Abstract
Patient: Male, 73-year-old
Final Diagnosis: T cell lymphoma
Symptoms: Dyspnea
Medication: —
Clinical Procedure: Thoracentesis
Specialty: Oncology
Objective:
Rare disease
Background:
Primary effusion lymphoma (PEL) is a rare and aggressive non-Hodgkin lymphoma (NHL) that is responsible for 1% of all lymphomas not related to human immunodeficiency virus (HIV). PEL is characterized by human herpesvirus-8 (HHV-8) positivity in the absence of overt tumor burden that does not exhibit typical B cell or T cell immunophenotype characteristics. The exact mechanism of development is unknown, but it is hypothesized to develop from post-germinal B cell origin. Although it is most common in HIV patients, other immuno-compromising comorbidities can be seen in conjunction with PEL, including liver cirrhosis.
Case Report:
We present the case of a 73-year-old HIV-seronegative man with alcohol-induced liver cirrhosis who was found to have T cell PEL of the pleural space diagnosed by thoracentesis.
Conclusions:
Little is known regarding oncogenesis of T cell PEL, and few studies exist regarding appropriate treatment regimens for PEL as a whole, prompting need for further investigation and discussion to improve survival rates. Even in the absence of active HIV infection, PEL should be considered as a potential cause of pleural effusion in cirrhotic patients in order to prompt earlier treatment for the best chance of survival.
MeSH Keywords: Liver Cirrhosis, Alcoholic; Lymphoma, Primary Effusion; Pleural Effusion, Malignant
Background
Primary effusion lymphoma (PEL) is a rare and aggressive large B cell non-Hodgkin’s lymphoma (NHL). PEL most commonly occurs in immunocompromised patients with human immunodeficiency virus (HIV), patients who received solid-organ transplants, and certain patients with liver cirrhosis. PEL is responsible for 4% of HIV-related to NHL and about 1% of all lymphomas not related to HIV. PEL is defined by the seroprevalence of human herpesvirus-8 (HHV-8), also referred to as Kaposi sarcoma-associated herpesvirus (KSHV). Epstein-Barr virus (EBV) co-infection with HHV-8 is found in 60–90% of PEL patients, unless they are a non-HIV-infected elderly from an HHV8-endemic area [1,2], such as patients of Mediterranean descent [3]. There is also a significant sex predilection favoring males, with a rate of 92% [4]. The diagnosis of PEL is multimodal and uses virologic, immunophenotypic, morphologic, and molecular criteria [5]. PEL is characterized by malignant effusions, most commonly in the body cavities such as the pleura, pericardium, and peritoneum, without any detectable neoplastic masses [1]. Typically, only 1 body cavity is affected, with the most common site being the pleura (in 55%) [4]. The detection of HHV-8 in the malignant cells is essential for establishing the diagnosis [5,6]. The main mode of detection is via fluorescence in-situ hybridization (FISH) for HHV-8, which is made possible by intracellular expression of latency-associated nuclear antigen (LANA) and is significantly more accurate than serology for HHV-8 [7]. Polymerase chain reaction (PCR) for HHV-8 allows for quantitative results that can be correlated with viral copies and infected cells, which can aid in diagnosis of PEL [7]. The exact incidence of PEL in liver cirrhosis patients is unknown, but has been described in elderly patients with liver cirrhosis [8]. Association with hepatitis C virus (HCV) has been documented [3], but there is no known association of HHV-8-positive PEL with alcohol-induced liver cirrhosis and PEL. Additionally, a similar association with end-stage renal disease and PEL has been described, indicating an association with volume overload states [9]. Only a few cases of PEL with T cell antigen expression have been recorded among HHV-8-positive patients [6]. Here, we present a rare case of T cell primary effusion lymphoma in an HIV-seronegative patient with alcohol-induced liver cirrhosis.
