Abstract
Idiopathic pulmonary fibrosis (IPF) is a devastating disease characterized as progressive and irreversible fibrosis in the interstitium of lung tissues. There is still an unmet need to develop a novel therapeutic drug for IPF. We have previously demonstrated that periostin, a matricellular protein, plays an important role in the pathogenesis of pulmonary fibrosis. However, the underlying mechanism of how periostin causes pulmonary fibrosis remains unclear. In this study, we sought to learn whether the cross-talk between TGF-β (transforming growth factor-β), a central mediator in pulmonary fibrosis, and periostin in lung fibroblasts leads to generation of pulmonary fibrosis and whether inhibitors for integrin αVβ3, a periostin receptor, can block pulmonary fibrosis in model mice and the TGF-β signals in fibroblasts from patients with IPF. We found that cross-talk exists between TGF-β and periostin signals via αVβ3/β5 converging into Smad3. This cross-talk is necessary for the expression of TGF-β downstream effector molecules important for pulmonary fibrosis. Moreover, we identified several potent integrin low-molecular-weight inhibitors capable of blocking cross-talk with TGF-β signaling. One of the compounds, CP4715, attenuated bleomycin-induced pulmonary fibrosis in vivo in mice and the TGF-β signals in vitro in fibroblasts from patients with IPF. These results suggest that the cross-talk between TGF-β and periostin can be targeted for pulmonary fibrosis and that CP4715 can be a potential therapeutic agent to block this cross-talk.
Keywords: transforming growth factor-β, periostin, integrin, idiopathic pulmonary fibrosis, inhibitor
Idiopathic pulmonary fibrosis (IPF) is a disease of unknown etiology characterized as progressive and irreversible fibrosis in the interstitium of lung tissue (1). IPF is a devastating disease; the median survival of patients with IPF is 3–5 years. Only two drugs, pirfenidone and nintedanib, have thus far been approved by the U.S. Food and Drug Administration for the treatment of IPF. However, the efficacy of these drugs is limited. Therefore, there is still an unmet need to develop an effective therapeutic drug for treating IPF.
TGF-β (transforming growth factor-β) is a central mediator in the pathogenesis of pulmonary fibrosis (2–4). It enhances the differentiation of fibroblasts into myofibroblasts, the synthesis of extracellular matrix (ECM) proteins, the inhibition of matrix degradation by matrix metalloproteinases, and the epithelial-to-mesenchymal transition. In contrast, it is known that TGF-β is a pleiotropic cytokine that has various physiological roles, including immunosuppressive effects (5). TGF-β1–deficient mice die soon after birth with autoimmune disorder–like lesions (6). Although a phase 1 study of administration of anti–TGF-β antibody (Ab; fresolimumab, GC1008) to patients with IPF was initiated (NCT00125385), its development was halted without explanation. Therefore, identifying an approach to blocking the TGF-β signals important for pulmonary fibrosis without interfering with the physiological functions of TGF-β would be promising for developing therapeutic drugs for pulmonary fibrosis.
Periostin (encoded by the POSTN gene) is a matricellular protein belonging to the fasciclin family (7). Periostin exerts its actions by binding with several integrin molecules on the surface of target cells. It is expressed and accumulates in the lesions of many inflammatory and fibrotic diseases (7). In patients with IPF, periostin is highly expressed in lung tissues, particularly in fibroblastic foci, probably via localized TGF-β induction (8, 9). Elevated serum periostin in patients with IPF is well correlated with decline of lung function, suggesting that serum periostin is a biomarker for IPF, predicting a poor prognosis (8, 10). Moreover, we found that periostin-deficient mice were protected from bleomycin (BLM)-induced pulmonary fibrosis (11). These results suggest that periostin is a key effector molecule in the pathogenesis of pulmonary fibrosis. However, the underlying mechanism by which periostin triggers lung fibrosis remains unclear.
In this study, we sought to determine whether the cross-talk between TGF-β and periostin specifically results in the generation of pulmonary fibrosis. Taking advantage of this known aspect of cross-talk between TGF-β and periostin, we searched for inhibitors of integrin αVβ3, a periostin receptor, to potentially block TGF-β and periostin cross-talk and then examined whether such an integrin αVβ3 inhibitor would also block the TGF-β signals followed by impairment of pulmonary fibrosis.
Methods
Cell Culture
The details of the purchased cells are described in the data supplement. Four strains of fibroblasts were cultured from the explanted lungs of patients with IPF undergoing lung transplant as we previously described (12, 13). IPF lung tissues were obtained under a protocol approved by the University of Pittsburgh Institutional Review Board.
RNAi-mediated Knockdown
The details of the RNAi-mediated knockdown method are described in the data supplement.
DNA Microarray Analysis
The details of the DNA microarray analysis method are described in the data supplement.
qRT-PCR
The details of the qRT-PCR method are described in the data supplement. Primers for qRT-PCR are described in Table E1 in the data supplement.
ELISA
The details of the ELISA method are described in the data supplement.
Transient Transfection
The details of the transient transfection method are described in the data supplement. All gene modification experiments were performed after obtaining approval of the safety committee of Saga University (30-05-1).
Western Blotting
The details of the western blotting method are described in the data supplement.
Generation of Stable TGF-β Reporter Cell Lines and Luciferase Assay
The details of this method are described in the data supplement.
Flow Cytometry
The details of the flow cytometry method are described in the data supplement.
Integrin αVβ3 Inhibitors
Integrin inhibitors and their derivatives were prepared as previously described (14–17).
Cell Adhesion Assay
Cell adhesion assay was performed as previously described (18, 19). The details of the cell adhesion assay method are described in the data supplement.
Immunohistochemistry
The details of the immunohistochemistry method are described in the data supplement.
Confocal Microscopy
The details of the confocal microscopy method are described in the data supplement.
Mice
All experiments were performed following the guidelines for care and use of experimental animals required by the Japanese Association for Laboratory Animal Science (1987) and were approved by the Saga University Animal Care and Use Committee (Saga, Japan; 27-040-0). The details of the used mice and the method for generation of lung fibrosis are described in the data supplement.
Hydroxyproline Measurement
The details of the hydroxyproline measurement method are described in the data supplement.
Lung Tissues
The lung tissues used for immunohistochemistry were prepared from four patients with usual interstitial pneumonia (UIP) and two healthy subjects. Four patients were histologically diagnosed with UIP at Nippon Medical School. Three were male, and one was female. Their ages ranged from 48 to 73 years. The lung tissues were obtained from two healthy subjects undergoing surgery for pneumothorax. Both were male, ages 50 and 83 years, respectively.
