Prenatal surgery for myelomeningocele reduces hydrocephalus and improves motor function in young children, but this does not translate into an improvement in adaptive behavior.
Abstract
BACKGROUND AND OBJECTIVES:
The Management of Myelomeningocele Study (MOMS), a randomized trial of prenatal versus postnatal repair for myelomeningocele, found that prenatal surgery resulted in reduced hindbrain herniation and need for shunt diversion at 12 months of age and better motor function at 30 months. In this study, we compared adaptive behavior and other outcomes at school age (5.9–10.3 years) between prenatal versus postnatal surgery groups.
METHODS:
Follow-up cohort study of 161 children enrolled in MOMS. Assessments included neuropsychological and physical evaluations. Children were evaluated at a MOMS center or at a home visit by trained blinded examiners.
RESULTS:
The Vineland composite score was not different between surgery groups (89.0 ± 9.6 in the prenatal group versus 87.5 ± 12.0 in the postnatal group; P = .35). Children in the prenatal group walked without orthotics or assistive devices more often (29% vs 11%; P = .06), had higher mean percentage scores on the Functional Rehabilitation Evaluation of Sensori-Neurologic Outcomes (92 ± 9 vs 85 ± 18; P < .001), lower rates of hindbrain herniation (60% vs 87%; P < .001), had fewer shunts placed for hydrocephalus (49% vs 85%; P < .001) and, among those with shunts, fewer shunt revisions (47% vs 70%; P = .02) than those in the postnatal group. Parents of children repaired prenatally reported higher mean quality of life z scores (0.15 ± 0.67 vs 0.11 ± 0.73; P = .008) and lower mean family impact scores (32.5 ± 7.8 vs 37.0 ± 8.9; P = .002).
CONCLUSIONS:
There was no significant difference between surgery groups in overall adaptive behavior. Long-term benefits of prenatal surgery included improved mobility and independent functioning and fewer surgeries for shunt placement and revision, with no strong evidence of improved cognitive functioning.
What’s Known on This Subject:
Prenatal surgery was found to reduce hydrocephalus and hindbrain herniation in infants with myelomeningocele as well as improve motor function at 30 months of age.
What This Study Adds:
Among school-aged children with myelomeningocele whose mothers were randomly assigned to surgery before birth versus standard postnatal repair, there were no significant differences in adaptive behavior, but motor function and quality of life were better.
Myelomeningocele is a complex congenital anomaly resulting from incomplete neural tube closure early in embryonic development that affects ∼1 in 1500 births in the United States.1,2 Individuals with myelomeningocele have lower extremity sensory and motor dysfunction as well as long-term neurodevelopmental sequelae that affect ambulation and bladder and bowel control and lead to learning difficulties.3–7 Neurocognitive differences are, in part, the result of specific congenital intracranial abnormalities and hydrocephalus. The Chiari II malformation is characterized by varying degrees of hindbrain herniation, cerebellar malformation, and midbrain anomalies. This malformation can obstruct the flow of cerebrospinal fluid, which may contribute to the development of hydrocephalus and the need for shunt diversion.8–10
Prenatal myelomeningocele repair was first performed in 1997.11 Observational data from the first 200 cases suggested that prenatal repair decreased hindbrain herniation and the need for ventricular shunting.12–14 The Management of Myelomeningocele Study (MOMS) was a randomized controlled trial used to compare the safety and efficacy of prenatal repair of myelomeningocele with standard postnatal repair. The trial began in 2003 but was stopped early for efficacy in 2010 after 183 maternal-fetal dyads had been randomly assigned because stopping criteria had been met for both primary outcomes.15 The first primary outcome was a composite measure of death or the need for ventriculoperitoneal shunting for hydrocephalus that was based on prespecified criteria developed by a consensus group of nationally recognized experts. In the final results, we found that 97.8% of infants in the postnatal repair group met the definition of hydrocephalus compared with 72.5% of the infants in the prenatal group. The actual rate of shunt placement was 83.7% in the postnatal and 44.0% in prenatal group.16 Shunts were not always placed when the criteria were met because of a more conservative approach to hydrocephalus management among some neurosurgeons. The second primary outcome of MOMS was a composite of a ranked score from the Bayley II Mental Development Index and the difference between the anatomic and functional levels of the lesion evaluated at 30 months. Prenatal repair was superior to postnatal repair for this outcome. Children in the prenatal group also had improved ambulation and self-care skills at 30 months.17
To evaluate the long-term impacts of prenatal surgery compared to standard postnatal repair, a follow-up of the Management of Myelomeningocele Study (MOMS2) was conducted when the children were of school age. We hypothesized that the benefits of prenatal surgery would result in better adaptive behavior indicated by higher scores on the Vineland Adaptive Behavior Scales. We also hypothesized that children would continue to have improved motor function and fewer brain anomalies requiring surgical intervention compared with children in the standard postnatal repair group. Other prespecified objectives were to determine if prenatal surgery improves neurocognitive and behavioral outcomes and lessens the negative impact on families.
