Abstract
Objective
Long-term efficacy of metformin in polycystic ovarian syndrome (PCOS) apart from in those with impaired glucose tolerance or diabetes remains unproven. We aimed to evaluate the impact of metformin in overweight-obese patients with PCOS and normal baseline glycemic homeostasis.
Methods
A 10-year longitudinal follow-up of a retrospective cohort comprising 159 patients with PCOS defined by Rotterdam criteria, BMI ≥25 kg/m2 and normal initial glucose homeostasis (age 28.4 ± 6.4 years, BMI 34.9 ± 6.6 kg/m2) that had been receiving metformin 1000 mg BID. Collection data contained 6085 time-points including anthropometric, hormonal and metabolic parameters.
Results
After the first year body mass (BM) decreased for 3.9 ± 6.8 kg (P < 0.001) and remained stable during the following 3 years. Menstrual frequency (MF) increased to 3.0 ± 3.9 bleeds/year (P < 0.001) after first year to over 11 bleeds/year in the following years. The total testosterone and androstenedione decreased to 15.4 ± 47.9% and 11.3 ± 46.4% within first year, with further decrease in total testosterone and androstenedione to 37.8 ± 61.8 and 24.8 ± 40.5% at the fifth year of the follow-up. The total conversion rate to prediabetes and diabetes was extremely low throughout observation period. Less than 25% of patients continued with metformin for more than 5 years with further dropout to only 6% on metformin therapy at the tenth year of follow-up.
Conclusions
Long-term metformin treatment of overweight-obese women with PCOS and normal baseline glycemic homeostasis resulted in reduction and stabilization of BM, improvements of MF and androgen profile and low conversion rate to diabetes.
Keywords: metformin, PCOS, obesity, diabetes, impaired glucose tolerance
Introduction
Polycystic ovarian syndrome (PCOS) brings significant heterogeneity of cardio-metabolic risk at the time of the confirmed diagnosis (1). Obesity, menstrual irregularity and hyperandrogenism are recognized as the most important clinical predictors of progressive metabolic burden (2). Metformin that has been used in PCOS over the past few decades (3, 4) may decrease the metabolic risk (1), yet the majority of therapeutic studies in this population have been small and have used metformin for a relatively brief period (5, 6).
Low-quality evidence supports the use of metformin for women with PCOS and glucose intolerance when lifestyle intervention (LSI) is insufficient and for the management of menstrual irregularity if women are unable to take oral contraceptives (OCPs) (7, 8). The latest international guidelines update recommends to consider metformin in addition to LSI also in women with PCOS with BMI ≥25 kg/m2, independent of the presence of glucose disturbances and menstrual irregularity (7). However, there is no clear answer for how long metformin should be prescribed in these subsets of patients, who would clearly benefit from long-term use of metformin in PCOS, and whether long-term treatment with metformin is of benefit above and beyond lifestyle modification (1, 8, 9).
The possibilities of preventing or delaying cardiometabolic diseases in adults at high risk had been hypothesized for many years. The largest and longest trial in general population at high risk of developing type 2 diabetes (T2D) included 3234 participants with impaired glucose tolerance, elevated fasting plasma glucose and BMI of ≥24 kg/m2, and metformin reduced the incidence of T2D by 31% compared with placebo after an average follow-up of 2.8 years and by 18% over 10 and 15 years post randomization (10). The subgroups that benefited the most included subjects with obesity, higher baseline fasting glucose or HbA1c and women with history of gestational diabetes mellitus (10, 11). Furthermore, a recent systemic review demonstrated that adults using metformin experienced and maintained greater decreases in weight/BMI when compared with subjects on placebo, irrespective of duration of intervention and of the prescribed daily dosage (12). One of the major determinants of the efficacy of metformin used as a preventive measures seems to be adherence (12) that is usually observed to be low in long-term settings.
We evaluated the long-term efficacy of metformin in overweight-obese patients with PCOS and normal baseline glycemic homeostasis on body mass (BM), menstrual frequencies (MF), metabolic and hormonal outcomes. This allows us to summarize our perspective in future research that can help to identify the optimal candidates and optimal duration for treatment with metformin in overweight-obese PCOS with normal baseline glucose homeostasis, where currently a long-term efficacy of metformin remains unproven.
Materials and methods
Study design
We conducted a retrospective review of medical records of all women with PCOS referred to a specialized endocrinology outpatient clinics at the Department of Endocrinology, Diabetes and Metabolism in University Medical Centre, from January 2006 to December 2008. Study protocol was approved by the National Ethics Committee and registered with ClinicalTrials.gov identifier NCT04043221.