Statistical Analysis
Results are presented as mean ± SD. Statistical analyses were performed using the unpaired Student’s t test or log-rank test. P < 0.05 was considered statistically significant.
Results
Identification of Signature Molecules of Pulmonary Fibrosis Induced by the Cross-Talk between TGF-β and Periostin in Lung Fibroblasts
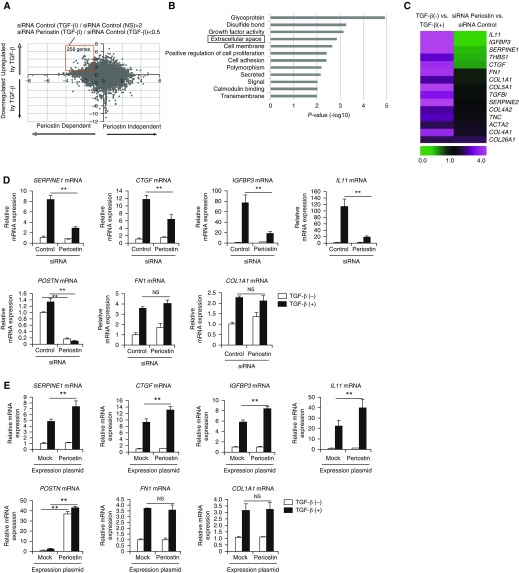
To explore the possibility that cross-talk exists between TGF-β and periostin in lung fibroblasts leading to pulmonary fibrosis, we first compared the gene expression profiles of normal human lung fibroblasts with or without TGF-β stimulation and with or without periostin knockdown using DNA microarray analysis (Figure 1A). On the basis of upregulation by stimulation of TGF-β and downregulation by POSTN knockdown, we divided the genes into four groups (Figure 1A): 1) TGF-β inducible and periostin dependent, 2) TGF-β inducible and periostin independent, 3) TGF-β noninducible and periostin dependent, and 4) TGF-β noninducible and periostin independent. From the upper left panel in Figure 1A, we selected 258 genes upregulated by more than twofold with TGF-β stimulation and downregulated by less than half with periostin knockdown as the definite cross-talk genes between TGF-β and periostin (red box in Figure 1A).
Figure 1.
Identification of signature molecules of pulmonary fibrosis induced by the cross-talk between TGF-β (transforming growth factor-β) and periostin in lung fibroblasts. (A) A dot plot of the genes showing dependency of either TGF-β or periostin analyzed by DNA microarray. We incubated normal human lung fibroblasts (NHLFs) with or without 1 ng/ml of TGF-β for 24 hours in the presence of 5 nM of either control siRNA or periostin siRNA, then subjected them to DNA microarray. The longitudinal axis represents the induction of the genes upregulated or downregulated by TGF-β. The horizontal axis represents the induction of the genes upregulated or downregulated by siRNA for periostin. The red square represents the genes upregulated by TGF-β by more than twofold and downregulated by periostin knockdown by less than half (258 genes). (B) Highly ranked Gene Ontology terms in the definite cross-talk genes between TGF-β and periostin in lung fibroblasts are depicted. “Extracellular space” is boxed. (C) Heat maps of the genes in the Gene Ontology term “extracellular space” are depicted. The ratios of control siRNA/TGF-β− to control siRNA/TGF-β+ (left) and control siRNA/TGF-β+ to periostin siRNA/TGF-β+ (right) are shown. (D) Effects of periostin knockdown on expression at mRNA levels of SERPINE1, CTGF, IGFBP3, IL11, POSTN, FN1, and COL1A1. NHLFs were stimulated with or without 1 ng/ml of TGF-β for 24 hours in the presence of control or periostin siRNA (n = 3). The values were adjusted by GAPDH expression. (E) MRC-5 cells were transiently transfected with the expression plasmid encoding periostin and then treated with or without 3 ng/ml of TGF-β for 24 hours. The mRNA expression of SERPINE1, CTGF, IGFBP3, IL11, POSTN, FN1, and COL1A1 is depicted. The values were adjusted by GAPDH expression. The same experiments were performed three times. **P < 0.01. NS = not significant.
We then applied the DAVID tool to the definite cross-talk genes between TGF-β and periostin to enrich biological functions in this group. In Gene Ontology terms highly correlated with TGF-β–inducible and periostin-dependent genes, we focused on “extracellular space” (a Gene Ontology term) because genes related to fibrosis were involved in this group (Figure 1B). The extracellular space group contained SERPINE1 (serpin family E member 1)/PAI-1, CCN2/CTGF (CCN family member 2/connective tissue growth factor), IGFBP-3 (insulin-like growth factor binding protein 3), and IL-11 (Figure 1C), all of which have already been shown to be highly expressed in patients with IPF (13, 20–23) and to individually play important roles in the pathogenesis of pulmonary fibrosis (24–29). In contrast, some TGF-β–inducible molecules, such as Col1α1 (collagen, type I, α1) and FN1 (fibronectin), known to be correlated with pulmonary fibrosis (30), were not downregulated by periostin knockdown and were classified as TGF-β–inducible and periostin-independent genes. In the following experiments, we denoted SERPINE1, CTGF, IGFBP-3, and IL-11 as signature molecules of pulmonary fibrosis induced by the cross-talk between TGF-β and periostin.
We investigated the requirement of periostin for expression of the signature molecules of pulmonary fibrosis induced by the cross-talk between TGF-β and periostin. TGF-β upregulated expression of all investigated molecules at the mRNA level―SERPINE1, CTGF, IGFBP3, IL11 (IL-11), FN1, and COL1A1 (collagen, type I, α1)―in both normal human lung fibroblasts and MRC-5 cells (Figures 1D and E1), whereas periostin knockdown downregulated expression of SERPINE1, CTGF, IGFBP3, and IL11, but not expression of FN1 and COL1A1, at mRNA levels and expression of IGFBP3 and IL-11 at protein levels.
We next examined the reciprocal effects of periostin on expression of the signature molecules of pulmonary fibrosis in lung fibroblasts by forced expression of periostin. We transfected the plasmid-encoding human periostin or control plasmid into MRC-5 cells followed by stimulation with TGF-β. Exogenous overexpression of periostin enhanced expression of SERPINE1, CTGF, IGFBP3, and IL11, but not of FN1 or COL1A1, compared with control-transfected cells stimulated by TGF-β at mRNA levels and expression of IGFBP3 and IL-11 at protein levels (Figures 1E and E1). These results confirm that periostin is required for the expression of the signature molecules of pulmonary fibrosis induced by the cross-talk between TGF-β and periostin in lung fibroblasts.