Methods
The study was conducted at the 3 centers that participated in the original MOMS trial (Children’s Hospital of Philadelphia, Vanderbilt University, and the University of California, San Francisco) along with the independent data-coordinating center at The George Washington University Biostatistics Center and the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Institutional review board approval was obtained at each clinical site and the data-coordinating center. Caregivers gave written informed consent, and children gave assent per local regulations.
Study Procedures
Families in the original trial were contacted and offered enrollment in the follow-up study. Participation consisted of a single comprehensive study visit when the child was between 5 and 10 years old; however, the goal was not to enroll children before 6 years of age. This visit took place at 1 of the clinical centers or, if the parent or caregiver declined to travel, at home. At the study visits, children underwent neurodevelopmental and behavioral evaluation by study-designated psychologists and a physical examination and functioning assessment by a study-designated physical therapist. A parent or caregiver also completed the Vineland Adaptive Behavior Scales II semistructured interview with a study psychologist. At the home visits, neurodevelopmental, behavioral, and physical evaluations were conducted by study examiners; and the Vineland Adaptive Behavior Scales II was conducted by telephone by a study psychologist. In addition, the parent or caregiver was asked for a medical history of the child and to complete various validated questionnaires. For quality control, all psychologists and physical therapists were trained centrally and certified by providing an acceptable videotaped examination on a test subject. Additional videotapes were submitted periodically to maintain certification. All of the physical therapists and psychologists were blinded to the treatment group, and before testing the parents or caregivers were asked to refrain from mentioning their child’s surgical repair group.
Children who came to the clinical center underwent a brain and spine MRI and urologic testing. A committee of 3 radiologists not involved in the conduct or clinical reading of the study MRIs reviewed the imaging. Common radiologic signs associated with spina bifida were determined by consensus. Urologic and detailed radiologic results will be reported separately.
Study Outcomes
The primary outcome was the composite score from the Vineland Adaptive Behavior Scales, Second Edition Interview. Adaptive behavior represents habitual performance of activities needed for communication, social, and daily living in the everyday environment. Secondary neuropsychological and behavioral outcomes included scores from the Kaufman Brief Intelligence Test, Second Edition (KBIT-2); the Woodcock-Johnson Tests of Achievement, Third Edition (WJ-III), subtests of reading and math achievement; the California Verbal Learning Test–Children’s Version, a multitrial list learning test; the Children’s Memory Scale (CMS) visual memory subtests; the Beery-Buktenica Developmental Test of Visual-Motor Integration; the Purdue Pegboard Test; and the Developmental Neuropsychological Assessment, Second Edition (NEPSY-II), word generation subtest. Parent or caregivers completed the Behavior Rating Inventory of Executive Function (BRIEF); the Swanson, Nolan, Achenbach, and Pelham rating of inattention and hyperactivity-impulsivity (SNAP-IV); the Child Behavior Checklist (CBCL), a rating of behavior and emotional functioning; the Parenting Stress Index; the Parkin Spina Bifida Health-Related Quality of Life; and the Stein Impact on Family questionnaires. Anthropometric measurements, the child’s walking status, and 34 items from the Functional Rehabilitation Evaluation of Sensori-Neurologic Outcomes (FRESNO, a validated measure of motor, self-care, social, communication, and cognitive functioning)18 were also included as secondary outcomes. The items chosen from the FRESNO evaluate the amount of assistance required by the child to complete activities of daily living and advanced motor skills. Walking independently was defined as walking without assistive devices or orthotics. We evaluated the frequency of neurosurgical procedures in the children. The presence of hindbrain herniation from the brain MRI and the presence of syringomyelia and epidermoid cysts from the spine MRI are included because these features have implications for surgery.
Statistical Analyses
At the time of study planning, we estimated that with a potential sample size of 177 children (the number known to be alive at the time of calculation), there was ∼90% power to detect a 0.5 SD difference in the primary outcome. Analyses were performed according to the intention-to-treat principle. For the neuropsychological testing, we calculated means and SDs by study group. Children who were unable to perform any of the neuropsychological tests because they were low functioning were assigned the lowest possible score.19 We also conducted sensitivity analyses excluding these children. For the Purdue Pegboard Test, the number of pegs with the preferred hand, nonpreferred hand, and pairs of pegs with both hands were separately converted into z scores by using normative data,20 and the average z score for the 3 tests was reported. Each item on the FRESNO scale was scored as 0 (dependent), 1 (requires assistance from another person), or 2 (can do it independently), and the total score was expressed as a percentage of the maximum score of 68. For the Parkin Spina Bifida Health-Related Quality of Life assessment, we estimated z scores from normative data.21 We compared all continuous variables using the Wilcoxon test and categorical variables using the χ2 test or Fisher’s exact test as appropriate. A general linear model was run for the primary outcome, adjusting for sex, which was known to be significantly different between groups in the trial. For walking status, the Cochrane-Armitage test for trend was used. Sensitivity analyses were conducted to determine if the participants differed from those children who were enrolled in MOMS but did not participate in this study. Nominal 2-sided P values of <.05 were considered to indicate statistical significance; no adjustments were made for multiple comparisons.
