Abstract
Background
To investigate the efficacy of neoadjuvant chemotherapy (NCT), neoadjuvant endocrine therapy (NET) and neoadjuvant chemoendocrine therapy (NCET) on the tumour response, including pathological complete response (pCR) rate and overall response rate (ORR), in postmenopausal women with hormone receptor (HR)-positive breast cancer.
Methods
Based on a PRISMA-IPD statement, the PubMed, Embase and Cochrane Library databases were used to identify eligible trials published from inception to 7 May 2019. Pooled odds ratio (OR) with 95% confidential interval (CI) was calculated to assess the pCR rate and ORR of tumours among those three treatments via fixed- or random-effect Mantel-Haenszel models in terms of a Heterogeneity Chi2 test with a significant level of p < 0.1. All statistical tests were performed by the software of StataSE, version 12.0.
Results
The analysed data consisted of 10 eligible clinical trials with 971 unique HR-positive breast cancer patients. The pooled results indicated that the pCR rate of those patients undergoing NET was significantly lower than those undergoing NCT (pooled OR, 0.48; 95% CI, 0.26–0.90), whereas the difference of ORR between both therapies was not statistically significant (pooled OR, 1.05; 95% CI, 0.73–1.52). The combined paradigm of NCET compared with the monotherapy of NET or NCT did not present a significantly improved pCR rate or ORR (pooled OR, 2.61; 95% CI, 0.94–7.25; and 2.25; 95% CI, 0.39–13.05; respectively).
Conclusion
Postmenopausal HR-positive breast cancer patients after NCT may have better tumour response than those after NET, while those undergoing NCET may not manifest the apparently improved clinical efficacies compared to those receiving monotherapy.
Keywords: Neoadjuvant endocrine therapy, Neoadjuvant chemotherapy, Neoadjuvant chemoendocrine therapy, Tumour response, Breast cancer
Background
The initial clinical experience of neoadjuvant therapy in breast cancer was to use it in locally advanced, inoperable tumours, showing excellent tumour response and ameliorative local control [1], as well as equivalent overall survival (OS) and disease-free survival (DFS) compared with adjuvant therapy [2, 3]. Neoadjuvant therapy for hormone receptor (HR)-positive breast cancer includes neoadjuvant chemotherapy (NCT), neoadjuvant endocrinotherapy (NET) and neoadjuvant chemoendocrine therapy (NCET). After these treatments, unresectable tumours can be made amenable to mastectomy, and the initially operable ones in requirement of mastectomy may be operated on in order to avoid sacrificing the breast via lumpectomy, thereby greatly extending long-term survival and improving the quality of life.
The National Surgical Adjuvant Breast and Bowel Project (NSABP) B-18 study found a positive correlation between higher pathological complete response (pCR) rate and improved relapse-free survival in HR-positive breast cancer patients who underwent adjuvant or neoadjuvant chemotherapy [4]. Similarly, a multicenter phase II study by the Japan Breast Cancer Research Group (JBCRG) demonstrated that the achievement of pCR in HR-positive breast cancer women who received NCT predicted favourable DFS [5]. These results suggest that pCR, overall response rate (ORR) and other biomarkers can be used as valid surrogate endpoints to predict long-term survival in patients after neoadjuvant treatment.
Some studies collectively confirmed that the pCR rate of HR-positive breast tumours following NCT was lower than that of HR-negative breast cancers [5–7]. Moreover, the majority of breast carcinoma patients with HR-positive tumours are postmenopausal and elderly and, therefore, have difficulty tolerating the adverse effects of NCT. These phenomena predispose them to choose NET, which has lower toxicity and similar clinical efficacy compared with NCT [8, 9]. However, a poor clinical response rate to NET is shown in highly aggressive (Ki67 > 10%) HR-positive breast tumours when compared to NCT [9], suggesting that these two treatment strategies are not equally effective in certain cases.
NCET, which concurrently applies NET in combination with NCT has attained promising antitumour activity in animal models and human bodies with breast cancer, resulting in more shrinkage of tumour volume and reduction of tumour cell proliferation compared to NET [10–12]. Many clinical studies have investigated the ORR of both modalities in treating HR-positive breast tumours but have reported somewhat different findings [11, 13, 14]. Herein, we set out to investigate these three neoadjuvant therapy scenarios to determine which one achieved the greatest tumour response rate in treating postmenopausal HR-positive breast carcinoma. Four comparison groups were designed, including the pCR rate and ORR of NET vs NCT and those of NCET vs NET or NCT alone.
Methods
Search strategy
According to the PRISMA-IPD Statement [15], electronic searching was performed in databases including PubMed, Cochrane Library and Embase using the following retrieval strategy: ((neoadjuvant endocrine therapy) OR (neoadjuvant endocrine treatment) OR (neoadjuvant endocrinotherapy) OR (neoadjuvant chemotherapy) OR (neoadjuvant therapy) OR (neoadjuvant chemoendocrine therapy) OR (neoadjuvant chemo-endocrine therapy) OR (neoadjuvant chemoendocrine treatment) OR (neoadjuvant chemo-endocrine treatment)) AND ((breast neoplasms) OR (breast cancer) OR (breast tumour) OR (breast tumour) OR (breast carcinoma)) AND (post-menopausal OR postmenopausal) AND ((hormone receptor positive) OR (oestrogen receptor positive) OR (estrogen receptor positive) OR (hormone receptor rich) OR (oestrogen receptor rich) OR (estrogen receptor rich) OR Luminal) AND (pCR OR (pathological complete response) OR ORR OR (overall response) OR (clinical response) OR (tumour response) OR (tumour response)). No restrictions were required during retrieval. Citations were terminated searching as of 7 May 2019.
Inclusion criteria
English publication;
Retrospective or prospective clinical trials;
Postmenopausal women with HR-positive breast cancer;
Publication reported the comparison of tumour response rate between either NET with NCT or NCET with NET or NCT alone. Tumour response rate involved pCR rate and ORR. The definition of pCR was that no malignant tumour cells were pathologically detected at the original tumour site. ORR referred to the merger value of complete response rate and partial response rate that were assessed by solid tumours after neoadjuvant therapy. The tumour response was evaluated in light of Response Evaluation Criteria in Solid Tumours, version 1.1 (RECIST v1.1) or World Health Organization (WHO) assessment criteria.