Activation of Smad3 by the Cross-Talk between TGF-β and Periostin
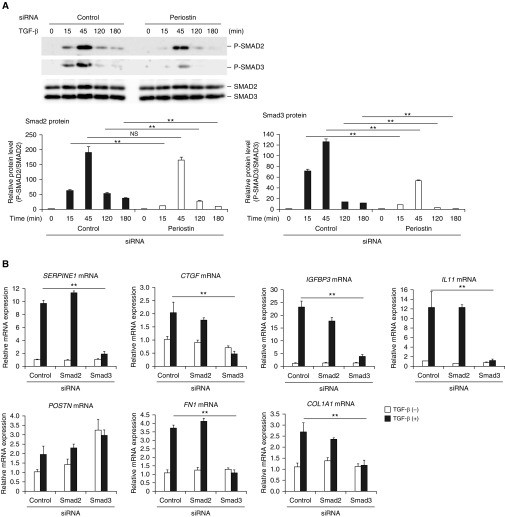
We explored how the signal pathways of periostin and TGF-β interact with each other. To do so, we examined whether activation of Smads, transcription factors critical for TGF-β signaling, was affected by the periostin signals. Knockdown of periostin slightly decreased TGF-β–induced phosphorylation of Smad2 and significantly decreased phosphorylation of Smad3 (Figure 2A), whereas overexpression of periostin upregulated phosphorylation of Smad3 (Figure E2), suggesting that the cross-talk between TGF-β and periostin signals merges upstream of Smad2/3 and that activation of Smad2/3 is affected by periostin signaling.
Figure 2.
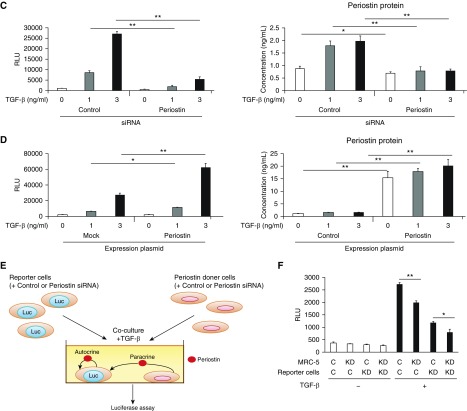
Activation of Smad3 by the cross-talk between TGF-β and periostin in lung fibroblasts. (A) Effects of periostin knockdown on Smad2 and Smad3 phosphorylation. MRC-5 cells were stimulated with 3 ng/ml of TGF-β for the indicated times in the presence of control siRNA or periostin siRNA. Densitometric quantification of Smad2 and Smad3 phosphorylation. The data were normalized to total Smad2 or Smad3 and expressed as the fold change compared with the first group at time 0. The same experiments were performed three times. (B) Effects of knockdown of either Smad2 or Smad3 on expression of SERPINE1, CTGF, IGFBP3, IL11, POSTN, FN1, and COL1A1 mRNA. MRC-5 cells were stimulated with or without 3 ng/ml of TGF-β for 24 hours in the presence of control siRNA or siRNA for Smad2 or Smad3 (n = 3). The values were adjusted by GAPDH expression. (C and D) Effect of knockdown (C) or overexpression (D) of periostin on the luciferase (Luc) activity in the reporter cells and periostin concentrations in the cell supernatants. The reporter cells were stimulated with 3 ng/ml of TGF-β in the presence of control siRNA or siRNA for periostin (C) or in the presence of control plasmid or the expression plasmid encoding periostin (D). All experiments were performed three times. (E) A scheme of the coculture system using the reporter cells. (F) Either periostin-producing cells (MRC-5 cells) or Smad3-binding element (SBE)-luciferase reporter cells were transfected with siRNA for control (C) or periostin (KD) and then cocultured at the ratio of 3:1. Cocultured cells were stimulated with or without 3 ng/ml of TGF-β for 24 hours, and luciferase assay was performed. All experiments were performed three times. *P < 0.05 and **P < 0.01. RLU = relative luminescence units.
We next evaluated which Smad, Smad2 or 3, was more important for the expression of the signature molecules of pulmonary fibrosis induced by the cross-talk between TGF-β and periostin using knockdown of either Smad2 or 3. Knockdown of Smad3, but not of Smad2, downregulated expression of SERPINE1, CTGF, IGFBP3, and IL11 (Figure 2B), suggesting that activation of Smad3 rather than Smad2 is more important for expression of the signature molecules of pulmonary fibrosis induced by this cross-talk.
To evaluate the effects of the cross-talk on Smad3 activation, we stably transfected a luciferase gene under the control of Smad3-binding element into WI-26 VA4 cells. Consistent with the data on Smad3 phosphorylation, the luciferase activity induced by TGF-β was downregulated by periostin knockdown (Figure 2C). Conversely, plasmid-mediated expression of periostin enhanced the luciferase activity (Figure 2D). These results demonstrate that the reporter cells detect Smad3 activation by the cross-talk between TGF-β and periostin.
We then examined the level of activity of periostin on target cells with a coculture system (Figure 2E). In this system, we cocultured the reporter cells with periostin-producing cells and measured the luciferase activity. We evaluated the effects of periostin produced in a paracrine or autocrine manner by knockdown of periostin in the periostin-producing cells or the reporter cells, respectively. Knockdown of periostin in both types of cells decreased luciferase activity (Figure 2F). These results demonstrate that periostin secreted from the producing cells or the reporter cells acts on target cells in both a paracrine and an autocrine manner.
Downregulation of the TGF-β Signals in BLM-challenged Postn−/− Mice
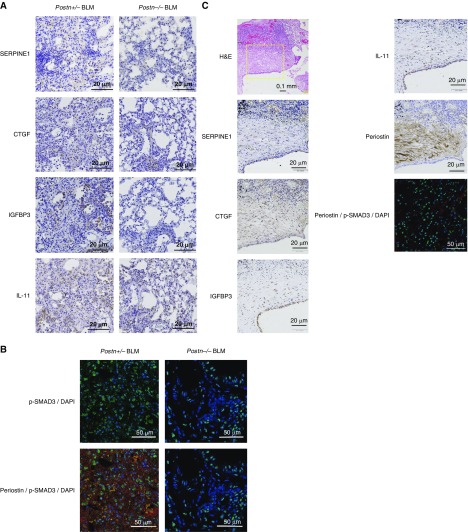
Although we previously demonstrated that a genetic deficiency of periostin impaired BLM-induced pulmonary fibrosis and improved survival in mice (11), it was unclear whether the TGF-β signal was downregulated in these mice. Therefore, we examined expression of the signature molecules of pulmonary fibrosis—SERPINE1, CTGF, IGFBP-3, and IL-11—and activation of Smad3 using BLM-challenged, periostin-deficient mice (Figures 3A and 3B). In BLM-treated Postn+/− mice, increased expression of SERPINE1, CTGF, IGFBP-3, and IL-11 was seen in reactive bronchial/pulmonary epithelial cells and, in the case of CTGF, also in the fibrotic regions. Moreover, phosphorylation and nuclear localization of Smad3 were upregulated in BLM-administered Postn+/− mice. In contrast, all of these changes were decreased in periostin-deficient mice in accordance with decreased fibrosis. These results are consistent with the in vitro finding that the cross-talk between TGF-β and periostin leads to expression of the signature molecules of pulmonary fibrosis via Smad3.
