Abstract
Objective
Thyroid hormones have been implicated to play a role in cardiovascular disease, along with studies linking thyroid hormone to kidney function. The aim of this study is to investigate whether kidney function modifies the association of subclinical thyroid dysfunction and the risk of cardiovascular outcomes.
Methods
In total, 5804 patients were included in the PROspective Study of Pravastatin in the Elderly at Risk (PROSPER). For the current analysis, 426 were excluded because of overt thyroid disease at baseline or 6 months, 266 because of inconsistent thyroid function at baseline and 6 months, 294 because of medication use that could influence thyroid function, and 16 because of missing kidney or thyroid values. Participants with normal fT4 were classified, based on TSH both at inclusion and 6 months, into three groups: subclinical hypothyroidism (TSH >4.5 mIU/L); euthyroidism (TSH = 0.45–4.5 mIU/L); and subclinical hyperthyroidism (TSH <0.45 mIU/L). Strata of kidney function were made based on estimated glomerular filtration rate into three clinically relevant groups: <45, 45–60, and >60 mL/min/1.73 m2. The primary endpoint consists of death from coronary heart disease, non-fatal myocardial infarction and (non)fatal stroke.
Results
Mean age was 75.3 years, and 49.0% patients were male. Mean follow-up was 3.2 years. Of all participants, 109 subjects (2.2%) had subclinical hypothyroidism, 4573 (94.0%) had euthyroidism, and 182 (3.7%) subclinical hyperthyroidism. For patients with subclinical hypothyroidism, euthyroidism, and subclinical hyperthyroidism, primary outcome occurred in 9 (8.3%), 712 (15.6%), and 23 (12.6%) patients, respectively. No statistically significant relationship was found between subclinical thyroid dysfunction and primary endpoint with adjusted hazard ratios of 0.51 (0.24–1.07) comparing subclinical hyperthyroidism and 0.90 (0.58–1.39) comparing subclinical hypothyroidism with euthyroidism. Neither was this relationship present in any of the strata of kidney function, nor did kidney function interact with subclinical thyroid dysfunction in the association with primary endpoint (P interaction = 0.602 for subclinical hyperthyroidism and 0.388 for subclinical hypothyroidism).
Conclusions
In this secondary analysis from PROSPER, we found no evidence that the potential association between thyroid hormones and cardiovascular disease is modified by kidney function in older patients with subclinical thyroid dysfunction.
Keywords: cardiovascular disease, kidney function, older patients, thyroid function
Introduction
Subclinical hyperthyroidism has been linked to atrial fibrillation and coronary artery calcification, whereas subclinical hypothyroidism has been associated with hypercholesterolemia and atherosclerosis (1, 2). Despite these observations, data from large prospective cohorts regarding thyroid function and cardiovascular events and mortality are conflicting (3, 4). The discrepancies between the study results may be explained by a small number of participants with subclinical thyroid disease and variations in the definitions of subclinical thyroid disease among different studies. In an attempt to tackle these limitations, recent large, individual participant data meta-analyses were performed and associations were found between increasing thyroid-stimulating hormone (TSH) levels and increased risk of stroke, high circulating free thyroxin (fT4) and increased risk of atrial fibrillation, and subclinical hypo- and hyperthyroidism and increased risk of coronary heart disease and mortality (5, 6, 7).
Chronic kidney disease (CKD) is accompanied by a substantial cardiovascular disease risk (8, 9). Associations of thyroid hormones and CKD have also been described. Multiple cross-sectional studies have linked lower thyroid function to a lower estimated glomerular filtration rate (eGFR) and increased prevalence of CKD (10, 11, 12). Longitudinal studies have presented conflicting results, linking kidney function decline to low (13) or high thyroid function (14). Whereas a recent individual participant data analysis found no role for thyroid hormones in renal function decline, it does not exclude reverse causality, indicating that previously found cross-sectional associations between kidney and thyroid may be explained by kidney dysfunction causing thyroid hormone changes (15). Whether or not kidney function causally alters thyroid hormones, several studies have shown an increased prevalence of subclinical thyroid disease and nonthyroidal illness in patients with CKD (16, 17), and this may be associated with higher mortality (18).
We hypothesize that there might be a role for kidney function in the relation between thyroid function and clinical cardiovascular outcomes, which may partly explain the conflicting results from large observational studies. Therefore, the aim of this study is to investigate whether kidney function modifies the association of subclinical thyroid dysfunction and the risk of cardiovascular outcomes in older patients.
