Abstract
Background
Patients with cancer admitted for an acute coronary syndrome (ACS) and/or percutaneous coronary intervention (PCI) represent a growing and high-risk population. The influence of co-existing cancer on mortality remains unclear in such patients. We aimed to assess the impact of cancer on early and late, all-cause and cardiac mortality in the setting of ACS and/or PCI.
Methods
We performed a systematic review and meta-analysis of studies comparing outcomes of patients with and without a history of cancer admitted for ACS and/or PCI.
Results
Six studies including 294,528 ACS patients and three studies including 39,973 PCI patients were selected for our meta-analysis. Patients with cancer had increased rates of in-hospital all-cause death (RR 1.74 [1.22; 2.47]), cardiac death (RR 2.44 [1.73; 3.44]) and bleeding (RR 1.64 [1.35; 1.98]) as well as one-year all-cause death (RR 2.62 [1.2; 5.73]) and cardiac death (RR 1.89 [1.25; 2.86]) in ACS studies. Rates of long term all-cause (RR 1.96 [1.52; 2.53]) but not cardiac death were higher in cancer patients admitted for PCI.
Conclusion
Cancer patients represent a high-risk population both in the acute phase and at long-term after an ACS or PCI. The magnitude of the risk of mortality should however be tempered by the heterogeneity among studies. Early and long term optimal management of such patients should be promoted in clinical practice.
Keywords: Cancer, Prognosis, Acute coronary syndrome, Percutaneous coronary intervention
Introduction
Coronary artery disease and cancer are the leading causes of mortality worldwide [1]. Cancer-related mortality has declined over the past decades due to earlier detection and advances in treatment [2, 3]. Consequently, patients with a history of cancer represent a growing population in general.
Patients with coronary artery disease and cancer often share common risk factors such as advanced age, sedentary lifestyle and smoking [4]. Anticancer therapies such as radiotherapy [5, 6] or drugs [7] are associated with an increased risk of coronary disease including myocardial infarction. Therefore, rates of history of cancer in patients admitted for an acute coronary syndrome (ACS) or elective percutaneous coronary intervention (PCI) are increasing in clinical practice but the data about the impact of a history of cancer on all-cause and more specifically cardiac mortality remain limited.
The aim of this study was to assess the in-hospital and long-term mortality among patients with and without a history of cancer using a systematic review and meta-analytic approach of published data in the setting of ACS and/or PCI.
Methods
Search strategy and studies’ selection
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines for the systematic review and meta-analysis. We conducted a systematic literature review by formal searches of the electronic databases MEDLINE (source PubMed) and the Cochrane Controlled Clinical Trials Register Database through September 2018. Relevant trials were identified by a combination of medical subject headings including the following terms: acute coronary syndrome, myocardial infarction, acute myocardial infarction, non-ST elevation myocardial infarction, ST elevation myocardial infarction, cancer, neoplasm, mortality, outcomes and prognosis. References from reviews and selected articles were also reviewed for potential relevant citations. Studies were selected by 2 independent reviewers (VR and LV).
We restricted our analysis to the trials that met all of the following inclusion criteria: (1) comparison between patients with history of cancer, active or not (cancer group) and non-cancer patients; (2) patients admitted for an ACS and/or PCI; (3) available data on mortality. We excluded studies with no comparison between cancer and non-cancer patients.
Outcomes
The primary outcomes assessed by the study were all-cause and cardiac in-hospital mortality. One study [8] reported 30-day mortality which was considered as early hence gathered with in-hospital mortality in the analysis. In-hospital bleeding, as defined in each study, was also included in the analysis. Long-term mortality was based on the longest follow-up available for each study.
Assessment of risk of bias
We used the Newcastle-Ottawa Scale for assessment of risk of bias. This scale assesses risk of bias in the following 3 domains: selection of the study groups, comparability of groups, and ascertainment of exposure. Studies with scores of less than 4 were considered to have a high risk of bias, those with scores of 4 to 6 an intermediate risk of bias, and those with scores of 7 or more a low risk of bias.
Statistical analysis
The total numbers of patients experiencing or not the outcomes of interest in each arm extracted directly from the publications were used for the analyses. Results are presented as relative risks (RR) with 95% confidence intervals (CI). Outcomes from individual studies were combined using the Mantel–Haenzel fixed and random-effect models. Heterogeneity across studies was studied by the Cochran’s Q statistic with a p value set at 0.1. The I2 was also taken into account regardless of the p value. An I2 of ≥50% was the pre-specified threshold considered too high to provide consistent analysis. The random-effect model was considered for the primary analysis. A fixed effect-model was also reported in figures, considered as a sensitivity analysis only. Tests were two-tailed and a p-value < 0.05 was considered statistically significant. Funnel plots and Egger’s regression test were used to assess publication bias. R software version 3.5.2 (2018-12-20) for MacOS (R Foundation for Statistical Computing) with Meta package was used for the analysis.
