [Google Scholar]
- 82. Danne T, Cariou B, Banks P. et al. HbA1c and hypoglycemia reductions at 24 and 52 weeks with sotagliflozin in combination with insulin in adults with type 1 diabetes: the European inTandem2 Study. Diabetes Care 2018; 41: 1981–1990 [DOI] [PubMed] [Google Scholar]
- 83. Buse JB, Garg SK, Rosenstock J. et al. Sotagliflozin in combination with optimized insulin therapy in adults with type 1 diabetes: the North American inTandem1 Study. Diabetes Care 2018; 41: 1970–1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Mathieu C, Dandona P, Phillip M. et al. Glucose variables in type 1 diabetes studies with dapagliflozin: pooled analysis of continuous glucose monitoring data from DEPICT-1 and -2. Diabetes Care 2019; 42: 1081–1087 [DOI] [PubMed] [Google Scholar]
- 85. Danne T, Cariou B, Buse JB. et al. Improved time in range and glycemic variability with sotagliflozin in combination with insulin in adults with type 1 diabetes: a pooled analysis of 24-week continuous glucose monitoring data from the inTandem program. Diabetes Care 2019; 42: 919–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Famulla S, Pieber TR, Eilbracht J. et al. Glucose exposure and variability with empagliflozin as adjunct to insulin in patients with type 1 diabetes: continuous glucose monitoring data from a 4-week, randomized, placebo-controlled trial (EASE-1). Diabetes Technol Ther 2017; 19: 49–60 [DOI] [PubMed] [Google Scholar]
- 87. Beck RW, Bergenstal RM, Riddlesworth TD. et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care 2019; 42: 400–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lu J, Ma X, Zhou J. et al. Association of time in range, as assessed by continuous glucose monitoring, with diabetic retinopathy in type 2 diabetes. Diabetes Care 2018; 41: 2370–2376 [DOI] [PubMed] [Google Scholar]
- 89. Wiviott SD, Raz I, Bonaca MP. et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019; 380: 347–357 [DOI] [PubMed] [Google Scholar]
- 90. Zinman B, Wanner C, Lachin JM. et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128 [DOI] [PubMed] [Google Scholar]
- 91. Perkovic V, Jardine MJ, Neal B. et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019; 380: 2295–2306 [DOI] [PubMed] [Google Scholar]
- 92. Neal B, Perkovic V, Mahaffey KW. et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377: 644–657 [DOI] [PubMed] [Google Scholar]
- 93. Danne T, Garg S, Peters AL. et al. International consensus on risk management of diabetic ketoacidosis in patients with type 1 diabetes treated with sodium-glucose cotransporter (SGLT) inhibitors. Diabetes Care 2019; 42: 1147–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]