Materials and methods
All subjects were participants of the ‘PROspective study of Pravastatin in the Elderly at Risk’ (PROSPER), a double-blind, randomized, placebo-controlled trial, designed to investigate the relationship between statin treatment and the risk of cardiovascular and cerebrovascular events. In summary, 5804 older participants (70–82 years) were enrolled in Ireland, Scotland, and The Netherlands. Patients were included if they had a history of, or an increased risk for, vascular disease and a baseline cholesterol between 4.0–9.0 mmol/L. A history of vascular disease included stroke, transient ischemic attack, myocardial infarction, arterial surgery, or amputation for vascular disease less than 6 months before study entry. Increased risk for vascular disease included current smoking, hypertension, diabetes mellitus or fasting blood glucose levels over 7 mmol/L. Detailed description of this population, including all in- and exclusion criteria, has been published previously (19). The study was approved by the institutional ethics review boards of centres of Cork University (Ireland), Glasgow University (Scotland), and Leiden University Medical Center (The Netherlands). Consent has been obtained from each patient or subject after full explanation of the purpose and nature of all procedures used.
Thyroid function
TSH was measured at baseline in all participants and fT4 levels were only determined in case of abnormal TSH levels. Measurements were performed in three accredited laboratory centers (Cork in Ireland, Glasgow in Scotland, and Leiden in The Netherlands). TSH and fT4 levels were measured using state-of-the-art immunoassays (third-generation assays with a functional sensitivity <0.05 mIU/L). For both measurements, estimated inter- and intra-assay coefficients of variation were less than 5%. TSH and fT4 were determined in all participants after 6 months of follow-up in available frozen plasma samples, which were stored at the University of Glasgow. The same electrochemiluminescence immunodetection method on a Roche Elecsys 2010 was used. The limits of detection were <0.005 mIU/L for TSH and 0.3 pmol/L for free T4. A reference range between 0.45 and 4.50 mIU/L was used for TSH and 12–22 pmol/L for fT4.
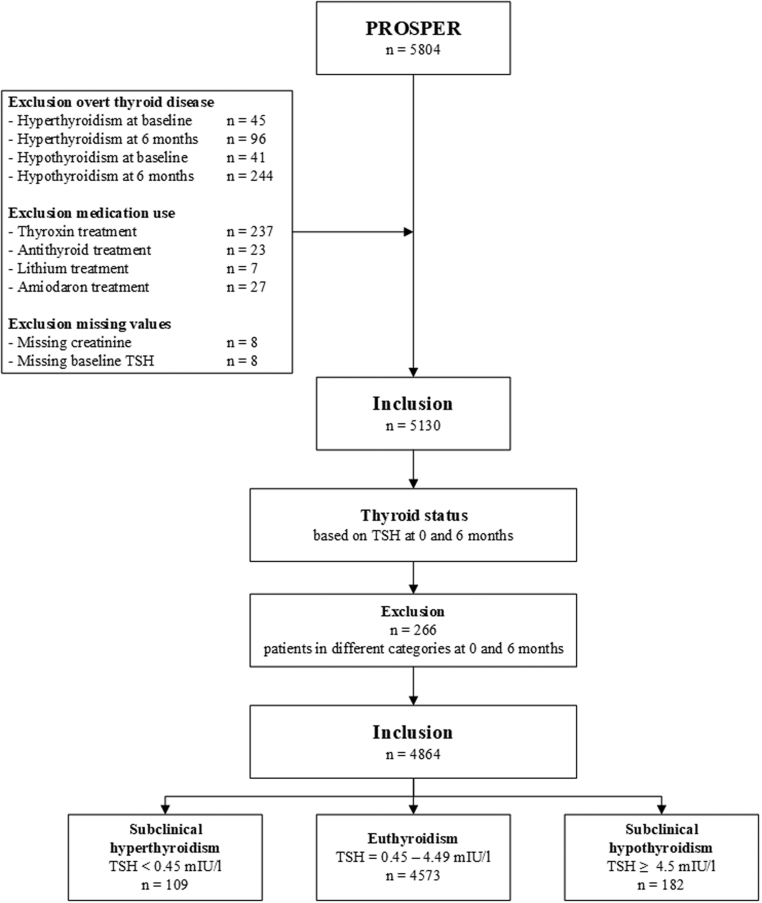
Participants with overt hyperthyroidism or hypothyroidism were excluded from the initial PROSPER trial. In the current study, participants were excluded when biochemical data regarding TSH was missing. In addition, participants using antithyroid medication, thyroxine supplementation, amiodarone, or lithium were also excluded from the final analyses, see Fig. 1. Participants were classified into three groups: subclinical hyperthyroidism, euthyroidism, and subclinical hypothyroidism. Subclinical hyperthyroidism was defined at TSH levels <0.45 mIU/L with normal fT4 levels. Euthyroidism was defined as normal TSH levels (0.45–4.5 mIU/L). Subclinical hypothyroidism was defined as TSH levels >4.5 mIU/L with normal fT4 levels.
