Abstract
Objective
Insulin resistance is a major pathophysiological link between obesity and its metabolic complications. Weight loss (WL) is an effective tool to prevent obesity-related diseases; however, the mechanisms of an improvement in insulin sensitivity (IS) after weight-reducing interventions are not completely understood. The aim of the present study was to analyze the relationships between IS and adipose tissue (AT) expression of the genes involved in the regulation of lipolysis in obese subjects after WL.
Methods
Fifty-two obese subjects underwent weight-reducing dietary intervention program. The control group comprised 20 normal-weight subjects, examined at baseline only. Hyperinsulinemic-euglycemic clamp and s.c. AT biopsy with subsequent gene expression analysis were performed before and after the program.
Results
AT expression of genes encoding lipases (PNPLA2, LIPE and MGLL) and lipid-droplet proteins enhancing (ABHD5) and inhibiting lipolysis (PLIN1 and CIDEA) were decreased in obese individuals in comparison with normal-weight individuals. The group of 38 obese participants completed dietary intervention program and clamp studies, which resulted in a significant WL and an improvement in mean IS. However, in nine subjects from this group IS did not improve in response to WL. AT expression of PNPLA2, LIPE and PLIN1 increased only in the group without IS improvement.
Conclusions
Excessive lipolysis may prevent an improvement in IS during WL. The change in AT PNPLA2 and LIPE expression was a negative predictor of the change in IS after WL.
Keywords: weight loss, insulin sensitivity, adipose tissue, hormone-sensitive lipase, adipose triglyceride lipase
Introduction
Obesity predisposes to the development of type 2 diabetes, dyslipidemia, hypertension, atherosclerosis, several types of cancer and other disorders. Insulin resistance is a major pathophysiological link between obesity and its complications (1). Adipose tissue (AT) function and the balance between lipid storage and mobilization play an important role in regulating insulin action. Inability of AT to store fat leads to free fatty acids (FFA) overflow to other tissues and to insulin resistance (2).
Lipolysis is a process of hydrolysis of triacylglycerols (TG) into fatty acids and glycerol, which results in a release of FFA from AT into circulation. In the first step, the hydrolysis of TG into diacylglycerol and fatty acid is catalyzed by the enzyme adipose triglyceride lipase (ATGL), encoded by PNPLA2. Diacylglycerol is further hydrolyzed to monoacylglycerol and fatty acid with hormone-sensitive lipase (HSL), encoded by LIPE, and monoacylglycerol is ultimately hydrolyzed to glycerol and fatty acid with monoacylglycerol lipase (MGLL) (2). Lipolysis is stimulated by catecholamines, which act through β1-3 adrenergic receptors (encoded by ADRB1-3), in humans mostly through β1, 2 receptors and natriuretic peptides, whereas it is inhibited mainly by insulin (2, 3). An important mediator of hormonal regulation of lipolysis is phosphodiesterase 3B (PDE3B), which may inhibit lipolysis in response to insulin signal transduction and may counteract the lipolytic effect of an increased adrenergic signaling (2, 3).
In adipocytes, TGs are packed in lipid droplets, and proteins associated with lipid droplet surface play an important role in the regulation of lipolysis (3). Among these proteins, perilipin (PLIN), Cell Death–Inducing DFFA (DNA Fragmentation Factor-α)-Like Effector A (CIDEA) and caveolin 1 (CAV1) inhibit lipolysis through stabilization of lipid droplet and protection against TG hydrolysis, whereas G0/G1 switch gene 2 (G0S2) inhibits ATGL activity (4, 5, 6, 7, 8). In contrast, comparative gene identification 58 (CGI-58, also known as alpha/beta-hydrolase domain-containing protein 5, ABHD5) is ATGL coactivator and thus enhances lipolysis (4). ABHD5 bound to PLIN is unable to activate ATGL. Upon hormonal stimulation, PLIN is phosphorylated, and ABHD5 is dissociated from PLIN and may interact with ATGL promoting its activation (4).
The inter-relationships between AT lipolysis and insulin sensitivity are complex and still not fully understood and the existing data are contradictory. Genetic adipose tissue-specific ATGL deletion (9), HSL haploinsufficiency (10) or pharmacological ATGL or HSL inhibition (10, 11) improved glucose tolerance and insulin sensitivity in mice, without increasing body weight. MGLL deficiency attenuated high-fat diet-induced insulin resistance in mice (12). G0S2 overexpression (8) and ABHD5 knockdown in mice (13) also protected against diet-induced insulin resistance and glucose intolerance. In contrast, mice with G0S2 knockout display increased AT lipase activity and lipolysis, and they are lean, resistant to high-fat diet-induced weight gain and are glucose tolerant and insulin sensitive (14).
In human adipocytes, HSL gene silencing led to improved insulin-stimulated glucose uptake (10). On the other hand, genetic or pharmacological ATGL or HSL knockdown in human multipotent adipose-derived stem cells disrupted mitochondrial respiration, impaired insulin signaling and decreased insulin-stimulated glucose uptake (15).
In humans, white AT lipolytic rate is positively associated with insulin resistance (10). However, a defect in lipolysis (16) and decreased AT HSL and ATGL protein expression and/or activity (16, 17, 18), as well as mRNA expression (19, 20, 21), were reported in obesity and insulin resistance, although the opposite findings were also described (22). Null mutation in HSL gene leads to impaired AT lipolysis, dyslipidemia, hepatic steatosis, insulin resistance and diabetes (23). Decreased AT expression of MGLL, PLIN1, CIDEA and G0S2 in different conditions associated with insulin resistance was observed (5, 24, 25, 26). The differences in human studies may come from different characteristics of the study groups and different clinical situations. Thus, there is need for further studies on the association between AT lipases and insulin sensitivity in different conditions/interventions in humans.
Weight loss is an effective tool to prevent obesity-associated insulin resistance and related comorbidities; however, the precise mechanism of an improvement in insulin sensitivity remains unclear. In our previous study we demonstrated that diet-induced moderate (11.3%) weight loss resulted in a significant increase in insulin sensitivity by 27%, without any effect on AT inflammation (27). During weight loss, AT mobilizes fat stores to provide FFA as a source of energy to other organs. Lipolysis plays a crucial role in this process. Data on the effect of weight loss on AT lipase and lipid droplet protein expression are conflicting. Furthermore, it remains unclear to what extent they are related to the concurrent change in insulin sensitivity.
We hypothesized that the changes in AT expression of genes associated with lipolysis may be related to the concurrent improvement in insulin sensitivity during weight loss. To test this hypothesis, we measured AT lipolysis gene expression in the samples obtained before and after weight loss in obese subjects and analyzed them in relation to the concurrent change in insulin sensitivity.