Case Report
A 73-year-old Hispanic man with a past medical history of end-stage renal disease (ESRD) on hemodialysis secondary to uncontrolled hypertension and type 2 diabetes mellitus and liver cirrhosis with ascites secondary to alcohol abuse presented to the hospital for progressive dyspnea of 2-weeks duration. Associated symptoms included a 30-pound (lb) weight loss and left-sided, pleuritic chest pain. Vitals on initial presentation were within normal limits. Physical examination revealed decreased breath sounds at lung bases, bilaterally, as well as an abdominal fluid wave. Initial complete blood count (CBC) showed mild leukopenia of 3.1×103/ul (4.1–10.4×103/ul) and thrombocytopenia, and a platelet count of 122×103/uL (145–355×103/ul). A peripheral blood smear showed mild anisopoikilocytosis and mild thrombocytopenia. White blood cells were normal in morphology. Creatinine was elevated to 4.65 mg/dl (0.70–1.30 mg/dL) and brain natriuretic peptide was 1275 pg/ml (0–100 pg/ml), due to ESRD. A chest radiograph revealed opacification of the right hemithorax and a small left pleural effusion. Subsequent computed tomography (CT) angiography of the chest revealed large right-sided pleural effusion with atelectasis of the entire right lung and moderate-sized left-sided pleural effusion with atelectasis of the majority of the left lower lobe (Figure 1). A right-sided chest tube was placed, which drained 4 L (liters) of serosanguinous fluid. The pleural fluid was positive for exudative effusion, revealing a lactate dehydrogenase (LDH) of 2795 U/L and protein of 5.0 g/dL. The pleural fluid was negative for fungi, acid-fast bacilli, gram stain, and anaerobic culture. The initial cytology was negative for malignancy. Due to enlarging left-sided pleural effusion visualized on chest radiography and increasing oxygen requirements, the patient underwent left-sided thoracentesis. Serous fluid (1.5 L) was drained. The LDH of the left side was 450 U/L and the differential revealed 74% atypical cells in the pleural fluid. In-situ hybridization was positive for HHV-8, and polymerase chain reaction (PCR) assay for T cell receptor gamma gene rearrangement was positive. These findings were consistent with CD30-positive large T cell lymphoma, HHV-8 associated (Figures 2, 3). Immunohistochemical staining was positive for CD3, CD4, CD30, and vimentin. Cells were non-reactive for CD45, CD20, and ALK-1. Pleural fluid was negative for Epstein-Barr virus. The results were concerning for immunodeficiency, so human immunodeficiency virus (HIV) antigen and antibody combo immunoassay was completed and was non-reactive. A follow-up study with HIV RNA quantification assay results also did not reveal copies of HIV. The blood T cell lymphocyte profile revealed a low CD4 cell count of 169.14 cells/uL (492.00–1656.00 cells/uL) and low CD8 count of 135.39 cells/uL (205.00–1068.00 uL); however, CD4% and CD8% values were within normal range. Hepatitis A IgM, hepatitis B core IgM, and surface antigen were negative and hepatitis C was negative. Additional hepatitis testing was sent to a laboratory for analysis, including hepatitis B e antigen, which was negative, and a quantitative hepatitis B antibody, which was 39.1 mIU/mL (Mayo Clinical Laboratory), indicating prior vaccination for hepatitis B. Further CT scans of chest, abdomen, and pelvis, as well as a magnetic resonance imaging (MRI) of the brain, were negative for any overt masses or lymphadenopathy. Echocardiogram showed a normal ejection fraction without evidence of pericardial effusion. Due to the patient’s multiple precluding comorbidities and poor Eastern Cooperative Oncology Group (ECOG) status, he was deemed a poor candidate for cytotoxic chemotherapy. The patient had bilateral pleural catheters placed by Interventional Radiology for palliation of symptoms and was discharged home with hospice services.
Figure 1.
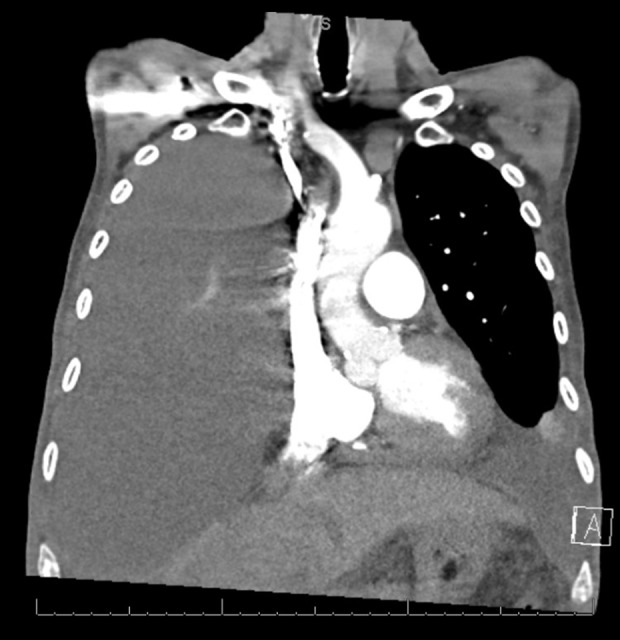
Right-sided pleural effusion with atelectasis of the entire right lung and moderate-sized left-sided pleural effusion with atelectasis of the majority of the left lower lobe.