Figure 3.
Downregulation of the TGF-β signals in bleomycin (BLM)-challenged Postn−/− mice, expression of signature molecules of pulmonary fibrosis and periostin, and activation of Smad3 in the lung tissues of patients with idiopathic pulmonary fibrosis (IPF). (A and B) BLM was administered intratracheally into periostin-deficient (Postn−/−) mice or their heterozygous littermates (Postn+/−) on Day 0. Lung tissue was prepared on Day 21. Expression of signature molecules of pulmonary fibrosis―SERPINE1, CTGF, IGFBP-3, and IL-11 (A)―and phosphorylated Smad3 (B) in the lung tissues of BLM-administered mice are depicted. The photographs of phosphorylated Smad3 (green) and DAPI (blue) with or without periostin (red) have been merged. Scale bars: 20 μm and 50 μm. (C) Hematoxylin and eosin (H&E) staining and immunostaining of SERPINE1, CTGF, IGFBP-3, IL-11, periostin, and phosphorylated Smad3 of the lung tissues of patients with usual interstitial pneumonia (UIP). Four patients with UIP were investigated; representative data are shown. The immunostains are high-magnitude images of the boxed region in the H&E staining. The photographs of phosphorylated Smad3 (green), DAPI (blue), and periostin (red) have been merged. Scale bars: 0.1 mm, 20 μm and 50 μm.
Expression of Signature Molecules of Pulmonary Fibrosis and Periostin and Activation of Smad3 in the Lung Tissues of Patients with IPF
We next confirmed the enhanced expression of pulmonary fibrosis―SERPINE1, CTGF, IGFBP-3, and IL-11―in the lung tissues of patients with IPF (Figure 3C). SERPINE1, IGFBP-3, and IL-11 were all clearly expressed in reactive bronchial/pulmonary epithelial cells, as well as in fibroblasts in the case of CTGF and IGFBP-3, in patients with UIP. CTGF was expressed in the interstitium, particularly in fibroblastic foci, in patients with UIP. Periostin was strongly expressed in the interstitium of the lung tissues, particularly in fibroblastic foci, of patients with UIP as previously described (8). Moreover, significant phosphorylation and nuclear localization of Smad3 was observed in patients with UIP. Periostin was well colocalized with the cells that had phosphorylated Smad3 (Figure 3C). All of these molecules and activation of Smad3 were not or were only minimally observed in the lung tissues of healthy subjects. These results are consistent with the in vitro and model mouse data.
Requirement of Integrin αvβ3/β5 for Cross-Talk between TGF-β and Periostin
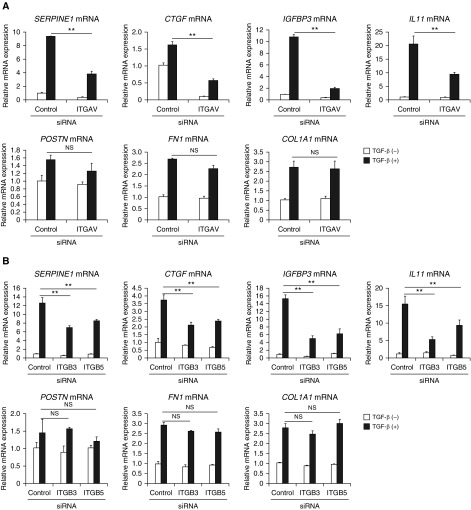
It is still controversial which integrin is being used for the periostin receptor (31). We previously found that αVβ3 integrin is the main receptor for periostin and that αVβ5 integrin acts as a complementary receptor in keratinocytes (32). Therefore, we examined the requirement for αVβ3/β5 integrin in the cross-talk between TGF-β and periostin in lung fibroblasts by knockdown of integrin αV and αV-coupled β3 and β5 integrins. We confirmed expression of αVβ3/β5 integrin on MRC-5 and WI-26 VA4 cells (Figure E3). Knockdown of integrin αV significantly, and knockdown of integrin β3 or β5 partially and almost equally, downregulated expression of SERPINE1, CTGF, IGFBP3, and IL11, but not of FN1 or COL1A1 (Figures 4A and 4B). These results suggest that periostin uses mainly αV and almost equally β3 and β5 as its partners for the cross-talk with TGF-β in lung fibroblasts.
Figure 4.
Requirement of integrin αvβ3/β5 for the cross-talk between TGF-β and periostin in lung fibroblasts. (A and B) Effects of knockdown of integrin αV (A) and integrin β3 or β5 (B) on expression of SERPINE1, CTGF, IGFBP3, IL11, POSTN, FN1, and COL1A1. MRC-5 cells were stimulated with or without 3 ng/ml of TGF-β for 24 hours in the presence of control siRNA or integrin αV (ITGAV) or integrin β3 (ITGB3) or β5 (ITGB5) siRNA (n = 3). The values were adjusted by GAPDH expression. The same experiments were performed three times. **P < 0.01.
Search for Integrin αVβ3 Inhibitors That Block the Cross-Talk between TGF-β and Periostin
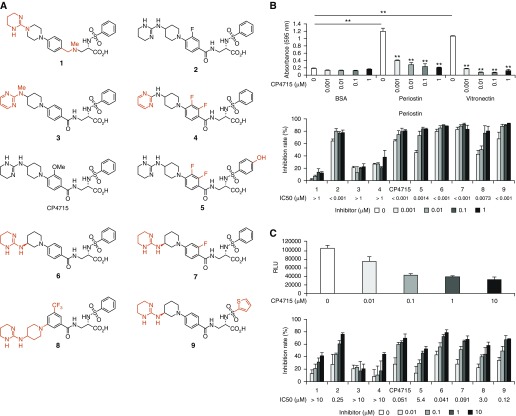
Given that the cross-talk between TGF-β and periostin in lung fibroblasts is important for expression of the signature molecules of pulmonary fibrosis, we searched for integrin αVβ3 inhibitors to downregulate the TGF-β signals cross-talking with periostin. We evaluated the inhibitory effects of 10 compounds that had previously shown inhibitory activities against integrin and their derivatives on the adhesion of periostin to integrin αVβ3 and on the luciferase activity in the reporter cells harboring the Smad3-binding element–conjugated luciferase gene (References 14–17 and Figure 5A). We confirmed expression of αVβ3/β5 integrin on SW480 cells or SW480 cells transfected with integrin β3 used for an adhesion assay (Figure E4). Inhibition of the adhesion activity showed almost the same trend as that of the luciferase activity, suggesting that inhibiting the binding of periostin to integrin αVβ3 leads to blockade of the TGF-β signals (Figures 5B and 5C). It is of note that the half-maximal inhibitory concentration values for the luciferase activity were almost 10-fold higher than those of the adhesion activity.