Figure 1.
Flowchart of study population. Inclusion and exclusion criteria of the current substudy of PROSPER (the PROspective Study of Pravastatin in the Elderly at Risk).
Subclinical thyroid dysfunction may spontaneously resolve over time (20), and euthyroid participants may have developed subclinical thyroid dysfunction during the study follow-up period. As only participants with persistent subclinical thyroid dysfunction are of interest in this study, participants with the same thyroid function classification, based on TSH, at both baseline and 6 months of follow-up were included in the final study population.
Kidney function
Serum creatinine levels were measured at central laboratories, one in each of the three participating countries. Participants with baseline creatinine levels over 200 µmol/L were excluded from PROSPER and for the current study, and participants were also excluded when biochemical data were missing regarding kidney function. GFR was estimated using the Modification of Diet in Renal Disease equation: eGFR = 186 × Scr(−1.154) × age(−0.203) (×0.742 (if female)), where Scr denotes serum creatinine level in mg/dL. It is assumed that all participants were of Northern European descent (21). Participants were stratified by eGFR into three clinically relevant groups: eGFR <45 mL/min/1.73 m2, eGFR 45–60 mL/min/1.73 m2, and eGFR >60 mL/min/1.73 m2 (22).
Endpoint
The primary endpoint of PROSPER is a combined endpoint which consists of death from coronary heart disease, non-fatal myocardial infarction, and fatal or non-fatal stroke. All clinical endpoints were adjudicated by an expert study endpoints committee blinded to randomised study medication and using predefined criteria.
Statistical analysis
Baseline characteristics were reported for the three different classifications of thyroid function. Data are presented as mean ± s.d. or median (interquartile range) depending on the distribution of data. One-way ANOVA, Kruskal Wallis, or Pearson chi-square tests were used to assess differences in baseline characteristics. Cox proportional hazard analysis was performed to assess the risk of the primary endpoint attributed to subclinical thyroid dysfunction within different subgroups of eGFR, including an interaction analysis between thyroid function and kidney function and the risk of primary endpoint. Analyses were adjusted for multiple prespecified variables including country, age, gender, use of pravastatin, history of vascular disease, history of diabetes mellitus, history of hypertension, current smoking, alcohol in units/week, BMI, total cholesterol/HDL ratio, and albumin. The data were analysed using IBM SPSS Statistics, version 23. P values were considered statistically significant if lower than 0.05 for baseline characteristics and hazard ratios (HR, 95% CI), or lower than 0.10 for interaction analyses.
Role of the funding sources
The Prospective Study of Pravastatin in the Elderly at Risk (PROSPER) trial was supported by an unrestricted investigator-initiated grant from Bristol-Myers Squibb, USA. The funding source had no involvement in study design; in the collection, analysis, and interpretation of data; in writing of the report; and in the decision to submit the paper for publication.
Results
Of the 5804 randomised participants, 4864 were suitable for inclusion in the current analysis (Fig. 1 and Supplementary materials, see section on supplementary materials given at the end of this article). A proportion of the participants with subclinical thyroid dysfunction had a spontaneous resolution when biochemical measurements were repeated after 6 months or vice versa (5.2%). Participants had a mean age of 75.3 years and 49.0% were male. Mean follow-up was 3.2 years. Of all participants, 109 subjects (2.2%) had subclinical hypothyroidism, 4573 (94.0%) had euthyroidism, and 182 (3.7%) subclinical hyperthyroidism.
Baseline characteristics are shown in Table 1 per thyroid function group and overall. Age and gender were comparable between groups. Furthermore, eGFR category did not differ (P = 0.417). A history of vascular disease was present in 33 (30.3%), 2059 (45.0%), and 69 (37.9%) patients with subclinical hyperthyroidism, euthyroidism, and subclinical hypothyroidism, respectively (P = 0.002). Use of pravastatin, beta-blockers, and antiarrhythmics were comparable. Patients with subclinical hyperthyroidism had highest blood pressure, both systolic with a mean difference of 6 mmHg (P = 0.015) and diastolic with a mean difference of 3 mmHg (P = 0.001).
Table 1.