Figure 2.

Pleural fluid. H&E stain showing CD30-positive atypical T lymphocytes.
Figure 3.
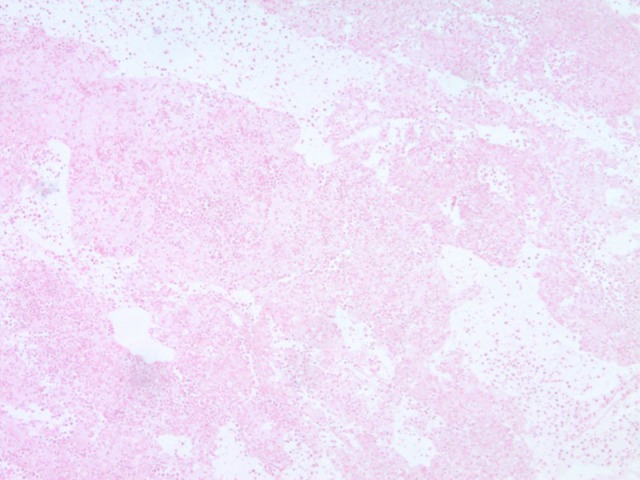
EBER-negative staining of pleural fluid cytology.
Discussion
Diagnosis of primary effusion lymphoma is made by identifying atypical cells with null immunophenotype and concomitant HHV-8 positivity, without obvious signs of lymph node enlargement or solid tumor masses [5]. Imaging modalities of PEL include CT scan, and subsequent imaging with positron emission tomography (PET) scan can be used if there is suspicion of disease in distant sites [5]. Although these modalities can help identify malignancy in other body cavities, all PEL are stage IV by nature, even if involving only a single body cavity [5]. Because of the HHV-8 positivity, PEL was previously classified as an AIDS-defining illness [5]. After infection of a host with HHV-8, there are 5 latent gene products that contribute to the development of PEL: LANA-1, LANA-2/viral interferon regulatory factor-3 (vIRF-3), viral homolog of FLICE-inhibitory protein (v-FLIP), viral homolog of cyclin D (v-Cyclin), and Kaposin (K12) [1,10]. The most important oncogene is LANA-1, which enters into host daughter cells during mitosis by binding to the nuclear chromosome [1,10]. Subsequently, it binds to TP53 and inhibits TP53-dependent apoptosis, as well as binding retinoblastoma (RB) and inducing cell proliferation [1,10]. Often, patients are co-infected with HHV-8 and EBV. The pathogenesis of EBV infection in PEL is similar to the way EBV predisposes patients to lymphomatous transformation. Chronic inflammation caused by longstanding EBV infection can cause cells to evade the host immune system, escape immune surveillance, and undergo malignant transformation [1,11]. PEL must be differentiated from other forms of effusion causing lymphoma. Differential diagnosis PEL includes extranodal diffuse large B cell lymphoma (DLBCL), extranodal Burkitt’s lymphoma, HHV-8/KSHV-unrelated PEL-like lymphoma, and pyothorax-associated lymphoma [1,10]. Diffuse large B cell lymphoma will appear morphologically different from PEL, which appears as anaplastic large-cell lymphoma and will be positive for CD20 [10]. Extranodal Burkitt’s lymphoma patients are c-Myc-positive. HHV-8/KSHV-unrelated PEL-like lymphoma patients are negative for HHV-8 [10]. Pyothorax-associated lymphoma is a non-Hodgkin B cell lymphoma often found in Japanese patients, resulting from treatment of tuberculosis with artificial pneumothorax, which shows a stronger association with EBV [10]. Our patient had a CD20-negative and CD30-positive T cell lymphoma, positive for HHV-8, making primary effusion lymphoma the most appropriate diagnosis. Usually, PEL cells are of post-germinal center B cell origin and over 90% exhibit a null lymphocyte phenotype (CD 45+), but typical T cell (CD 3, CD 4, CD 8) and B cell (immunoglobulin, CD 19, CD 20, CD 79a) markers are not expressed; thus, the designation of ‘null immunophenotype.’ Lymphocyte activation markers (CD 30, CD 38, CD 71, and human leukocyte antigen DR) and plasma cell differentiation (CD 138) markers are usually present [5,6]. There are only a few reports, including ours, of cases of PEL with a T cell phenotype [6]. Because traditional PEL is has a B cell origin, the oncogenesis of T cell PEL remains unclear and warrants further research [6]. The prognosis of PEL is relatively poor compared to HIV-related Burkitt’s lymphoma and diffuse large B cell lymphoma [8]. The survival rate for patients with PEL is typically less than 6 months and PEL is usually chemo-therapy-resistant [10]. Patients often have recurrence within 6–8 months [2]. Two studies have sought to identify prognostic features in PEL [1]. The first was a retrospective study published in 2005 assessed 28 patients with HIV who were diagnosed with PEL [12]. The study identified 2 factors associated with shorter survival: performance