Subjects and methods
Study groups
We examined 52 subjects with marked overweight or obesity (BMI >28 kg/m2, 27 males and 25 females, age 32.0 ± 7.9 years). The cut-off point of 28 kg/m2 instead of 25 kg/m2 was chosen to enhance the probability of achieving greater body weight reduction. Twenty normal-weight subjects (8 males and 12 females, age 23.5 ± 1.8 years) served as a control group. The detailed description of the study groups has been described previously (27). All participants were nonsmokers, had no serious diseases, morbid obesity (thus, the BMI range in the overweight/obese group was 28–40 kg/m2), impaired fasting glucose, impaired glucose tolerance or diabetes and were not taking any drugs. Body weight of the participants had remained stable (±1 kg) for at least 3 months prior to the study. Participants underwent clinical examination, anthropometric measurements and appropriate laboratory tests (27, 28). All the studies were performed after overnight fast. All human studies have been approved by the Local Ethics Committee of the Medical University of Białystok. All persons gave their written informed consent prior to their inclusion in the study.
Study protocol
The detailed study protocol has been described previously (27). Overweight/obese subjects underwent a 12-week dietary intervention program, which consisted of individually planned low-calorie diet (20 kcal per kg of proper body weight). The ideal body weight was calculated for the Broca formula. Before the program, none of the participants were on a special diet (like vegetarian, vegan, gluten-free, etc.). The dietary habits were assessed with the questionnaire. The sources of energy in the diet were carbohydrate 55–60%, fat 25% and protein 15–20%. Participants received a detailed instruction about low-calorie diet. Each participant received the detailed menu for every day of the 2-week period. The body weight changes were assessed every 2 weeks. The compliance was also assessed every 2 weeks by qualified dieticians – each participant reported non-adherence to the prescribed diet in a diary. Furthermore, at each visit, participants presented the detailed meal diary from the selected 2 days in every 2-week period. Physical activity was assessed by a questionnaire and all subjects were sedentary. Subjects were advised to maintain their daily physical activity, without specific advice to increase it. Physical activity was assessed with a questionnaire every 2 weeks and was unchanged during the program. All analyses were performed before and after dietary intervention.
Normal-weight subjects were examined only at baseline. Forty out of 52 subjects completed the program. Twelve individuals did not complete the program due to the non-adherence to the prescribed diets. Insulin sensitivity was assessed in 38 subjects before and after the program, because two individuals were not willing to undergo the clamp study at the end of the program.
Measurement of insulin sensitivity
Insulin sensitivity was measured with the 2 h hyperinsulinemic-euglycemic clamp (27, 28). On the morning of the study, two venous catheters were inserted into antecubital veins, one for the infusion of insulin and glucose and the other in the contralateral hand for blood sampling, with that hand heated to ~60°C. Insulin (Actrapid HM; Novo Nordisk) was given as a primed-continuous i.v. infusion for 120 min at 40 mU × m−2 × min−1, resulting in constant hyperinsulinemia of ~80 μIU/mL. Arterialized blood glucose was obtained every 5 min, and 20% dextrose (1.11 mol/L) infusion was adjusted to maintain plasma glucose levels at 5.0 mmol/L. The rate of whole-body glucose uptake (M value) was calculated as the mean glucose infusion rate during the last 40 min of the clamp, corrected for the glucose space, and normalized for fat-free mass (ffm).
Subcutaneous AT biopsy
Subcutaneous AT biopsy from the umbilical region was performed using biopsy needle under local anesthesia with 1% lidocaine, after small skin incision of less than 1 cm (27, 28). Tissue were collected to 1 mL of RNA stabilization reagent (Allprotect tissue reagent; QIAGEN GmbH) and were kept at −80°C until analyses.
Isolation of RNA from adipose tissue and determination of gene expression
Total RNA was isolated from AT as described (27, 28). AT mRNA expression was analyzed with quantitative Real-Time PCR. The samples were quantified with the Light Cycler 480 II Real-Time PCR Instrument (Roche Diagnostics, GmbH) using Roche LightCycler480 Probes Master (Roche Diagnostics GmbH). Data were analyzed with LC480 Software 1.5.0 SP3. LIPE and PNPLA2 mRNA expression was measured using primers in combination with predesigned mono-color hydrolysis probes from the Universal Probe Library Roche (UPL) (Roche Diagnostics GmbH): LIPE forward 5′-cttcaccgtggctcttcg, reverse 5′-ggtaggctgccatgatgc, UPL probe #66; PNPLA2 forward 5′-ctccaccaacatccacgag, reverse 5′-ccctgcttgcacatctctc, UPL probe #89. For other genes, the validated PrimePCR Probe Assays were purchased from Bio-Rad: MGLL (qHsaCEP0058399), PLIN (qHsaCEP0053667), ABHD5 (qHsaCEP0057650), CIDEA (qHsaCEP0024748), CAV1 (qHsaCIP00333230), G0S2 (qHsaCEP0051262) and PGK1 (qHsaCEP0050174), ADRB1 (qHsaCEP0032252), ADRB2 (qHsaCEP0032222), ADRB3 (qHsaCEP0055256), PDE3B (qHsaCEP0052820). All samples were run in triplicate and average values were calculated. All results were normalized to the levels of PGK1, since its expression was the most stable among a few housekeeping genes tests. Relative quantification was calculated using the ΔΔCt formula cycle threshold.
Statistical analysis
The statistics were performed with the STATISTICA 12.5 (Statsoft, Krakow, Poland). All data are presented as mean ± s.d. The variables which did not have normal distribution were log-transformed before analyses. For the purpose of data presentation, absolute values are shown in the Results. Differences between the groups were analyzed with the unpaired Student’s t-test. Differences in the estimated parameters before and after weight loss program in the entire obese group were assessed with the paired Student’s t-test. Differences before and after weight loss program in the subgroups of subjects with and without the concurrent improvement in insulin sensitivity were analyzed with repeated-measures ANOVA with time vs group interaction. For all the observed differences, Benjamini–Hochberg correction for multiple comparisons was applied. To assess the effect of the initial insulin sensitivity and the decrease in body weight during the intervention between subjects, in whom insulin sensitivity increased and did not increase, we used analysis of covariance. To assess the differences among the initial BMI quartiles, we used one-way ANOVA with post hoc Tukey test. Differences in categorical variables were assessed with the chi-square test. Relationships between variables were studied with the Pearson product moment correlation analysis and with multiple regression analysis. The level of significance was accepted at P value lower than 0.05.
Results
Baseline (before dietary intervention) differences between normal-weight and obese subjects
Obese subjects had lower baseline insulin sensitivity in comparison with normal-weight subjects (6.59 ± 2.97 vs 9.77 ± 3.36 mg/kg ffm/min, P < 0.0001). Differences in the baseline AT expression of the studied genes are shown in Table 1. Obese subjects had lower AT LIPE, MGLL, ABHD5, CIDEA, PDE3B (P < 0.001), PNPLA2 (P = 0.002), PLIN expression (P = 0.01) and ADRB1-3 expression (P < 0.005) (Table 1). All the differences remained significant after adjustment for age. AT G0S2 and CAV1 expression did not differ between the groups (Table 1).
Table 1.