Baseline characteristics split by baseline thyroid status and overall.
| Subcl hyperth (n = 109) | Euthyroidism (n = 4573) | Subcl hypoth (n = 182) | Total (n = 4864) | P value | |
|---|---|---|---|---|---|
| Age (years; mean ± s.d.) | 74.7 ± 3.2 | 75.3 ± 3.4 | 75.2 ± 3.5 | 75.3 ± 3.4 | 0.160 |
| Male gender, n (%) | 64 (58.7) | 2226 (48.7) | 95 (52.2) | 2385 (49.0) | 0.080 |
| Education (years; mean ± s.d.) | 15.1 ± 1.9 | 15.1 ± 2.1 | 15.1 ± 2.1 | 15.1 ± 2.1 | 0.966 |
| History of hypertension, n (%) | 70 (64.2) | 2804 (61.3) | 110 (60.4) | 2984 (61.3) | 0.801 |
| History of diabetes, n (%) | 9 (8.3) | 490 (10.7) | 20 (11.0) | 519 (10.7) | 0.706 |
| History of vascular disease, n (%) | 33 (30.3) | 2059 (45.0) | 69 (37.9) | 2161 (44.4) | 0.002 |
| eGFR categories, n (%) | 0.417 | ||||
| <45 mL/min/1.73 m2 | 14 (12.8) | 615 (13.4) | 27 (14.8) | 656 (13.5) | |
| <45–60 mL/min/1.73 m2 | 35 (32.1) | 1830 (40.0) | 68 (37.4) | 1933 (39.7) | |
| >60 mL/min/1.73 m2 | 60 (55.0) | 2128 (46.5) | 87 (47.8) | 2275 (46.8) | |
| Smoking status, n (%) | 0.665 | ||||
| Never | 32 (29.4) | 1545 (33.8) | 54 (29.7) | 1631 (33.5) | |
| Former | 46 (42.2) | 1776 (38.8) | 73 (40.1) | 1895 (39.0) | |
| Current | 31 (28.4) | 1252 (27.4) | 55 (30.2) | 1338 (27.5) | |
| Alcohol in units/week, n (%) | 6.2 ± 9.2 | 5.3 ± 9.3 | 6.4 ± 12.1 | 5.3 ± 9.4 | 0.173 |
| Medication use, n (%) | |||||
| Pravastatin | 56 (51.4) | 2292 (50.1) | 90 (49.5) | 2438 (50.1) | 0.951 |
| Aspirin | 28 (25.7) | 1672 (36.6) | 61 (33.5) | 1761 (36.2) | 0.049 |
| Βeta-blockers | 25 (22.9) | 1191 (26.0) | 58 (31.9) | 1274 (26.2) | 0.159 |
| Antiarrhythmics | 3 (2.8) | 117 (2.6) | 4 (2.2) | 124 (2.5) | 0.946 |
| Objective measures (mean ± s.d.) | |||||
| SBP (mmHg) | 161 ± 21 | 155 ± 22 | 155 ± 21 | 155 ± 22 | 0.015 |
| DBP (mmHg) | 88 ± 10 | 84 ± 12 | 84 ± 10 | 84 ± 12 | 0.001 |
| BMI (kg/m2) | 26.5 ± 4.3 | 26.8 ± 4.2 | 26.6 ± 4.0 | 26.8 ± 4.2 | 0.552 |
| Total cholesterol/HDL-ratio (mmol/L) | 4.7 ± 1.3 | 4.7 ± 1.3 | 4.7 ± 1.3 | 4.7 ± 1.3 | 0.967 |
| Glucose (mmol/L) | 5.4 ± 1.2 | 5.5 ± 1.5 | 5.5 ± 1.2 | 5.5 ± 1.5 | 0.922 |
| CRP (mg/L) | 4.0 ± 5.1 | 4.2 ± 6.5 | 3.7 ± 5.4 | 4.2 ± 6.5 | 0.560 |
| Urea (mg/dL) | 6.3 ± 1.8 | 6.3 ± 1.8 | 6.2 ± 1.6 | 6.3 ± 1.8 | 0.627 |
| Hb (mmol/L) | 8.9 ± 0.7 | 8.7 ± 0.8 | 8.8 ± 0.8 | 8.7 ± 0.8 | 0.018 |
BMI, body mass index; CRP, C-reactive protein; DBP, diastolic blood pressure; Hb, haemoglobin; HDL, high-density lipoprotein; SBP, systolic blood pressure; s.d., standard deviation; Subcl hyper, subclinical hyperthyroidism; Subl hypo, subclinical hypothyrodism.
Subclinical thyroid dysfunction
Table 2 shows the univariate and multivariate association between thyroid function groups and the primary endpoint. Primary endpoint occurred in 9 (8.3%), 712 (15.6%), and 23 (12.6%) patients with subclinical hyperthyroidism, euthyroidism, and subclinical hypothyroidism, respectively. Multivariate adjusted HRs (95% CI) were 0.51 (0.24–1.07) comparing subclinical hyperthyroidism with euthyroidism and 0.90 (0.58–1.39) comparing subclinical hypothyroidism with euthyroidism.
Table 2.