Exclusion criteria
Trials published in non–English;
Article type: review, case report, study protocol and conference paper;
Other details that did not meet the inclusion criteria.
Two reviewers independently screened the titles and abstracts of citations and scrutinized the full text of all potential studies. Only the satisfactory ones were retained. If there were some inconsistencies, they were figured out by discussion.
Data abstraction
The following information was abstracted from included studies by two co-authors who utilized the software of Excel version 2016: first author, publication year, study duration, original nation, median age, median follow-up, detailed regimen of treatment, total sample size, the event number of pCR and clinical response after individual therapy. If some unconformities existed, they were resolved by the third co-author. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Statistical analysis
The comparisons of pCR and ORR between the study cohort and the control cohort were presented by the value of pooled odds ratio (OR). The crude ORs with 95% confidence intervals (CIs) in all included studies were independently calculated then pooled together. If the publication did not provide the number of events, the calculation was in terms of the endpoint percentage or other information. The heterogeneity among different trials was assessed by Heterogeneity Chi2 test with a significance level of p < 0.1 [16]. When the heterogeneity test appeared to indicate no statistical significance (p ≥ 0.1), a fixed-effect Mantel-Haenszel model was utilized to pool the data; if not, a random-effect Mantel-Haenszel model was used [16]. The publication bias was investigated by Egger’s test with a significance level of p < 0.05. The quality of all RCTs was evaluated in terms of the instrument presented by Jadad and colleagues (Additional file 1: Table S1, page 1) [17]. All statistical tests were analysed using the software StataSE version 12.0 (StataCorp LP, College Station, TX, USA).
Results
Search results
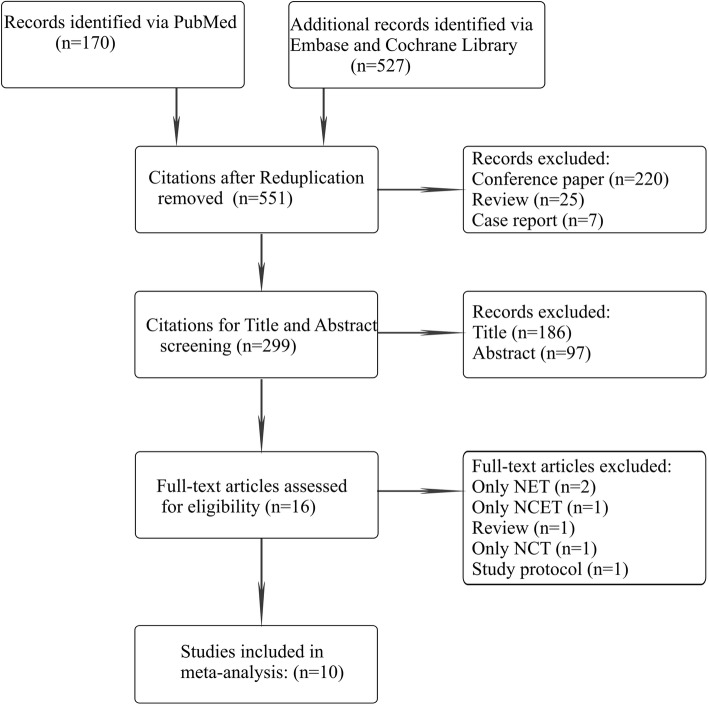
Following systematic retrieval, a total of 697 potential citations were identified. Citations that were duplicates (n = 146) or categorized as review (n = 25), case report (n = 7) and conference papers (n = 220) were excluded. The remaining 299 citations were entered into title and abstract screening, and 283 citations were removed. Therefore, sixteen studies were left for full-text scrutinizing, and 6 of them were omitted because of only NET (n = 2), only NCET (n = 1), only NCT (n = 1), review (n = 1) or study protocol (n = 1). Ultimately, ten eligible clinical trials with 971 unique HR-positive breast cancer patients met the inclusion criteria [8, 11, 13, 14, 18–23]. The PRISMA flow diagram of the inclusion process was outlined in Fig. 1.
Fig. 1.
The PRISMA flow diagram of the inclusion process. Abbreviations; NCET, neoadjuvant chemoendocrine therapy; NCT, neoadjuvant chemotherapy
Characteristics of eligible studies
Of the included trials, the publication year ranged from 2007 to 2018; four recorded the types of surgery after neoadjuvant treatment, in which only one assessed the nodes management; three reported the toxicity after treatments that focused on NCET vs NET or NCT monotherapy; the baseline clinical stages were mainly in T1/2 (n = 3), T3/4 (n = 3) and unknown (n = 4); the lengths of neoadjuvant treatment were different, ranging from 9 to 24 weeks; the HER2 status was negative (n = 4), or negative/positive (n = 5) or undescribed (n = 1), however, the number of patients with HER2-positive disease was scarce, with a total number of 29 (ranged from 2 to 18). Table 1 also represented other detailed characteristics of included studies. The treatment regimens for NET, NCT and NCET strategies were outlined in Table 2.
Table 1.