Study cohort
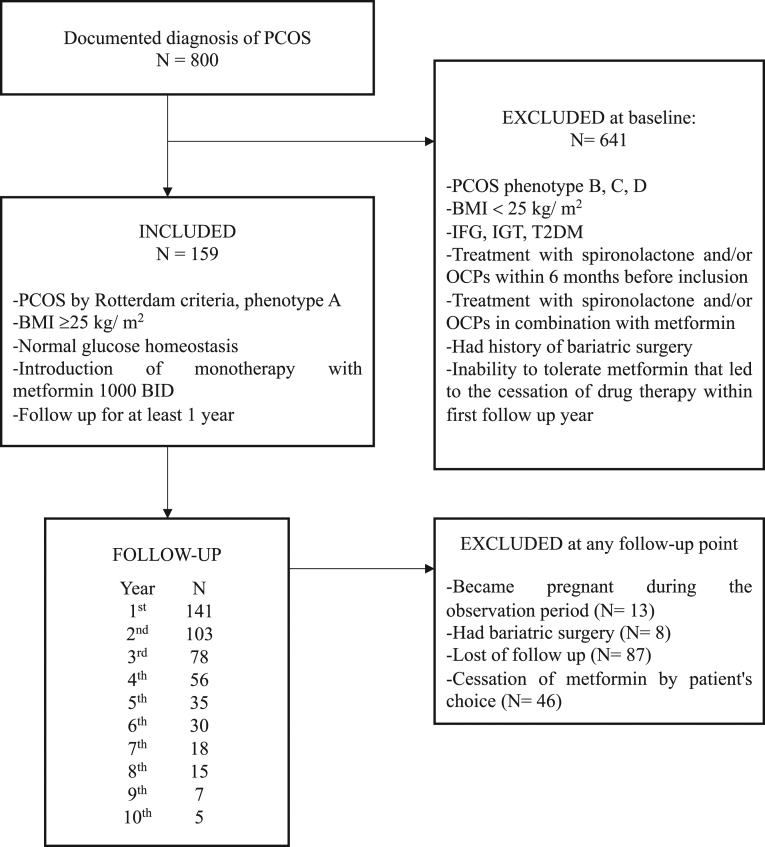
We identified 800 patients diagnosed with PCOS referred to first endocrine check-up during the selected 3-year period. The medical records of all patients were reviewed by the authors to confirm the diagnosis of PCOS based on the Rotterdam criteria and phenotype themfrom A to D. Phenotype A is defined as concomitant presence of hyperandrogenism, ovulatory dysfunction and polycystic ovarian morphology (PCOM) and represents the classical form of the syndrome. Phenotype B presents hyperandrogenism and ovulatory dysfunction, without PCOM. Phenotype C is the so-called ‘ovulatory’ PCOS (hyperandrogenism and PCOM only) and phenotype D is often referred to as ‘nonhyperandrogenic’ PCOS (ovulatory dysfunction and PCOM only) (13). After the review of the medical records we recruited the patients. Eligibility criteria included phenotype A, BMI ≥25 kg/m2, normal glucose homeostasis, introduction of monotherapy with metformin 1000 mg BID and follow-up period for at least 1 year. Patients were excluded from the study if they showed the following: (a) phenotype B, C or D; (b) BMI <25 kg/m2; (c) impaired fasting glucose (IFG), impaired glucose tolerance (IGT) or T2Dat baseline; (d) had been treated with spironolactone and/or OCPs within the last 6 months before recruitment; (e) had history of bariatric surgery; (f) became pregnant during the observation period or (g) had the inability to tolerate metformin that led to the cessation of drug therapy within first follow-up year (Fig. 1).
Figure 1.
Flowchart of the study. PCOS, polycystic ovarian syndrome; N numerus; BMI body mass index; BID, bis in die, twice a day; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; T2DM, type 2 diabetes mellitus.
Data collection
Each patient’s age and height were recorded at baseline. In addition, each patient’s weight, blood pressure, menstrual frequency, fasting glucose, androstenedione, dehydroepiandrosterone sulphate (DHEAS), free and total testosterone, follicle-stimulating hormone (FSH), luteinizing hormone (LH) were identified at baseline and at every follow-up visit at 1-year intervals where available. Oral glucose tolerance test was performed at baseline and then rescreened periodically over 2–3 years. Waist circumference, Ferriman–Gallwey score, serum insulin levels and lipid profile were not consistently assessed as part of the standard clinical care in women with PCOS at that time and sufficient data are unavailable for analysis.
Glucose levels were determined using a standard glucose oxidase method (Beckman Coulter Glucose Analyzer, Beckman Coulter Inc CA, USA). Androstenedione and DHEAS were measured by specific double antibody RIA using 125 I-labeled hormones (Diagnostic Systems Laboratories, Webster, Tx). Total and free testosterone levels were measured by coated tube RIA (DiaSorin, S. p. A, Salluggia, Italy and Diagnostic Products Corporation, LA, respectively). LH and FSH were measured using immunometric assay (Diagnostic Products Corporation, LA). Intra-assay coefficient of variation (CV) for androstenedione ranges from 5.0 to 7.5% and inter-assay CV from 4.1 to 11.3%, and intra-assay CV for DHEAS from 4.9 to 9.8% and inter-assay CV from 7.9 to 13.0%. Intra-assay CV for free testosterone is 7.7–19.3% and inter-assay CV is 6.4–13.2%. Intra-assay CV for total testosterone is 5.1–16.3% and inter-assay CV is 7.2–24.3%. Intra-assay CV for SHBG is 2.5–5.3% and inter-assay CV is 4–6.6%. BMI was calculated as the weight in kilograms divided by square of height in meters. Menstrual regularity was defined as number of bleeds per year using self reported menstrual intervals based on dairy review. According to World Health Organization (WHO) criteria (available from: http://www.who.int/diabetes/publications/diagnosis_diabetes2006/en/), during OGTT, normal glucose tolerance (NGT) was defined as fasting glucose levels below 6.1 mmol/L and 2-h glucose in 75 g-OGTT <7.8 mmol/L, impaired fasting glucose (IFG) as fasting glucose between 6.1 and 6.9 mmol/L and 2-h glucose in 75g-OGTT <7.8 mmol/L, impaired glucose tolerance (IGT) was identified by 2-h glucose levels in 75 g-OGTT between 7.8 and 11 mmol/L and T2D as fasting glucose level ≥7.0 mmol/L or 2-h glucose ≥11.1 mmol/L. Conversion was viewed as worsening of category from NGT to IFG, IGT or T2D, a change from IFG to T2D or a change from IGT to T2D over time, and reversion was viewed as an improvement from IFG, IGT or T2D to NGT or from T2D to IGT or IFG.
Metformin therapy
All participants began treatment with metformin at baseline. Metformin was commenced at 500 mg once daily, with 500 mg increments weekly up to 2000 mg in most patients. As part of the routine clinical care, all patients were counseled regarding lifestyle changes at every visit.
Statistical analysis
Continuous variables are represented as mean ± s.d., while categorical variables are described using frequencies. Nonparametric Wilcoxon signed-rank test was used for comparison of clinical parameters for related samples. Spearman’s rho was used to calculate correlations between continuous variables. Nonparametric Mann–Whitney or Kruskal–Wallis tests were used to compare the distribution of continuous variables among different groups. P values of <0.05 were considered statistically significant. IBM SPSS Statistics, version 21.0 (IBM Corporation) was used for all statistical analyses.
Results
During the selected 3-year period, 800 women with the diagnosis of PCOS underwent assessment, 159 among them fulfilled the eligibility criteria. The mean values of their baseline characteristics are outlined in Table 1. Longitudinal follow-up included 6085 time-points with a subset of anthropometric, reproductive, hormonal and metabolic parameters.
Table 1.