Results
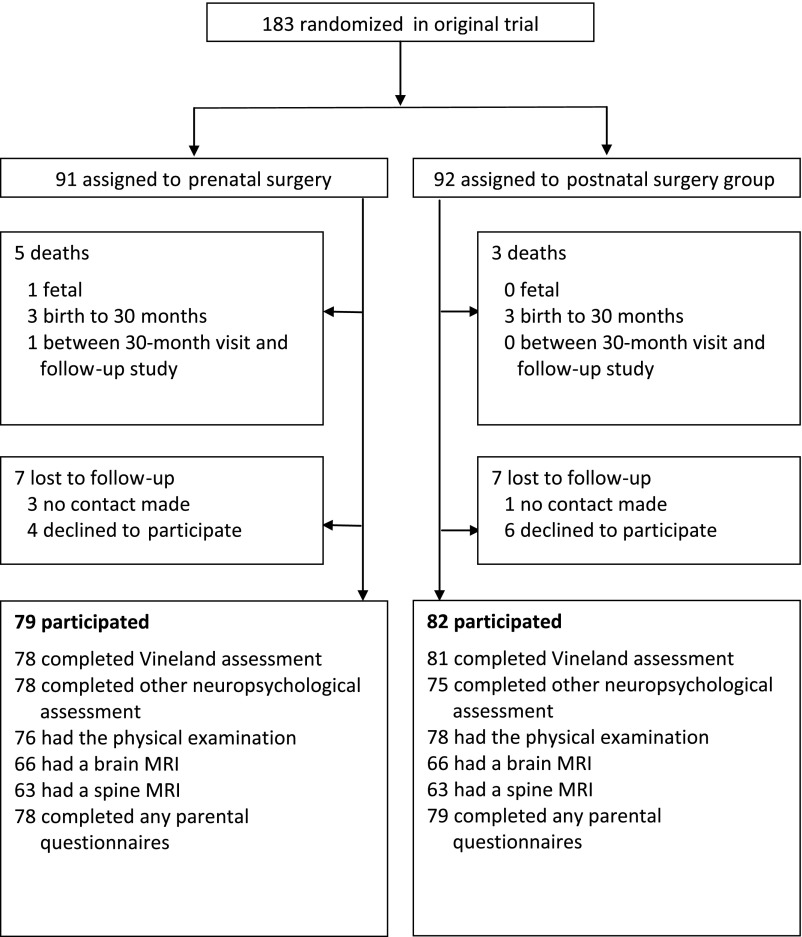
Study visits took place from June 2011 until April 2017, when the youngest children turned 6 years old. A total of 161 families participated in MOMS2. Of the original 183 child participants from MOMS, 8 died after random assignment (see Fig 1). Of the remaining 175 families, 10 declined to participate and 4 could not be contacted before 11 years of age. The Vineland composite score was assessed in 159 (90.9%) of the 175 children (78 in the prenatal surgery group and 81 children in the postnatal group). A total of 147 families had a study visit at 1 of the MOMS centers, 10 families had home visits, and 4 agreed to Vineland interviews by telephone only. Baseline characteristics for the MOMS2 population are detailed in Table 1. On average, the children were 7.8 years of age at the time of the study visit (range 5.9–10.3). As observed in the MOMS trial, children in the prenatal group were less likely to be female (43% vs 65%; P = .006) and were born an average 3 weeks earlier than those in the postnatal group (34.4 weeks versus 37.4 weeks; P < .001). There were no significant differences in baseline characteristics between the children who did and did not participate in the study, with the exception of gestational age at birth (mean age in the study population was 35.9 ± 2.5 weeks versus 34.4 ± 3.1 weeks in nonparticipants: P = .03) The average scores on the Vineland subscales were not different between the 2 surgery groups and neither was the composite score (89.0 ± 9.6 in the prenatal group versus 87.5 ± 12.0 in the postnatal group; P = .35; Table 2). These results were similar when adjusted for sex (P = .35).
FIGURE 1.
Enrollment and follow-up.
TABLE 1.