Results
A total of 9 studies were selected for the meta-analysis: 6 studies [8–13] in the setting of ACS and 3 studies [14–16] in the setting of PCI (elective or for ACS), including 294,528 and 39,973 patients respectively. The review process is depicted in Fig. 1. Most studies were registries [9, 11–14, 16], one was obtained from databases [8] and the two remaining from retrospective cohorts [10, 15]. Two studies reported only pair-matched comparison results [11, 15] and two others used propensity scores [8, 14]. In one study [13] results were available in matched and unmatched groups: our principal meta-analysis was performed using the unmatched group as more endpoints were available and most included studies were not matched (the sensitivity analysis with the matched group of this study is reported in the Additional file 1). For one PCI study [16], the results of the subgroup of patients who underwent PCI for ACS were available and included in the ACS meta-analysis (named Nakastuma-AMI).
Fig. 1.
Flow diagram of meta-analysis studies’ selection
The major characteristics of the patients of each study are detailed in Table 1 for ACS studies and Table 2 for PCI studies. Overall, cancer patients represented 8.1% (5.6 to 23.4%) and 6.5% (3.3 to 9.1%) of all patients in the ACS and PCI studies respectively. Duration of long-term follow-up ranged between 5.3 and 11 years [8, 14–16].
Table 1.
Demographic characteristics of patients with acute coronary syndrome in selected studies
| Velders et al. | Ianaccone et al. | Wang et al. | Kurisu et al. | Rohrmann et al. | Gong et al. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics, n (%) | Cancer n = 208 |
No cancer n = 3215 |
Cancer n = 858 |
No cancer n = 13,773 |
Cancer n = 263 |
No cancer n = 2083 |
Cancer n = 18 |
No cancer n = 59 |
Cancer n = 1981 |
No cancer n = 1981 |
Cancer n = 22,907 |
No cancer n = 247,182 |
| Age (years) | 69.6 ± 11 | 62.8 ± 12.4 | 70.8 ± 10.3 | 62.8 ± 12.6 | 72.0 ± 10.7 | 63.3 ± 13.7 | 70.4 ± 8.1 | 68.8 ± 11.8 | 73.1 ± 11.6 | 73.1 ± 10.9 | n/a | n/a |
| Men | 141 (67.8) | 2427 (75.5) | 612 (71.3) | 10,633 (77.2) | 178 (67.7) | 1480 (71.1) | 13 (72.2) | 44 (74.6) | 1432 (72.3) | 1443 (72.8) | 13,996 (61.1) | 153,747 (62.2) |
| Systemic hypertension | 88 (42.7) | 1135 (35.4) | 558 (65) | 7961 (57.8) | 199 (75.7) | 1429 (68.5) | 11(61.1) | 37 (62.7) | 1309/1879 (69.7) | 1313/1878 (69.9) | 13,790 (60.2) | 147,568 (59.7) |
| Diabetes mellitus | 23 (11) | 361 (11.2) | 246 (28.7) | 3237 (23.5) | 44 (16.7) | 381 (18.3) | 7 (38.9) | 25 (42.4) | 439/1894 (23.2) | 476/1890 (25.2) | 6849 (29.9) | 72,177 (29.2) |
| Hyperlipidemia | 46 (22.4) | 738 (23.1) | 414 (48.2) | 7327 (53.2) | 186 (70.7) | 1536 (73.7) | n/a | n/a | 974/1704 (57.2) | 1033/1737 (59.5) | 8980 (39.2) | 97,390 (39.4) |
| Active smoker | 61 (31) | 1487 (46.8) | n/a | n/a | 169 (64.3) | 1422 (68.3) | n/a | n/a | 456/1728 (26.4) | 494/1733 (28.5) | n/a | n/a |
| History of | ||||||||||||