status and HAART. The study was limited by the small sample size and variation of treatment regimens used [12]. The second was a retrospective study of 104 patients, which suggested that patients with involvement of a single body cavity compared to multiple body cavities have longer survival [1]. Treatment is therefore based upon the patient’s performance status, other comorbidities, and addressing the patient’s goals of care [2]. PEL is typically treated with NHL chemotherapy regimens, such as CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) and with highly active antiretroviral therapy (HAART) if the patient is HIV-positive. Other treatment options that show favorable outcomes are dose-adjusted EPOCH (etoposide, cyclophosphamide, doxorubicin, vincristine, and prednisone) and CDE (cyclophosphamide, doxorubicin, and etoposide) [10]. New studies show that inhibition of a protein complex that controls transcription of DNA (NF-κB) with proteosome inhibitors, such as bortezomib, can lead to prolonged remission [13]. Additionally, brentuximab-vedotin, which is a CD30-directed antibody-drug conjugate, is approved for use in CD30-positive peripheral T cell lymphomas and has shown promising early results in PEL [14]. In addition, radiation has been shown to prolong survival in some patients who have failed chemotherapy and can be used for either chemotherapy refractory PEL or PEL associated with solid tumor masses [10]. Unfortunately, our patient was HIV-negative; therefore, HAART was not indicated. In addition, he had multiple comorbidities, making him a poor candidate for aggressive chemotherapy. Because of the limited number of cases and poor prognosis associated with PEL, limited studies exist on treatment options; therefore, treatments are largely based on case series and expert consensus opinion [4,10]. There is a documented association between volume overload states, such as end-stage renal disease and cirrhosis, and PEL [9], although the pathology is largely unknown. It is possible that fluid overload states predispose cells to malignant transformation by chronic serosal stimulation [11]. The incidence of PEL in cirrhotic patients remains uncertain, but there is a documented correlation between PEL and cirrhosis, usually caused by hepatitis C virus as opposed to alcohol-induced cirrhosis, as seen in our case [3,6]. Cirrhosis, regardless of the underlying cause, has been associated with low CD4 T cell counts and normal CD4% [15], which our patient exhibited. This pattern suggests that cirrhosis-induced lymphopenia may explain why there is an established correlation between PEL and cirrhosis. There has been 1 previous case report [16] of PEL associated with alcohol-induced liver cirrhosis, but the subject was negative for HHV-8, which is a fundamental element of PEL [4,6]. Even in the absence of active HIV infection, PEL should be considered as a potential cause of pleural effusion in cirrhotic patients in order to prompt earlier treatment for the best chance of survival.
Conclusions
Only a few case reports of T cell PEL exist to date, and little is known regarding the oncogenesis of T cell PEL, prompting the need for further research. Additionally, although PEL rarely occurs in the absence of HIV, there is a documented correlation between PEL and volume overload states, including both ESRD and liver cirrhosis [9]. Cirrhosis-induced lymphopenia may be the reason PEL is seen in cirrhotic patients in the absence of HIV [15]. Additionally, thoracenteses are often not performed in ESRD patients because the effusions resolve with hemodialysis. Therefore, it is possible that some malignant pleural effusions are missed, and that the incidence of PEL is actually higher than reported. Therefore, it is important to consider primary effusion lymphoma in patients with risk factors of volume overload states like cirrhosis and pleural effusions refractory to dialysis [9].