Baseline characteristic and longitudinal follow-up to 7th year of therapy with metformin of clinical parameters in women with PCOS, treated with metformin.a
| Clinical parameter mean ± s.d. | Baseline characteristic | Years of metformin therapy | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
| n = 159 | n = 141 | n = 103 | n = 78 | n = 56 | n = 35 | n = 30 | n = 18 | |
| Age (years) | 28.4 ± 6.4 | |||||||
| Weight (kg) | 96.1 ± 19.2 | 92.4 ± 18 | 91.1 ± 18.1 | 92.7 ± 19.2 | 92.7 ± 19.5 | 95.1 ± 18.1 | 93 ± 15.3 | 90.4 ± 13.6 |
| BMI (kg/m2) | 34.9 ± 6.6 | 33.5 ± 6.3 | 33.2 ± 6.6 | 33.5 ± 6.6 | 33.4 ± 6.6 | 34.5 ± 6.6 | 34 ± 6.2 | 32.8 ± 4.8 |
| FPG (mmol/L) | 4.9 ± 0.5 | 4.9 ± 0.7 | 4.9 ± 0.6 | 4.9 ± 0.8 | 5.2 ± 0.8 | 5.5 ± 1.4 | 5.2 ± 1 | 5.6 ± 1.6 |
| MF (number of cycles/year) | 7.6 ± 3.8 | 10.8 ± 2.7 | 10.6 ± 3 | 11.3 ± 1.7 | 10.6 ± 2.9 | 11 ± 2.4 | 11 ± 2.9 | 11.6 ± 0.6 |
| DHEAS (μmol/L) | 6.5 ± 3.2 | 7.3 ± 9.2 | 6.9 ± 3 | 6.4 ± 3.4 | 6.8 ± 3.6 | 5.8 ± 3.2 | 6.3 ± 3.2 | 9.5 ± 10.9 |
| Total testosterone (nmol/L) | 2 ± 1 | 1.5 ± 0.8 | 1.6 ± 1 | 1.3 ± 0.9 | 1.3 ± 0.8 | 1.4 ± 1.2 | 1.7 ± 1.3 | 2 ± 2.3 |
| Free testosterone (pmol/L) | 6.6 ± 4.3 | 5.2 ± 3.6 | 5.3 ± 3.7 | 5.9 ± 4.1 | 6.1 ± 4.1 | 4.9 ± 2.8 | 5.2 ± 3.7 | 4.5 ± 2.8 |
| Androstenedione (nmol/L) | 10 ± 9.9 | 7.3 ± 3.5 | 8.3 ± 4.4 | 6.8 ± 3.5 | 7.4 ± 4.4 | 7.4 ± 4.4 | 6.9 ± 3.6 | 5.9 ± 2 |
| FSH (IU/L) | 5.4 ± 5.8 | 5.1 ± 2.3 | 5.2 ± 2.5 | 5.2 ± 2.2 | 5.7 ± 3.2 | 7.8 ± 12.6 | 5.3 ± 2.6 | 6.5 ± 2.6 |
| LH (IU/L) | 9.1 ± 7.3 | 7.5 ± 8.5 | 8 ± 7.2 | 7.1 ± 6.5 | 7.9 ± 8.8 | 12.3 ± 12.1 | 6.6 ± 5.1 | 8.6 ± 10.3 |
aThe data analyses beyond 7 years are truncated because less than 10% of the participants continued with metformin therapy for more than 7 years.
BMI, body mass index; DHEAS, DHEA sulphate; FBG, fasting plasma glucose; FSH, follicle-stimulating hormone; LH, luteinizing hormone; MF, menstrual frequencies; s.d., standard deviation.
Anthropometric parameters
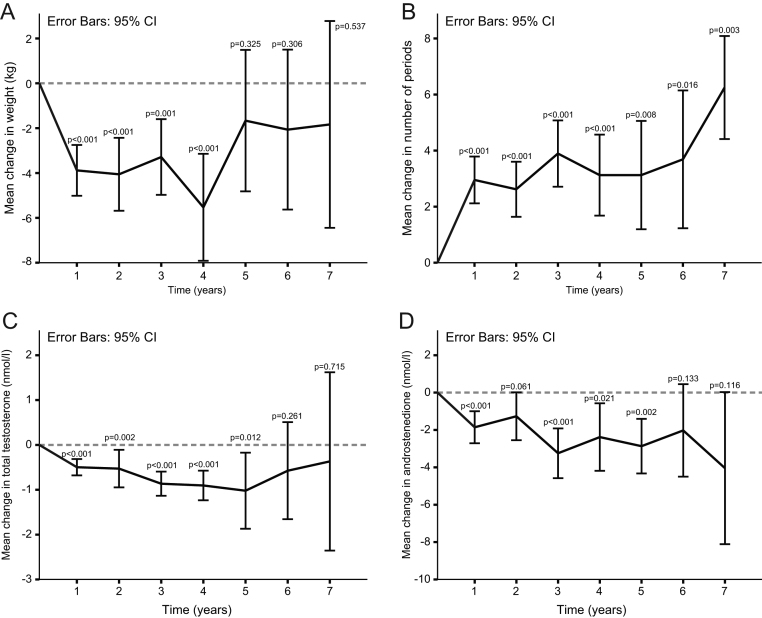
BM decreased for 3.9 ± 6.8 kg (P < 0.001) in the first year of follow-up. BMI decreased for 1.5 ± 2.3 kg/m2 (P < 0.001) in the first year of follow-up. Altogether in the first year, 104 patients lost weight, 8 remained the same and 28 patients gained weight. Change of the BM after the first year was inversely correlated with baseline BM (Spearman’s rho = −0.337; P < 0.001) and BMI (Spearman’s rho = −0.308; P < 0.001). Patients who lost weight in the first year had significantly less frequent menstrual bleeding at baseline (Spearman’s rho = 0.190; P = 0.045). Twenty-five patients gained weight after 1 year, 60% of those who had 11 or 12 menstrual bleedings at baseline. There was no correlation between body weight change and fasting plasma glucose level or hormonal status (level of LH, FSH, DHEAS, androstenedione, total and free testosterone). Patients who lost weight in the first year had significantly less frequent menstrual bleeding at baseline (6.9 ± 3.8 vs 9.2 ± 3.6 numbers of bleedings/year (P = 0.003)) when compared to patients that gained weight or remained stable. The two groups did not differ at baseline BM, BMI, fasting plasma glucose level and hormonal status (level of LH, FSH, DHEAS, androstenedione, total and free testosterone). Decrease of BM and BMI remained significant up to visit (V) 4 when compared with baseline. From V5 up to V10, no significant change in BM was observed when compared with baseline (Fig. 2A).