| Myocardial infarction | 35 (17) | 335 (10.4) | 132 (15.4) | 1584 (11.5) | 44 (16.7) | 301 (14.5) | n/a | n/a | 444/1981 (22.4) | 404/1981 (20.4) | n/a | n/a |
| PCI | 21 (10.2) | 267 (8.3) | 125 (14.6) | 1708 (12.4) | 55 (20.9) | 330 (15.8) | n/a | n/a | n/a | n/a | 916 (4) | 9640 (3.9) |
| CABG | 5 (2.4) | 79 (2.5) | 40 (4.7) | 427 (3.1) | 32 (12.2) | 146 (7) | n/a | n/a | n/a | n/a | n/a | n/a |
| Stroke | 24 (11.7) | 191 (5.9) | 71 (8.3) | 744 (5.4) | 39 (14.8) | 161 (7.7) | n/a | n/a | 164/1981 (8.3) | 153/1981 (7.7) | 1558 (6.8) | 15,573 (6.3) |
| Anemia | 53 (11.8) | 152 (5.2) | n/a | n/a | n/a | n/a | 9 (50) | 4 (6.8) | 312/807 (38.7) | 192/777 (24.7) | 4971 (21.7) | 49,931 (20.2) |
| Clinical presentation | ||||||||||||
| STEMI | 208 (100) | 3215 (100) | 440 (51.3) | 8043 (58.4) | 263 (100) | 2083 (100) | n/a | n/a | 1033/1981 (52.1) | 987/1981 (49.8) | n/a | n/a |
| NSTEMI | 0 | 0 | 279 (32.5) | 3912 (28.4) | 0 | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
| Unstable angina | 0 | 0 | 139 (16.2) | 1818 (13.2) | 0 | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
| Number of diseased vessels | ||||||||||||
| 1 vessel | 87 (41.8) | 1522 (47.4) | n/a | n/a | 244 (92,8) | 1959 (94) | 9 (50) | 35 (59.3) | n/a | n/a | n/a | n/a |
| 2 vessels | 71 (34.1) | 1005 (31.3) | n/a | n/a | 19 (7.2) | 119 (5.7) | 7 (38.9) | 17 (28.8) | n/a | n/a | n/a | n/a |
| 3 vessels | 50 (24) | 685 (21.3) | n/a | n/a | 0 | 5 (0.2) | 2 (11.1) | 7 (11.9) | n/a | n/a | n/a | n/a |
| Procedure | ||||||||||||
| Stenting | 192 (92.3) | 3082 (95.9) | 812 (94.6) | 13,332 (96.8) | n/a | n/a | 16 (88.9) | 54 (91.5) | n/a | n/a | n/a | n/a |
| Drug-eluting stent | 124 (60.8) | 2263 (70.8) | 304 (35.4) | 5523 (40.1) | 110 (41.8) | 1138 (54.6) | 1 (5.6) | 7 (11.9) | n/a | n/a | n/a | n/a |
| Bare metal stent | 67 (32.8) | 812 (25.4) | 508 (59.2) | 7809 (56.7) | n/a | n/a | 15 (83.3) | 47 (79.7) | n/a | n/a | n/a | n/a |
| Treatments at discharge | ||||||||||||
| Beta-blockers | 160 (84.2) | 2812 (90.5) | 640 (74.6) | 11,198 (81.3) | n/a | n/a | 2 (14.3) | 27 (48.2) | 1183/1960 (60.4) | 1192/1957 (60.9) | 7193 (31.4) | 75,638 (30.6) |
| ACE/ARBs | 139 (73.2) | 2220 (71.5) | 605 (70.6) | 10,344 (75.1) | n/a | n/a | 6 (42.9) | 43 (76.8) | 995/1961 (50.7) | 1038/1962 (52.9) | 8705 (38) | 90,963 (36.8) |
| Statins | 171 (90) | 2897 (93.2) | 780 (90.9) | 12,878 (93.5) | n/a | n/a | 8 (57.1) | 41 (73.2) | 1322/1958 (67.5) | 1384/1965 (70.4) | 5933 (25.9) | 62,537 (25.3) |
| Aspirin | 187 (98.4) | 3097 (99.3) | 850 (99.1) | 13,690 (99.4) | n/a | n/a | 13 (92.9) | 56 (100) | 1829/1973 (92.7) | 1858/1975 (94.1) | n/a | n/a |
| P2Y12 inhibitors | 186 (97.9) | 3058 (98) | 766 (89.3) | 11,996 (87.1) | n/a | n/a | n/a | n/a | 1380/1963 (70.3) | 1465/1971 (74.3) | 962 (4.2) | 10,135 (4.1) |
ACE Angiotensin-Converting Enzyme; ARBs Angiotensin Receptor Blockers; CABG Coronary Artery Bypass Grafting; NSTEMI Non ST-Elevation Myocardial Infarction; PCI Percutaneous Coronary Intervention; STEMI ST-Elevation Myocardial Infarction; n/a not available
Table 2.