Baseline (before dietary intervention) AT gene expression (A.U.) in normal-weight (n = 20) and obese subjects (n = 52).
| Normal-weight | Obese | |
|---|---|---|
| PNPLA2 | 1.37 ± 0.41 | 1.00 ± 0.36a |
| LIPE | 1.60 ± 0.44 | 1.05 ± 0.25a |
| MGLL | 1.29 ± 0.44 | 0.96 ± 0.25a |
| PLIN | 1.19 ± 0.24 | 1.00 ± 0.25a |
| ABHD5 | 2.69 ± 1.65 | 1.00 ± 0.36a |
| CIDEA | 3.05 ± 1.42 | 1.00 ± 0.42a |
| CAV1 | 0.99 ± 0.18 | 1.00 ± 0.25 |
| G0S2 | 1.37 ± 1.04 | 1.00 ± 0.62 |
| ADRB1 | 1.74 ± 1.04 | 1.00 ± 0.59a |
| ADRB2 | 1.52 ± 0.83 | 1.00 ± 0.42a |
| ADRB3 | 1.72 ± 0.89 | 1.00 ± 0.63a |
| PDE3B | 1.58 ± 0.40 | 1.00 ± 0.41a |
a*P < 0.05 vs the normal-weight group.
A.U., arbitrary units.
The effect of diet-induced weight loss on AT gene expression in the entire overweight/obese group
All 40 subjects who completed the program had a reduction in body weight. The range of body weight reduction was from 5.5 kg to 21.8 kg.
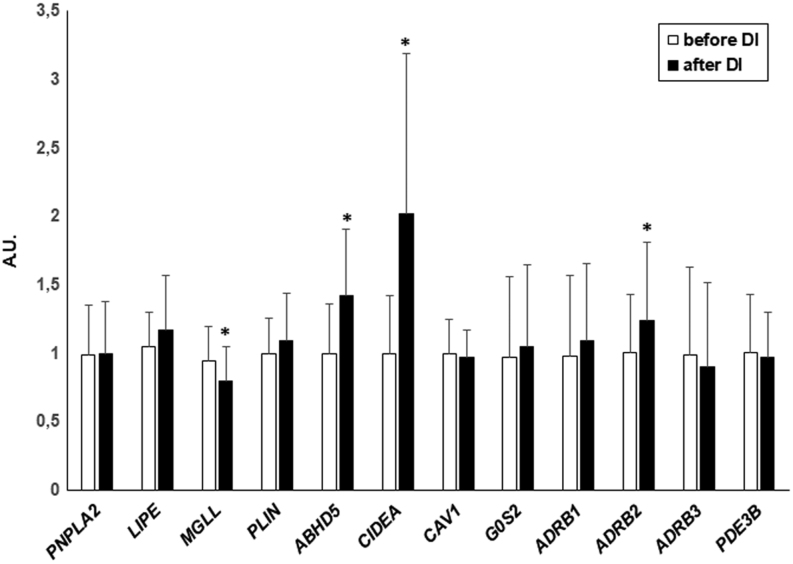
Weight loss resulted in an increase in AT expression of ABHD5, CIDEA (P < 0.0001) and ADRB2 (P = 0.017) and a decrease in the expression of MGLL (P = 0.01) (Fig. 1). The expression of LIPE and PLIN increased after weight loss; however, it lost its significance after correction for multiple comparisons. The expression of other genes studied did not change in response to weight loss in the entire study group (Fig. 1).
Figure 1.
The effect of diet-induced weight loss on AT gene expression in the entire obese group (n = 38). Data are shown as mean ± s.d. *P < 0.05 vs before dietary intervention. A.U., arbitrary units.
Next, we divided the obese group according to the initial BMI quartiles (lower quartile – 29.9, median – 32.7, upper quartile – 35.2 kg/m2). Weight loss and the change in insulin sensitivity did not differ among the BMI quartiles. However, we observed the relationship between the initial BMI and the change in BMI after weight loss (r = -0.35, P = 0.032), that is, the higher the initial BMI, the greater decrease in BMI in response to weight loss. An increase in AT CIDEA expression was higher in the lowest vs the highest initial BMI quartile (P = 0.039). The change in AT expression of other analysed genes did not differ among the BMI quartiles.
The effect of diet-induced weight loss on anthropometric and metabolic parameters in subjects in whom insulin sensitivity increased vs subjects in whom insulin sensitivity did not increase
Although mean insulin sensitivity increased during the dietary intervention, in 9 out of 38 subjects it not only did not improve, but even worsened in response to weight loss. The frequency of no improvement in insulin sensitivity was similar in males and females (chi-square P = 0.57).
Individuals without an improvement in insulin sensitivity had higher initial M value (P = 0.001); however, they did not differ in any other of the initial anthropometric and metabolic parameters from subjects, in whom insulin sensitivity increased after the dietary intervention (Table 2). Furthermore, body weight, waist circumference, body fat, serum insulin and total cholesterol reduction during the dietary intervention was similar in both groups (P < 0.05) (Table 2). Serum triglycerides (TG) decreased in the group with an increase in insulin sensitivity (P = 0.0007) (Table 2). Fasting serum FFA increased in the group, in which insulin sensitivity did not improve (P = 0.01) (Table 2). There was a significant correlation between initial insulin sensitivity and the change in M value after weight loss (r = −0.44, P = 0.006).
Table 2.
The effect of diet-induced weight loss on anthropometric and metabolic parameters in obese subjects, in whom insulin sensitivity increased (Obese - improve, n = 29) or did not increase (Obese - no improve, n = 9).
| Obese – improve | Obese – no improve | |||
|---|---|---|---|---|
| Before | After | Before | After | |
| Body weight (kg) | 99.1 ± 15.7 | 87.7 ± 13.9a | 100 ± 11.9 | 88.9 ± 12.0a |
| BMI (kg/m2) | 33.3 ± 3.1 | 29.5 ± 2.8a | 31.3 ± 2.6 | 27.9 ± 3.1a |
| Waist (cm) | 107 ± 10.1 | 98.3 ± 8.9a | 105 ± 7.4 | 96.2 ± 7.9a |
| Fat mass (kg) | 38.9 ± 8.4 | 29.5 ± 7.5a | 36.9 ± 6.5 | 27.5 ± 7.9a |
| Fasting plasma glucose (mg/dL) | 88.2 ± 4.5 | 86.1 ± 6.5 | 87.3 ± 8.2 | 86.3 ± 8.8 |
| Fasting serum insulin (μIU/mL) | 15.7 ± 5.9 | 10.9 ± 3.2a | 13.5 ± 5.0 | 10.3 ± 1.9a |
| M (mg/kg ffm/min) | 5.63 ± 2.49 | 8.55 ± 3.42a | 9.16 ± 3.00b | 7.19 ± 2.21a |
| Fasting serum FFA (mmol/L) | 0.71 ± 0.21 | 0.76 ± 0.35 | 0.58 ± 0.08 | 0.89 ± 0.26a |
| Cholesterol (mg/dL) | 192 ± 25.7 | 171 ± 31.0a | 176 ± 27.5 | 159 ± 29.8a |
| TG (mg/dL) | 114 ± 58.6 | 80.2 ± 33.1a | 94.0 ± 39.7 | 72.6 ± 21.7 |
| HDL-cholesterol (mg/dL) | 52.5 ± 10.5 | 49.9 ± 10.5 | 51.1 ± 8.3 | 48.6 ± 5.5 |
| LDL-cholesterol (mg/dL) | 123 ± 25.4 | 110 ± 29.4 | 112 ± 36.4 | 103 ± 31.1 |
aP < 0.05 vs before dietary intervention; bP < 0.05 vs improve in the respective time-point.