Figure 2.
Change in body weight (A), number of menstrual cycles (periods) (B), total plasma testosterone levels (C) and plasma androstenedione levels (D) during the time. Values are means with 95% CI shown by vertical lines. Comparisons to pretreatment values were calculated using Wilcoxon test for paired samples. *The values beyond 7 years are truncated because less than 10% of the participants continued with metformin therapy for more than 7 years.
Menstrual regularity
Menstrual frequency increased from 7.6 ± 3.8 to 10.8 ± 2.7 bleeds/year (P < 0.001) after first year to over 11 bleeds/year from V5 up to V7 (Fig. 2B and Table 1). The increase in menstrual frequency was statistically significant from V1–V7 when compared to baseline (Fig. 2B).
We also stratified patients by age and evaluated menstrual frequency in patients younger than 35 years at baseline and patients 35 years old or older at baseline. In patients younger than 35 years, menstrual frequency increased from 7.4 ± 3.9 to 10.7 ± 2.9 bleeds/year (P < 0.001) after first year and to 11 bleeds/year or more from V5 up to V7. In patients ≥35 years, menstrual frequency increased from 9.0 ± 3.1 to 11.1 ± 1 bleeds/year (P < 0.026) after first year.
Change in menstrual frequency after first year negatively correlated with the menstrual frequency at baseline (Spearman’s rho = −0.803; P < 0.001) and age (Spearman’s rho = −0.221; P = 0.044). Correlation with menstrual frequency at baseline was significant both in patients younger than 35 years (Spearman’s rho = −0.792; P < 0.001) and in patients ≥35 years (Spearman’s rho = −0.805; P = 0.009).
Increased menstrual frequency after first year correlated with the decreases in BM (P = 0.008) and decrease in total testosterone (P = 0.035), with no correlation with the change in LH/FSH ratio after first year. Correlation with BM and total testosterone was significant only in patients younger than 35 years (P = 0.023 and P = 0.015, respectively) and not in patients ≥35 years (P = 0.062 and P = 0.639, respectively).
Patients in whom menstrual regularity improved after first year had significantly less frequent bleedings (5.8 ± 3.3 vs 11.0 ± 2.2 numbers of bleedings/year (P < 0.001)) and lower DHEAS (5.9 ± 3.0 vs 7.7 ± 3.7 μmol/L, P = 0.040) at baseline when compared with those with no improvement in menstrual frequencies. After stratification by age, only association of number of bleedings/year in patients younger than 35 years was significant (5.3 ± 3.2 vs 11.0 ± 2.3 number of bleedings/year (P < 0.001)).
Metabolic parameters
Mean levels of fasting glucose did not change significantly after first year follow-up and not during the following years. The mean values were in the normal range throughout the follow-up (Table 1). Patients in whom fasting glucose significantly decreased after first year had significantly higher fasting glucose at baseline (5.0 ± 0.4 vs 4.8 ± 0.5 mmol/l (P = 0.007)) and significantly lower menstrual frequency (6.3 ± 3.7 vs 8.5 ± 3.7 numbers of bleedings/year (P = 0.006)) when compared with women in whom fasting glucose increased or remained unchanged after first year. Fasting glucose after first year was negatively correlated with fasting glucose at baseline (Spearman’s rho = −0.326; P = 0.001) and positively correlated with the number of menstrual bleeding per year (Spearman’s rho = −0.305; P = 0.005).
An overall conversion rate to IFG was 6.2% (10 women), to IGT 1.2% (2 women), to IFG and IGT 1.2% (2 women) and to T2D was 4.4% (7 women), among them 3% (5 women) developed T2D from previously NGT and 2 from IFG. The highest conversion rate was observed in the first year, when 4 women developed IFG (one of them developed T2D in the second year), 2 women developed IGT, 2 women developed IFG and IGT (one of them developed T2D in fourth year) and one woman developed T2D. In the second year IFG developed in 2 women, in the third year in one, in fourth year in 2 and in sixth year in one woman. T2D was developed from previously NGT in 2 women in first year, in 1 woman in fourth year and in 2 women in fifth year. Only in one patient remission from T2D (which occurred in second year after developing IFG in first year) to IFG was observed in sixth year, but in seventh year T2D reoccurred.
Hormonal parameters
Total testosterone and androstenedione decreased for 15.4 ± 47.9% and 11.3 ± 46.4% respectively after the first year (Fig. 2C and D), with further decrease in total testosterone and androstenedione for 37.8 ± 61.8 and 24.8 ± 40.5% from the initial values at the fifth year of the follow-up. After a decrease in the first year, value of total testosterone remained stable over the treatment period (Fig. 1D). The changes of total testosterone and androstenedione after first year were inversely correlated with baseline total testosterone and androstenedione (Spearman’s rho = −0.541, P < 0.001 and Spearman’s rho = −0.302, P = 0.009 respectively). In addition, the change of androstenedione after first year was inversely correlated with baseline androstenedione (Spearman’s rho = −0.582; P < 0.001). In comparison with the women in whom the total testosterone and androstenedione remained unchanged or increased, women in whom total testosterone and androstenedione significantly decreased had higher androstenedione at baseline (10.2 ± 3.7 vs 6.7 ± 3.4 nmol/l (P < 0.001) in patients with significant decrease of androstenedione and 10.3 ± 3.8 vs 7.4 ± 3.4 nmol/l (P = 0.001) in patients with significant decrease of total testosterone). Women in whom total testosterone significantly decreased after first year were significantly younger (28.2 ± 7.0 vs 31.5 ± 5.6 years) and also had significantly higher testosterone at baseline, when compared with non-responders in this regard. Values of LH and FSH decreased slightly over the treatment period, although the difference was not statistically significant yet. Values of DHEAS did not changed significantly during follow-up period. (Table 1).