Figure 5.
Search of integrin inhibitors to inhibit the cross-talk between TGF-β and periostin. (A) Structures of integrin inhibitor–related molecules. The different portions from CP4715 are depicted in red. (B and C) Effects of each compound on the adhesion of periostin or vitronectin to integrin αVβ3 (B) or on the luciferase activity in the reporter cells harboring the SBE-conjugated luciferase gene (C). Half-maximal inhibitory concentration values of each compound are depicted. **P < 0.01.
The inhibitory effect on the adhesion of periostin to integrin αVβ3 was compound 2 = CP4715 = 6 = 7 = 9 > 5 > 8 > > 1 = 3 = 4, whereas the inhibitory effect on the luciferase activity was CP4715 = 6 > 7 = 9 > 2 > 8 > 5 > >1 = 3 = 4 (Figures 5B and 5C). These data suggest that a tetrahydropyrimidin-2-yl-amino moiety at the N-terminus and a benzoyl moiety at the center of a molecule are required to block cross-talk. Taken together, those integrin antagonistic derivatives show blockade of cross-talk between TGF-β and periostin in proportion to the antagonistic activities against integrin αVβ3.
In Vivo Attenuation of BLM-induced Pulmonary Fibrosis by Integrin αVβ3 Inhibitor CP4715
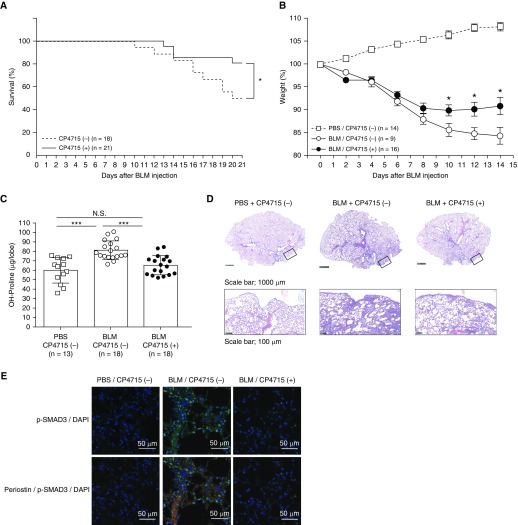
Given that several integrin inhibitors downregulate the TGF-β signals, we explored the possibility that blocking the periostin/integrin pathway would lead to impairment of pulmonary fibrosis in vivo. We chose CP4715, one of the integrin inhibitors showing strong inhibitory effects on the TGF-β signals in vitro. We administered CP4715 continuously into the mice using osmotic pumps implanted in their backs (Figure E5), and we confirmed that CP4715 was administered from the pumps up to Day 10. When we followed the mice 3 weeks after BLM administration, half of the control mice had died, whereas CP4715-treated mice showed a better survival rate (50% vs. 80%; P < 0.05) (Figure 6A). Accordingly, treatment with CP4715 reduced BLM-induced weight loss up to 14 days after BLM administration (85.6% vs. 89.9%, 90.1% vs. 84.7%, and 90.8% vs. 84.2% at Days 10, 12, and 14, respectively; P < 0.05) (Figure 6B). Moreover, when we examined hydroxyproline content on Day 10, treatment with CP4715 significantly reduced the BLM-induced increase of hydroxyproline (82.0 ± 10.8 μg vs. 66.5 ± 11.0 μg; P < 0.001) (Figure 6C). Histological and gross examinations showed that CP4715 attenuated pulmonary fibrosis and activation of Smad3 (Figures 6D, 6E, and E6). These results demonstrate the beneficial effects of CP4715 on BLM-induced pulmonary fibrosis in vivo.
Figure 6.
Effect of CP4715 on BLM-induced pulmonary fibrosis in mice. Nine-week-old C57BL/6 mice and periostin-deficient (Postn−/−) mice or their heterozygous littermates (Postn+/−) weighing less than 28.5 g were used. Postn−/− mice were prepared as previously described (11). Murine lung fibrosis was induced by a single administration of 3 mg/kg of BLM via the oropharyngeal aspiration route on Day 0. Two osmotic pumps filled with 200 μl of CP4715 (50 mg/ml) or an equal volume of vehicle (50% DMSO) were subcutaneously implanted into C57BL/6 mice at 5 days before BLM instillation, and CP4715 was released at 0.5 μl/h. (A–E) The mortality (A) and weight loss (B) of the mice were evaluated 3 weeks or 2 weeks after BLM administration, respectively, and hydroxyproline measurement (C), Masson trichrome staining (D), and immunostaining with indicated antibodies (E) were performed on Day 10. In D, the upper and lower lines represent low and high magnifications, respectively. The boxes represent the portions shown in the high magnifications. Scale bars: 1,000 μm and 100 μm. In E, the photographs of phosphorylated Smad3 (green) and DAPI (blue) with or without periostin (red) have been merged. *P < 0.05 and ***P < 0.001. Scale bars: 50 μm.
In Vitro Attenuation of the TGF-β Signals in Lung Fibroblasts Derived from Patients with IPF
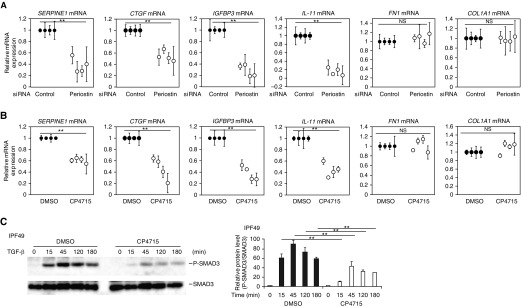
We then examined whether CP4715 downregulates the TGF-β signals in lung fibroblasts derived from patients with IPF. We confirmed that even in lung fibroblasts derived from patients with IPF, expression of SERPINE1, CTGF, IGFBP3, and IL11, but not of FN1 and COL1A1, is dependent on periostin by periostin knockdown (Figure 7A). CP4715 significantly decreased expression of SERPINE1, CTGF, IGFBP3, and IL11, but not of FN1 and COL1A1, in lung fibroblasts derived from all four patients with IPF (Figure 7B). Moreover, CP4715 also decreased phosphorylation of both Smad2 and Smad3 induced by TGF-β in these cells (Figures 7C and E7). These results support the potentially beneficial effects of CP4715 in patients with IPF.