The details of included studies
| Characteristic | Studies, No. (%) (N = 10) | Postmenopausal HR+ Breast Cancer Patients, No. (%) (N = 971) |
|---|---|---|
| Study type | ||
| Randomized clinical trial | 7 (70.0) | 676 (69.6) |
| Retrospective | 2 (20.0) | 239 (24.6) |
| Prospective | 1 (10.0) | 56 (5.8) |
| Publication date, median (range), y | 2015 (2012–2018) | |
| Follow-up, median (range), moa | 48.1 (17.3–74) | |
| Mean age, median (range), ya | 60.9 (49–70) | |
| Original nation | ||
| the United States | 3 (30.0) | 460 (47.4) |
| Korea | 1 (10.0) | 25 (2.6) |
| Japan | 3 (30.0) | 141 (14.5) |
| the United Kingdom | 1 (10.0) | 44 (4.5) |
| Russia | 1 (10.0) | 239 (24.6) |
| Iran | 1 (10.0) | 62 (6.4) |
| Assessment of type of surgery | ||
| Yes | 4 (40.0) | 378 (38.9) |
| No | 6 (60.0) | 593 (61.1) |
| Nodes management | ||
| Yes | 1 (10.0) | 221 (22.8) |
| No | 9 (90.0) | 750 (77.2) |
| Toxicity | ||
| NET vs NCT | 0 (0.0) | 0 (0.0) |
| NECT vs NET | 2 (20.0) | 113 (11.6) |
| NECT vs NCT | 1 (10.0) | 62 (6.4) |
| Not assessed | 7 (70.0) | 796 (82.0) |
| Assessment criteria of tumour response | ||
| RECIST v1.1 | 5 (50.0) | 244 (25.1) |
| WHO | 1 (10.0) | 239 (24.6) |
| Not assessed | 4 (40.0) | 488 (50.3) |
| Mainly baseline clinical stage | ||
| T1/2 | 3 (30.0) | 183 (18.8) |
| T3/4 | 3 (30.0) | 441 (45.4) |
| Not assessed | 4 (40.0) | 347 (35.7) |
| Length of neoadjuvant treatment | ||
| 9–18 ws | 4 (40.0) | 547 (56.3) |
| 18–23 ws | 1 (10.0) | 44 (4.5) |
| 24 ws | 3 (30.0) | 141 (14.5) |
| Not assessed | 2 (20.0) | 239 (24.6) |
| HER2 status | ||
| Negative | 4 (40.0) | 352 (36.3) |
| Negative/Positive | 5 (50.0) | 351/29 (36.1/3.0)b |
| Not assessed | 1 (10.0) | 239 (24.6) |
| Research contents | ||
| NET vs NCT | 6 (60.0) | 768 (79.1) |
| NECT vs monotherapy | 4 (40.0) | 203 (20.9) |
Abbreviations: NET Neoadjuvant endocrine therapy, NCT Neoadjuvant chemotherapy, NECT Neoadjuvant chemoendocrine therapy, RECIST v1.1 Response Evaluation Criteria in Solid Tumours, version 1.1, WHO World Health Organization
aThe median value is calculated by available data
bThese data before and after the semicolon respectively represent the number of HER2-positive disease and HER2-negative disease patients
Table 2.
The treatment regimen of neoadjuvant therapy
| Study | Year | NET | NCT | NCET |
|---|---|---|---|---|
| Chae [24] | 2016 | Letrozole qd | FEC, a switch to docetaxel if PD or SD | |
| Wright [25] | 2015 | AIs or tamoxifen qd | PAT or AT | |
| Palmieri [26] | 2014 | Letrozole qd | FE100C or FE75C, a switch to docetaxel if PD or SD | |
| Semiglazov [27] | 2007 | Exemestane or anastrozole qd | Doxorubicin + paclitaxel | |
| Marcus [28] | 2013 | AIs or tamoxifen qd | Anthracycline-based or non-anthracycline-based | |
| Ellis [29] | 2017 | AIs qda | Anthracycline-based or non-anthracycline-based | |
| Nakayama [30] | 2018 | Anastrozole qd | Anastrozole qd + UFT | |
| Sato [31] | 2018 | Exemestane qd | Exemestane qd + cyclophosphamide | |
| Sugiu [32] | 2015 | FEC-T | Exemestane qd + EFC-T | |
| Mohammad [33] | 2012 | FAC | Letrozole qd + FAC |
Explanation of regimen: FEC, 5-fluorouracil, epirubicin, and cyclophosphamide; FE100C, 5-fluorouracil 500 mg/m2, cyclophosphamide 500 mg/m2, epirubicin 100 mg/m2; FE75C, 5-fluorouracil 600 mg/m2, cyclophosphamide 600 mg/m2, epirubicin 75 mg/m2; UFT, tegafur/uracil combination in 1:4 M ratio; 270 mg/m2/day in two divided doses; FEC-T, 80 mg/m2 of paclitaxel followed by a combination of fluorouracil 500 mg/m2, epirubicin 100 mg/m2 and cyclophosphamide 500 mg/m2; FAC, 5-Fluorouracil 600 mg/m2, doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2
Abbreviations: NET Neoadjuvant endocrine therapy, NCT Neoadjuvant chemotherapy, NCET Neoadjuvant chemoendocrine therapy, AIs Aromatase inhibitors, PD Progressive disease, SD Stable disease
aThese aromatase inhibitors include letrozole, anastrozole and exemestane
NET vs NCT
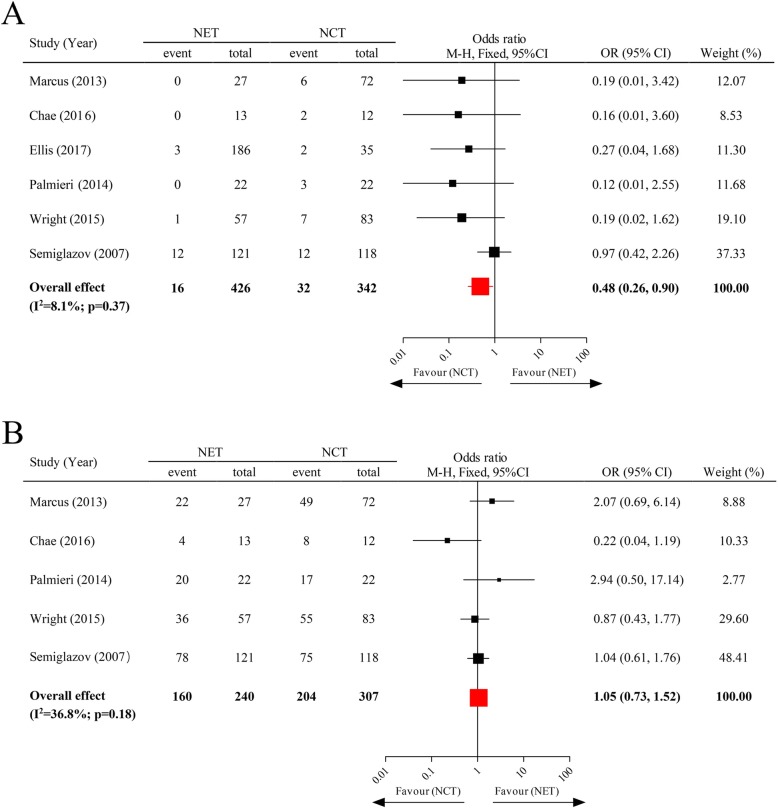
Six clinical trials were involved in analysing the pCR of NET vs NCT, with a total number of 768 subjects. Of these, the American College of Surgeons Oncology Group (ACOSOG) Z1031 trial [20] did not investigate the ORR of breast tumour after neoadjuvant therapy, thereby giving rise to only 5 articles for analysis of the ORR of NET vs NCT. The pooled results indicated that the pCR rate of postmenopausal, HR-positive breast tumour patients after receiving NET was significantly lower than those that underwent NCT (pooled OR = 0.48; 95% CI, 0.26–0.90), whereas no significant difference of ORR existed between those that underwent these two treatment paradigms (pooled OR = 1.05; 95% CI, 0.73–1.52) (Fig. 2).