Adherence
From 159 subjects, the dropout rate was 10.4% (18 patients) in the first year, 35.3% (56 patients) in the second year, 51% (81 patients) in the third year, 64.2% (103 patients) in the fourth and 78% (124 patients) in the fifth year. Only 22% (35 subjects) continued with metformin therapy until fifth year of follow-up. About 18% (30 subjects) continuously received metformin for more than 5 years with further dropout to 5.6% (9 subjects) on metformin therapy at the tenth year of follow-up.
The drop out in this cohort was not associated with metformin intolerance, adverse effects, bariatric surgery or pregnancy since those were the exclusion criteria. As reported by the patients, the main reason for discontinuation after the third year was the lack of motivation. As seen from the patient’s perspective, maximal treatment response achieved after first year was followed by a steady state and then the benefits of the steady state and other potential beneficial effects of long-term treatment were not sufficiently discussed with them.
There were no statistically significant changes in the baseline characteristic between patients that dropped out after third year and the patients who were continuing with the therapy after the third year. No baseline characteristics were associated with drop out or non-drop out status.
Adverse events
Among the 800 women, 3% had intolerable side effects that resulted in cessation of therapy within the first year. They were not included in the study. Although several women that were eligible for the study reported mild-to-moderate nausea and diarrhea at first year of follow-up, the symptoms were transient and disappeared after 4–8 weeks. No case of lactic acidosis or significant anemia due to vitamin B12 deficiency was documented within the study group during the observational period. Development of mild anemia with normal mean corpuscular volume (MCV) values were documented in five patients, all had normal levels of vitamin B12.
Discussion
Long-term metformin treatment of women with PCOS, BMI ≥25 kg/m2 and normal baseline glucose homeostasis resulted in significant treatment response after the first year, with weight loss, increased menstrual regularity and improved androgen profile. In majority of women that subsequently remained on therapy, an overall beneficial steady state was observed throughout the follow-up. Conversion rate to IFG and T2D was low during the observation period, with the highest conversion observed within the first year. Those with higher baseline BM, BMI, less frequent menstrual bleedings and higher levels of androgens were identified as subgroups where metformin’s effect seemed to be enhanced. Notably, after 2 years, remarkable drop out that had not been related to metformin intolerance or side effects was seen in this real-life setting.
Impact on body weight
With regard to weight loss, it is often questioned whether long-term treatment with metformin is of benefit above and beyond LSI in overweight-obese patients with PCOS (1, 6, 8, 9, 14). In our study, mean BM decreased for 3.7% after the first year and remained stable up to fourth year of follow-up. A meta-analysis of 630 participants with PCOS treated with metformin for 6 months reported no evidence of its effect on BMI (15). On the other hand, another meta-analysis comparing the effect of metformin with or without LSI to LSI with or without placebo concluded that metformin in combination with LSI was associated with lower BMI (6, 16, 17). Similarly, and in line with our observation, when only participants with BMI ≥25 kg/m2 were combined in a subgroup analysis, it was demonstrated that metformin offered additive benefits for weight and BMI when compared to LSI alone (18). Also, in concordance with our observation, metformin added to LSI offered success in sustainability of weight reduction over 4 years in prospective cohort of 74 PCOS women (19), whereas LSI alone is in general not very successful in preventing body weight regain after initial weight loss (20, 21, 22, 23, 24). Given that PCOS is characterized by progressive weight gain from menarche (25, 26, 27), the difficulty in stabilization of BM is expected to be even more pronounced in PCOS when compared to general population. Potential goal of long-term treatment with metformin in overweight-obese women with PCOS should therefore be a stabilization of BM through the years rather than weight reduction itself.
The amount of weight loss in our study correlated with the initial body weight and baseline number of menstrual bleedings per year. Women who lost weight in the first year had higher BM and less frequent menstrual bleeding at baseline. Greater capacity to lose weight in patients with higher BMI than in patients with slighter obesity was confirmed also in another real-life setting investigated metformin use in PCOS (28). Interestingly, correlation with BM and total testosterone was significant only in our patients younger than 35 years and not in patients ≥35 years. This finding may be explained by the lower levels of testosterone in women older than 35 years, which may reduce the strength of the association between the two parameters. The data on the relationship between adiposity and total testosterone in PCOS from other studies are scarce and controversial (29).
Impact on menstrual regularity
Metformin use in PCOS is not consistently associated with improvements in menstrual regularity. In a Cohrane review that included a meta-analysis of 38 RCT of 3495 women with PCOS, metformin therapy only marginally improved menstrual pattern (15). In our cohort menstrual frequency increased after first year and normalized in the majority of patients in the following years. In the 24-month study conducted with prospective cohort, metformin was also associated with improvements in the menstrual cycle in overweight and normal weight women with PCOS (30). Our patients in whom menstrual regularity improved after first year had less frequent bleedings at baseline when compared with those with no improvement in menstrual frequencies. Increased menstrual frequency correlated with the decrease in BM and total testosterone, whereas no correlation with the change in LH/FSH ratio was demonstrated. Weight, insulin resistance, testosterone and LH/FSH disturbances are established determinants of menstrual regularity (31); yet, the relative contributions of those parameters on menstrual regularity across the different phenotypes of PCOS and the consequences of metformin impact on these different spectrums are currently unknown (30). As menstrual irregularity in PCOS typically improves with time, we also stratified patients by age and confirmed the increase in patients younger than 35 years and in patients ≥35 years old at baseline.
Impact on androgens
Metformin lowered testosterone levels by approximately 20–25% in women with PCOS (5). In accordance with these reports (5), mean total testosterone and androstenedione decreased up to 15% after the first year, with further decrease in total testosterone and androstenedione from 25 to up to 40% from the initial values at the fifth year of the follow-up. Importantly, the potential effect of aging that results in improvement of hyperandogenism as well as the well-known methodological difficulties related to RIA assays should be taken into account when interpreting the observed impact on the androgen status. In comparison with the women in whom androgens remained unchanged or increased, women in whom androgens decreased had worst androgen profile at baseline. It is believed that metformin lowered testosterone levels by reducing hyperinsulinemia (32, 33). In addition, it might have a direct inhibitory effect on ovarian steroidogenesis (34, 35, 36) through inhibition of mitochondrial complex I (34). Given that an androgen excess plays an important role in favoring the expansion of visceral fat and development of metabolic syndrome and T2D (37), the improved androgen profile observed throughout long-term metformin treatment should be considered as an independent cardiometabolic risk reduction outcome in these population.