Demographic and Baseline Clinical Characteristics
| Characteristic | Prenatal Surgery (n = 79) | Postnatal Surgery (n = 82) |
|---|---|---|
| Age of child at MOMS2 visit, y | 7.8 ± 1.3 | 7.9 ± 1.2 |
| Female sex, n (%) | 34 (43) | 53 (65) |
| Child race or ethnic group, n (%)a | ||
| White non-Hispanic | 72 (91) | 69 (84) |
| African American non-Hispanic | 1 (1) | 1 (1) |
| Hispanic | 3 (4) | 5 (6) |
| Other or not reported | 3 (4) | 7 (9) |
| Anatomic lesion level, n (%) | ||
| Thoracic | 2 (3) | 1 (1) |
| L1–L2 | 12 (15) | 14 (17) |
| L3–L4 | 38 (48) | 41 (50) |
| L5–S2 | 27 (34) | 26 (32) |
| Gestational age at birth, wk | 34.4 ± 2.6 | 37.4 ± 1.0 |
Data are presented as mean ± SD or n (%). The only between-group comparisons that were significant were female sex (P = .006) and gestational age at birth (P < .001).
Race or ethnic group was self-reported.
TABLE 2.
Primary and Neuropsychological Outcomes
| Prenatal Surgery | Postnatal Surgery | ||||
|---|---|---|---|---|---|
| n | Mean ± SD | n | Mean ± SD | P | |
| Vineland Adaptive Behavior Scales IIa | 78 | 89.0 ± 9.6 | 81 | 87.5 ± 12.0 | .35 |
| Communicationa | 78 | 94.3 ± 12.2 | 81 | 92.4 ± 15.0 | — |
| Daily livinga | 78 | 87.6 ± 10.8 | 81 | 84.3 ± 12.4 | — |
| Socializationa | 78 | 96.2 ± 10.9 | 81 | 96.2 ± 12.4 | — |
| KBIT-2 | |||||
| Composite scorea | 78 | 99.0 ± 16.2 | 80 | 91.6 ± 22.5 | .05 |
| Verbal learninga | 78 | 101.3 ± 15.1 | 80 | 95.9 ± 22.2 | — |
| Nonverbal reasoninga | 78 | 96.3 ± 17.4 | 80 | 88.0 ± 21.5 | — |
| California Verbal Learning Test–Children’s Version (trials 1–5)b | 76 | 41.5 ± 10.6 | 75 | 39.4 ± 11.2 | .35 |
| WJ-III | |||||
| Reading compositea,c | 77 | 100.5 ± 17.2 | 79 | 93.8 ± 19.8 | .01 |
| Math calculationsa | 77 | 91.9 ± 21.2 | 77 | 88.7 ± 22.3 | .42 |
| CMS | |||||
| Dot learningd | 78 | 8.2 ± 3.0 | 77 | 7.0 ± 3.2 | .04 |
| Faces immediated | 72 | 7.7 ± 2.7 | 71 | 7.4 ± 4.1 | .34 |
| Beery Visual-Motor Integrationa | 78 | 85.9 ± 10.8 | 79 | 82.5 ± 14.6 | .26 |
| Purdue Pegboarde | 78 | −2.1 ± 1.2 | 78 | −2.9 ± 1.6 | <.001 |
| Developmental Neuropsychological Assessment, Second Edition, word generationd | 77 | 8.1 ± 2.7 | 80 | 7.3 ± 3.3 | .17 |
P values from the Wilcoxon test. —, not applicable.
Standard score metric mean 100; SD 15.
T score metric mean 50; SD 10.
Average of the following tests: Letter-Word Identification and Passage Comprehension.
Scale score, mean 10; SD 3 for each subtest.
Average z score for pegs placed with preferred hand, nonpreferred hand, and both hands.
There were 5 children in the postnatal surgery group and none in the prenatal surgery group who were unable to perform the neuropsychological tests because they were low functioning and could not perform any of the tests. As shown in Table 2, on average, children in the prenatal group scored higher on the KBIT-2 (99.0 ± 16.2 vs 91.6 ± 22.5; P = .05), the reading composite of the WJ-III (100.5 ± 17.2 vs 93.8 ± 19.8; P = .01), the dot-learning subtest of the CMS (8.2 ± 3.0 vs 7.0 ± 3.2; P = .04), and the Purdue Pegboard compared with children in the postnatal group (−2.1 ± 1.2 vs −2.9 ± 1.6; P ≤ .001). However, when the 5 low-functioning children were removed from the analysis, only the reading composite of the Woodcock-Johnson and the Purdue Pegboard remained statistically significant.
As shown in Table 3, children in the prenatal surgery group were ∼3.8 cm taller (P = .05) when adjusting for sex (P = .024). They demonstrated higher overall sensorimotor functioning on the FRESNO, with an average of 92% ± 9% of the maximum compared with 85% ± 18% of the maximum in the postnatal group (P = .001). As in the 30-month follow-up, children in the prenatal group walked independently more commonly (29% in the prenatal group versus 11% in the postnatal group [P = .006]; P < .001 when considered as a trend from walking without orthotics to not walking at all as in Table 3). Since birth, children in the prenatal group less commonly had shunts for hydrocephalus (49% in the prenatal group versus 85% in the postnatal group; P < .001), and they were less likely to require shunt revisions. Among only the children who had shunts placed for hydrocephalus, children in the prenatal surgery group also less commonly needed shunt revisions (47% vs 70% of those in the postnatal group; P = .02). There was no significant difference between the 2 groups in the proportion of children receiving Chiari decompression surgeries but more children in the prenatal surgery group had tethered cord release (27% in the prenatal group versus 15% in the postnatal group, P=0.03) . As shown in Table 3, children in the postnatal group more commonly had evidence of hindbrain herniation and syringomyelia on MRI. Although 11% of children in the prenatal group had epidermoid cysts identified on MRI compared with 3% of children in the postnatal group, this difference was not statistically significant.
