Pulmonary hypertension (PH) is a progressive, enigmatic disease of the lung vasculature that is clinically defined by elevated mean pulmonary arterial pressure and pulmonary vascular resistance that results in significant and often fatal right ventricular (RV) failure (1). The term “PH” encompasses multiple heterogeneous etiologies that are classified into five groups by the World Health Organization. Historically, studies have focused predominantly on the most severe subtype, Group 1 pulmonary arterial hypertension (PAH). Broadly, panvascular remodeling in PAH is attributed to injury or dysfunction within the pulmonary vessels, and a growing number of heritable mutations are being recognized as initiating triggers for this disease (1). Furthermore, a “sex paradox” has been described for this disease whereby women exhibit increased susceptibility to PAH but better survival as compared with men, for incompletely defined reasons (2). The vasodilatory medications currently used to treat PAH fail to prevent or reverse disease progression (3), making identification of novel therapeutic targets crucial.
An increasing number of studies support a link between metabolic reprogramming and progressive tissue dysfunction in the pulmonary vasculature. For example, tissues from animal and human models of PH were shown to exhibit attenuated oxidative phosphorylation (OXPHOS) and increased glycolysis in aerobic conditions (4, 5), a phenomenon that was first described in proliferating cancer cells known as the Warburg effect (6, 7). Importantly, results obtained with emerging metabolic therapies, such as dichloroacetate, a drug previously used in patients with cancer and mitochondrial diseases (8), suggest that targeting metabolic dysfunction may be a reasonable strategy for certain patients with Group 1 PAH. However, evidence of genetic mutations that primarily drive metabolic reprogramming in Group 1 PAH is limited. Furthermore, any metabolic differences between female and male patients that influence PAH pathogenesis remain largely unknown.
In recent years and in line with this “metabolic theory,” pulmonary endothelial deficiency of iron-sulfur (Fe-S) clusters—bioinorganic cofactors required for enzymatic redox function—has been shown to promote PH (9–11). Specifically, deficiencies of certain Fe-S biogenesis genes, ISCU1/2 (iron-sulfur duster assembly protein) and BOLA3 (BolA family member 3), were found to attenuate OXPHOS and ultimately drive PH in preclinical models (10, 11). Like many other Fe-S biogenesis genes, rare (but naturally occurring) human mutations in these genes have been linked to mitochondrial syndromes (12). In turn, such metabolic diseases, specifically driven by ISCU1/2 (10) and BOLA3 (13), have been associated with PH. Similarly, a mutation (Gly206Cys) near the Fe-S binding motif on the mitochondrial scaffolding NFU1 gene results in multiple mitochondrial dysfunctions syndrome 1 (MMDS1), which is often complicated by early death and PAH (14, 15). Other clinical links between PH and metabolic diseases have been reported as well, but proof that genetic mutations in any Fe-S biogenesis genes directly drive PH pathogenesis has been elusive. As such, the clinical classification of PH associated with metabolic syndromes in general has been relegated to WHO Group 5 PH (PH due to unknown causes), reflecting the fact that the multifactorial mechanisms underlying this association have yet to be defined.
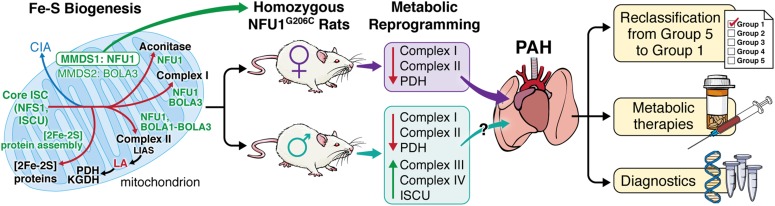
In a study presented in this issue of the Journal, Niihori and colleagues (pp. 231–242) obtained more definitive proof regarding the causative link between NFU1 mutations and PAH by generating homozygous NFU1G206C mutant Sprague Dawley rats (16). Compared with wild-type controls, homozygous females exhibited hemodynamic changes consistent with PAH, including increased RV systolic pressure (RVSP), Fulton’s index (a surrogate for RV hypertrophy), occlusive vessel remodeling, and vessel rarefaction. These changes were accompanied by decreased NFU1 hexamer oligomerization, activity of pyruvate dehydrogenase (PDH), and expression and activity of complexes I and II. Conversely, the majority of homozygous males did not exhibit increased RVSP or Fulton’s index, despite similar evidence of pulmonary vessel remodeling and a metabolic shift away from OXPHOS (i.e., decreased PDH and complex II expression and activity). The authors partially addressed these sex-based differences by assessing compensatory changes in Fe-S biogenesis and mitochondrial respiration. To this point, they found that homozygous males exhibited normal hexamer formation, increased ISCU1/2 expression, and increased activity of complexes III and IV.