The effect of diet-induced weight loss on AT gene expression in subjects in whom insulin sensitivity increased vs subjects in whom insulin sensitivity did not increase
AT expression PNPLA2 (P = 0.01), LIPE (P < 0.001) and PLIN (P = 0.007) increased after weight loss only in subjects without an improvement in insulin sensitivity and did not change in subjects with an improvement in insulin sensitivity (Fig. 2). The expression of MGLL decreased significantly only in subjects with an improvement in insulin sensitivity (P = 0.002) (Fig. 2). The difference in response (Δ) between the groups without improvement and with improvement in insulin sensitivity in PNPLA2 (0.23 ± 0.19 vs −0.06 ± 0.26 A.U., P = 0.016) and LIPE (0.43 ± 0.25 vs 0.02 ± 0.33 A.U., P = 0.013) was significant, whereas the difference in MGLL and PLIN response lost its significance after correction for multiple comparisons (P = 0.052 and P = 0.059 after correction, respectively). Furthermore, the difference in PNPLA2 and LIPE response to weight loss remained significant after adjustment for the initial insulin sensitivity and for the concurrent decrease in BMI and the changes in FFA (P < 0.05).
Figure 2.
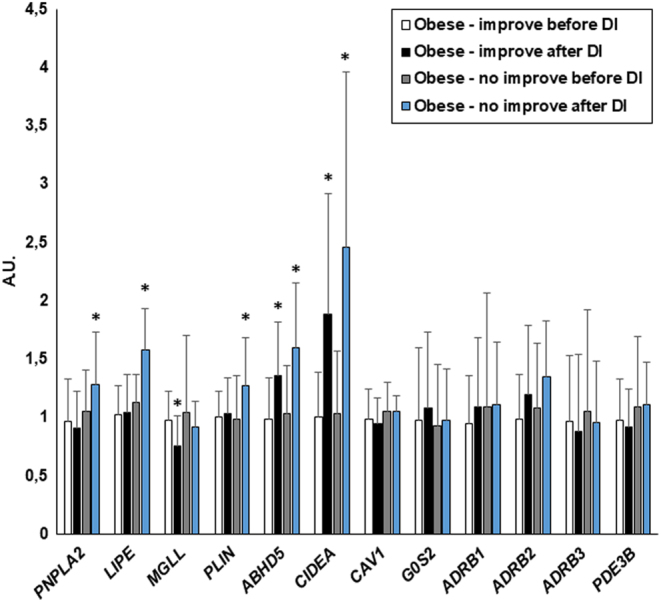
The effect of diet-induced weight loss on AT gene expression in obese subjects, in whom insulin sensitivity increased (Obese - improve, n = 29) or did not increase (Obese - no improve, n = 9). Data are shown as mean ± s.d. *P < 0.05 vs before dietary intervention. A.U., arbitrary units.
AT expression of ABHD5 and CIDEA increased similarly in both groups after the intervention (P < 0.005), whereas the expression of G0S2, CAV1, ADRB1, ADRB3 and PDE3B did not change in any of the groups (Fig. 2). AT ADRB2 expression did not change in the groups with and without increase in insulin sensitivity analyzed separately.
The correlation between the analyzed genes and insulin sensitivity
At baseline, before dietary intervention, AT PNPLA2 (r = 0.30, P = 0.019), LIPE (r = 0.45, P < 0.001), PLIN (r = 0.30, P = 0.021), ABHD5 (r = 0.26, P = 0.042) and CIDEA (r = 0.35, P = 0.006) expression was positively related to insulin sensitivity in the entire study group. Only correlation of LIPE with insulin sensitivity remained significant after adjustment for BMI (β = 0.29, P = 0.04). Baseline LIPE (r = 0.42, P = 0.007) expression was also positively related to insulin sensitivity within the group of obese subjects.
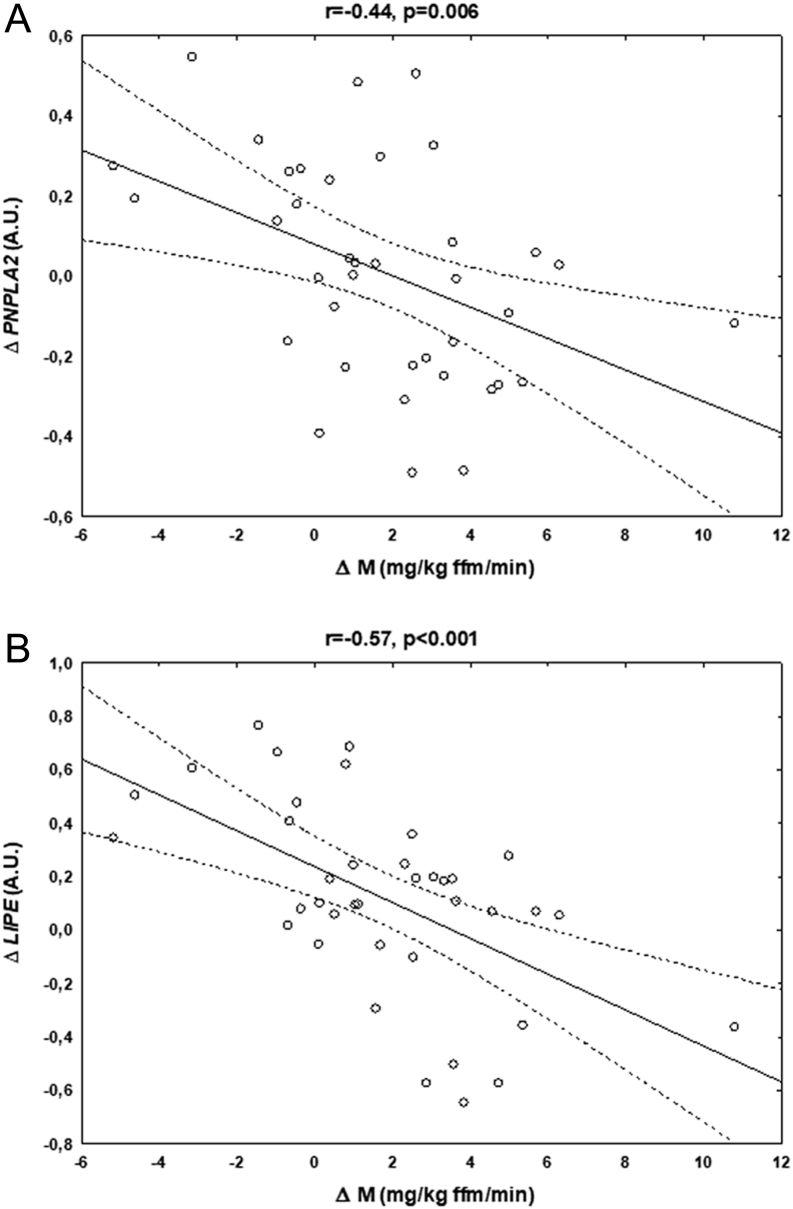
The changes in the AT PNPLA2 (r = −0.44, P = 0.006; Fig. 3A) and LIPE (r = −0.57, P < 0.001; Fig. 3B) after weight loss were inversely related to the concurrent changes in insulin sensitivity (i.e. the higher increase in the respective gene expression, the lower increase in insulin sensitivity) in the entire overweight/obese group. Both correlations remained significant after adjustment for initial insulin sensitivity and the concurrent change in BMI (PNPLA2, β = -0.33, P = 0.046; LIPE, β = -0.45, P = 0.007), as well as for the concurrent changes in FFA (P < 0.05). In contrast, the relationship between initial insulin sensitivity and the change in this parameter during the intervention remained significant after adjustment for the change in PNPLA2 (β = -0.34, P = 0.036), but not in LIPE expression. All the associations remained significant after exclusion of one subject with the highest increase in insulin sensitivity (data not shown).