Univariate and multivariate influence of thyroid status on the primary endpointa.
| Thyroid status based on TSH at baseline and 6 months | ||
|---|---|---|
| Subcl hyperth vs euth (n = 109 vs 4573) | Subcl hypo vs euth (n = 182 vs 4573) | |
| No. of events (%) | 9 (8.3) vs 712 (15.6) | 23 (12.6) vs 721 (15.6) |
| Univariate HR (95% CI) | 0.52 (0.27–1.00) | 0.80 (0.52–1.22) |
| Age and sex adjusted HR (95% CI) | 0.52 (0.27–0.99)b | 0.30 (0.53–1.22) |
| Multivariate adjusted HR (95% CI) | 0.51 (0.24–1.07) | 0.90 (0.58–1.39) |
Multivariate adjusted analysis include prespecified variables: country, age, gender, use of pravastatin, history of vascular disease, history of diabetes mellitus, history of hypertension, current smoking, alcohol in units/week, BMI, total cholesterol/HDL-ratio, albumin.
aPrimary endpoint includes coronary heart disease death or non-fatal myocardial infarction or fatal or non-fatal stroke; bP-value of hazard ratio <0.05.
CI, confidence interval; HR, hazard ratio.
The univariate and multivariate association of thyroid status with primary endpoint over strata of baseline eGFR category is shown in Table 3. In the prespecified eGFR categories 656 subjects (13.5%) had an eGFR <45 mL/min/1.73 m2, 1933 (39.7%) 45-60 mL/min/1.73 m2, and 2275 (46.8%) >60 mL/min/1.73 m2. The levels of TSH and fT4 did not differ between this categories. Mean ± s.e. TSH was 2.1 ± 0.06; 2.1 ± 0.03; and 2.0 ± 0.03 in patients with eGFR <45 mL/min/1.73 m2; eGFR 45–60 mL/min/1.73 m2; and eGFR >60 mL/min/1.73 m2, respectively (P = 0.383). Mean ± s.e. fT4 was 16.4 ± 0.3; 16.5 ± 0.1; and 16.7 ± 0.1 in patients with eGFR <45 mL/min/1.73 m2; eGFR 45–60 mL/min/1.73 m2; and eGFR >60 mL/min/1.73 m2, respectively (P = 0.413). When comparing subclinical hyperthyroidism with euthyroidism, multivariate adjusted HRs (95% CI) were 1.07 (0.26–4.40), 0.25 (0.03–1.76), and 0.54 (0.20–1.45) in eGFR <45 mL/min/1.73 m2, 45–60 mL/min/1.73 m2, and >60 mL/min/1.73 m2, respectively, without a significant interaction (P interaction = 0.602). When comparing subclinical hypothyroidism with euthyroidism multivariate adjusted HRs (95% CI) were 1.41 (0.57–3.51), 0.24 (0.06–0.95), and 1.43 (0.83–2.46) in eGFR <45 mL/min/1.73 m2, 45–60 mL/min/1.73 m2, and >60 mL/min/1.73 m2, respectively, also without a significant interaction (P-interaction = 0.388).
Table 3.
Univariate and multivariate influence of thyroid status on the primary endpointa split by baseline eGFR category.
| eGFR <45, n = 656 | eGFR 45–60, n = 1933 | eGFR >60, n = 2275 | ||||
|---|---|---|---|---|---|---|
| Subcl hyperth vs euth (n = 14 vs 615) | Subcl hypo vs euth (n = 27 vs 615) | Subcl hyperth vs euth (n = 35 vs 1830) | Subcl hypo vs euth (n = 68 vs 1830) | Subcl hyperth vs euth (n = 60 vs 2128) | Subcl hypo vs euth (n = 87 vs 2128) | |
| No. of events (%) | 6 (33.3) vs 123 (18.8) | 8 (20.5) vs 123 (18.8) | 4 (12.9) vs 280 (15.0) | 8 (7.8) vs 280 (15.0) | 4 (9.8) vs 338 (16.0) | 16 (13.0) vs 338 (16.0) |
| Univariate HR (95% CI) | 0.72 (0.18–2.93) | 1.13 (0.50–2.56) | 0.37 (0.09–1.47) | 0.28 (0.09–0.88)b | 0.55 (0.23–1.33) | 1.10 (0.64–1.87) |
| Age and sex adjusted HR (95% CI) | 0.79 (0.20–3.12) | 1.18 (0.52–2.69) | 0.36 (0.09–1.43) | 0.27 (0.09–0.86)b | 0.54 (0.22–1.31) | 1.10 (0.65–1.89) |
| Multivariate adjusted HR (95% CI) | 1.07 (0.26–4.40) | 1.41 (0.57–3.51) | 0.25 (0.03–1.76) | 0.24 (0.06–0.95)b | 0.54 (0.20–1.45) | 1.43 (0.83–2.46) |
Multivariate adjusted analysis include prespecified variables: country, age, gender, use of pravastatin, history of vascular disease, history of diabetes mellitus, history of hypertension, current smoking, alcohol in units/week, BMI, total cholesterol/HDL-ratio, albumin.
aPrimary endpoint includes coronary heart disease death or non-fatal myocardial infarction or fatal or non-fatal stroke; bP-value of hazard ratio <0.05.