Impact on metabolic features
Few studies aimed to clarify the relationship between PCOS and T2D independent of obesity in longitudinal population-based cohorts. The most recent Australian Longitudinal Study on Women’s Health database reported that women with PCOS are at an increased risk of T2D, irrespective of age and BMI (2). There have been very limited studies of the natural history of glucose homeostasis in this population. In an elegant study by Legro et al., there was a nearly two-fold increase in the rates of conversion for subjects with PCOS and baseline NGT compared with the reference population, but there was also a significant chance for a spontaneous reversion rate to normal glucose tolerance (38). Prospective studies investigating the impact of metformin on T2D risk specifically in women with PCOS are lacking (6). Nonetheless, considering that these women are at high risk for developing T2D (39, 40), it has been suggested that they will benefit from metformin therapy in case of glucose intolerance (7). One of the longest retrospective study with 50 patients followed by a mean treatment period of 43.3 months demonstrated a 11-fold decrease in the annual conversion rate from NGT to IGT and complete prevention of the development of T2D (40). In our cohort the mean fasting glucose was within normal range throughout the longitudinal follow-up. Conversion to IGT and T2D was low, yet OGTT was not performed annually, but as recommended by national guidelines rescreened periodically at 2–3 years, meaning that this conversion risk could be underestimated.
Limitations and strengths
We acknowledge that our study has several limitations. The major one is its retrospective nature. It is clear that prospective randomized design represents the gold standard, but such a study would be extremely long lasting and laborious to achieve. Lack of randomization with placebo represents another possible bias, yet placebo arm in this group would have hardly been a realistic or possible approach. Moreover, the possible bias might be related to the process of ageing, in particular when interpreting the improvements of menstrual irregularity and hyperandrogenism as both improve by age. Furthermore, lifestyle measures that had been promoted by the lifestyle advice might contribute to at least some of the benefits including low rates of conversion to IGT and T2DM. Another point of concern is the high rate of drop-outs and the effects that may have on the study outcome. However, this attrition rate was similar to that from other studies on PCOS (16, 30).
The main strength of this study is the long-term longitudinal follow-up assessing the effectiveness of treatment with metformin in real life setting that is insufficiently studied in PCOS. Attempts were made to achieve clinical homogeneity of the included patients by reviewing the medical records of all 800 patients that had been referred to our clinics from 2006 to 2008. We included only those with phenotype A, BMI ≥25 kg/m2 with normal baseline fasting glucose level and normal glucose tolerance. All patients were managed in the single center using a standardized treatment protocol with 2000 mg metformin that had been introduced in all overweight-obese women regardless of their glycemic status unless contraindicated since 2006, and thus any possible selection bias had been eliminated. This offers very important and rarely available insight into the long-term longitudinal follow-up in this subset of patients that have not been, in general, characterized as candidates for metformin treatment until the latest recommendations update (7).
Conclusions
We conclude that, in agreement with the latest recommendations (7), metformin should not be withheld from treatment of PCOS in overweight-obese women with normal fasting glucose and normal glucose tolerance. We suggest that treatment decisions for metformin are based on BMI, oligomenorrhea and biochemical hyperandrogenism regardless of the glycemic status. The overweight-obese and oligomenorrheic women should be prioritized treatment candidates. The high rate of drop outs not related to intolerance and side effects could be decreased in clinical practice by discussing the realistic treatment goals and potential benefits of long-term intervention.
Unanswered questions and future perspective
We encourage future designs to investigate the stabilization of BM through the years as one of the main treatment benefits of long-term treatment with metformin in overweight-obese PCOS. The separate impact of metformin on visceral and s.c. fat depots is another topic that deserves further attention. The protective effect of the long-term use of metformin in reducing the risk of unopposed endometrial proliferation and endometrium cancer should also be evaluated.
The next important question is for how long metformin should be applied to reach the homeostasis that can sustain weight and glucose metabolism after metformin withdrawal. We suggest to compare the consequences of metformin withdrawal after long-term therapy as opposed to the consequences of metformin withdrawal immediately after the maximum treatment effect is achieved, usually after the first year. Intermittent regimens vs continuing long-term interventions with metformin represent another issue to be addressed.
The heterogeneity in response to metformin represents another exciting research field. Traditional as well as nontraditional risk cardiometabolic markers including chronic inflammation, oxidative stress, homeostasis and fibrinolysis imbalance, gut microbiota dysbiosis and epigenetic alterations sympathetic nervous system dysfunction (41) should be considered as potential predictors for responders and non-responders. Furthermore, large-scale genome wide studies are also imperative to identify the best responders.
The further research needs to firstly identify and then prioritize those groups who will benefit most from being treated with metformin. The treatment goals and duration of therapy should be clearly defined, in particular, in overweight-obese women with PCOS and normal initial glucose homeostasis where its long-term use is currently more difficult to advocate. Individually tailored approaches might lead to better adherence that would provide better insights into a long-term cost–benefit profile. Continuing follow-ups in randomized prospective trials and in real life settings would provide further information on putative long-term benefits of metformin and whether they are homogeneous across subgroups.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial, or not-for profit sector.