Demographic characteristics of patients admitted for percutaneous coronary intervention in selected studies
| Hess et al. | Nakatsuma et al. | Landes et al. (*) | ||||
|---|---|---|---|---|---|---|
| Characteristics, n (%) | Cancer n = 496 |
No cancer n = 14,512 |
Cancer n = 1109 |
No cancer n = 11,071 |
Cancer n = 969 |
No cancer n = 969 |
| Age (years) | 68 (61.8) | 62 (53.7) | 73.2 ± 8.5 | 67.8 ± 11.1 | 76.6 ± 10.1 | 76.9 ± 9.2 |
| Men | 354 (71.4) | 9586 (66.1) | 825 (74.4) | 7976 (72) | 700 (72.2) | 700 (72.2) |
| Systemic hypertension | 334 (67.3) | 9478 (65.3) | 904 (81.5) | 9100 (82.2) | 843 (87) | 843 (87) |
| Diabetes mellitus | 129 (26) | 4013 (27.7) | 440 (39.7) | 4154 (37.5) | 318 (45.7) | 318 (45.7) |
| Hyperlipidemia | n/a | n/a | n/a | n/a | ||
| Active smoker | 238 (48) | 7712 (53.1) | 230 (20.7) | 3648 (33) | 230 (23.7) | 204 (21.1) |
| History of | ||||||
| Myocardial infarction | 246 (49.6) | 7414 (51.1) | 119 (10.7) | 1141 (10.3) | n/a | n/a |
| PCI | n/a | n/a | n/a | n/a | n/a | n/a |
| CABG | n/a | n/a | n/a | n/a | 185 (19.1) | 196 (20.2) |
| Stroke | 66 (13.3) | 1196 (8.2) | 142 (12.8) | 1149 (10.4) | 87 (9) | 63 (6.5) |
| Anemia | n/a | n/a | 228 (20.6) | 1165 (10.5) | n/a | n/a |
| Presentation | ||||||
| ACS | 329 (66.6) | 10,481 (72.4) | 317 (28.6) | 2992 (27) | 592 (61.1) | 527 (54.4) |
| Number of diseased vessels | ||||||
| 1 vessel | 255 (55.8) | 8839 (65.4) | n/a | n/a | 163 (16.9) | 175 (18.1) |
| 2 vessels | 131 (28.7) | 3095 (22.9) | n/a | n/a | 320 (33) | 310 (32) |
| 3 vessels | 71 (15.5) | 1574 (11.7) | n/a | n/a | 485 (50.1) | 369 (40.9) |
| Procedure | ||||||
| Drug-eluting stent | 164 (54.5) | 4209 (62.1) | 570 (51.4) | 6218 (56.2) | 392 (40.5) | 465 (48) |
| Bare metal stent | n/a | n/a | n/a | n/a | 529 (54.6) | 464 (47.9) |
| Treatment at discharge | ||||||
| Beta-blockers | 447 (90.1) | 13,077 (90.1) | 294 (27) | 3410 (31) | n/a | n/a |
| ACE/ARBs | 418 (84.3) | 10,992 (75.7) | 571 (51) | 6573 (59) | n/a | n/a |
| Statins | n/a | n/a | 487 (44) | 5816 (53) | n/a | n/a |
| Aspirin | 489 (98.6) | 14,355 (98.9) | 1092 (98) | 10,936 (99) | n/a | n/a |
| P2Y12 Inhibitors | 437 (88.1) | 11,023 (76) | 136 (13) | 1018 (9.3) | n/a | n/a |
ACE Angiotensin-Converting Enzyme; ACS Acute Coronary Syndrome; ARBs Angiotensin Receptor Blockers; CABG Coronary Artery Bypass Grafting; PCI Percutaneous Coronary Intervention
(*) data of the matched patients
The analysis showed increased rates of in-hospital all-cause death (RR 1.74 [1.22; 2.47]; Fig. 2a), cardiac death (RR 2.44 [1.73; 3.44]; Fig. 2b) and bleeding (RR 1.64 [1.35; 1.98]; Fig. 2f) as well as one-year all-cause death (RR 2.62 [1.2; 5.73]; Fig. 2c) and cardiac death (RR 1.89 [1.25; 2.86]; Fig. 2d) in the cancer group compared to the non-cancer group in ACS studies. Long-term all-cause death did not significantly differ (Fig. 2e) between groups. The consistency was low for the in hospital and one-year all-cause death outcomes (I2 = 87 and 98% respectively). When the matched group of the study by Wang et al. [13] was used, comparisons showed important heterogeneity for in-hospital and long-term all-cause and cardiac death (Additional file 1: Figure S1).
Fig. 2.