CI, confidence interval; HR, hazard ratio.
Discussion
In this secondary analysis of the PROSPER study, we aimed to explore whether kidney function explains the association of thyroid function and the risk of cardiovascular events. In these older patients at high risk of cardiovascular disease, no relationship was found between subclinical thyroid dysfunction and cardiovascular events, nor was this relationship present after stratification by kidney function. Furthermore, kidney function did not interact with subclinical thyroid dysfunction in relation with cardiovascular events.
Although the association of thyroid dysfunction on cardiovascular events and mortality has been studied in patients with CKD (23, 24), this is the first time this association has been studied over different strata of CKD to explore a possible interaction between thyroid and kidney function, which we did not find.
There are two hypotheses regarding the interaction of kidney and thyroid. First, thyroid hormone changes may predispose to kidney dysfunction, due to possible alterations in kidney hemodynamics and structure, both direct and indirect via the cardiovascular system (25). Secondly, kidney dysfunction may predispose to thyroid hormone changes, via influence on the metabolism of thyroid hormones or due to chronic illness (as CKD) leading to nonthyroidal illness as potential underlying pathophysiological mechanisms (23, 26). A recent individual participant data analysis from 16 independent cohorts of the Thyroid Studies Collaboration found no role for thyroid hormones in kidney dysfunction (15). However, it does not exclude that previously found cross-sectional associations between kidney and thyroid (10, 11) may be explained by kidney dysfunction causing thyroid hormone changes. Another explanation could be that there might be a common pathway to thyroid and kidney dysfunction, due to shared cardiovascular risk factors, especially in older people.
The prevalence of subclinical thyroid dysfunction within this study (2.2% patients with subclinical hypothyroidism and 3.7% with subclinical hyperthyroidism) was lower than the prevalence of subclinical thyroid dysfunction among the general population within the same geographical location (27). In addition, contrary to what has been reported in literature, the prevalence of subclinical thyroid dysfunction did not change with increasing degrees of kidney dysfunction (28), nor did the levels of TSH and fT4 differ between groups stratified by kidney function (12).
Strengths and limitations
The presence of two thyroid measurements is a major strength which allowed us to more accurately assess subclinical thyroid disease by excluding those without persistent thyroid disease after 6 months of follow-up. In most studies regarding thyroid function and cardiovascular disease risk, all measurements are performed at baseline and compared to follow-up data. We showed that a proportion of participants with subclinical thyroid dysfunction have a spontaneous resolution when biochemical measurements are repeated after 6 months or vice versa (5.2%), explaining why for instance the TRUST study ‘Thyroid hormone Replacement for Untreated older adults with Subclinical hypothyroidism - a randomised placebo controlled Trial’ had a relatively long run-in phase, as patients with subclinical thyroid disease spontaneously resolved to euthyroidism before the trial started (20). Using two separate measurements, as in our study, thus filters the bias which is otherwise present in studies utilizing only baseline measurements. Furthermore, we excluded patients who used medication that could be of influence on thyroid function such as both thyroxin and antithyroid treatment, but also lithium and amiodaron, although amiodaron could have long-lasting effects and its previous use is unknown. Despite the large number of participants within the PROSPER study, the number of patients with subclinical thyroid dysfunction within this study was relatively small, limiting the power to find an association between subclinical thyroid disease and cardiovascular events, and an interaction between thyroid and kidney function, to a smaller magnitude. Whereas one of the exclusion criteria was a high creatinine (>200 µmol/L) at baseline, the influence of severe or end-stage CKD (eGFR <30 mL/min/1.73 m2) on the association of thyroid function and cardiovascular events is unknown. Furthermore, the larger proportion of euthyroid patients with a history of vascular disease compared to those with subclinical thyroid dysfunction may have masked the risk of cardiovascular events in subclinical thyroid dysfunction, but adjustment in linear regression models yielded similar results.
Conclusion
In this secondary analysis from PROSPER, kidney function did not interact in the association of thyroid function and cardiovascular disease. Therefore, we found no evidence that the potential association between thyroid hormones and cardiovascular disease is modified by kidney function in older patients with subclinical thyroid dysfunction.
Supplementary Material
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
The Prospective Study of Pravastatin in the Elderly at Risk (PROSPER) trial was supported by an unrestricted investigator-initiated grant from Bristol-Myers Squibb, USA.