TABLE 3.
Clinical and Radiologic Outcomes
| Prenatal Surgery | Postnatal Surgery | |||||
|---|---|---|---|---|---|---|
| n | Mean ± SD or Frequency (%) | n | Mean ± SD or Frequency (%) | RR (95% CI) | P | |
| Height or length, cm | 76 | 120.6 ± 10.9 | 77 | 116.8 ± 9.7 | — | .04 |
| Functional assessments | ||||||
| FRESNO % of maximum total scorea | 76 | 92 ± 9 | 78 | 85 ± 18 | — | <.001 |
| Walking status | 73 | 74 | — | <.001b | ||
| Walking without orthotics | — | 21 (29) | — | 8 (11) | — | — |
| Walking with orthotics only | — | 31 (42) | — | 25 (34) | — | — |
| Walking with assistive device | — | 16 (22) | — | 26 (35) | — | — |
| Unable to walk | — | 5 (7) | — | 15 (20) | — | — |
| Neurosurgeries (since birth) | ||||||
| Shunt placed | 78 | 38 (49) | 82 | 70 (85) | 0.6 (0.4–0.7) | <.001 |
| Shunt revision | 78 | 18 (23) | 82 | 49 (60) | 0.4 (0.2–0.6) | <.001 |
| Tethered cord release | 79 | 23 (27) | 82 | 12 (15) | 2.0 (1.1–3.7) | .03 |
| Chiari decompression | 79 | 3 (4) | 82 | 9 (11) | 0.3 (0.1–1.2) | .13 |
| MRI findings | ||||||
| Hindbrain herniation | 66 | 40 (61) | 62 | 54 (87) | 0.7 (0.6–0.9) | <.001 |
| Syringomyelia | 63 | 37 (59) | 59 | 48 (81) | 0.7 (0.6–0.9) | .007 |
| Epidermoid cyst | 63 | 7 (11) | 58 | 2 (3) | 3.2 (0.7–14.9) | .17 |
Data are presented as mean ± SD with P value from Wilcoxon test or n (%) with P value from χ2 or Fisher’s exact test. CI = confidence interval; RR, relative risk; —, not applicable.
Maximum score for 34 items addressing self-care and motor skills is 68.
Cochrane-Armitage test for trend.
As shown in Table 4, overall quality of life was significantly higher in the prenatal repair group on the caregiver-reported Parkin Spina Bifida Health-Related Quality of Life assessment, with a mean z score of 0.15 ± 0.68 in the prenatal and 0.11 ± 0.73 in the postnatal surgery group (P = .008). In addition, the prenatal group had a lower total Stein Impact on Family score (32.5 ± 7.8 vs 37.0 ± 8.9; P = .002). No statistically significant differences were found in the scales from the BRIEF, the CBCL, and the SNAP-IV.
TABLE 4.
Parental Questionnaire Outcomes
| Prenatal Surgery | Postnatal Surgery | ||||
|---|---|---|---|---|---|
| n | Mean ± SD | n | Mean ± SD | P | |
| Parkin Spina Bifida Health-Related Qualitya of Life | 76 | 0.15 ± 0.67 | 78 | 0.11 ± 0.73 | .008 |
| SNAP- IV | |||||
| Inattentionb | 78 | 1.14 ± 0.65 | 78 | 1.03 ± 0.63 | .51 |
| Hyperactivity-impulsivityb | 78 | 0.63 ± 0.48 | 78 | 0.58 ± 0.50 | .34 |
| Oppositional defianceb | 78 | 0.68 ± 0.64 | 78 | 0.53 ± 0.48 | .22 |
| BRIEF | |||||
| Global executive compositec | 75 | 54.3 ± 10.6 | 78 | 52.2 ± 10.5 | .20 |
| Behavioral regulation indexc | 76 | 50.3 ± 10.9 | 79 | 50.1 ± 11.2 | — |
| Metacognition indexc | 75 | 56.4 ± 11.3 | 78 | 53.3 ± 10.6 | — |
| CBCL | |||||
| Internalizing compositec | 77 | 47.0 ± 10.8 | 79 | 46.2 ± 10.5 | .67 |
| Externalizing compositec | 77 | 41.3 ± 9.9 | 79 | 39.0 ± 7.9 | .21 |
| Impact on familyd | |||||
| Total impact | 78 | 32.5 ± 7.8 | 78 | 37.0 ± 8.9 | .002 |
| Financial impact | 78 | 4.8 ± 2.1 | 78 | 5.7 ± 2.4 | — |
| Familial and/or social impact | 78 | 14.3 ± 3.7 | 78 | 16.1 ± 4.2 | — |
| Personal strain | 78 | 16.5 ± 4.6 | 78 | 18.8 ± 4.9 | — |
| Mastery (lack of) | 78 | 6.0 ± 1.9 | 78 | 5.9 ± 1.4 | — |
Data are presented as mean ± SD with P values from the Wilcoxon test. —, not applicable.