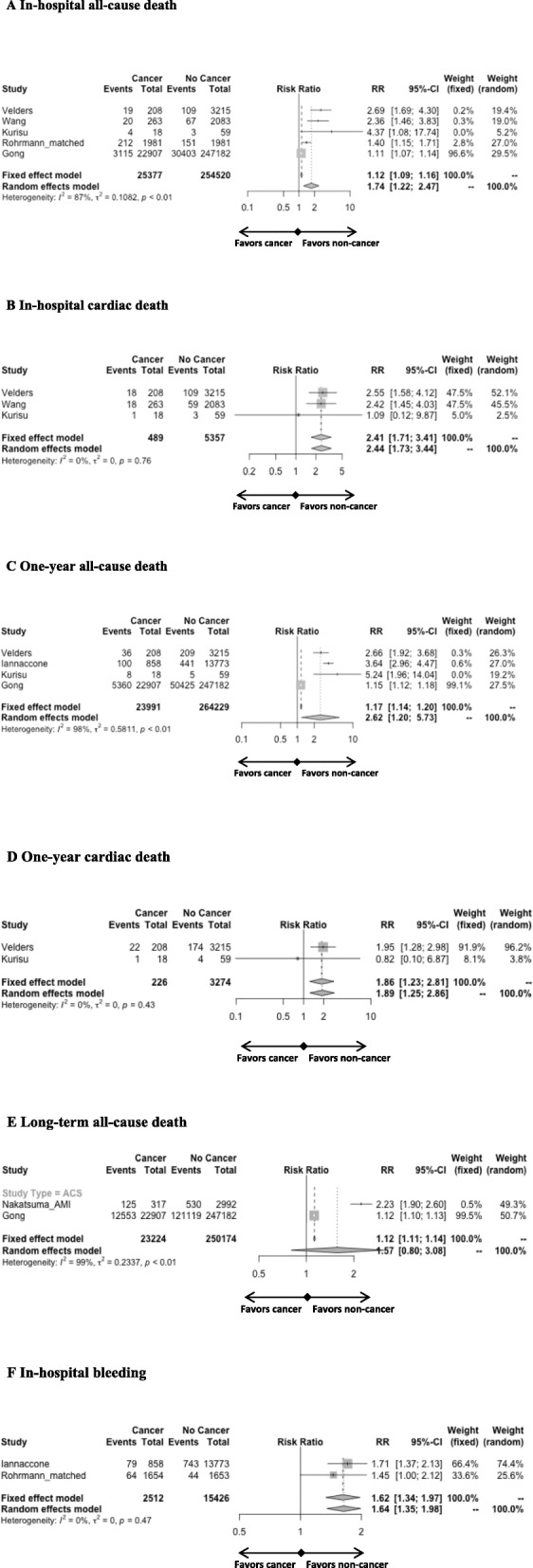
Forest plots of ACS studies comparing the impact of cancer versus no cancer on in hospital all-cause death (A), in-hospital cardiac death (B), one-year all-cause death (C), one-year cardiac death (D), long-term all-cause death (E) and in-hospital bleeding (F)
In the PCI studies, the meta-analysis showed only increased rates of long-term all-cause death (RR 1.96 [1.52; 2.53]; Fig. 3a) in the cancer group but with very low consistency (I2 = 97%). Long-term cardiac death did not significantly differ between groups (Fig. 3b).
Fig. 3.
Forest plots of PCI studies comparing the impact of cancer versus no cancer on long-term all-cause death (A) and long-term cardiac death (B)
Funnel plots showed publication bias for in-hospital and one-year all-cause death but not for cardiac death in all analyses and in-hospital bleeding (Additional file 1: Figure S2). The studies were judged to be at intermediate or low risk of bias using the adapted Newcastle-Ottawa Scale (Additional file 2).
Discussion
Our analysis shows that overall 8.1% of the patients admitted for an ACS have a history of cancer. Such patients are at higher risk of in-hospital and one-year, all-cause and cardiac mortality as well as in-hospital bleeding compared to those without cancer. However the results should be tempered because of high heterogeneity and publication bias with the exception of cardiac death, which was consistently increased in cancer patients. Among PCI studies, cancer patients were at higher risk of all-cause but not cardiac long-term mortality.
Cancer patients represent a growing and high-risk population in the setting of ACS. Our meta-analysis confirmed the worse, in-hospital and one-year, prognosis of cancer patients. Even if the magnitude of the relative risk of early and late all-cause mortality should be tempered by the heterogeneity among studies, all included studies consistently showed a worse prognosis in such patients. The heterogeneity among studies may be explained by the differences in sample size and statistical methods. Additionally, the small increase of all-cause death in cancer patients observed in the largest study by Gong et al. [8] -that differs from other results- is explained by their selection of cancer survivors only (without cancer treatment nor diagnosis within the last year). Patients with cancer are older, more often women and have more comorbidities including diabetes mellitus, chronic kidney disease, history of heart failure and stroke [8, 9, 11, 12], compared to non-cancer patients. These conditions are associated with poor prognosis in ACS patients [17–20]. Increased risk of mortality may also be explained by the prothrombotic state associated with cancer, due to reduced fibrinolysis and production of procoagulants -such as tissue factor- and inflammatory cytokines by the tumor [21] as well as tumor cell-induced platelet aggregation [22]. Moreover malignancy is associated with the risk of stent thrombosis [23]. Finally, patients with cancer are less likely to receive optimal guideline recommended medications [9, 11, 24].