Footnotes
Conflict of interest
None.
References:
- 1.Shimada K, Hayakawa F, Kiyoi H. Biology and management of primary effusion lymphoma. Blood. 2018;132(18):1879–88. doi: 10.1182/blood-2018-03-791426. [DOI] [PubMed] [Google Scholar]
- 2.Narkhede M, Arora S, Ujjani C. Primary effusion lymphoma: Current perspectives. Onco Targets Ther. 2018;11:3747–54. doi: 10.2147/OTT.S167392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsieh P, Huang S, Li D, et al. Primary effusion lymphoma involving both pleural and abdominal cavities in a patient with hepatitis B virus-related liver cirrhosis. J Formos Med Assoc. 2007;106(6):504–8. doi: 10.1016/S0929-6646(09)60302-8. [DOI] [PubMed] [Google Scholar]
- 4.Kalogeraki A, Haniotis V, Karvelas-Kalogerakis M, et al. Primary effusion lymphoma with aberrant T-cell phenotype in an iatrogenically immuno-suppressed renal transplant male: Cytologic diagnosis in peritoneal fluid. Diagn Cytopathol. 2014;43(2):144–48. doi: 10.1002/dc.23149. [DOI] [PubMed] [Google Scholar]
- 5.Chen YB, Rahemtullah A, Hochberg E. Primary effusion lymphoma. Oncologist. 2007;12(5):569–76. doi: 10.1634/theoncologist.12-5-569. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi T, Mori D, Ureshino H, et al. Primary effusion lymphoma-like lymphoma with a T cell phenotype. Ann Hematol. 2018;97:717–18. doi: 10.1007/s00277-017-3200-x. [DOI] [PubMed] [Google Scholar]
- 7.Borie R, Cadranel J, Guihot A, et al. Pulmonary manifestations of human herpes virus-8 during infection. Eur Respir J. 2013;42(4):1105–18. doi: 10.1183/09031936.00154212. [DOI] [PubMed] [Google Scholar]
- 8.Klepfish A, Zuckermann B, Schattner A. Primary effusion lymphoma in the absence of HIV infection – clinical presentation and management. QJM. 2014;108(6):481–88. doi: 10.1093/qjmed/hcu232. [DOI] [PubMed] [Google Scholar]
- 9.Sasaki Y, Isegawa T, Shimabukuro A, et al. Primary effusion lymphoma in an elderly HIV-negative patient with hemodialysis: Importance of evaluation for pleural effusion in patients receiving hemodialysis. Case Rep Nephrol Urol. 2014;4(2):95–102. doi: 10.1159/000363223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okada S, Goto H, Yotsumoto M. Current status of treatment for primary effusion lymphoma. Intractable Rare Dis Res. 2014;3(3):65–74. doi: 10.5582/irdr.2014.01010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alexanian S, Said J, Lones M, Pullarkat ST. KSHV/HHV8-negative effusion-based lymphoma, a distinct entity associated with fluid overload states. Am J Surg Pathol. 2013;37(2):241–49. doi: 10.1097/PAS.0b013e318267fabc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boulanger E, Gérard L, Gebarre J, et al. Prognostic factors and outcome of human herpesvirus 8-associated primary effusion lymphoma in patients with AIDS. J Clin Oncol. 2005;23(19):4372–80. doi: 10.1200/JCO.2005.07.084. [DOI] [PubMed] [Google Scholar]
- 13.Patel S, Xiao P. Primary effusion lymphoma. Arch Pathol Lab Med. 2013;137:1152–54. doi: 10.5858/arpa.2012-0294-RS. [DOI] [PubMed] [Google Scholar]
- 14.Chang VA, Wang H, Reid EG. Activity of brentuximab vedotin in AIDS-related primary effusion lymphoma. Blood Adv. 2019;3(5):766–68. doi: 10.1182/bloodadvances.2018026351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGovern BH, Golan Y, Lopez M, et al. The impact of cirrhosis on CD4+ T cell counts in HIV-seronegative patients. Clin Infect Dis. 2007;44(3):431–37. doi: 10.1086/509580. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez J, Romaguera JE, Katz RL, et al. Primary effusion lymphoma in an HIV-negative patient with no serologic evidence of Kaposi’s sarcoma virus. Leuk Lymphoma. 2001;41:185–89. doi: 10.3109/10428190109057969. [DOI] [PubMed] [Google Scholar]