Fig. 2.
The pCR rate and ORR of neoadjuvant endocrine therapy vs neoadjuvant chemotherapy. Abbreviations: NET, neoadjuvant endocrine therapy; NCT, neoadjuvant chemotherapy
NCET vs Monotherapy of NET or NCT
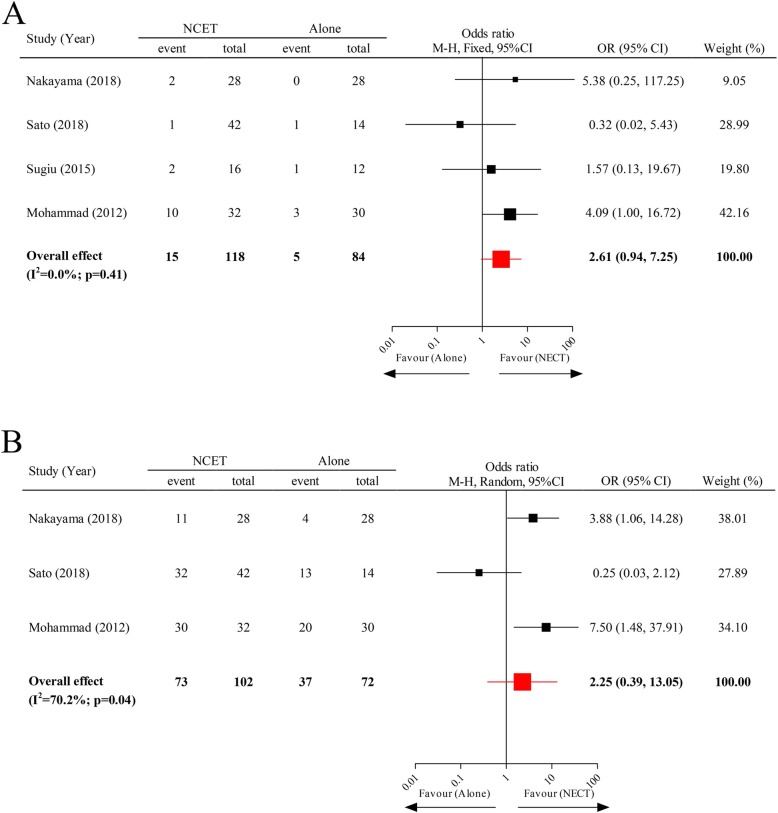
To investigate this comparison, three RCTs and one retrospective study (total sample size of 203) were ultimately collected. Irrespective of HR-positive breast cancer patients receiving combined treatment of NCET or monotherapy of NET or NCT, the differences in pCR rate and ORR between them were not statistically significant (pooled OR = 2.61; 95% CI, 0.94–7.25; and 2.25; 95% CI, 0.39–13.05; respectively) (Fig. 3).
Fig. 3.
The pCR rate and ORR of neoadjuvant endocrine therapy vs neoadjuvant chemoendocrine therapy. Abbreviations: NET, neoadjuvant endocrine therapy; NCET, neoadjuvant chemoendocrine therapy
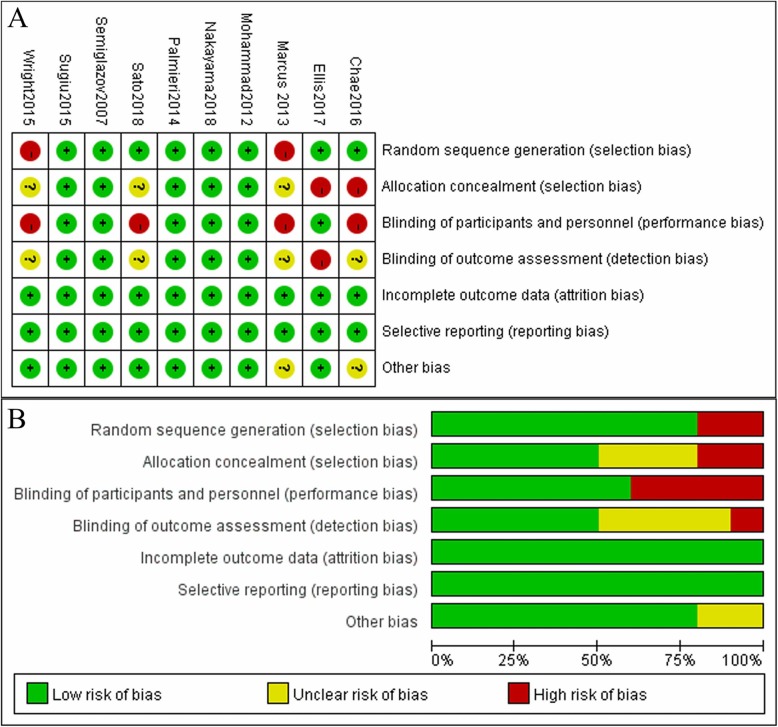
Risk of bias in included studies
Figure 4A and B showed the judgements of risk of bias summary and risk of bias graph, respectively, of the analysed studies for each “Risk of bias” domain. The detailed risk of bias assessments was provided in Additional file 1: Table S2, page 2.
