Acute respiratory distress syndrome (ARDS) is a life-threatening disease characterized by pulmonary edema and excessive alveolar inflammation with bilateral pulmonary infiltrates (1). The cardinal features of ARDS consist of recruitment of inflammatory cells, including neutrophils and macrophages, in addition to epithelial damage and excessive inflammation in the lung (1). Because no specific therapy is available for ARDS, management consists of treatment of the underlying cause, supportive care, and prevention of hospital-acquired infections (2). Thus, the identification of novel approaches for treatment of patients suffering from ARDS is needed.
The innate immune response is initiated by signals generated from cytosolic and membrane-bound pattern recognition receptors (PRRs) (3, 4). Toll-like receptors (TLRs) are germ-line–encoded PRRs that are expressed in both mucosal epithelial cells and myeloid cells. They sense diverse pathogen-associated molecular patterns derived from pathogens, and numerous damage-associated molecular patterns containing molecules released by damaged host cells. Paradoxically, TLR activation is beneficial to the host and can also result in excessive inflammation and severe organ damage (3). Unlike TLR2 and TLR4, TLR8 is an endosomal PRR that senses single-stranded nucleic acids, such as exogenous viral single-stranded RNA and endogenous RNA (including siRNAs, microRNAs [miRNAs], and their products), to induce proinflammatory responses via activation of the NF-κB signaling cascade (5).
In eukaryotic cells, post-translational protein modifications, including ubiquitination, are critical mechanisms that broaden protein functions and organize signaling networks. Ubiquitination is an enzymatic action that results in the attachment of a ubiquitin protein to a target protein, resulting in both inactivation of the substrate protein and its identification for rapid degradation by the proteasome (6). The process of ubiquitination is controlled by three main types of ligases: ubiquitin-activating enzymes (E1), ubiquitin-conjugating enzymes (E2), and ubiquitin ligases (E3) (6). The identification of ligases that govern the stability of TLRs may have clinical implications for pulmonary diseases.
In previous studies, Londino and colleagues and Evankovich and colleagues demonstrated the role of E3 ubiquitin-protein ligases in the stability of two critical mediators of inflammation: IFNGR1 (IFN-γ receptor 1) (in human epithelial and monocytic cell lines) (6) and RAGE (receptor for advanced glycation end products) (in a human bronchial epithelial cell line) (7). In addition, they elucidated the role of the TRIM21 E3 ubiquitin-protein ligase in human lung microvascular endothelial cells and in a mouse model of LPS-induced acute lung injury (8). In this issue of the Journal, Evankovich and colleagues (pp. 157–167) report the importance of the E3 ubiquitin-protein ligase RNF216 (Ring Finger protein 216, a.k.a. TRIAD3) in TLR8 stability in response to circulating miRNAs as assessed in blood samples from patients with ARDS and the THP-1 (human monocyte) cell line (9). Whole-blood samples from subjects with ARDS showed a decrease in TLR8, but not TLR2, protein levels compared with those obtained from healthy control subjects. Moreover, the authors confirmed that miRNA mimetics had no effect on TLR8 mRNA expression. In addition, they found that pretreatment with a proteasome inhibitor (MG-132) blocked the decrease in TLR8 protein in cells after stimulation with a TLR8 agonist (R848), which was confirmed in human peripheral blood–derived monocytes. Using cycloheximide, they determined that the half-life of TLR8 protein is 1 hour in cells. Together, these findings suggest that TLR8 is rapidly degraded after stimulation, likely through the proteasome.