Author contribution statement
L E Z, D M v V, S S, S P M, J W J, and S T performed conception and design of the study, analysis and interpretation of data, drafted the manuscript, provided intellectual content of critical importance to the work described, and approved the final manuscript. M v B performed analysis and interpretation of data, provided intellectual content of critical importance to the work described, and approved the final manuscript. D J S and I F provided intellectual content of critical importance to the work described and approved the final manuscript.
References
- 1.Peixoto de Miranda EJF, Bittencourt MS, Staniak HL, Pereira AC, Foppa M, Santos IS, Lotufo PA, Bensenor IM. Thyrotrophin levels and coronary artery calcification: cross-sectional results of the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Clinical Endocrinology 2017. 597–604. ( 10.1111/cen.13393) [DOI] [PubMed] [Google Scholar]
- 2.Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocrine Reviews 2008. 76–131. ( 10.1210/er.2006-0043) [DOI] [PubMed] [Google Scholar]
- 3.Cappola AR, Fried LP, Arnold AM, Danese MD, Kuller LH, Burke GL, Tracy RP, Ladenson PW. Thyroid status, cardiovascular risk, and mortality in older adults. JAMA 2006. 1033–1041. ( 10.1001/jama.295.9.1033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodondi N, Newman AB, Vittinghoff E, de Rekeneire N, Satterfield S, Harris TB, Bauer DC. Subclinical hypothyroidism and the risk of heart failure, other cardiovascular events, and death. Archives of Internal Medicine 2005. 2460–2466. ( 10.1001/archinte.165.21.2460) [DOI] [PubMed] [Google Scholar]
- 5.Collet TH, Gussekloo J, Bauer DC, den Elzen WP, Cappola AR, Balmer P, Iervasi G, Asvold BO, Sgarbi JA, Volzke H, et al. Subclinical hyperthyroidism and the risk of coronary heart disease and mortality. Archives of Internal Medicine 2012. 799–809. ( 10.1001/archinternmed.2012.402) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodondi N, den Elzen WP, Bauer DC, Cappola AR, Razvi S, Walsh JP, Asvold BO, Iervasi G, Imaizumi M, Collet TH, et al. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA 2010. 1365–1374. ( 10.1001/jama.2010.1361) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaker L, Baumgartner C, den Elzen WP, Ikram MA, Blum MR, Collet TH, Bakker SJ, Dehghan A, Drechsler C, Luben RN, et al. Subclinical hypothyroidism and the risk of stroke events and fatal stroke: an individual participant data analysis. Journal of Clinical Endocrinology and Metabolism 2015. 2181–2191. ( 10.1210/jc.2015-1438) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. New England Journal of Medicine 2004. 1296–1305. ( 10.1056/NEJMoa041031) [DOI] [PubMed] [Google Scholar]
- 9.Ford I, Bezlyak V, Stott DJ, Sattar N, Packard CJ, Perry I, Buckley BM, Jukema JW, de Craen AJ, Westendorp RG, et al. Reduced glomerular filtration rate and its association with clinical outcome in older patients at risk of vascular events: secondary analysis. PLOS Medicine 2009. e16 ( 10.1371/journal.pmed.1000016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asvold BO, Bjoro T, Vatten LJ. Association of thyroid function with estimated glomerular filtration rate in a population-based study: the HUNT study. European Journal of Endocrinology 2011. 101–105. ( 10.1530/EJE-10-0705) [DOI] [PubMed] [Google Scholar]
- 11.Peixoto de Miranda ÉJF, Bittencourt MS, Goulart AC, Santos IS, de Oliveira Titan SM, Ladeira RM, Barreto SM, Lotufo PA, Bensenor IJM. Thyrotropin levels are associated with chronic kidney disease among healthy subjects in cross-sectional analysis of the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Clinical and Experimental Nephrology 2017. 1035–1043. ( 10.1007/s10157-017-1400-2) [DOI] [PubMed] [Google Scholar]
- 12.Iglesias P, Bajo MA, Selgas R, Diez JJ. Thyroid dysfunction and kidney disease: an update. Reviews in Endocrine and Metabolic Disorders 2017. 131–144. ( 10.1007/s11154-016-9395-7) [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Chang Y, Ryu S, Cho J, Lee WY, Rhee EJ, Kwon MJ, Pastor-Barriuso R, Rampal S, Han WK, et al. Thyroid hormone levels and incident chronic kidney disease in euthyroid individuals: the Kangbuk Samsung Health Study. International Journal of Epidemiology 2014. 1624–1632. ( 10.1093/ije/dyu126) [DOI] [PubMed] [Google Scholar]