Fig. 4.
The judgements of risk of bias summary and risk of bias graph
Heterogeneity and publication bias
Most of the studies in this meta-analysis did not appear to contain heterogeneity, as their Heterogeneity Chi2 tests were not statistically significant (p > 0.1); thus, the fixed-effect model was adopted to calculate the value of pooled OR. There was an exception, which was the evaluation of the ORR between NCET with NET or NCT alone, in which the value of that was computed by the random-effect model. Whilst its publication bias was not significant according to the results of Egger’s test (p = 0.452) (Additional file 1: Table S3, page 3).
Discussion
The popularity of neoadjuvant therapy in the treatment of breast cancer greatly increases the proportion of breast-conserving surgeries, prolongs the long-term survival of patients and improves their quality of life. With an increasing body of clinical studies proving that endocrine therapy drugs, such as tamoxifen and, subsequently, emergent fulvestrant and aromatase inhibitors (AIs), have preeminent curative effects on HR-positive breast cancers, a consensus has been reached that endocrinotherapy is preferred to chemotherapy for such patients because the former treatment is less toxic and easier tolerated than the latter one. Otherwise, for patients with endocrine drug-resistance, rapid tumour progression or visceral crisis NCT, which has high efficiency in tumour regression, is selected because NET takes effect slowly, commonly needing 3–4 months to achieve observable tumour shrinkage [34]. However, it is not recommended to integrate chemotherapy into endocrine therapy to treat HR-positive breast tumours. Our results demonstrated that postmenopausal HR-positive breast cancer patients undergoing NCT were prone to obtain pCR compared to those that received NET, but there was no difference in ORR between the two treatment paradigms, and NCET was not more efficient than NET or NCT alone in achieving pCR or tumour clinical response.
Ki67 is the most commonly used biomarker in endocrine therapy trials and the tool to differentiate HR-positive breast cancer into Luminal A and Luminal B molecular subtype diseases, in parallel, which can also be utilized to predict tumor response and long-term outcomes after endocrine therapy in neoadjuvant setting [20, 35, 36]. The ACOSOG Z1031A trial [20] registered 245 postmenopausal HR-positive breast cancer patients who accepted the breast biopsy after 2 weeks of NET to ascertain Ki67 index. Of those patients, 35 switched to 4 weeks of NCT because of Ki67>10% who experienced high a pCR rate of 5.7% and were more likely to undergo mastectomy and axillary lymph node dissection than those patients continuously received 4 weeks of NET, indicating that chemotherapy efficacy was lower than expected in HR-positive breast cancers resistant to NET. Nevertheless, a randomized controlled trial of Palmieri et al. who did not distinguish HR-positive breast tumour patients in light of the Ki67 index found a similar breast-conserving surgery rate between those patients undergoing NET and NCT [21]. Overall, the biomarker Ki67 may impact on the decision of extent of breast surgery.
Earlier studies reported the promising prognosis in HR-positive breast cancer patients who underwent endocrine therapy with tamoxifen [37–39]. Currently, fulvestrant is the only and so-called “pure anti-oestrogen” oestrogen receptor downregulator without any agonist effects. In advanced HR-positive breast tumour patients, fulvestrant is often used in combination with a cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitor, which can significantly prolong progression-free survival compared to fulvestrant alone (9.2 months vs 3.5 months; P = 0.007) [40]; in patients sensitive to endocrine therapy, this combination therapy also brings about longer OS than fulvestrant monotherapy (39.7 months vs 29.7 months; Hazard ratio, 0.92; 95% CI, 0.55–0.94) [41]. In postmenopausal women, the most oestrogen is produced via androstenedione synthesized from adrenal gland enters into peripheral adipose tissue that is converted to oestrogen by 17-hydroxysteroid dehydrogenase [42]. AIs can inhibit the activity of 17-hydroxysteroid dehydrogenase and result in preventing the conversion of androstenedione to oestrogen. A large number of clinical studies indicated that the usage of the third generation AIs in postmenopausal HR-positive breast cancer patients outperformed tamoxifen in favourable long-term survival, improved tumour response and increased the breast-conserving surgery rate [43–47].
Although there have been a large number of studies concluding the increased clinical response rate of tumours in the combined strategy of chemotherapy and endocrinotherapy compared to chemotherapy alone to treat advanced HR-positive breast tumours [48–50], there are a paucity of clinical trials comparing the clinical utility of the combination to endocrinotherapy or chemotherapy in a neoadjuvant setting. Of these, most indicate no differences of pCR rate and ORR between postmenopausal HR-positive breast cancer women who undergo NCET and monotherapy of NET or NCT, which is consistent with our results. One probable explanation is that endocrine therapy works through a cytostatic mechanism to prevent cell division and downsize tumour volume; in contrast, chemotherapeutic medicines will be more effective when the cycling of cells in patients is more rapid [51]. Consequently, in theory, the coexistence of chemotherapy and endocrine agents in NCET may give rise to an antagonistic effect that does not reinforce the efficacy of this combined therapy. This conclusion is consolidated by a systematic review of 20 studies with 3490 unique patients that NET as monotherapy manifested a similar clinical response rate but significantly attenuated toxicity when compared with NCET [52].
Justifiably, the results of some clinical trials showed that the safety of NCET was less than that of NET alone [11, 14], especially in high-grade (Grade ≥ 3) adverse events (AEs) including increased aspartate transaminase, increased alanine aminotransferase, increased γ-glutamyl transpeptidase, back pain, and hypertriglyceridemia, as well as arthralgia but without treatment-related death. However, compared with NCT arm, the high-grade AEs in NCET arm was no significant difference [13].
Admittedly, this meta-analysis had some limitations. First, the inclusion criteria stipulated that only English publications were eligible, which might lead to selection bias and publication bias; undeniably, there was publication bias in comparing the pCR rate between NET and NCT (Additional file 1: Table S2, page 2). Second, since few clinical trials evaluated the comparison of tumour response rate between postmenopausal HR-positive breast tumours after combination of NCET with monotherapy of NET or NCT, only 4 valid studies were involved to analyse this comparison, and considerable heterogeneity existed in the meta-analysis comparing ORR between both paradigms, which might cause resultant bias. Finally, there might be clinical heterogeneity by virtue of the differences in HER2 status of patients, assessment criteria for tumour response, and treatment regimen in analysed studies.