Figure 7.
Effect of CP4715 on TGF-β signals in lung fibroblasts derived from patients with IPF. (A–C) Effects of periostin knockdown (A) or CP4715 (B and C) on expression at mRNA levels of SERPINE1, CTGF, IGFBP3, IL11, POSTN, FN1, and COL1A1 (A and B) or on phosphorylation of Smad3 (C). Lung fibroblasts derived from four patients with IPF were stimulated with or without 1 ng/ml of TGF-β for 24 hours in the presence of control or periostin siRNA (A) or 1 μM CP4715 from 24 hours before the TGF-β stimulation (B and C). In C, the data of one clone of lung fibroblasts derived from patients with IPF (IPF49) are depicted. The values were adjusted by GAPDH expression (A and B). **P < 0.01.
Discussion
In this study, we demonstrated that the cross-talk between TGF-β and periostin in lung fibroblasts causes expression of several signature molecules of pulmonary fibrosis, such as SERPINE1, CTGF, IGFBP-3, and IL-11 (Figure E8). Smad3 and integrins αVβ3/β5 are involved in the cross-talk between the TGF-β and periostin signals. SERPINE1-deficient mice are protected from BLM-induced pulmonary fibrosis with less fibrin deposition (24). Deficiency of CTGF or administration of anti-CTGF Abs impairs BLM-induced skin or pulmonary fibrosis in mice (25, 26). IGFBP-3 exerts profibrotic effects independently of insulin-like growth factor, inducing expression of ECM proteins such as collagen type I, fibronectin, tenascin C, and syndecan 2 (13, 27, 29). Moreover, expression of IL-11 in fibroblasts alone is enough to induce the phenotypes of pulmonary fibrosis in mice (22). Thus, the cross-talk between TGF-β and periostin in lung fibroblasts would be critical for the onset of pulmonary fibrosis regulating expression of these signature molecules.
Interaction of the periostin signals with TGF-β signals has been reported in a fibrosis mechanism associated with scleroderma (33, 34), carcinogenesis (35), muscular dystrophy (36), bronchopulmonary dysplasia (37), and asthma (38). In this study, we elucidated that the periostin/integrin αVβ3/β5 pathway enhances the TGF-β/Smad2/3 pathway in lung fibroblasts, which leads to pulmonary fibrosis. Because periostin is a downstream effector molecule of TGF-β in lung fibroblasts, the cross-talk between TGF-β and periostin could be seen as an amplifying system of TGF-β via periostin. It was of great interest that there exist both periostin-dependent and periostin-independent genes in TGF-β–inducible group genes (Figure 1A). It is widely known that the TGF-β signals are balanced by canonical (Smad-based) and noncanonical (non–Smad-based) pathways (2, 3). There may be a gradation in the contribution of these pathways to the expression of TGF-β–inducible genes, and the expression of periostin-dependent genes may be more dependent on activation of Smad3 that is critical for pulmonary fibrosis (39). Alternatively, because it is known that Smad3 uses different cofactors for the targeted genes, periostin may differentially activate cofactors of Smad3 (40, 41).
Integrin αVβ6 binds to a latency-associated peptide of TGF-β, followed by its activation (42). Accordingly, anti–integrin αVβ6 Ab improved BLM-induced pulmonary fibrosis in mice (43). A phase 2 study of anti–integrin αVβ6 Ab (STX-100) for patients with IPF is now underway (NCT01371305). Our present study shows that integrin αVβ3 shows promise for developing therapeutic agents against pulmonary fibrosis. CP4715 or its related molecules have the potential to be such agents. Targeting integrin αVβ3 for pulmonary fibrosis would have the following advantages. First, the cross-talk between TGF-β and periostin widely controls the TGF-β signals, including expression of the signature molecules of pulmonary fibrosis depicted in the present study. Actually, anti-CTGF Ab (FG-3019) showed good efficacy in an open-label phase 2 trial in patients with IPF (44), and a double-blind phase 2 trial is now underway (NCT01890265). Targeting integrin αVβ3 would involve the blockade of CTGF. Second, Postn−/− mice manifested no immunological reactions (11, 32), and in this study, CP4715-treated mice showed no inflammatory reactions. These results suggest that targeting integrin αVβ3 is unlikely to seriously affect immunological reactions impacted by targeting TGF-β signals. In contrast, integrin αVβ3 has an ability to bind to several ligands other than periostin, such as vitronectin, fibrinogen, laminin, and collagen (45). In future studies, we will examine whether adverse effects could be caused by blocking the binding of other ligands to integrin αVβ3 and whether it will be advantageous to develop additional derivatives with increased selectivity for lung periostin.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Dr. Dovie R. Wylie for critical review of the manuscript and Prof. Yasuhiko Nishioka (Tokushima University) for critical comments. The authors also thank Tomoyo Yoshida, Maki Watanabe, and Takashi Arai for technical assistance.
Footnotes
Supported in part by the Japan Society for the Promotion of Science grant-in-aid for scientific research (KAKENHI) JP16H05343 (K.I.) and National Institutes of Health grant HL135657 (S.J.C.).