The use of early invasive strategy, and PCI if needed, is associated with improved outcome after ACS [25, 26]. A less frequent use of PCI or drug eluting stents in patients with a history of cancer admitted for ACS has been reported [9, 11, 12] but current data remain conflicting [8]. A recent study reported that optimal medical therapy was prescribed in only one third of cancer patients at discharge [27]. The higher comorbidities associated with cancer such as renal impairment, asthenia and anemia [8, 9, 11, 16] may contribute to the suboptimal use of invasive strategy and evidence-based recommended medication. Moreover, the use of potent antithrombotic treatments may be limited in such patients because of the higher early bleeding risk found in our study. Explanations for higher bleeding risk may include the tumour-bleeding risk, the more frequent need for surgery, the drug-induced bone marrow toxicity and malnutrition [28] as well as comorbidities especially older age and renal impairment. Extensive data have shown that bleeding in ACS is associated with high risk of mortality [29, 30].
Considering the growing number of cancer patients admitted for ACS and their higher risk of death and bleeding, optimal management of these patients is crucial in clinical practice. The main cause of in-hospital mortality after ACS remains cardiac death [12, 13]. A reported, increased use of invasive coronary strategies and pharmacotherapies in such patients has been associated with a decline of mortality over the same time period [8]. A tailored approach appears important to reduce both the risk of cardiac death and bleeding during the acute phase. A multidisciplinary approach with a cardio-oncologist may be helpful for the early and long term management of such complex patient population [31]. The higher rates of long term all-cause but not cardiac death in cancer patients admitted for PCI highlights the fact that non-cardiovascular comorbidities may be of greater prognostic importance over the years after an ACS [32] as cancer patients will mostly die of cancer at long-term [13].
Limitations
Our meta-analysis was not performed on individual patient data. We pooled together studies with a large degree of clinical heterogeneity which is mirrored by the statistical heterogeneity across some outcomes. Cancer patients are excluded from most trials and limited data are available from observational studies. This point is a limit but also a justification for our meta-analysis. Cancer drugs may have influenced the hemorrhagic risk because of their potential different interactions with antiplatelet agents but these data were not available for more detailed analysis. Finally data on the cancer type or the time interval between cancer diagnosis and ACS which may highly impact the prognosis [12] were not available for further analysis.
Conclusion
Cancer patients represent a growing and high-risk patient population admitted for ACS. Our study showed that this population is at higher risk of in-hospital and one-year, all-cause and cardiac mortality as well as higher in-hospital bleeding risk compared to non-cancer patients. Even if the magnitude of the risk of all-cause mortality should be tempered by the heterogeneity among studies, all studies show higher risks of mortality in such patients. The consistent results with respect to the risk of cardiac mortality, especially the twice higher risk of in-hospital cardiac death, support optimal management of these patients with a tailored pharmaceutical and invasive strategy in the acute phase.
Supplementary information
Additional file 1: Figure S1. Forest plots of acute coronary syndrome (ACS) studies comparing the impact of cancer versus absence of cancer on in hospital all-cause death (A), in-hospital cardiac death (B), long-term all-cause death (C) and long-term cardiac death (CD) including the matched comparison for the study of Wang et al. Figure S2. Funnel plots in the acute coronary syndrome studies (panel A = In-hospital all-cause death; panel B = In-hospital cardiac death; panel C = One-year all-cause death; panel D = One-year cardiac death; panel E = In-hospital bleeding). Egger’s test was not applicable for one-year cardiac death and in-hospital bleeding as there were only 2 studies.
Additional file 2: Table S1. Newcastle-Ottawa Scale for risk of bias assessment of studies included in the meta-Analysis.
Acknowledgements
not applicable.
Abbreviations
- ACS
Acute coronary syndrome
- CI
Confidence intervals
- PCI
Percutaneous coronary intervention
- PRISMA
Preferred reporting items for systematic reviews and meta-analysis
- RR
Relative risks
Authors’ contributions
VR and LV: extraction, analysis and interpretation of data, drafting of the manuscript; KB PA AL MB RS: analysis of data, manuscript revision; JA and FB: design and revision; FB: statistical analysis; All: final approval of the manuscript submitted.
Funding
none.
Availability of data and materials
All data analyzed during this study are included in this published article and its supplementary information files.
Ethics approval and consent to participate
not applicable.
Consent for publication
not applicable.
Competing interests
The authors have no conflicts of interest to declare.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12872-020-01352-0.