- 14.Chaker L, Sedaghat S, Hoorn EJ, Elzen WP, Gussekloo J, Hofman A, Ikram MA, Franco OH, Dehghan A, Peeters RP. The association of thyroid function and the risk of kidney function decline: a population-based cohort study. European Journal of Endocrinology 2016. 653–660. ( 10.1530/EJE-16-0537) [DOI] [PubMed] [Google Scholar]
- 15.Meuwese CL, van Diepen M, Cappola AR, Sarnak MJ, Shlipak MG, Bauer DC, Fried LP, Iacoviello M, Vaes B, Degryse J, et al. Low thyroid function is not associated with an accelerated deterioration in renal function. Nephrology Dialysis Transplantation 2019. 650–659. ( 10.1093/ndt/gfy071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rhee CM, Alexander EK, Bhan I, Brunelli SM. Hypothyroidism and mortality among dialysis patients. Clinical Journal of the American Society of Nephrology 2013. 593–601. ( 10.2215/CJN.06920712) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Afsar B, Yilmaz MI, Siriopol D, Unal HU, Saglam M, Karaman M, Gezer M, Sonmez A, Eyileten T, Aydin I, et al. Thyroid function and cardiovascular events in chronic kidney disease patients. Journal of Nephrology 2017. 235–242. ( 10.1007/s40620-016-0300-y) [DOI] [PubMed] [Google Scholar]
- 18.Rhee CM, You AS, Nguyen DV, Brunelli SM, Budoff MJ, Streja E, Nakata T, Kovesdy CP, Brent GA, Kalantar-Zadeh K. Thyroid status and mortality in a prospective hemodialysis cohort. Journal of Clinical Endocrinology and Metabolism 2017. 1568–1577. ( 10.1210/jc.2016-3616) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shepherd J, Blauw GJ, Murphy MB, Cobbe SM, Bollen EL, Buckley BM, Ford I, Jukema JW, Hyland M, Gaw A, et al. The design of a prospective study of pravastatin in the Elderly at Risk (PROSPER). PROSPER Study Group. PROspective Study of pravastatin in the Elderly at Risk. American Journal of Cardiology 1999. 1192–1197. ( 10.1016/s0002-9149(99)00533-0) [DOI] [PubMed] [Google Scholar]
- 20.Stott DJ, Rodondi N, Kearney PM, Ford I, Westendorp RGJ, Mooijaart SP, Sattar N, Aubert CE, Aujesky D, Bauer DC, et al. Thyroid hormone therapy for older adults with subclinical hypothyroidism. New England Journal of Medicine 2017. 2534–2544. ( 10.1056/NEJMoa1603825) [DOI] [PubMed] [Google Scholar]
- 21.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Annals of Internal Medicine 1999. 461–470. ( 10.7326/0003-4819-130-6-199903160-00002) [DOI] [PubMed] [Google Scholar]
- 22.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. American Journal of Kidney Diseases 2002. (Supplement 1) S1–S266. [PubMed] [Google Scholar]
- 23.Rhee CM, Brent GA, Kovesdy CP, Soldin OP, Nguyen D, Budoff MJ, Brunelli SM, Kalantar-Zadeh K. Thyroid functional disease: an under-recognized cardiovascular risk factor in kidney disease patients. Nephrology, Dialysis, Transplantation 2015. 724–737. ( 10.1093/ndt/gfu024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tatar E, Kircelli F, Ok E. The contribution of thyroid dysfunction on cardiovascular disease in patients with chronic kidney disease. Atherosclerosis 2013. 26–31. ( 10.1016/j.atherosclerosis.2012.10.068) [DOI] [PubMed] [Google Scholar]
- 25.Mariani LH, Berns JS. The renal manifestations of thyroid disease. Journal of the American Society of Nephrology 2012. 22–26. ( 10.1681/ASN.2010070766) [DOI] [PubMed] [Google Scholar]
- 26.Kaptein EM. Thyroid hormone metabolism and thyroid diseases in chronic renal failure. Endocrine Reviews 1996. 45–63. ( 10.1210/edrv-17-1-45) [DOI] [PubMed] [Google Scholar]
- 27.Parle JV, Franklyn JA, Cross KW, Jones SC, Sheppard MC. Prevalence and follow-up of abnormal thyrotrophin (TSH) concentrations in the elderly in the United Kingdom. Clinical Endocrinology 1991. 77–83. ( 10.1111/j.1365-2265.1991.tb01739.x) [DOI] [PubMed] [Google Scholar]
- 28.Chonchol M, Lippi G, Salvagno G, Zoppini G, Muggeo M, Targher G. Prevalence of subclinical hypothyroidism in patients with chronic kidney disease. Clinical Journal of the American Society of Nephrology 2008. 1296–1300. ( 10.2215/CJN.00800208) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a