z score based on the population mean for 5- to 12-y-old children 168 ± 24.21
Ten items for each subscale rated on a 4-point scale from 0 (“not at all”) to 3 (“very much”) and averaged. These mean scores are well below clinically significant levels.
T score metric mean 50; SD 10.
Items are rated on a 4-point scale from 1 (“strongly agree”) to 4 (“strongly disagree”). Low scores indicate low impact. For total impact, financial impact, familial and/or social impact, personal strain, and lack of mastery, minimum and maximum scores are 19, 76; 3, 12; 9, 36; 10, 40; and 4, 16, respectively.
Discussion
There were no significant differences between the prenatal and postnatal surgery groups on the primary outcome measure, the Vineland composite score, obtained from a semistructured, nondirective caregiver interview. These null results were apparent for the child’s habitual completion of everyday activities involving communication, daily living, and socialization. The null results on the composite score are likely due to the specificity of the beneficial effects of prenatal surgery on motor skills observed in the 30-month assessment, which persisted in this long-term follow-up.17 In addition to a higher rate of ambulation, the FRESNO scale revealed significantly better sensorimotor functioning in the prenatal group, reflecting a lower severity of disability. The Parkin Quality of Life measure, specific to the disabling effects of spina bifida, such as physical and cognitive functioning, revealed significantly higher quality of life in the prenatal group. Cognitive differences between the groups were not robust with the exception of fine motor dexterity on the pegboard task.
The prenatal surgery group continued to show a lower rate of shunt placements and a higher frequency of independent ambulation. Parents in the prenatal group reported less effect of myelomeningocele on a family impact measure, family and social impact, and less personal strain, consistent with previous data from the MOMS cohort.22 Contributing to the reduced impact may be the lower rate of neurosurgical surgeries in the prenatal group (except for tethered cord release). It is not likely that these results are due to biased parental self-reports because the findings consistently implicate improved motor functions, not cognitive functions, and are pervasively higher on parent reports of the prenatal group.
The improvements in motor and neurologic outcomes likely reflect the reduced rate of hindbrain herniation and the neuroprotective effect of limiting exposure to amniotic fluid in utero observed in the prenatal group in the 12- and 30-month follow-up.16,17,23-27 The impact of the Chiari II malformation on gross motor functions was reduced, as was the severity of hydrocephalus necessitating shunting. Notably, the motor improvements were not restricted to the lower limbs, as the prenatal group showed better performance on a fine motor (pegboard) task associated with cerebellar functioning. This task requires reaching and precise, rhythmic pace of picking up and inserting small pegs for success. These are skills commonly impaired in children with myelomeningocele.
As in the initial follow-up at 12 and 30 months, the effects of prenatal surgery did not extend to cognitive functions or behavior.15,17 Children in the prenatal group had higher reading skills, but reading ability, often preserved in myelomeningocele, was average in both groups.6 Although children in the prenatal group scored higher on the KBIT-2, a measure of nonverbal and verbal intelligence, the difference was not significant if the 5 low-functioning children were excluded, in which case the means for both groups were in the average range. Similarly, for the dot-learning subscale, the means were in the low average range with the 5 low-functioning children removed.
Additionally, there were no differences on a variety of parent-rated assessments of attention, executive functions, and behavior. The pattern of cognitive results, with better reading than math skills, verbal memory performance ∼1 SD below average, impaired visuomotor integration, and higher verbal than nonverbal IQ scores, were consistent with other studies using similar measures in predominantly non-Hispanic and middle-class children with Chiari II malformations, shunted hydrocephalus, and lower-level spinal lesions like the current sample.6,25
This project has limitations. First, we were unable to recruit all of the families involved in MOMS, although we note that the children from the 14 families who declined participation or were unreachable were similar to the children in the study at baseline except for gestational age at birth. Second, although every attempt was made to keep examiners blinded to the child’s random assignment in MOMS, parents were not blinded to their child’s surgical group, which could have biased their responses on questionnaires. Similarly, local treating providers may have been biased in the way they counseled parents regarding surgical interventions, and families may have been biased in their responses to this counseling by knowing the surgical group status. Third, there is the possibility of higher cognitive functioning in the subset of children in the prenatal group who either did not develop or were not shunted for hydrocephalus. This subgroup requires separate secondary evaluation because the averages within the prenatal group may mask potentially beneficial effects of prenatal surgery on cognitive outcomes and adaptive behavior. Fourth, 1 or more of the differences in the secondary outcomes could have been due to type 1 error. Lastly, the study sample was not demographically similar to all children with myelomeningocele in the United States, which limits the generalizability of the findings.