This is the first study that systematically investigates the tumour response rate in postmenopausal HR-positive breast tumours after NET, NCT or NCET, finding some clinically promising outcomes. Our results further consolidate the consensus that NET is the preferred alternative for the treatment of these patients, with the exception of those that needs rapid reduction of tumour volume or who are resistant to endocrinotherapy.
Conclusion
Postmenopausal HR-positive breast cancer patients may benefit more tumour response from NCT than NET, but may be devoid of the improved prognostic outcomes from NCET when compared to NET or NCT alone.
Supplementary information
Additional file 1: Table S1. Study quality of eligible randomized controlled trials. Table S2. The detailed risk of bias assessments. Table S3. The publication bias by Egger’s test.
Acknowledgements
Not applicable.
Abbreviations
- ACOSOG
American College of Surgeons Oncology Group
- AEs
Adverse events
- AIs
Aromatase inhibitors
- CDK4/6
Cyclin-dependent kinase 4 and 6
- CIs
Confidence intervals
- DFS
Disease-free survival
- HER2
Human epidermal growth factor receptor 2
- HR
Hormone receptor
- NCET
Neoadjuvant chemoendocrine therapy
- NCT
Neoadjuvant chemotherapy
- NET
Neoadjuvant endocrinotherapy
- NSABP
National Surgical Adjuvant Breast and Bowel Project
- OR
Odds ratio
- ORR
Overall response rate
- OS
Overall survival
- pCR
Pathological complete response
Authors’ contributions
YW: Writing manuscript; Charting all figures; Drawing the Tables. LH: Writing manuscript; Article selection; Data abstract. YS: Article selection; Data abstract. QW: Article selection; Data abstract. HW: Article selection; Data abstract. BZ: Article selection; Data abstract. XM: Conception/Design; Final approval of manuscript. All authors reviewed and approved the manuscript prior to submission.
Funding
No funding.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yaling Wang and Lin He contributed equally to this work.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12905-020-0879-y.
References
- 1.Hortobagyi GN, Ames FC, Buzdar AU, et al. Management of stage III primary breast cancer with primary chemotherapy, surgery, and radiation therapy. Cancer. 1988;62(12):2507–2516. doi: 10.1002/1097-0142(19881215)62:12<2507::AID-CNCR2820621210>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 2.Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and bowel project B-18. J Natl Cancer Inst Monogr. 2001;30:96–102. doi: 10.1093/oxfordjournals.jncimonographs.a003469. [DOI] [PubMed] [Google Scholar]
- 3.Mauri D, Pavlidis N, Ioannidis JP. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst. 2005;97(3):188–194. doi: 10.1093/jnci/dji021. [DOI] [PubMed] [Google Scholar]
- 4.Fisher B, Brown A, Mamounas E, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and bowel project B-18. J Clin Oncol. 1997;15(7):2483–2493. doi: 10.1200/JCO.1997.15.7.2483. [DOI] [PubMed] [Google Scholar]
- 5.Toi M, Nakamura S, Kuroi K, et al. Phase II study of preoperative sequential FEC and docetaxel predicts of pathological response and disease free survival. Breast Cancer Res Treat. 2008;110(3):531–539. doi: 10.1007/s10549-007-9744-z. [DOI] [PubMed] [Google Scholar]
- 6.Bear HD, Anderson S, Brown A, et al. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and bowel project protocol B-27. J Clin Oncol. 2003;21(22):4165–4174. doi: 10.1200/JCO.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Bear HD, Anderson S, Smith RE, et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National Surgical Adjuvant Breast and bowel project protocol B-27. J Clin Oncol. 2006;24(13):2019–2027. doi: 10.1200/JCO.2005.04.1665. [DOI] [PubMed] [Google Scholar]
- 8.Semiglazov VF, Semiglazov VV, Dashyan GA, et al. Phase 2 randomized trial of primary endocrine therapy versus chemotherapy in postmenopausal patients with estrogen receptor-positive breast cancer. Cancer. 2007;110(2):244–254. doi: 10.1002/cncr.22789. [DOI] [PubMed] [Google Scholar]
- 9.Alba E, Calvo L, Albanell J, et al. Chemotherapy (CT) and hormonotherapy (HT) as neoadjuvant treatment in luminal breast cancer patients: results from the GEICAM/2006-03, a multicenter, randomized, phase-II study. Ann Oncol. 2012;23(12):3069–3074. doi: 10.1093/annonc/mds132. [DOI] [PubMed] [Google Scholar]
- 10.Nukatsuka M, Saito H, Nakagawa F, et al. Oral fluoropyrimidine may augment the efficacy of aromatase inhibitor via the down-regulation of estrogen receptor in estrogen-responsive breast cancer xenografts. Breast Cancer Res Treat. 2011;128(2):381–390. doi: 10.1007/s10549-010-1141-3. [DOI] [PubMed] [Google Scholar]
- 11.Nakayama T, Sagara Y, Takashima T, et al. Randomized phase II study of anastrozole plus tegafur-uracil as neoadjuvant therapy for ER-positive breast cancer in postmenopausal Japanese women (neo-ACET BC) Cancer Chemother Pharmacol. 2018;81(4):755–762. doi: 10.1007/s00280-018-3544-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bottini A, Generali D, Brizzi MP, et al. Randomized phase II trial of letrozole and letrozole plus low-dose metronomic oral cyclophosphamide as primary systemic treatment in elderly breast cancer patients. J Clin Oncol. 2006;24(22):3623–3628. doi: 10.1200/JCO.2005.04.5773. [DOI] [PubMed] [Google Scholar]
- 13.Mohammadianpanah M, Ashouri Y, Hoseini S, et al. The efficacy and safety of neoadjuvant chemotherapy +/− letrozole in postmenopausal women with locally advanced breast cancer: a randomized phase III clinical trial. Breast Cancer Res Treat. 2012;132(3):853–861. doi: 10.1007/s10549-011-1814-6. [DOI] [PubMed] [Google Scholar]