Author Contributions: Y.N., S.N., T.Y., and Y.H. performed the experiments and evaluated the data together with K.I. Y. Yokozaki prepared the transfectants for the SW480 cells. Y.T., Y. Yamaguchi, and C.F.-B. obtained lung tissues of patients with idiopathic pulmonary fibrosis and normal donor lung tissues and cultured primary lung fibroblasts. K.A. and S.M. prepared the integrin inhibitors. S.J.C. prepared the periostin-deficient mice. Y.N., S.J.C., and K.I. wrote the manuscript. All authors edited the manuscript.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2019-0245OC on September 10, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Martinez FJ, Collard HR, Pardo A, Raghu G, Richeldi L, Selman M, et al. Idiopathic pulmonary fibrosis. Nat Rev Dis Primers. 2017;3:17074. doi: 10.1038/nrdp.2017.74. [DOI] [PubMed] [Google Scholar]
- 2.Ask K, Martin GE, Kolb M, Gauldie J. Targeting genes for treatment in idiopathic pulmonary fibrosis: challenges and opportunities, promises and pitfalls. Proc Am Thorac Soc. 2006;3:389–393. doi: 10.1513/pats.200602-021TK. [DOI] [PubMed] [Google Scholar]
- 3.Chanda D, Otoupalova E, Smith SR, Volckaert T, De Langhe SP, Thannickal VJ. Developmental pathways in the pathogenesis of lung fibrosis. Mol Aspects Med. 2019;65:56–69. doi: 10.1016/j.mam.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-β: the master regulator of fibrosis. Nat Rev Nephrol. 2016;12:325–338. doi: 10.1038/nrneph.2016.48. [DOI] [PubMed] [Google Scholar]
- 5.Travis MA, Sheppard D. TGF-β activation and function in immunity. Annu Rev Immunol. 2014;32:51–82. doi: 10.1146/annurev-immunol-032713-120257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kulkarni AB, Huh CG, Becker D, Geiser A, Lyght M, Flanders KC, et al. Transforming growth factor β1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci USA. 1993;90:770–774. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Izuhara K, Nunomura S, Nanri Y, Ono J, Takai M, Kawaguchi A.Periostin: an emerging biomarker for allergic diseases Allergy 2019742116–2128 [DOI] [PubMed] [Google Scholar]
- 8.Okamoto M, Hoshino T, Kitasato Y, Sakazaki Y, Kawayama T, Fujimoto K, et al. Periostin, a matrix protein, is a novel biomarker for idiopathic interstitial pneumonias. Eur Respir J. 2011;37:1119–1127. doi: 10.1183/09031936.00059810. [DOI] [PubMed] [Google Scholar]
- 9.Takayama G, Arima K, Kanaji T, Toda S, Tanaka H, Shoji S, et al. Periostin: a novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J Allergy Clin Immunol. 2006;118:98–104. doi: 10.1016/j.jaci.2006.02.046. [DOI] [PubMed] [Google Scholar]
- 10.Ohta S, Okamoto M, Fujimoto K, Sakamoto N, Takahashi K, Yamamoto H, et al. Consortium for Development of Diagnostics for Pulmonary Fibrosis Patients (CoDD-PF) The usefulness of monomeric periostin as a biomarker for idiopathic pulmonary fibrosis. PLoS One. 2017;12:e0174547. doi: 10.1371/journal.pone.0174547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uchida M, Shiraishi H, Ohta S, Arima K, Taniguchi K, Suzuki S, et al. Periostin, a matricellular protein, plays a role in the induction of chemokines in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2012;46:677–686. doi: 10.1165/rcmb.2011-0115OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsu E, Shi H, Jordan RM, Lyons-Weiler J, Pilewski JM, Feghali-Bostwick CA. Lung tissues in patients with systemic sclerosis have gene expression patterns unique to pulmonary fibrosis and pulmonary hypertension. Arthritis Rheum. 2011;63:783–794. doi: 10.1002/art.30159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pilewski JM, Liu L, Henry AC, Knauer AV, Feghali-Bostwick CA. Insulin-like growth factor binding proteins 3 and 5 are overexpressed in idiopathic pulmonary fibrosis and contribute to extracellular matrix deposition. Am J Pathol. 2005;166:399–407. doi: 10.1016/S0002-9440(10)62263-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kubota D, Ishikawa M, Yamamoto M, Murakami S, Hachisu M, Katano K, et al. Tricyclic pharmacophore-based molecules as novel integrin αvβ3 antagonists. Part 1: design and synthesis of a lead compound exhibiting αvβ3/αIIbβ3 dual antagonistic activity. Bioorg Med Chem. 2006;14:2089–2108. doi: 10.1016/j.bmc.2005.10.060. [DOI] [PubMed] [Google Scholar]
- 15.Ishikawa M, Kubota D, Yamamoto M, Kuroda C, Iguchi M, Koyanagi A, et al. Tricyclic pharmacophore-based molecules as novel integrin α(v)β3 antagonists. Part 2: synthesis of potent αvβ3/αIIbβ3 dual antagonists. Bioorg Med Chem. 2006;14:2109–2130. doi: 10.1016/j.bmc.2005.10.061. [DOI] [PubMed] [Google Scholar]
- 16.Ishikawa M, Hiraiwa Y, Kubota D, Tsushima M, Watanabe T, Murakami S, et al. Tricyclic pharmacophore-based molecules as novel integrin αvβ3 antagonists. Part III: synthesis of potent antagonists with αvβ3/αIIbβ3 dual activity and improved water solubility. Bioorg Med Chem. 2006;14:2131–2150. doi: 10.1016/j.bmc.2005.10.055. [DOI] [PubMed] [Google Scholar]
- 17.Kubota D, Ishikawa M, Ishikawa M, Yahata N, Murakami S, Fujishima K, et al. Tricyclic pharmacophore-based molecules as novel integrin αvβ3 antagonists. Part IV: preliminary control of αvβ3 selectivity by meta-oriented substitution. Bioorg Med Chem. 2006;14:4158–4181. doi: 10.1016/j.bmc.2006.01.062. [DOI] [PubMed] [Google Scholar]
- 18.Yokosaki Y, Monis H, Chen J, Sheppard D. Differential effects of the integrins α9β1, αvβ3, and αvβ6 on cell proliferative responses to tenascin: roles of the β subunit extracellular and cytoplasmic domains. J Biol Chem. 1996;271:24144–24150. doi: 10.1074/jbc.271.39.24144. [DOI] [PubMed] [Google Scholar]
- 19.Ono J, Takai M, Kamei A, Nunomura S, Nanri Y, Yoshihara T, et al. Periostin forms a functional complex with IgA in human serum. Allergol Int. doi: 10.1016/j.alit.2019.05.014. [online ahead of print] 1 Jul 2019; DOI: 10.1016/j.alit.2019.05.014. [DOI] [PubMed] [Google Scholar]
- 20.Kotani I, Sato A, Hayakawa H, Urano T, Takada Y, Takada A. Increased procoagulant and antifibrinolytic activities in the lungs with idiopathic pulmonary fibrosis. Thromb Res. 1995;77:493–504. doi: 10.1016/0049-3848(95)00025-9. [DOI] [PubMed] [Google Scholar]
- 21.Allen JT, Knight RA, Bloor CA, Spiteri MA. Enhanced insulin-like growth factor binding protein-related protein 2 (connective tissue growth factor) expression in patients with idiopathic pulmonary fibrosis and pulmonary sarcoidosis. Am J Respir Cell Mol Biol. 1999;21:693–700. doi: 10.1165/ajrcmb.21.6.3719. [DOI] [PubMed] [Google Scholar]