Figure 3.
Correlations between the changes in insulin sensitivity (Δ M) and the concurrent changes in PNPLA2 (A) and LIPE (B) expression after diet-induced weight loss in obese subjects (n = 38). A.U., arbitrary units.
Post-intervention insulin sensitivity was related to post-intervention AT CIDEA expression (r = 0.38, P = 0.019); however, this association disappeared after adjustment for BMI in the respective time-point.
Discussion
The most important finding of the present study is that obese subjects, in whom insulin sensitivity does not increase in response to weight loss, demonstrate higher initial insulin sensitivity (Table 2) and an increase in AT HSL and ATGL expression after dietary intervention (Fig. 2). Only the change in gene expression encoding HSL and ATGL independently predicted the changes in insulin sensitivity during the weight loss program in multiple regression analysis.
The decrease in body weight and body fat was very similar in the groups of subjects with and without an improvement in insulin sensitivity. Subjects without an improvement in insulin sensitivity had higher initial M value and exhibited a decrease in their insulin sensitivity after the dietary intervention program. Subjects with lower initial insulin sensitivity achieved greater metabolic benefit from lifestyle intervention (29). Findings similar to ours regarding worsening (29, 30), or the lack of improvement in insulin sensitivity (31) after weight loss in subjects with high initial insulin sensitivity, were observed by other researchers. In the cited studies, AT gene expression was not reported. In our study, initial insulin sensitivity value was not an independent predictor of the subsequent change in insulin sensitivity, therefore, changes in AT gene expression seem to be of particular importance.
At baseline, before the intervention, obese subjects had lower AT expression of the genes associated with lipolysis. Our data are in agreement with other studies, which also reported lower AT lipase expression in obesity (16, 17, 18, 19, 20, 21). Furthermore, decreased AT expression of PLIN (25), ABHD5 (32, 33) and CIDEA (6, 33) in obesity was also reported. Decrease in AT G0S2 (33, 34) and an increase in CAV1 (35) were previously reported; however, we were unable to confirm these findings in our study. Our study subjects were young and had uncomplicated obesity, which may potentially explain the difference in the results. Interestingly, AT expression of the factors increasing (PNPLA2, LIPE, MGLL, ABHD5) and decreasing lipolysis (PLIN, CIDEA) were simultaneously decreased in obese subjects. This finding may suggest that the balance between pro- and anti-lipolytic factors is maintained in obesity; however, it is set at the altered level.
Before the dietary intervention, expression of PNPLA2, LIPE, PLIN, ABHD5 and CIDEA was positively related to insulin sensitivity; however, most of these correlations (except LIPE) lost their significance after adjustment for BMI. In other studies, AT HSL and ATGL were associated with different measures of insulin sensitivity, independent of adiposity (19, 21). Similar findings were reported for AT PLIN and CIDEA (5, 36). Although we did observe positive correlations between the estimated genes and clamp-derived insulin sensitivity, most of these associations were dependent on BMI. However, it should be noted that our study group were young and that obese subjects did not have overt metabolic disturbances and had relatively mild degree of insulin resistance (decrease by approx. 30% in comparison to normal-weight subjects), which may potentially influence the results. Anyway, our results do not indicate the genes associated with lipolysis as primary factors in obesity-related insulin resistance, with the exception of HSL.
We did not observe a significant increase in AT PNPLA2 and LIPE expression in the entire study group. Data on the effect of weight loss on AT lipases are conflicting. Jocken et al. reported a decrease in AT HSL and ATGL mRNA and protein expression after a 10-week hypocaloric diet, whereas insulin resistance, measured as HOMA-IR, did not significantly change (19). In another study, both HSL and ATGL s.c. AT expression decreased after a 2-month low-calorie diet, which was preceded by a 1 month very low-calorie diet (37). No correlations between the change in lipase expression and anthropometric and metabolic parameters were found (37). In contrast, there are also data showing a normalization of an initially low AT HSL with an unchanged ATGL expression after weight loss (38) and an increase in ATGL after a 10-month weight maintenance period, but not during the preceding weight loss program (39). An increase in AT ATGL protein expression after 5 weeks of a very low-calorie diet followed by a 3-week weight maintenance diet (40) and an increase in AT HSL and ATGL mRNA expression after bariatric surgery-induced weight loss (41) were reported. In the latter study, post-intervention lipase expression displayed some relationships with beneficial metabolic profile; however, no BMI or other adjustments were reported (41). All the aforementioned studies differ regarding subjects’ characteristics, type and duration of weight-reducing intervention, the degree of weight loss and the method of AT sampling, which likely may contribute to the observed differences in the results.
In our study, the change in AT PNPLA2 and LIPE expression was associated with the concurrent change in insulin sensitivity independently of other estimated variables. It is postulated that a decreased lipase expression and an impaired AT lipolysis may contribute to the development and maintenance of obesity (16, 19). However, this explanation is rather unlikely, as HSL-deficient animals are resistant to diet-induced obesity (42), and humans with HSL null mutation display an impaired lipolysis and metabolic disturbances; however, their BMI is usually within the range of overweight and not obese (24). Alternatively, it was suggested that lipase downregulation in obesity may be a compensatory mechanism to prevent an excessive FFA outflow and to restrain obesity-related insulin resistance (16, 19). Girousse et al. demonstrated that a decrease in AT lipolysis correlated with the concurrent improvement in insulin sensitivity 2 years after bariatric surgery. Lipase expression was not reported in the clinical part of this work, whereas initially lipolysis correlated positively with insulin resistance across a wide range of BMI (10). Another issue is the predisposition to weight regain, which has been linked to the defect in lipolysis in recent animal study (43). Our data show that AT lipase expression response to weight loss should be analyzed together with the concurrent changes in insulin sensitivity as the differences in individual response exist. Our results also suggest that the relation of AT lipase expression with insulin action may be different in the baseline conditions and in the state of a negative energy balance.
It is possible that worsening in insulin sensitivity is associated mainly with an FFA excess and with lipotoxicitiy during weight loss, as suggested by an increase in the genes encoding ATGL and HSL and lack of decrease in MGLL. The changes in PNPLA2 and LIPE expression were related to the change in insulin sensitivity independent of change in FFA; however, fasting FFA measurement does not reflect possible lipotoxicity effect during the 3-month intervention. It was also demonstrated that lipolysis during weight loss may influence inflammatory response in AT in mice (44). However, AT inflammation was not decreased by weight loss in our previous report (27) and it was unchanged or even worsened when insulin sensitivity improved after ATGL inhibition in experimental studies (9, 10). Therefore, we propose that sustained lipotoxic effect may be responsible for the lack of improvement in insulin sensitivity during dietary intervention. It should be noted that one cannot establish the cause-effect relationship between different changes occurring simultaneously during weight loss on the basis of our results, and an increase in lipase expression may simply reflect an impaired inhibitory effect of insulin due to the lack of improvement in insulin sensitivity. However, despite this lack of improvement, insulin sensitivity remained still relatively good in this group, which argues against such an interpretation of our results.