- 14.Sato N, Masuda N, Morimoto T, Ueno T. Neoadjuvant endocrine therapy with exemestane followed by response-guided combination therapy with low-dose cyclophosphamide in postmenopausal patients with estrogen receptor-positive breast cancer: a multicenter, open-label, phase II study. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stewart LA, Clarke M, Rovers M, et al. Preferred reporting items for systematic review and meta-analyses of individual participant data: the PRISMA-IPD statement. Jama. 2015;313(16):1657–1665. doi: 10.1001/jama.2015.3656. [DOI] [PubMed] [Google Scholar]
- 16.Sutton AJAK, Jones DR. Methods for metaanalysis in medical research. Wiley, Hoboken: Wiley series in probability and statistics-applied probability and statistics section; 2000. [Google Scholar]
- 17.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 18.Marcus DM, Switchenko JM, Prabhu R, et al. Neoadjuvant hormonal therapy is associated with comparable outcomes to Neoadjuvant chemotherapy in post-menopausal women with estrogen receptor-positive breast Cancer. Front Oncol. 2013;3:317. doi: 10.3389/fonc.2013.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chae SY, Kim SB, Ahn SH, et al. A randomized feasibility study of (18)F-Fluoroestradiol PET to predict pathologic response to Neoadjuvant therapy in estrogen receptor-rich postmenopausal breast Cancer. J Nucl Med. 2017;58(4):563–568. doi: 10.2967/jnumed.116.178368. [DOI] [PubMed] [Google Scholar]
- 20.Ellis MJ, Suman VJ, Hoog J, et al. Ki67 proliferation index as a tool for chemotherapy decisions during and after Neoadjuvant aromatase inhibitor treatment of breast Cancer: results from the American College of Surgeons oncology group Z1031 trial (Alliance) J Clin Oncol. 2017;35(10):1061–1069. doi: 10.1200/JCO.2016.69.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palmieri C, Cleator S, Kilburn LS, et al. NEOCENT: a randomised feasibility and translational study comparing neoadjuvant endocrine therapy with chemotherapy in ER-rich postmenopausal primary breast cancer. Breast Cancer Res Treat. 2014;148(3):581–590. doi: 10.1007/s10549-014-3183-4. [DOI] [PubMed] [Google Scholar]
- 22.Wright JL, Saigal K, Reis IM, et al. Locoregional and overall recurrence after Neaodjuvant endocrine therapy versus chemotherapy in postmenopausal women with estrogen receptor+ HER2- breast Cancer. Am J Clin Oncol. 2017;40(5):490–497. doi: 10.1097/COC.0000000000000194. [DOI] [PubMed] [Google Scholar]
- 23.Sugiu K, Iwamoto T, Kelly CM, et al. Neoadjuvant chemotherapy with or without concurrent hormone therapy in estrogen receptor-positive breast Cancer: NACED-randomized multicenter phase II trial. Acta Med Okayama. 2015;69(5):291–299. doi: 10.18926/AMO/53675. [DOI] [PubMed] [Google Scholar]
- 24.Chae SY, et al. A randomized feasibility study of (18)F-Fluoroestradiol PET to predict pathologic response to Neoadjuvant therapy in estrogen receptor-rich postmenopausal breast Cancer. J Nucl Med. 2017;58:563–568. doi: 10.2967/jnumed.116.178368. [DOI] [PubMed] [Google Scholar]
- 25.Wright JL, et al. Locoregional and overall recurrence after Neaodjuvant endocrine therapy versus chemotherapy in postmenopausal women with estrogen receptor+ HER2- breast Cancer. Am J Clin Oncol. 2017;40:490–497. doi: 10.1097/coc.0000000000000194. [DOI] [PubMed] [Google Scholar]
- 26.Palmieri C, et al. NEOCENT: a randomised feasibility and translational study comparing neoadjuvant endocrine therapy with chemotherapy in ER-rich postmenopausal primary breast cancer. Breast Cancer Res Treat. 2014;148:581–590. doi: 10.1007/s10549-014-3183-4. [DOI] [PubMed] [Google Scholar]
- 27.Semiglazov VF, et al. Phase 2 randomized trial of primary endocrine therapy versus chemotherapy in postmenopausal patients with estrogen receptor-positive breast cancer. Cancer. 2007;110:244–254. doi: 10.1002/cncr.22789. [DOI] [PubMed] [Google Scholar]
- 28.Marcus DM, et al. Neoadjuvant hormonal therapy is associated with comparable outcomes to Neoadjuvant chemotherapy in post-menopausal women with estrogen receptor-positive breast Cancer. Front Oncol. 2013;3:317. doi: 10.3389/fonc.2013.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ellis MJ, et al. Ki67 proliferation index as a tool for chemotherapy decisions during and after Neoadjuvant aromatase inhibitor treatment of breast Cancer: results from the American College of Surgeons oncology group Z1031 trial (Alliance) J Clin Oncol. 2017;35:1061–1069. doi: 10.1200/jco.2016.69.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakayama T, et al. Randomized phase II study of anastrozole plus tegafur-uracil as neoadjuvant therapy for ER-positive breast cancer in postmenopausal Japanese women (neo-ACET BC) Cancer Chemother Pharmacol. 2018;81:755–762. doi: 10.1007/s00280-018-3544-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sato N, Masuda N, Morimoto T, Ueno T. Neoadjuvant endocrine therapy with exemestane followed by response-guided combination therapy with low-dose cyclophosphamide in postmenopausal patients with estrogen receptor-positive breast cancer: a multicenter, open-label, phase II study. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sugiu K, et al. Neoadjuvant chemotherapy with or without concurrent hormone therapy in estrogen receptor-positive breast Cancer: NACED-randomized multicenter phase II trial. Acta Med Okayama. 2015;69:291–299. doi: 10.18926/AMO/53675. [DOI] [PubMed] [Google Scholar]