Conclusions
In this study, we found enduring effects of prenatal surgery for myelomeningocele with improved motor functioning and a reduction in Chiari II malformations and hydrocephalus, as well as better gross and fine motor functioning and quality of life, with reduced negative impacts on families. These findings, on the most rigorously studied group of children with myelomeningocele, add to the growing body of literature demonstrating the benefits of prenatal surgery. Providers should consider these findings when counseling expectant mothers to ensure that families considering prenatal surgery for their fetus understand the potential risks and benefits. Regardless of surgery type (pre- or postnatal repair), children with myelomeningocele are at risk for significant learning and adaptation challenges, requiring intense contact with their health care team and robust follow-up to maximize their functional potential.
Acknowledgments
We acknowledge the following:
The Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania: Alan W. Flake, MD; Holly L. Hedrick, MD; Julie S. Moldenhauer, MD; Jamie Koh, RN, MSN; and Janet Jackson-Coty, PT;
University of California San Francisco, San Francisco, California: Jody Farrell, RN, PNP; Monica Rivera, PT; Tamara Ryan, RN; Larry Rand, MD; and Anita Moon-Grady, MD;
Vanderbilt University Medical Center, Nashville, Tennessee: Mary Dabrowiak, RN; Tracy Perry; and Laura Flynn, PT;
The George Washington University Biostatistics Center, Washington, District of Columbia: Jonathan Rowland, BS; Gary Heinrich, BS; and Kristen Holloway, MS;
The University of Houston, Houston, Texas: Rosalva McPherson; and
The Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Maryland: Rosemary Higgins, MD.
Glossary
- BRIEF
Behavior Rating Inventory of Executive Function
- CBCL
Child Behavior Checklist
- CMS
Children’s Memory Scale
- FRESNO
Functional Rehabilitation Evaluation of Sensori-Neurologic Outcomes
- KBIT-2
Kaufman Brief Intelligence Test, Second Edition
- MOMS
Management of Myelomeningocele Study
- MOMS2
follow-up of the Management of Myelomeningocele Study
- SNAP-IV
Swanson, Nolan, Achenbach, and Pelham rating of inattention and hyperactivity-impulsivity
- WJ-III
Woodcock-Johnson Tests of Achievement, Third Edition
Footnotes
Drs Houtrow and Fletcher, Ms Burrows, and Dr Thom made substantial contributions to the conception and design of the study, acquisition of data, and analysis and interpretation of data and drafted the manuscript; Drs Adzick, Lee, Glenn, Farmer, Howell, Brock, Walker, and Gupta made substantial contributions to the conception and design; Drs Thomas, Cooper, Bilaniuk, and Pruthi made substantial contributions to the acquisition and analysis and interpretation of data; Dr MacPherson made substantial contributions to the analysis and interpretation of data; and all authors revised the article critically for important intellectual content, approved the final manuscript as submitted, and agree to be accountable for all aspects of the work.
This trial has been registered at www.clinicaltrials.gov (identifier NCT00060606).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Funded by the National Institutes of Health Eunice Kennedy Shriver National Institute of Child Health and Human Development (U01 HD06854: follow-up of the Management of Myelomeningocele Study). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Parker SE, Mai CT, Canfield MA, et al. ; National Birth Defects Prevention Network . Updated national birth prevalence estimates for selected birth defects in the United States, 2004-2006. Birth Defects Res A Clin Mol Teratol. 2010;88(12):1008–1016 [DOI] [PubMed] [Google Scholar]
- 2.Lupo PJ, Agopian AJ, Castillo H, et al. Genetic epidemiology of neural tube defects. J Pediatr Rehabil Med. 2017;10(3–4):189–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Houtrow A, Dicianno BE. Advances in spina bifida care: from the womb to adulthood. Curr Phys Med Rehabil Rep. 2014;2:71–78 [Google Scholar]
- 4.Norrlin S, Strinnholm M, Carlsson M, Dahl M. Factors of significance for mobility in children with myelomeningocele. Acta Paediatr. 2003;92(2):204–210 [DOI] [PubMed] [Google Scholar]