Overall, the NFU1G206C rat constitutes the first preclinical model of PAH driven by a human Fe-S biogenesis gene mutation, and thus allows previously elusive evidence to be obtained regarding the direct causative relationship between Fe-S biogenesis and this vascular disease (Figure 1). Interestingly, although patients with MMDS1 uniformly die at a young age, with failure to thrive and substantial neurologic dysfunction (13–15), the authors did not report whether these rats reproduce all of these phenotypic features, although certainly these rats remain viable to adulthood. Although this may suggest an incomplete recapitulation of MMDS1, the spontaneous development of PAH in NFU1G206C rats provides additional support for a causative vascular link between this mutation and PAH that is independent of other confounding systemic disease features. The observation that heterozygous females showed a trend toward increased hemodynamic and histologic manifestations of PAH suggests the possibility that asymptomatic heterozygous carriers of the NFU1G206C mutation could be at risk for developing PAH. At the molecular level, beyond the reported OXPHOS deficiency in the pulmonary vasculature of these rats, it remains to be seen whether alterations in the breadth of nonmitochondrial, non-Warburg, Fe-S–dependent functions in the cytoplasm or nucleus may also impact pulmonary vascular function. Moreover, although prior mechanistic work has implicated ISCU1/2 and BOLA3 deficiency specifically in endothelial cells in PAH (9–11), further studies will be important to determine how multiple cell types in the pulmonary vessel, right ventricle, and/or even hematopoietic lineages may contribute to NFU1-specific disease, and how that may relate to the pulmonary, but not systemic, alteration of blood vessel function in these rats. Guided by this work, investigators may be able to develop rat models of known human mutations in other Fe-S biogenesis genes, which will be crucial for understanding the in vivo landscape of Fe-S pathobiology in PAH.
Figure 1.
Human mutation of the iron-sulfur (Fe-S) scaffolding gene NFU1 in rats drives sex-dependent metabolic changes and pulmonary arterial hypertension (PAH). NFU1 is a mitochondrial scaffolding protein that is involved in the multistep process of Fe-S biosynthesis, and genetic mutation in patients with mitochondrial multiple dysfunctions syndrome 1 (MMDS1) has previously been associated with pulmonary hypertension. Niihori and colleagues used CRISPR/Cas9 to generate human missense mutations in Sprague Dawley rats (16). In female homozygous rats, they observed mitochondrial deficiencies in electron transport chain complexes I and II, and increased right ventricular systolic pressure and right ventricular hypertrophy. Although certain metabolic phenotypic aberrations persisted, male homozygous rats exhibited compensatory mechanisms that seemed to prevent or delay disease formation. Although the complete mechanism that accounts for this sex dimorphism is not yet clear, these data demonstrate that the NFU1 mutation is a primary driver of pulmonary vascular disease, supporting the classification of Fe-S genetic mutations into heritable causes of WHO Group 1 PAH, bolstering the use of emerging metabolic therapies in PAH, and informing the diagnostic and prognostic benefits of sex-driven differences in metabolism. BOLA3 = BolA family member 3; CIA = cytoplasmic iron-sulfur assembly; Core ISC = core iron-sulfur cluster machinery; ISCU = iron-sulfur duster assembly protein; KGDH = α-ketoglutarate dehydrogenase; LA = lipoic acid; LIAS = lipoic acid synthetase; NFS1 = a mitochondrial cysteine desulfurase; NFU1 = mitochondrial iron-sulfur scaffold; PDH = pyruvate dehydrogenase. Illustration by Jill K. Gregory.
These findings also provide an exciting model to probe for a potential metabolic basis for the sex dimorphism in PAH. However, some important questions remain to be addressed. First, it is unknown whether female patients with MMDS1 are predisposed to developing PAH, and therefore the exact applicability of this rodent sex discrepancy to human disease is unclear. Second, at the molecular level, whether these sex differences in the rodent are linked to sex hormone and/or intrinsic sex chromosome variations is yet to be defined. Third, although this study reports an increased susceptibility to PAH in female NFU1G206C rats, the findings do not address a potential male-specific effect of NFU1 mutation on survival, as observed in humans (2). Interestingly, even though RVSP was not elevated in male NFU1G206C rats, end-diastolic RV pressure was significantly increased in the homozygous males, potentially signifying NFU1-dependent RV dysfunction. Serial hemodynamic and histologic assessments of the RV–pulmonary artery circuit over the course of PAH development could help investigators determine whether male rats eventually develop increased RVSP, what their prognosis might be, and whether any sex-driven differences in survival are related to metabolic reprogramming.