- 33.Mohammadianpanah M, et al. The efficacy and safety of neoadjuvant chemotherapy +/− letrozole in postmenopausal women with locally advanced breast cancer: a randomized phase III clinical trial. Breast Cancer Res Treat. 2012;132:853–861. doi: 10.1007/s10549-011-1814-6. [DOI] [PubMed] [Google Scholar]
- 34.Dixon JM, Anderson TJ, Miller WR. Neoadjuvant endocrine therapy of breast cancer: a surgical perspective. Eur J Cancer. 2002;38(17):2214–2221. doi: 10.1016/S0959-8049(02)00265-4. [DOI] [PubMed] [Google Scholar]
- 35.Dowsett M, Smith I, Robertson J, et al. Endocrine therapy, new biologicals, and new study designs for presurgical studies in breast cancer. J Natl Cancer Inst Monogr. 2011;2011(43):120–123. doi: 10.1093/jncimonographs/lgr034. [DOI] [PubMed] [Google Scholar]
- 36.Dowsett M, Smith IE, Ebbs SR, et al. Short-term changes in Ki-67 during neoadjuvant treatment of primary breast cancer with anastrozole or tamoxifen alone or combined correlate with recurrence-free survival. Clin Cancer Res. 2005;11(2 Pt 2):951s–958s. [PubMed] [Google Scholar]
- 37.Preece PE, Wood RA, Mackie CR, Cuschieri A. Tamoxifen as initial sole treatment of localised breast cancer in elderly women: a pilot study. Br Med J (Clin Res Ed) 1982;284(6319):869–870. doi: 10.1136/bmj.284.6319.869-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Helleberg A, Lundgren B, Norin T, Sander S. Treatment of early localized breast cancer in elderly patients by Tamoxifen. Br J Radiol. 1982;55(655):511–515. doi: 10.1259/0007-1285-55-655-511. [DOI] [PubMed] [Google Scholar]
- 39.Bradbeer JW, Kyngdon J. Primary treatment of breast cancer in elderly women with Tamoxifen. Clin Oncol. 1983;9(1):31–34. [PubMed] [Google Scholar]
- 40.Iwata H, Im SA, Masuda N, et al. PALOMA-3: phase III trial of Fulvestrant with or without Palbociclib in premenopausal and postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast Cancer that progressed on prior endocrine therapy-safety and efficacy in Asian patients. J Glo Oncol. 2017;3(4):289–303. doi: 10.1200/JGO.2016.008318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turner NC, Slamon DJ, Ro J, et al. Overall survival with Palbociclib and Fulvestrant in advanced breast Cancer. N Engl J Med. 2018;379(20):1926–1936. doi: 10.1056/NEJMoa1810527. [DOI] [PubMed] [Google Scholar]
- 42.Judd HL, Judd GE, Lucas WE, Yen SS. Endocrine function of the postmenopausal ovary: concentration of androgens and estrogens in ovarian and peripheral vein blood. J Clin Endocrinol Metab. 1974;39(6):1020–1024. doi: 10.1210/jcem-39-6-1020. [DOI] [PubMed] [Google Scholar]
- 43.Mouridsen H, Gershanovich M, Sun Y, et al. Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: analysis of survival and update of efficacy from the international Letrozole breast Cancer group. J Clin Oncol. 2003;21(11):2101–2109. doi: 10.1200/JCO.2003.04.194. [DOI] [PubMed] [Google Scholar]
- 44.Ellis MJ, Coop A, Singh B, et al. Letrozole is more effective neoadjuvant endocrine therapy than tamoxifen for ErbB-1- and/or ErbB-2-positive, estrogen receptor-positive primary breast cancer: evidence from a phase III randomized trial. J Clin Oncol. 2001;19(18):3808–3816. doi: 10.1200/JCO.2001.19.18.3808. [DOI] [PubMed] [Google Scholar]
- 45.Bonneterre J, Buzdar A, Nabholtz JM, et al. Anastrozole is superior to tamoxifen as first-line therapy in hormone receptor positive advanced breast carcinoma. Cancer. 2001;92(9):2247–2258. doi: 10.1002/1097-0142(20011101)92:9<2247::AID-CNCR1570>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 46.Tubiana-Hulin MSF, Becette V. San Antonio Breast Cancer Symposium. 2003. Phase II study of neo-adjuvant exemestane in postmenopuasal patients with operable breast cancer. [Google Scholar]
- 47.Semiglazov VKA, Semiglazov V, et al. Exemestane vs tamoxifen as neoadjuvant endocrine therapy for postmenopausal women with ER+ breast cancer (T2N1–2, T3N0–1,T4N0M0). Orlando: In ASCO annual meeting; 2005. [Google Scholar]
- 48.Brunner KW, Sonntag RW, Alberto P, et al. Combined chemo- and hormonal therapy in advanced breast cancer. Cancer. 1977;39(6 Suppl):2923–2933. doi: 10.1002/1097-0142(197706)39:6<2923::AID-CNCR2820390679>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 49.Cocconi G, De Lisi V, Boni C, et al. Chemotherapy versus combination of chemotherapy and endocrine therapy in advanced breast cancer. A prospective randomized study. Cancer. 1983;51(4):581–588. doi: 10.1002/1097-0142(19830215)51:4<581::AID-CNCR2820510404>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 50.Mouridsen HT, Rose C, Engelsmann E, Sylvester R, Rotmensz N. Combined cytotoxic and endocrine therapy in postmenopausal patients with advanced breast cancer. A randomized EORTC study of CMF vs CMF + tamoxifen. J Steroid Biochem. 1985;23(6b):1141–1146. doi: 10.1016/0022-4731(85)90033-0. [DOI] [PubMed] [Google Scholar]
- 51.Pritchard KI. Combining endocrine agents with chemotherapy: which patients and what sequence? Cancer. 2008;112(3 Suppl):718–722. doi: 10.1002/cncr.23189. [DOI] [PubMed] [Google Scholar]
- 52.Spring LM, Gupta A, Reynolds KL, et al. Neoadjuvant endocrine therapy for estrogen receptor-positive breast Cancer: a systematic review and meta-analysis. JAMA Oncology. 2016;2(11):1477–1486. doi: 10.1001/jamaoncol.2016.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Study quality of eligible randomized controlled trials. Table S2. The detailed risk of bias assessments. Table S3. The publication bias by Egger’s test.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.