An increase in AT PLIN, the gene encoding perilipin, a protein that stabilizes lipid droplet and decreases lipolysis, in the group with a worsening of insulin sensitivity may reflect the inter-relationships of this factor with lipases and/or may represent a compensatory mechanism aimed to balance an increase in lipase expression. In contrast to PNPLA2 and LIPE, the change in PLIN was not related to the change in insulin sensitivity.
Weight loss is usually associated with the beneficial metabolic changes. Thus, it may seem paradoxical that some of the changes observed in the present study during weight loss prevented an increase in insulin sensitivity. One may hypothesize that the most beneficial for metabolic health would be to mobilize fat stores efficiently in response to the weight-reducing intervention without subsequent maintaining prolonged lipolytic response. Our data support the notion that the regulation of lipolysis may uncouple adiposity from insulin sensitivity (8) especially in the conditions of weight loss. Our results also suggest that inhibition of AT lipolysis as a treatment option for insulin resistance (2) may have a particular benefit during weight-reducing interventions.
In the entire study group, we observed an increase in AT ABHD5 and CIDEA and a decrease in MGLL expression. An increase in AT CIDEA after diet- or bariatric surgery-induced weight loss was observed previously (6, 36, 45, 46), and some of the studies report also an increase in ABHD5 and PLIN (41, 47) and the latter one was not observed in our study when the entire group was analyzed. AT ABHD5 and CIDEA expression increased similarly in the group with and without an improvement in insulin sensitivity and was not related to the concurrent change in M value. In the study of Montastier et al. (48), changes in adipose tissue CIDEA paralleled variations in insulin sensitivity independently of changes in BMI during diet induced weight loss and weight maintenance program. Our data suggest that low AT CIDEA is related rather to obesity per se and secondarily to insulin resistance.
It should be noted that our dietary intervention consisted of calorie restriction and balanced diet, without any specific modification in diet composition. Physical activity maintained stable throughout the program. Furthermore, we observed a decrease in fasting serum insulin in both groups and triglycerides in the group with an improvement in insulin sensitivity. In the group without an improvement in insulin sensitivity, serum FFA increased after weight loss. These changes are typical for diet-induced weight loss. Thus, it is reasonable to assume that the metabolic alterations reported in the present study, including AT lipase expression, are due to the calorie restriction and associated weight loss. It should also be noted that that an increase in AT lipase expression was not related to the changes in adrenergic receptors and PDE3B expression and the mechanism of an increase in AT PNPLA2 and LIPE expression specifically in the group without improvement in insulin sensitivity should be studied further.
We did not measure AT protein expression, which may be considered as a limitation of the present study. Such measurement was impossible due to the limited tissue availability. An excellent correlation between AT lipase mRNA and protein expression was demonstrated, both at baseline and after weight loss (19). It was also impossible to analyze adipocyte size; however, in the study of Verhoef et al. (39) the changes in the adipocyte size were not paralleled by the changes in ATGL during weight loss and maintenance. The group without an improvement in insulin sensitivity comprised of only nine subjects. However, the proportion of subjects with and without an improvement in insulin sensitivity is similar to other aforementioned studies (29, 30, 31). Furthermore, the change in PNPLA2 and LIPE expression was inversely associated with the concurrent change in insulin sensitivity in the entire study group.
In conclusion, our data suggest that excessive lipolysis may prevent an improvement in insulin sensitivity after weight loss. The change in AT PNPLA2 and LIPE expression was a negative predictor of the change in insulin sensitivity after weight loss.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
Supported by the National Science Centre, Poland (grant NCN number 2001/03/B/NZ7/04980), by The Grant UDA-POIG.01.03.01-00-128/08; from the Program Innovative Economy 2007–2013; part-financed by the European Union within the European Regional Development Fund, and by the statutory funds of the Medical University of Białystok, Poland (N/ST/ZB/16/001/1173 and N/ST/ZB/16/002/1173). Monika Karczewska-Kupczewska was supported by REFRESH Project (grant FP7-REGPOT-2010-1 264103).
References
- 1.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006. 840–846. ( 10.1038/nature05482) [DOI] [PubMed] [Google Scholar]
- 2.Morigny P, Houssier M, Mouisel E, Langin D. Adipocyte lipolysis and insulin resistance. Biochimie 2016. 259–266. ( 10.1016/j.biochi.2015.10.024) [DOI] [PubMed] [Google Scholar]
- 3.Lafontan M, Langin D. Lipolysis and lipid mobilization in human adipose tissue. Progress in Lipid Research 2009. 275–297. ( 10.1016/j.plipres.2009.05.001) [DOI] [PubMed] [Google Scholar]
- 4.Granneman JG, Moore HP, Krishnamoorthy R, Rathod M. Perilipin controls lipolysis by regulating the interactions of AB-hydrolase containing 5 (Abhd5) and adipose triglyceride lipase (Atgl). Journal of Biological Chemistry 2009. 34538–34544. ( 10.1074/jbc.M109.068478) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puri V, Ranjit S, Konda S, Nicoloro SM, Straubhaar J, Chawla A, Chouinard M, Lin C, Burkart A, Corvera S, et al. Cidea is associated with lipid droplets and insulin sensitivity in humans. PNAS 2008. 7833–7838. ( 10.1073/pnas.0802063105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nordström EA, Rydén M, Backlund EC, Dahlman I, Kaaman M, Blomqvist L, Cannon B, Nedergaard J, Arner P. A human-specific role of cell death-inducing DFFA (DNA fragmentation factor-alpha)-like effector A (CIDEA) in adipocyte lipolysis and obesity. Diabetes 2005. 1726–1734. ( 10.2337/diabetes.54.6.1726) [DOI] [PubMed] [Google Scholar]
- 7.Cohen AW, Razani B, Schubert W, Williams TM, Wang XB, Iyengar P, Brasaemle DL, Scherer PE, Lisanti MP. Role of caveolin-1 in the modulation of lipolysis and lipid droplet formation. Diabetes 2004. 1261–1270. ( 10.2337/diabetes.53.5.1261) [DOI] [PubMed] [Google Scholar]