Several lines of evidence in the study by Evankovich and colleagues support the idea that TLR8 is ubiquitinated (9). First, TLR8 accumulates in cells after treatment with an upstream E1-activating enzyme inhibitor (MLN7243). Second, ectopically expressed V5-TLR8 levels in HEK293 cells were reduced after enhanced coexpression of ubiquitin. Finally, a polyubiquitin band was observed in lysates from V5-His-TLR8–transfected cells, and after enrichment for TLR8, a polyubiquitin smear was observed in lysates from MG132-treated cells. Additional experiments using the same TLR8-enriched cellular fractions revealed TLR8-enriched K48 (lys residue) polyubiquitination. It is important to note that the authors found that TLR8 is K48 polyubiquitinated by the E3 ubiquitin-protein ligase RNF216, followed by proteasomal degradation (Figure 1). This was shown by silencing RNF216 in cells, which resulted in an increase in TLR8 protein levels. This finding is in line with previous findings that RNF216 is responsible for ubiquitination and degradation of TLR4 and TLR9, but not TLR2, with a concomitant reduction in signaling cascades in HeLa (cervical cancer) cells transfected with human TLR4 and in HEK293 cells transfected with human TLR9 (10). It is intriguing that in the current investigation (9), whole plasma and RNA obtained from plasma of patients with ARDS were also found to activate TLR8 signaling in vitro. Nevertheless, TLR8 stimulation was not affected by treatment with RNase, suggesting that the RNAs present in the plasma are miRNAs and not free, uncomplexed RNA. Moreover, the gene expression profiles obtained in critically ill patients or patients with ARDS revealed reduced RNF216. This suggests that reduced RNF216 abundance may lead to enhanced stability of TLR8, which may ultimately orchestrate the hyperinflammatory response observed in patients with ARDS. A key limitation of this study is its heavy reliance on human (THP-1) and macrophage cell lines. Therefore, the data need to be validated in human primary cells. Nonetheless, RNF216 has been shown to regulate the stability of other TLRs, and it is possible that pharmacological inhibition of this protease could ameliorate TLR-mediated inflammatory signals. Because excessive inflammation is a feature of ARDS, such an intervention may be highly relevant from a public health point of view. However, blocking RNF216 could have off-target effects, as it has been reported to regulate RIG-like receptor signaling via degradation of the TNF receptor–associated factor 3 adapter molecule and to inhibit autophagy by beclin1 ubiquitination (11). In addition, RNF216 has been linked to Gordon Holmes syndrome, in which disordered ubiquitination leads to ataxia, dementia, and hypogonadotropism (12). Recently, Husain and colleagues reported that a loss-of-function mutation in RNF216 leads to defective Arc (activity-regulated cytoskeleton-associated protein) ubiquitination, thereby causing cognitive impairment in patients with Gordon Holmes syndrome (13). Thus, therapeutic approaches to modulate RNF216 as a means of ameliorating TLR8-mediated hyperinflammation in patients with ARDS will have to be carefully considered.
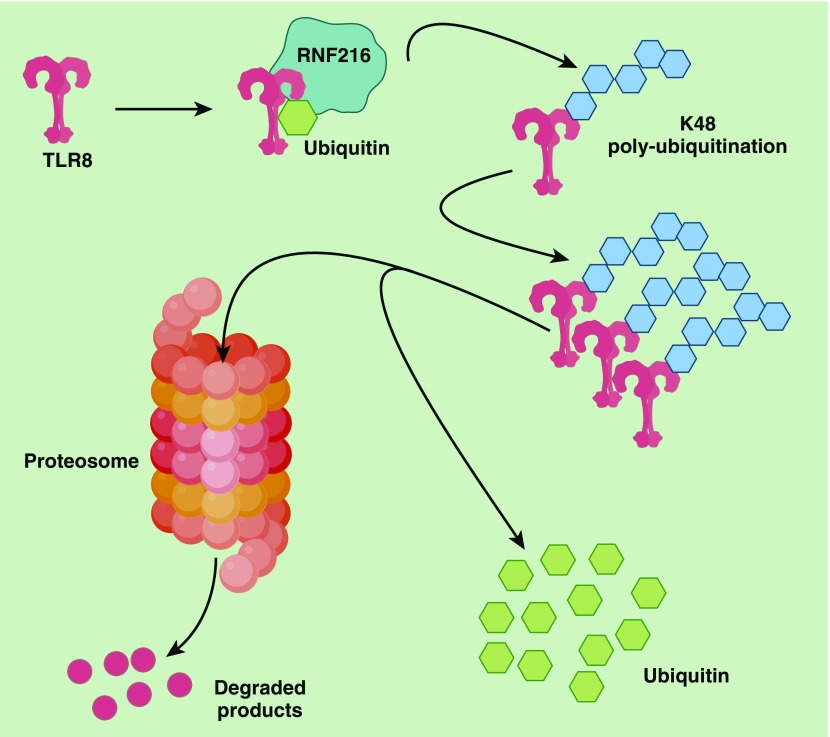
Figure 1.
TLR8 (Toll-like receptor 8) degradation directed by the E3 ligase RNF216. The E3 ligase RNF216 attaches ubiquitin onto the target protein (TLR8), which is followed by K48 polyubiquitination of TLR8. Polyubiquitinated TLR8 is ultimately degraded by the proteosome. RNF216 = Ring Finger protein 216.
The finding that the presence of miRNAs in plasma from patients with ARDS coincided with the activation of TLR8 signaling and downregulation of the E3 ubiquitin-protein ligase RNF216 in critically ill patients or patients with ARDS (9) could have clinical implications. Reduced expression of RNF216 in these patients could contribute to the prolonged stability of TLR8, thereby aggravating inflammation. However, these in vitro observations need be replicated in an in vivo model of acute lung injury to determine their true clinical relevance. In support of the results of this study, an endogenous ligand (miR-21) was previously found to activate TLR8 to induce neuropathic pain in murine dorsal root ganglion (14). Although TLR8 has long been considered to be nonfunctional in mice (15), recent studies have reported it to be functional (14). Thus, despite the fact that human TLR8 is known to be functional, it is still debatable whether murine TLR8 is functional.