References
- 1.Bates GW, Legro RS. Long-term management of polycystic ovarian syndrome (PCOS). Molecular and Cellular Endocrinology 2013. 91–97. ( 10.1016/j.mce.2012.10.029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kakoly NS, Earnest A, Teede HJ, Moran LJ, Joham AE. The impact of obesity on the incidence of Type 2 diabetes among women with polycystic ovary syndrome. Diabetes Care 2019. 560–567. ( 10.2337/dc18-1738) [DOI] [PubMed] [Google Scholar]
- 3.Velazquez EM, Mendoza S, Hamer T, Sosa F, Glueck CJ. Metformin therapy in polycystic ovary syndrome reduces hyperinsulinemia, insulin resistance, hyperandrogenemia, and systolic blood pressure, while facilitating normal menses and pregnancy. Metabolism: Clinical and Experimental 1994. 647–654. ( 10.1016/0026-0495(94)90209-7) [DOI] [PubMed] [Google Scholar]
- 4.Tang T, Glanville J, Hayden CJ, White D, Barth JH, Balen AH. Combined lifestyle modification and metformin in obese patients with polycystic ovary syndrome. A randomized, placebo-controlled, double-blind multicentre study. Human Reproduction 2006. 80–89. ( 10.1093/humrep/dei311) [DOI] [PubMed] [Google Scholar]
- 5.McCartney CR, Marshall JC. CLINICAL PRACTICE. Polycystic ovary syndrome. New England Journal of Medicine 2016. 54–64. ( 10.1056/NEJMcp1514916) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sam S, Ehrmann DA. Metformin therapy for the reproductive and metabolic consequences of polycystic ovary syndrome. Diabetologia 2017. 1656–1661. ( 10.1007/s00125-017-4306-3) [DOI] [PubMed] [Google Scholar]
- 7.Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, Piltonen T, Norman RJ. & International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Human Reproduction 2018. 1602–1618. ( 10.1093/humrep/dey256) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, Pasquali R, Welt CK. & Endocrine Society. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. Journal of Clinical Endocrinology and Metabolism 2013. 4565–4592. ( 10.1210/jc.2013-2350) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodman NF, Cobin RH, Futterweit W, Glueck JS, Legro RS, Carmina E, American Association of Clinical Endocrinologists (AACE), American College of Endocrinology (ACE) & Androgen Excess and PCOS Society (AES). American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome – Part 1. Endocrine Practice 2015. 1291–1300. ( 10.4158/EP15748.DSC) [DOI] [PubMed] [Google Scholar]
- 10.Aroda VR, Knowler WC, Crandall JP, Perreault L, Edelstein SL, Jeffries SL, Molitch ME, Pi-Sunyer X, Darwin C, Heckman-Stoddard BM, et al. Metformin for diabetes prevention: insights gained from the Diabetes Prevention Program/Diabetes Prevention Program Outcomes Study. Diabetologia 2017. 1601–1611. ( 10.1007/s00125-017-4361-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diabetes Prevention Program Research Group. Long-term effects of metformin on diabetes prevention: identification of subgroups that benefited most in the diabetes prevention program and diabetes prevention program outcomes study. Diabetes Care 2019. 601–608. ( 10.2337/dc18-1970) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lentferink YE, Knibbe CAJ, van der Vorst MMJ. Efficacy of metformin treatment with respect to weight reduction in children and adults with obesity: a systematic review. Drugs 2018. 1887–1901. ( 10.1007/s40265-018-1025-0) [DOI] [PubMed] [Google Scholar]
- 13.Azziz R. Introduction: determinants of polycystic ovary syndrome. Fertility and Sterility 2016. 4–5. ( 10.1016/j.fertnstert.2016.05.009) [DOI] [PubMed] [Google Scholar]
- 14.Jayasena CN, Franks S. The management of patients with polycystic ovary syndrome. Nature Reviews: Endocrinology 2014. 624–636. ( 10.1038/nrendo.2014.102) [DOI] [PubMed] [Google Scholar]
- 15.Tang T, Lord JM, Norman RJ, Yasmin E, Balen AH. Insulin-sensitising drugs (metformin, rosiglitazone, pioglitazone, D-chiro-inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility. Cochrane Database of Systematic Reviews 2012. CD003053 ( 10.1002/14651858.CD003053.pub5) [DOI] [PubMed] [Google Scholar]
- 16.Hoeger KM, Kochman L, Wixom N, Craig K, Miller RK, Guzick DS. A randomized, 48-week, placebo-controlled trial of intensive lifestyle modification and/or metformin therapy in overweight women with polycystic ovary syndrome: a pilot study. Fertility and Sterility 2004. 421–429. ( 10.1016/j.fertnstert.2004.02.104) [DOI] [PubMed] [Google Scholar]
- 17.Naderpoor N, Shorakae S, de Courten B, Misso ML, Moran LJ, Teede HJ. Metformin and lifestyle modification in polycystic ovary syndrome: systematic review and meta-analysis. Human Reproduction Update 2015. 560–574. ( 10.1093/humupd/dmv025) [DOI] [PubMed] [Google Scholar]
- 18.Teede H, Tassone EC, Piltonen T, Malhotra J, Mol BW, Peña A, Witchel SF, Joham A, McAllister V, Romualdi D, et al. Effect of the combined oral contraceptive pill and/or metformin in the management of polycystic ovary syndrome: a systematic review with meta-analyses. Clinical Endocrinology 2019. 479–489. ( 10.1111/cen.14013) [DOI] [PubMed] [Google Scholar]
- 19.Glueck CJ, Aregawi D, Agloria M, Winiarska M, Sieve L, Wang P. Sustainability of 8% weight loss, reduction of insulin resistance, and amelioration of atherogenic-metabolic risk factors over 4 years by metformin-diet in women with polycystic ovary syndrome. Metabolism: Clinical and Experimental 2006. 1582–1589. ( 10.1016/j.metabol.2006.08.001) [DOI] [PubMed] [Google Scholar]
- 20.Franks S. When should an insulin sensitizing agent be used in the treatment of polycystic ovary syndrome? Clinical Endocrinology 2011. 148–151. ( 10.1111/j.1365-2265.2010.03934.x) [DOI] [PubMed] [Google Scholar]