- 8.Heckmann BL, Zhang X, Xie X, Saarinen A, Lu X, Yang X, Liu J. Defective adipose lipolysis and altered global energy metabolism in mice with adipose overexpression of the lipolytic inhibitor G0/G1 switch gene 2 (G0S2). Journal of Biological Chemistry 2014. 1905–1916. ( 10.1074/jbc.M113.522011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schoiswohl G, Stefanovic-Racic M, Menke MN, Wills RC, Surlow BA, Basantani MK, Sitnick MT, Cai L, Yazbeck CF, Stolz DB, et al. Impact of reduced ATGL-mediated adipocyte lipolysis on obesity-associated insulin resistance and inflammation in male mice. Endocrinology 2015. 3610–3624. ( 10.1210/en.2015-1322) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Girousse A, Tavernier G, Valle C, Moro Cs, Mejhert N, Dinel AL, Houssier M, Roussel B, Besse-Patin A, Combes M, et al. Partial inhibition of adipose tissue lipolysis improves glucose metabolism and insulin sensitivity without alteration of fat mass. PLoS Biology 2013. e1001485 ( 10.1371/journal.pbio.1001485) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schweiger M, Romauch M, Schreiber R, Grabner GF, Hütter S, Kotzbeck P, Benedikt P, Eichmann TO, Yamada S, Knittelfelder O, et al. Pharmacological inhibition of adipose triglyceride lipase corrects high-fat diet-induced insulin resistance and hepatosteatosis in mice. Nature Communications 2017. 14859 ( 10.1038/ncomms14859) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taschler U, Radner FP, Heier C, Schreiber R, Schweiger M, Schoiswohl G, Preiss-Landl K, Jaeger D, Reiter B, Koefeler HC, et al. Monoglyceride lipase deficiency in mice impairs lipolysis and attenuates diet-induced insulin resistance. Journal of Biological Chemistry 2011. 17467–17477. ( 10.1074/jbc.M110.215434) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown JM, Betters JL, Lord C, Ma Y, Han X, Yang K, Alger HM, Melchior J, Sawyer J, Shah R, et al. CGI-58 knockdown in mice causes hepatic steatosis but prevents diet-induced obesity and glucose intolerance. Journal of Lipid Research 2010. 3306–3315. ( 10.1194/jlr.M010256) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El-Assaad W, El-Kouhen K, Mohammad AH, Yang J, Morita M, Gamache I, Mamer O, Avizonis D, Hermance N, Kersten S, et al. Deletion of the gene encoding G0/G 1 switch protein 2 (G0s2) alleviates high-fat-diet-induced weight gain and insulin resistance, and promotes browning of white adipose tissue in mice. Diabetologia 2015. 149–157. ( 10.1007/s00125-014-3429-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jocken JW, Goossens GH, Popeijus H, Essers Y, Hoebers N, Blaak EE. Contribution of lipase deficiency to mitochondrial dysfunction and insulin resistance in hMADS adipocytes. International Journal of Obesity 2016. 507–513. ( 10.1038/ijo.2015.211) [DOI] [PubMed] [Google Scholar]
- 16.Langin D, Dicker A, Tavernier G, Hoffstedt J, Mairal A, Rydén M, Arner E, Sicard A, Jenkins CM, Viguerie N, et al. Adipocyte lipases and defect of lipolysis in human obesity. Diabetes 2005. 3190–3197. ( 10.2337/diabetes.54.11.3190) [DOI] [PubMed] [Google Scholar]
- 17.Yao-Borengasser A, Varma V, Coker RH, Ranganathan G, Phanavanh B, Rasouli N, Kern PA. Adipose triglyceride lipase expression in human adipose tissue and muscle. Role in insulin resistance and response to training and pioglitazone. Metabolism: Clinical and Experimental 2011. 1012–1020. ( 10.1016/j.metabol.2010.10.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrer R, Pardina E, Rossell J, Baena-Fustegueras JA, Lecube A, Balibrea JM, Caubet E, González O, Vilallonga R, Fort JM, et al. Decreased lipases and fatty acid and glycerol transporter could explain reduced fat in diabetic morbidly obese. Obesity 2014. 2379–2387. ( 10.1002/oby.20861) [DOI] [PubMed] [Google Scholar]
- 19.Jocken JW, Langin D, Smit E, Saris WH, Valle C, Hul GB, Holm C, Arner P, Blaak EE. Adipose triglyceride lipase and hormone-sensitive lipase protein expression is decreased in the obese insulin-resistant state. Journal of Clinical Endocrinology and Metabolism 2007. 2292–2299. ( 10.1210/jc.2006-1318) [DOI] [PubMed] [Google Scholar]
- 20.Large V, Reynisdottir S, Langin D, Fredby K, Klannemark M, Holm C, Arner P. Decreased expression and function of adipocyte hormone-sensitive lipase in subcutaneous fat cells of obese subjects. Journal of Lipid Research 1999. 2059–2066. [PubMed] [Google Scholar]
- 21.Berndt J, Kralisch S, Klöting N, Ruschke K, Kern M, Fasshauer M, Schön MR, Stumvoll M, Blüher M. Adipose triglyceride lipase gene expression in human visceral obesity. Experimental and Clinical Endocrinology and Diabetes 2008. 203–210. ( 10.1055/s-2007-993148) [DOI] [PubMed] [Google Scholar]
- 22.Tinahones FJ, Garrido-Sanchez L, Miranda M, García-Almeida JM, Macias-Gonzalez M, Ceperuelo V, Gluckmann E, Rivas-Marin J, Vendrell J, García-Fuentes E. Obesity and insulin resistance-related changes in the expression of lipogenic and lipolytic genes in morbidly obese subjects. Obesity Surgery 2010. 1559–1567. ( 10.1007/s11695-010-0194-z) [DOI] [PubMed] [Google Scholar]
- 23.Albert JS, Yerges-Armstrong LM, Horenstein RB, Pollin TI, Sreenivasan UT, Chai S, Blaner WS, Snitker S, O'Connell JR, Gong DW, et al. Null mutation in hormone-sensitive lipase gene and risk of type 2 diabetes. New England Journal of Medicine 2014. 2307–2315. ( 10.1056/NEJMoa1315496) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sidibeh CO, Pereira MJ, Lau Börjesson J, Kamble PG, Skrtic S, Katsogiannos P, Sundbom M, Svensson MK, Eriksson JW. Role of cannabinoid receptor 1 in human adipose tissue for lipolysis regulation and insulin resistance. Endocrine 2017. 839–852. ( 10.1007/s12020-016-1172-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Sullivan S, Trujillo M, Lee MJ, Schneider SH, Brolin RE, Kang YH, Werber Y, Greenberg AS, Fried SK. Perilipin expression in human adipose tissues: effects of severe obesity, gender, and depot. Obesity Research 2003. 930–936. ( 10.1038/oby.2003.128) [DOI] [PubMed] [Google Scholar]
- 26.Nielsen TS, Kampmann U, Nielsen RR, Jessen N, Orskov L, Pedersen SB, Jørgensen JO, Lund S, Møller N. Reduced mRNA and protein expression of perilipin A and G0/G1 switch gene 2 (G0S2) in human adipose tissue in poorly controlled type 2 diabetes. Journal of Clinical Endocrinology and Metabolism 2012. E1348–E1352. ( 10.1210/jc.2012-1159) [DOI] [PubMed] [Google Scholar]