References
- 1.2016. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 388:1459–544. [DOI] [PMC free article] [PubMed]
- 2.Ryerson AB, Eheman CR, Altekruse SF, Ward JW, Jemal A, Sherman RL, et al. Annual report to the nation on the status of Cancer, 1975-2012, featuring the increasing incidence of liver cancer. Cancer. 2016;122:1312–1337. doi: 10.1002/cncr.29936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 4.Gu D, Kelly TN, Wu X, Chen J, Samet JM, Huang JF, et al. Mortality attributable to smoking in China. N Engl J Med. 2009;360:150–159. doi: 10.1056/NEJMsa0802902. [DOI] [PubMed] [Google Scholar]
- 5.Darby SC, Ewertz M, Mcgale P, Bennet AM, Blom-Goldman U, Bronnum D, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 6.Hooning MJ, Botma A, Aleman BM, Baaijens MH, Bartelink H, Klijn JG, et al. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst. 2007;99:365–375. doi: 10.1093/jnci/djk064. [DOI] [PubMed] [Google Scholar]
- 7.Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. 2009;53:2231–2247. doi: 10.1016/j.jacc.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 8.Gong IY, Yan AT, Ko DT, Earle CC, Cheung WY, Peacock S, et al. Temporal changes in treatments and outcomes after acute myocardial infarction among cancer survivors and patients without cancer, 1995 to 2013. Cancer. 2018;124:1269–1278. doi: 10.1002/cncr.31174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iannaccone M, D'ascenzo F, Vadala P, Wilton SB, Noussan P, Colombo F, et al. Prevalence and outcome of patients with cancer and acute coronary syndrome undergoing percutaneous coronary intervention: a BleeMACS substudy. Eur Heart J Acute Cardiovasc Care. 2018;7:631–638. doi: 10.1177/2048872617706501. [DOI] [PubMed] [Google Scholar]
- 10.Kurisu S, Iwasaki T, Ishibashi K, Mitsuba N, Dohi Y, Kihara Y. Comparison of treatment and outcome of acute myocardial infarction between cancer patients and non-cancer patients. Int J Cardiol. 2013;167:2335–2337. doi: 10.1016/j.ijcard.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 11.Rohrmann S, Witassek F, Erne P, Rickli H, Radovanovic D. Treatment of patients with myocardial infarction depends on history of cancer. Eur Heart J Acute Cardiovasc Care. 2018;7:639–645. doi: 10.1177/2048872617729636. [DOI] [PubMed] [Google Scholar]
- 12.Velders MA, Boden H, Hofma SH, Osanto S, Van Der Hoeven BL, Heestermans AA, et al. Outcome after ST elevation myocardial infarction in patients with cancer treated with primary percutaneous coronary intervention. Am J Cardiol. 2013;112:1867–1872. doi: 10.1016/j.amjcard.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 13.Wang F, Gulati R, Lennon RJ, Lewis BR, Park J, Sandhu GS, et al. Cancer history portends worse acute and long-term noncardiac (but not cardiac) mortality after primary percutaneous coronary intervention for acute ST-segment elevation myocardial infarction. Mayo Clin Proc. 2016;91:1680–1692. doi: 10.1016/j.mayocp.2016.06.029. [DOI] [PubMed] [Google Scholar]
- 14.Hess CN, Roe MT, Clare RM, Chiswell K, Kelly J, Tcheng JE, et al. Relationship between Cancer and cardiovascular outcomes following percutaneous coronary intervention. J Am Heart Assoc. 2015;4. [DOI] [PMC free article] [PubMed]
- 15.Landes U, Kornowski R, Bental T, Assali A, Vaknin-Assa H, Lev E, et al. Long-term outcomes after percutaneous coronary interventions in cancer survivors. Coron Artery Dis. 2017;28:5–10. doi: 10.1097/MCA.0000000000000429. [DOI] [PubMed] [Google Scholar]
- 16.Nakatsuma K, Shiomi H, Morimoto T, Watanabe H, Nakagawa Y, Furukawa Y, et al. Influence of a history of cancer on long-term cardiovascular outcomes after coronary stent implantation (an observation from coronary revascularization demonstrating outcome study-Kyoto registry Cohort-2) Eur Heart J Qual Care Clin Outcomes. 2018;4:200–207. doi: 10.1093/ehjqcco/qcy014. [DOI] [PubMed] [Google Scholar]
- 17.Alexander KP, Newby LK, Armstrong PW, Cannon CP, Gibler WB, Rich MW, et al. Acute coronary care in the elderly, part II: ST-segment-elevation myocardial infarction: a scientific statement for healthcare professionals from the American Heart Association Council on clinical cardiology: in collaboration with the Society of Geriatric Cardiology. Circulation. 2007;115:2570–2589. doi: 10.1161/CIRCULATIONAHA.107.182616. [DOI] [PubMed] [Google Scholar]
- 18.Granger CB, Goldberg RJ, Dabbous O, Pieper KS, Eagle KA, Cannon CP, et al. Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med. 2003;163:2345–2353. doi: 10.1001/archinte.163.19.2345. [DOI] [PubMed] [Google Scholar]