In conclusion, the current article demonstrates that 1) TLR8 is a short-lived protein that is proteasomally degraded via RNF216 after activation by RNA ligands that may control TLR8 levels in human cells to reduce excessive innate immune responses in ARDS, and 2) TLR8 stimulation by circulating RNA may be an important contributor of unwanted innate immunity leading to ARDS. Thus, it is critical to understand the mechanisms that govern the intensity and duration of TLR protein activation in the context of ARDS so that we can design better treatment and prevention methods to mitigate organ damage.
Supplementary Material
Footnotes
Originally Published in Press as DOI: 10.1165/rcmb.2019-0272ED on August 15, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med. 2017;377:562–572. doi: 10.1056/NEJMra1608077. [DOI] [PubMed] [Google Scholar]
- 2.Malhotra A. Low-tidal-volume ventilation in the acute respiratory distress syndrome. N Engl J Med. 2007;357:1113–1120. doi: 10.1056/NEJMct074213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baral P, Batra S, Zemans RL, Downey GP, Jeyaseelan S. Divergent functions of Toll-like receptors during bacterial lung infections. Am J Respir Crit Care Med. 2014;190:722–732. doi: 10.1164/rccm.201406-1101PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ravi Kumar S, Paudel S, Ghimire L, Bergeron S, Cai S, Zemans RL, et al. Emerging roles of inflammasomes in acute pneumonia. Am J Respir Crit Care Med. 2018;197:160–171. doi: 10.1164/rccm.201707-1391PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanji H, Ohto U, Shibata T, Taoka M, Yamauchi Y, Isobe T, et al. Toll-like receptor 8 senses degradation products of single-stranded RNA. Nat Struct Mol Biol. 2015;22:109–115. doi: 10.1038/nsmb.2943. [DOI] [PubMed] [Google Scholar]
- 6.Londino JD, Gulick DL, Lear TB, Suber TL, Weathington NM, Masa LS, et al. Post-translational modification of the interferon-gamma receptor alters its stability and signaling. Biochem J. 2017;474:3543–3557. doi: 10.1042/BCJ20170548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evankovich J, Lear T, Mckelvey A, Dunn S, Londino J, Liu Y, et al. Receptor for advanced glycation end products is targeted by FBXO10 for ubiquitination and degradation. FASEB J. 2017;31:3894–3903. doi: 10.1096/fj.201700031R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li L, Wei J, Mallampalli RK, Zhao Y, Zhao J.TRIM21 mitigates human lung microvascular endothelial cells’ inflammatory responses to LPS Am J Respir Cell Mol Biol 201961776–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evankovich J, Lear T, Baldwin C, Chen Y, White V, Villandre J, et al. Toll-like receptor 8 stability is regulated by ring finger 216 in response to circulating microRNAs Am J Respir Cell Mol Biol 202062157–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chuang TH, Ulevitch RJ. Triad3A, an E3 ubiquitin-protein ligase regulating Toll-like receptors. Nat Immunol. 2004;5:495–502. doi: 10.1038/ni1066. [DOI] [PubMed] [Google Scholar]
- 11.Xu C, Feng K, Zhao X, Huang S, Cheng Y, Qian L, et al. Regulation of autophagy by E3 ubiquitin ligase RNF216 through BECN1 ubiquitination. Autophagy. 2014;10:2239–2250. doi: 10.4161/15548627.2014.981792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Margolin DH, Kousi M, Chan YM, Lim ET, Schmahmann JD, Hadjivassiliou M, et al. Ataxia, dementia, and hypogonadotropism caused by disordered ubiquitination. N Engl J Med. 2013;368:1992–2003. doi: 10.1056/NEJMoa1215993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Husain N, Yuan Q, Yen YC, Pletnikova O, Sally DQ, Worley P, et al. TRIAD3/RNF216 mutations associated with Gordon Holmes syndrome lead to synaptic and cognitive impairments via Arc misregulation. Aging Cell. 2017;16:281–292. doi: 10.1111/acel.12551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang ZJ, Guo JS, Li SS, Wu XB, Cao DL, Jiang BC, et al. TLR8 and its endogenous ligand miR-21 contribute to neuropathic pain in murine DRG. J Exp Med. 2018;215:3019–3037. doi: 10.1084/jem.20180800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.