- 21.Norman RJ, Davies MJ, Lord J, Moran LJ. The role of lifestyle modification in polycystic ovary syndrome. Trends in Endocrinology and Metabolism 2002. 251–257. ( 10.1016/s1043-2760(02)00612-4) [DOI] [PubMed] [Google Scholar]
- 22.Velázquez E, Acosta A, Mendoza SG. Menstrual cyclicity after metformin therapy in polycystic ovary syndrome. Obstetrics and Gynecology 1997. 392–395. ( 10.1016/s0029-7844(97)00296-2) [DOI] [PubMed] [Google Scholar]
- 23.Kiddy DS, Hamilton-Fairley D, Bush A, Short F, Anyaoku V, Reed MJ, Franks S. Improvement in endocrine and ovarian function during dietary treatment of obese women with polycystic ovary syndrome. Clinical Endocrinology 1992. 105–111. ( 10.1111/j.1365-2265.1992.tb02909.x) [DOI] [PubMed] [Google Scholar]
- 24.Look AHEAD Research Group, Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, Coday M, Crow RS, Curtis JM, Egan CM, et al. Cardiovascular effects of intensive lifestyle intervention in Type 2 diabetes. New England Journal of Medicine 2013. 145–154. ( 10.1056/NEJMoa1212914) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glueck CJ, Dharashivkar S, Wang P, Zhu B, Gartside PS, Tracy T, Sieve L. Obesity and extreme obesity, manifest by ages 20–24 years, continuing through 32–41 years in women, should alert physicians to the diagnostic likelihood of polycystic ovary syndrome as a reversible underlying endocrinopathy. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2005. 206–212. ( 10.1016/j.ejogrb.2005.03.010) [DOI] [PubMed] [Google Scholar]
- 26.Glueck CJ, Wang P, Fontaine R, Tracy T, Sieve-Smith L. Metformin to restore normal menses in oligo-amenorrheic teenage girls with polycystic ovary syndrome (PCOS). Journal of Adolescent Health 2001. 160–169. ( 10.1016/s1054-139x(01)00202-6) [DOI] [PubMed] [Google Scholar]
- 27.Ollila MM, Piltonen T, Puukka K, Ruokonen A, Järvelin MR, Tapanainen JS, Franks S, Morin-Papunen L. Weight gain and dyslipidemia in early adulthood associate with polycystic ovary syndrome: prospective cohort study. Journal of Clinical Endocrinology and Metabolism 2016. 739–747. ( 10.1210/jc.2015-3543) [DOI] [PubMed] [Google Scholar]
- 28.Seifarth C, Schehler B, Schneider HJ. Effectiveness of metformin on weight loss in non-diabetic individuals with obesity. Experimental and Clinical Endocrinology and Diabetes 2013. 27–31. ( 10.1055/s-0032-1327734) [DOI] [PubMed] [Google Scholar]
- 29.Tosi F, Di Sarra D, Kaufman JM, Bonin C, Moretta R, Bonora E, Zanolin E, Moghetti P. Total body fat and central fat mass independently predict insulin resistance but not hyperandrogenemia in women with polycystic ovary syndrome. Journal of Clinical Endocrinology and Metabolism 2015. 661–669. ( 10.1210/jc.2014-2786) [DOI] [PubMed] [Google Scholar]
- 30.Yang PK, Hsu CY, Chen MJ, Lai MY, Li ZR, Chen CH, Chen SU, Ho HN. The efficacy of 24-month metformin for improving menses, hormones, and metabolic profiles in polycystic ovary syndrome. Journal of Clinical Endocrinology and Metabolism 2018. 890–899. ( 10.1210/jc.2017-01739) [DOI] [PubMed] [Google Scholar]
- 31.Van Anders SM, Watson NV. Menstrual cycle irregularities are associated with testosterone levels in healthy premenopausal women. American Journal of Human Biology 2006. 841–844. ( 10.1002/ajhb.20555) [DOI] [PubMed] [Google Scholar]
- 32.Nestler JE, Jakubowicz DJ. Decreases in ovarian cytochrome P450c17 alpha activity and serum free testosterone after reduction of insulin secretion in polycystic ovary syndrome. New England Journal of Medicine 1996. 617–623. ( 10.1056/NEJM199608293350902) [DOI] [PubMed] [Google Scholar]
- 33.Nestler JE, Jakubowicz DJ. Lean women with polycystic ovary syndrome respond to insulin reduction with decreases in ovarian P450c17 alpha activity and serum androgens. Journal of Clinical Endocrinology and Metabolism 1997. 4075–4079. ( 10.1210/jcem.82.12.4431) [DOI] [PubMed] [Google Scholar]
- 34.Hirsch A, Hahn D, Kempná P, Hofer G, Nuoffer JM, Mullis PE, Flück CE. Metformin inhibits human androgen production by regulating steroidogenic enzymes HSD3B2 and CYP17A1 and complex I activity of the respiratory chain. Endocrinology 2012. 4354–4366. ( 10.1210/en.2012-1145) [DOI] [PubMed] [Google Scholar]
- 35.Mansfield R, Galea R, Brincat M, Hole D, Mason H. Metformin has direct effects on human ovarian steroidogenesis. Fertility and Sterility 2003. 956–962. ( 10.1016/s0015-0282(02)04925-7) [DOI] [PubMed] [Google Scholar]
- 36.Attia GR, Rainey WE, Carr BR. Metformin directly inhibits androgen production in human thecal cells. Fertility and Sterility 2001. 517–524. ( 10.1016/s0015-0282(01)01975-6) [DOI] [PubMed] [Google Scholar]
- 37.Pasquali R, Oriolo C. Obesity and androgens in women. Frontiers of Hormone Research 2019. 120–134. ( 10.1159/000494908) [DOI] [PubMed] [Google Scholar]
- 38.Legro RS, Gnatuk CL, Kunselman AR, Dunaif A. Changes in glucose tolerance over time in women with polycystic ovary syndrome: a controlled study. Journal of Clinical Endocrinology and Metabolism 2005. 3236–3242. ( 10.1210/jc.2004-1843) [DOI] [PubMed] [Google Scholar]
- 39.Ehrmann DA, Barnes RB, Rosenfield RL, Cavaghan MK, Imperial J. Prevalence of impaired glucose tolerance and diabetes in women with polycystic ovary syndrome. Diabetes Care 1999. 141–146. ( 10.2337/diacare.22.1.141) [DOI] [PubMed] [Google Scholar]
- 40.Sharma ST, Wickham EP, 3rd, Nestler JE. Changes in glucose tolerance with metformin treatment in polycystic ovary syndrome: a retrospective analysis. Endocrine Practice 2007. 373–379. ( 10.4158/EP.13.4.373) [DOI] [PubMed] [Google Scholar]
- 41.Chiu WL, Boyle J, Vincent A, Teede H, Moran LJ. Cardiometabolic risks in polycystic ovary syndrome: non-traditional risk factors and the impact of obesity. Neuroendocrinology 2017. 412–424. ( 10.1159/000455233) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a