- 19.Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2018;39:119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 20.Piccolo R, Franzone A, Koskinas KC, Raber L, Pilgrim T, Valgimigli M, et al. Effect of diabetes mellitus on frequency of adverse events in patients with acute coronary syndromes undergoing percutaneous coronary intervention. Am J Cardiol. 2016;118:345–352. doi: 10.1016/j.amjcard.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 21.De Cicco M. The prothrombotic state in cancer: pathogenic mechanisms. Crit Rev Oncol Hematol. 2004;50:187–196. doi: 10.1016/j.critrevonc.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 22.Hara Y, Steiner M, Baldini MG. Characterization of the platelet-aggregating activity of tumor cells. Cancer Res. 1980;40:1217–1222. [PubMed] [Google Scholar]
- 23.Van Werkum JW, Heestermans AA, Zomer AC, Kelder JC, Suttorp MJ, Rensing BJ, et al. Predictors of coronary stent thrombosis: the Dutch stent thrombosis registry. J Am Coll Cardiol. 2009;53:1399–1409. doi: 10.1016/j.jacc.2008.12.055. [DOI] [PubMed] [Google Scholar]
- 24.Hu J, Xie Y, Shu Z, Yang W, Zhan S. Trends in the use of guideline-recommended medications and in-hospital mortality of patients with acute myocardial infarction in a Chinese population. PLoS One. 2015;10:e0118777. doi: 10.1371/journal.pone.0118777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bavry AA, Kumbhani DJ, Rassi AN, Bhatt DL, Askari AT. Benefit of early invasive therapy in acute coronary syndromes: a meta-analysis of contemporary randomized clinical trials. J Am Coll Cardiol. 2006;48:1319–1325. doi: 10.1016/j.jacc.2006.06.050. [DOI] [PubMed] [Google Scholar]
- 26.Puymirat E, Taldir G, Aissaoui N, Lemesle G, Lorgis L, Cuisset T, et al. Use of invasive strategy in non-ST-segment elevation myocardial infarction is a major determinant of improved long-term survival: FAST-MI (French registry of acute coronary syndrome) JACC Cardiovasc Interv. 2012;5:893–902. doi: 10.1016/j.jcin.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 27.Iannaccone M, F DA, De Filippo O, Gagliardi M, southern DA, Raposeiras-Roubin S, et al. Optimal medical therapy in patients with malignancy undergoing percutaneous coronary intervention for acute coronary syndrome: a BleeMACS sub-study. Am J Cardiovasc Drugs. 2017;17:61–71. doi: 10.1007/s40256-016-0196-x. [DOI] [PubMed] [Google Scholar]
- 28.Birgegard G, Aapro MS, Bokemeyer C, Dicato M, Drings P, Hornedo J, et al. Cancer-related anemia: pathogenesis, prevalence and treatment. Oncology. 2005;68(Suppl 1):3–11. doi: 10.1159/000083128. [DOI] [PubMed] [Google Scholar]
- 29.Boden H, Velders MA, Van Der Hoeven BL, Cannegieter SC, Schalij MJ. In-hospital major bleeding and its clinical relevance in patients with ST elevation myocardial infarction treated with primary percutaneous coronary intervention. Am J Cardiol. 2013;112:1533–1539. doi: 10.1016/j.amjcard.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 30.Moscucci M, Fox KA, Cannon CP, Klein W, Lopez-Sendon J, Montalescot G, et al. Predictors of major bleeding in acute coronary syndromes: the global registry of acute coronary events (GRACE) Eur Heart J. 2003;24:1815–1823. doi: 10.1016/S0195-668X(03)00485-8. [DOI] [PubMed] [Google Scholar]
- 31.Barac A, Murtagh G, Carver JR, Chen MH, Freeman AM, Herrmann J, et al. Cardiovascular health of patients with Cancer and Cancer survivors: a roadmap to the next level. J Am Coll Cardiol. 2015;65:2739–2746. doi: 10.1016/j.jacc.2015.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vagnarelli F, Taglieri N, Ortolani P, Norscini G, Cinti L, Bacchi Reggiani ML, et al. Long-term outcomes and causes of death after acute coronary syndrome in patients in the Bologna, Italy, area. Am J Cardiol. 2015;115:171–177. doi: 10.1016/j.amjcard.2014.10.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Forest plots of acute coronary syndrome (ACS) studies comparing the impact of cancer versus absence of cancer on in hospital all-cause death (A), in-hospital cardiac death (B), long-term all-cause death (C) and long-term cardiac death (CD) including the matched comparison for the study of Wang et al. Figure S2. Funnel plots in the acute coronary syndrome studies (panel A = In-hospital all-cause death; panel B = In-hospital cardiac death; panel C = One-year all-cause death; panel D = One-year cardiac death; panel E = In-hospital bleeding). Egger’s test was not applicable for one-year cardiac death and in-hospital bleeding as there were only 2 studies.
Additional file 2: Table S1. Newcastle-Ottawa Scale for risk of bias assessment of studies included in the meta-Analysis.
Data Availability Statement
All data analyzed during this study are included in this published article and its supplementary information files.