In summary, this study provides proof that homozygous NFU1G206C mutations drive the development of hemodynamic and histologic manifestations of PAH in vivo (16), corroborating the association between MMDS1 and PAH in humans, and augmenting previous findings on the importance of Fe-S deficiency in PH. Considering these data in aggregate, we propose that genetic mutations in NFU1 (now known to be causatively linked to PAH) and perhaps in other Fe-S biogenesis genes (i.e., ISCU and BOLA3) should be catalogued in the growing list of factors that result in a genetic predisposition to WHO Group 1 PAH rather than Group 5 PH. Although human mutations in Fe-S biogenesis genes are rare, the importance of this pathobiology should not be underestimated, particularly considering that Fe-S deficiency can also be driven by acquired exposures (such as hypoxia) (9, 11) and likely is prevalent, in general, across multiple subtypes of PH. Thus, these findings now shift our thinking and emphasize an exciting avenue that should be pursued for prognostic and therapeutic applications in PAH within the context of Fe-S biology.
Supplementary Material
Footnotes
Supported by National Institutes of Health grants F30 HL139017 (M.K.C.) and R01 HL124021, HL 122596, HL 138437, and UH2 TR002073 (S.Y.C.), and American Heart Association grant 18EIA33900027 (S.Y.C.).
Originally Published in Press as DOI: 10.1165/rcmb.2019-0309ED on September 17, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53:1801913. doi: 10.1183/13993003.01913-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Austin ED, Lahm T, West J, Tofovic SP, Johansen AK, Maclean MR, et al. Gender, sex hormones and pulmonary hypertension. Pulm Circ. 2013;3:294–314. doi: 10.4103/2045-8932.114756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lai Y-C, Potoka KC, Champion HC, Mora AL, Gladwin MT. Pulmonary arterial hypertension: the clinical syndrome. Circ Res. 2014;115:115–130. doi: 10.1161/CIRCRESAHA.115.301146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paulin R, Michelakis ED. The metabolic theory of pulmonary arterial hypertension. Circ Res. 2014;115:148–164. doi: 10.1161/CIRCRESAHA.115.301130. [DOI] [PubMed] [Google Scholar]
- 5.Culley MK, Chan SY. Mitochondrial metabolism in pulmonary hypertension: beyond mountains there are mountains. J Clin Invest. 2018;128:3704–3715. doi: 10.1172/JCI120847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 7.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michelakis ED, Gurtu V, Webster L, Barnes G, Watson G, Howard L, et al. Inhibition of pyruvate dehydrogenase kinase improves pulmonary arterial hypertension in genetically susceptible patients. Sci Transl Med. 2017;9:eaao4583. doi: 10.1126/scitranslmed.aao4583. [DOI] [PubMed] [Google Scholar]
- 9.Chan SY, Zhang YY, Hemann C, Mahoney CE, Zweier JL, Loscalzo J. MicroRNA-210 controls mitochondrial metabolism during hypoxia by repressing the iron-sulfur cluster assembly proteins ISCU1/2. Cell Metab. 2009;10:273–284. doi: 10.1016/j.cmet.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White K, Lu Y, Annis S, Hale AE, Chau BN, Dahlman JE, et al. Genetic and hypoxic alterations of the microRNA-210-ISCU1/2 axis promote iron-sulfur deficiency and pulmonary hypertension. EMBO Mol Med. 2015;7:695–713. doi: 10.15252/emmm.201404511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu Q, Tai YY, Tang Y, Zhao J, Negi V, Culley MK, et al. BOLA (BolA Family Member 3) deficiency controls endothelial metabolism and glycine homeostasis in pulmonary hypertension. Circulation. 2019;139:2238–2255. doi: 10.1161/CIRCULATIONAHA.118.035889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rouault TA. Mammalian iron-sulphur proteins: novel insights into biogenesis and function. Nat Rev Mol Cell Biol. 2015;16:45–55. doi: 10.1038/nrm3909. [DOI] [PubMed] [Google Scholar]
- 13.Cameron JM, Janer A, Levandovskiy V, Mackay N, Rouault TA, Tong W-H, et al. Mutations in iron-sulfur cluster scaffold genes NFU1 and BOLA3 cause a fatal deficiency of multiple respiratory chain and 2-oxoacid dehydrogenase enzymes. Am J Hum Genet. 2011;89:486–495. doi: 10.1016/j.ajhg.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Navarro-Sastre A, Tort F, Stehling O, Uzarska MA, Arranz JA, Del Toro M, et al. A fatal mitochondrial disease is associated with defective NFU1 function in the maturation of a subset of mitochondrial Fe-S proteins. Am J Hum Genet. 2011;89:656–667. doi: 10.1016/j.ajhg.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahting U, Mayr JA, Vanlander AV, Hardy SA, Santra S, Makowski C, et al. Clinical, biochemical, and genetic spectrum of seven patients with NFU1 deficiency. Front Genet. 2015;6:123. doi: 10.3389/fgene.2015.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niihori M, Eccles CA, Kurdyukov S, Zemskova M, Varghese MV, Stepanova AA, et al. Rats with a human mutation of NFU1 develop pulmonary hypertension Am J Respir Cell Mol Biol 202062231–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.