- 22.Ng B, Dong J, Viswanathan S, D’Agostino G, Viswanathan S, Widjaja AA, Lim WW, et al. Interleukin-11 is a therapeutic target in idiopathic pulmonary fibrosis. Sci Transl Med. 2019;11:eaaw1237. doi: 10.1126/scitranslmed.aaw1237. [DOI] [PubMed] [Google Scholar]
- 23.Pan LH, Yamauchi K, Uzuki M, Nakanishi T, Takigawa M, Inoue H, et al. Type II alveolar epithelial cells and interstitial fibroblasts express connective tissue growth factor in IPF. Eur Respir J. 2001;17:1220–1227. doi: 10.1183/09031936.01.00074101. [DOI] [PubMed] [Google Scholar]
- 24.Eitzman DT, McCoy RD, Zheng X, Fay WP, Shen T, Ginsburg D, et al. Bleomycin-induced pulmonary fibrosis in transgenic mice that either lack or overexpress the murine plasminogen activator inhibitor-1 gene. J Clin Invest. 1996;97:232–237. doi: 10.1172/JCI118396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu S, Shi-wen X, Abraham DJ, Leask A. CCN2 is required for bleomycin-induced skin fibrosis in mice. Arthritis Rheum. 2011;63:239–246. doi: 10.1002/art.30074. [DOI] [PubMed] [Google Scholar]
- 26.Ponticos M, Holmes AM, Shi-Wen X, Leoni P, Khan K, Rajkumar VS, et al. Pivotal role of connective tissue growth factor in lung fibrosis: MAPK-dependent transcriptional activation of type I collagen. Arthritis Rheum. 2009;60:2142–2155. doi: 10.1002/art.24620. [DOI] [PubMed] [Google Scholar]
- 27.Brissett M, Veraldi KL, Pilewski JM, Medsger TA, Jr, Feghali-Bostwick CA. Localized expression of tenascin in systemic sclerosis-associated pulmonary fibrosis and its regulation by insulin-like growth factor binding protein 3. Arthritis Rheum. 2012;64:272–280. doi: 10.1002/art.30647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schafer S, Viswanathan S, Widjaja AA, Lim WW, Moreno-Moral A, DeLaughter DM, et al. IL-11 is a crucial determinant of cardiovascular fibrosis. Nature. 2017;552:110–115. doi: 10.1038/nature24676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruiz XD, Mlakar LR, Yamaguchi Y, Su Y, Larregina AT, Pilewski JM, et al. Syndecan-2 is a novel target of insulin-like growth factor binding protein-3 and is over-expressed in fibrosis. PLoS One. 2012;7:e43049. doi: 10.1371/journal.pone.0043049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsu E, Yasuoka H, Feghali-Bostwick CA. Gene expression in pulmonary fibrosis. Crit Rev Eukaryot Gene Expr. 2008;18:47–56. doi: 10.1615/critreveukargeneexpr.v18.i1.40. [DOI] [PubMed] [Google Scholar]
- 31.Izuhara K, Arima K, Ohta S, Suzuki S, Inamitsu M, Yamamoto K. Periostin in allergic inflammation. Allergol Int. 2014;63:143–151. doi: 10.2332/allergolint.13-RAI-0663. [DOI] [PubMed] [Google Scholar]
- 32.Masuoka M, Shiraishi H, Ohta S, Suzuki S, Arima K, Aoki S, et al. Periostin promotes chronic allergic inflammation in response to Th2 cytokines. J Clin Invest. 2012;122:2590–2600. doi: 10.1172/JCI58978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang L, Serada S, Fujimoto M, Terao M, Kotobuki Y, Kitaba S, et al. Periostin facilitates skin sclerosis via PI3K/Akt dependent mechanism in a mouse model of scleroderma. PLoS One. 2012;7:e41994. doi: 10.1371/journal.pone.0041994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kanaoka M, Yamaguchi Y, Komitsu N, Feghali-Bostwick CA, Ogawa M, Arima K, et al. Pro-fibrotic phenotype of human skin fibroblasts induced by periostin via modulating TGF-β signaling. J Dermatol Sci. 2018;90:199–208. doi: 10.1016/j.jdermsci.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 35.Ouanouki A, Lamy S, Annabi B. Periostin, a signal transduction intermediate in TGF-β-induced EMT in U-87MG human glioblastoma cells, and its inhibition by anthocyanidins. Oncotarget. 2018;9:22023–22037. doi: 10.18632/oncotarget.25153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lorts A, Schwanekamp JA, Baudino TA, McNally EM, Molkentin JD. Deletion of periostin reduces muscular dystrophy and fibrosis in mice by modulating the transforming growth factor-β pathway. Proc Natl Acad Sci USA. 2012;109:10978–10983. doi: 10.1073/pnas.1204708109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bozyk PD, Bentley JK, Popova AP, Anyanwu AC, Linn MD, Goldsmith AM, et al. Neonatal periostin knockout mice are protected from hyperoxia-induced alveolar simplication. PLoS One. 2012;7:e31336. doi: 10.1371/journal.pone.0031336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sidhu SS, Yuan S, Innes AL, Kerr S, Woodruff PG, Hou L, et al. Roles of epithelial cell-derived periostin in TGF-β activation, collagen production, and collagen gel elasticity in asthma. Proc Natl Acad Sci USA. 2010;107:14170–14175. doi: 10.1073/pnas.1009426107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flanders KC. Smad3 as a mediator of the fibrotic response. Int J Exp Pathol. 2004;85:47–64. doi: 10.1111/j.0959-9673.2004.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ikushima H, Miyazono K. TGF-β signal transduction spreading to a wider field: a broad variety of mechanisms for context-dependent effects of TGF-β. Cell Tissue Res. 2012;347:37–49. doi: 10.1007/s00441-011-1179-5. [DOI] [PubMed] [Google Scholar]
- 41.Morikawa M, Koinuma D, Miyazono K, Heldin CH. Genome-wide mechanisms of Smad binding. Oncogene. 2013;32:1609–1615. doi: 10.1038/onc.2012.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, et al. The integrin αvβ6 binds and activates latent TGF β1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 43.Horan GS, Wood S, Ona V, Li DJ, Lukashev ME, Weinreb PH, et al. Partial inhibition of integrin αvβ6 prevents pulmonary fibrosis without exacerbating inflammation. Am J Respir Crit Care Med. 2008;177:56–65. doi: 10.1164/rccm.200706-805OC. [DOI] [PubMed] [Google Scholar]
- 44.Raghu G, Scholand MB, de Andrade J, Lancaster L, Mageto Y, Goldin J, et al. FG-3019 anti-connective tissue growth factor monoclonal antibody: results of an open-label clinical trial in idiopathic pulmonary fibrosis. Eur Respir J. 2016;47:1481–1491. doi: 10.1183/13993003.01030-2015. [DOI] [PubMed] [Google Scholar]
- 45.Bennett JS, Berger BW, Billings PC. The structure and function of platelet integrins. J Thromb Haemost. 2009;7:200–205. doi: 10.1111/j.1538-7836.2009.03378.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.