- 5.Dicianno BE, Karmarkar A, Houtrow A, et al. Factors associated with mobility outcomes in a National Spina Bifida Patient Registry. Am J Phys Med Rehabil. 2015;94(12):1015–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dennis M, Barnes MA. The cognitive phenotype of spina bifida meningomyelocele. Dev Disabil Res Rev. 2010;16(1):31–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fletcher JM, Copeland K, Frederick JA, et al. Spinal lesion level in spina bifida: a source of neural and cognitive heterogeneity. J Neurosurg. 2005;102(suppl 3):268–279 [DOI] [PubMed] [Google Scholar]
- 8.McLone DG, Dias MS. The Chiari II malformation: cause and impact. Childs Nerv Syst. 2003;19(7–8):540–550 [DOI] [PubMed] [Google Scholar]
- 9.McLone DG, Knepper PA. The cause of Chiari II malformation: a unified theory. Pediatr Neurosci. 1989;15(1):1–12 [DOI] [PubMed] [Google Scholar]
- 10.Dias MS, McLone DG. Hydrocephalus in the child with dysraphism. Neurosurg Clin N Am. 1993;4(4):715–726 [PubMed] [Google Scholar]
- 11.Bruner JP, Tulipan NE, Richards WO. Endoscopic coverage of fetal open myelomeningocele in utero. Am J Obstet Gynecol. 1997;176(1 pt 1):256–257 [DOI] [PubMed] [Google Scholar]
- 12.Sutton LN, Adzick NS, Bilaniuk LT, Johnson MP, Crombleholme TM, Flake AW. Improvement in hindbrain herniation demonstrated by serial fetal magnetic resonance imaging following fetal surgery for myelomeningocele. JAMA. 1999;282(19):1826–1831 [DOI] [PubMed] [Google Scholar]
- 13.Bruner JP, Tulipan N, Paschall RL, et al. Fetal surgery for myelomeningocele and the incidence of shunt-dependent hydrocephalus. JAMA. 1999;282(19):1819–1825 [DOI] [PubMed] [Google Scholar]
- 14.Heuer GG, Moldenhauer JS, Scott Adzick N. Prenatal surgery for myelomeningocele: review of the literature and future directions. Childs Nerv Syst. 2017;33(7):1149–1155 [DOI] [PubMed] [Google Scholar]
- 15.Adzick NS, Thom EA, Spong CY, et al. ; MOMS Investigators . A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med. 2011;364(11):993–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tulipan N, Wellons JC III, Thom EA, et al. ; MOMS Investigators . Prenatal surgery for myelomeningocele and the need for cerebrospinal fluid shunt placement. J Neurosurg Pediatr. 2015;16(6):613–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farmer DL, Thom EA, Brock JW III, et al. ; Management of Myelomeningocele Study Investigators . The Management of Myelomeningocele Study: full cohort 30-month pediatric outcomes. Am J Obstet Gynecol. 2018;218(2):256.e1-256.e13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roberts SD, Wells RD, Brown IS, et al. The FRESNO: a pediatric functional outcome measurement system. J Rehabil Outcomes Meas. 1999;3(1):11–19 [Google Scholar]
- 19.Lachin JM. Worst-rank score analysis with informatively missing observations in clinical trials. Control Clin Trials. 1999;20(5):408–422 [DOI] [PubMed] [Google Scholar]
- 20.Gardner RA, Broman M. The Purdue pegboard: normative data on 1334 school children. J Clin Child Psychol. 1979;8(3):156–162 [Google Scholar]
- 21.Parkin PC, Kirpalani HM, Rosenbaum PL, et al. Development of a health-related quality of life instrument for use in children with spina bifida. Qual Life Res. 1997;6(2):123–132 [DOI] [PubMed] [Google Scholar]
- 22.Antiel RM, Adzick NS, Thom EA, et al. ; Management of Myelomeningocele Study Investigators . Impact on family and parental stress of prenatal vs postnatal repair of myelomeningocele. Am J Obstet Gynecol. 2016;215(4):522.e1-522.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Danzer E, Johnson MP, Adzick NS. Fetal surgery for myelomeningocele: progress and perspectives. Dev Med Child Neurol. 2012;54(1):8–14 [DOI] [PubMed] [Google Scholar]
- 24.Del Bigio MR. Neuropathology and structural changes in hydrocephalus. Dev Disabil Res Rev. 2010;16(1):16–22 [DOI] [PubMed] [Google Scholar]
- 25.Heffez DS, Aryanpur J, Hutchins GM, Freeman JM. The paralysis associated with myelomeningocele: clinical and experimental data implicating a preventable spinal cord injury. Neurosurgery. 1990;26(6):987–992 [PubMed] [Google Scholar]
- 26.Meuli M, Meuli-Simmen C, Hutchins GM, et al. In utero surgery rescues neurological function at birth in sheep with spina bifida. Nat Med. 1995;1(4):342–347 [DOI] [PubMed] [Google Scholar]
- 27.Copp AJ, Adzick NS, Chitty LS, Fletcher JM, Holmbeck GN, Shaw GM. Spina bifida. Nat Rev Dis Primers. 2015;1:15007. [DOI] [PMC free article] [PubMed] [Google Scholar]