- 27.Strączkowski M, Nikołajuk A, Majewski R, Filarski R, Stefanowicz M, Matulewicz N, Karczewska-Kupczewska M. The effect of weight loss, with or without β-glucan addition, on adipose tissue inflammatory gene expression. Endocrine 2018. 275–284. ( 10.1007/s12020-018-1619-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matulewicz N, Stefanowicz M, Nikołajuk A, Karczewska-Kupczewska M. Markers of adipogenesis, but not inflammation in adipose tissue, are independently related to insulin sensitivity. Journal of Clinical Endocrinology and Metabolism 2017. 3040–3049. ( 10.1210/jc.2017-00597) [DOI] [PubMed] [Google Scholar]
- 29.Karelis AD, Messier V, Brochu M, Rabasa-Lhoret R. Metabolically healthy but obese women: effect of an energy-restricted diet. Diabetologia 2008. 1752–1754. ( 10.1007/s00125-008-1038-4) [DOI] [PubMed] [Google Scholar]
- 30.Gilardini L, Vallone L, Cottafava R, Redaelli G, Croci M, Conti A, Pasqualinotto L, Invitti C. Insulin sensitivity deteriorates after short-term lifestyle intervention in the insulin sensitive phenotype of obesity. Obesity Facts 2012. 68–76. ( 10.1159/000336926) [DOI] [PubMed] [Google Scholar]
- 31.Kantartzis K, Machann J, Schick F, Rittig K, Machicao F, Fritsche A, Häring HU, Stefan N. Effects of a lifestyle intervention in metabolically benign and malign obesity. Diabetologia 2011. 864–868. ( 10.1007/s00125-010-2006-3) [DOI] [PubMed] [Google Scholar]
- 32.Petridou A, Chatzinikolaou A, Avloniti A, Jamurtas A, Loules G, Papassotiriou I, Fatouros I, Mougios V. Increased triacylglycerol lipase activity in adipose tissue of lean and obese men during endurance exercise. Journal of Clinical Endocrinology and Metabolism 2017. 3945–3952. ( 10.1210/jc.2017-00168) [DOI] [PubMed] [Google Scholar]
- 33.Bak AM, Møller AB, Vendelbo MH, Nielsen TS, Viggers R, Rungby J, Pedersen SB, Jørgensen JO, Jessen N, Møller N. Differential regulation of lipid and protein metabolism in obese vs. lean subjects before and after a 72-h fast. American Journal of Physiology: Endocrinology and Metabolism 2016. E224–E235. ( 10.1152/ajpendo.00464.2015) [DOI] [PubMed] [Google Scholar]
- 34.Skopp A, May M, Janke J, Kielstein H, Wunder R, Flade-Kuthe R, Kuthe A, Jordan J, Engeli S. Regulation of G0/G1 switch gene 2 (G0S2) expression in human adipose tissue. Archives of Physiology and Biochemistry 2016. 47–53. ( 10.3109/13813455.2015.1122066) [DOI] [PubMed] [Google Scholar]
- 35.Singh P, Sharma P, Sahakyan KR, Davison DE, Sert-Kuniyoshi FH, Romero-Corral A, Swain JM, Jensen MD, Lopez-Jimenez F, Kara T, et al. Differential effects of leptin on adiponectin expression with weight gain versus obesity. International Journal of Obesity 2016. 266–274. ( 10.1038/ijo.2015.181) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gummesson A, Jernås M, Svensson PA, Larsson I, Glad CA, Schéle E, Gripeteg L, Sjöholm K, Lystig TC, Sjöström L, et al. Relations of adipose tissue CIDEA gene expression to basal metabolic rate, energy restriction, and obesity: population-based and dietary intervention studies. Journal of Clinical Endocrinology and Metabolism 2007. 4759–4765. ( 10.1210/jc.2007-1136) [DOI] [PubMed] [Google Scholar]
- 37.Koppo K, Valle C, Šiklová-Vítková M, Czudková E, de Glisezinski I, van de Voorde J, Langin D, Štich V. Expression of lipolytic genes in adipose tissue is differentially regulated during multiple phases of dietary intervention in obese women. Physiological Research 2013. 527–535. [DOI] [PubMed] [Google Scholar]
- 38.Mairal A, Langin D, Arner P, Hoffstedt J. Human adipose triglyceride lipase (PNPLA2) is not regulated by obesity and exhibits low in vitro triglyceride hydrolase activity. Diabetologia 2006. 1629–1636. ( 10.1007/s00125-006-0272-x) [DOI] [PubMed] [Google Scholar]
- 39.Verhoef SP, Camps SG, Bouwman FG, Mariman EC, Westerterp KR. Physiological response of adipocytes to weight loss and maintenance. PLoS ONE 2013. e58011 ( 10.1371/journal.pone.0058011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bouwman FG, Wang P, van Baak M, Saris WH, Mariman EC. Increased β-oxidation with improved glucose uptake capacity in adipose tissue from obese after weight loss and maintenance. Obesity 2014. 819–827. ( 10.1002/oby.20359) [DOI] [PubMed] [Google Scholar]
- 41.Karki S, Farb MG, Myers S, Apovian C, Hess DT, Gokce N. Effect of bariatric weight loss on the adipose lipolytic transcriptome in obese humans. Mediators of Inflammation 2015. 106237 ( 10.1155/2015/106237) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harada K, Shen WJ, Patel S, Natu V, Wang J, Osuga J, Ishibashi S, Kraemer FB. Resistance to high-fat diet-induced obesity and altered expression of adipose-specific genes in HSL-deficient mice. American Journal of Physiology: Endocrinology and Metabolism 2003. E1182–E1195. ( 10.1152/ajpendo.00259.2003) [DOI] [PubMed] [Google Scholar]
- 43.Kasher-Meron M, Youn DY, Zong H, Pessin JE. Lipolysis defect in white adipose tissue and rapid weight regain. American Journal of Physiology: Endocrinology and Metabolism 2019. E185–E193. ( 10.1152/ajpendo.00542.2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kosteli A, Sugaru E, Haemmerle G, Martin JF, Lei J, Zechner R, Ferrante AW., Jr Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. Journal of Clinical Investigation 2010. 3466–3479. ( 10.1172/JCI42845) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mardinoglu A, Heiker JT, Gärtner D, Björnson E, Schön MR, Flehmig G, Klöting N, Krohn K, Fasshauer M, Stumvoll M, et al. Extensive weight loss reveals distinct gene expression changes in human subcutaneous and visceral adipose tissue. Scientific Reports 2015. 14841 ( 10.1038/srep14841) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johansson LE, Danielsson AP, Parikh H, Klintenberg M, Norström F, Groop L, Ridderstråle M. Differential gene expression in adipose tissue from obese human subjects during weight loss and weight maintenance. American Journal of Clinical Nutrition 2012. 196–207. ( 10.3945/ajcn.111.020578) [DOI] [PubMed] [Google Scholar]
- 47.Moreno-Navarrete JM, Ortega F, Serrano M, Rodriguez-Hermosa JI, Ricart W, Mingrone G, Fernandez-Real JM. CIDEC/FSP27 and PLIN1 gene expression run in parallel to mitochondrial genes in human adipose tissue, both increasing after weight loss. International Journal of Obesity 2014. 865–872. ( 10.1038/ijo.2013.171) [DOI] [PubMed] [Google Scholar]
- 48.Montastier E, Déjean S, Le Gall C, Saris WH, Langin D, Viguerie N. Adipose tissue CIDEA is associated, independently of weight variation, to change in insulin resistance during a longitudinal weight control dietary program in obese individuals. PLoS ONE 2014. e98707 ( 10.1371/journal.pone.0098707) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a