Abstract
Disruption of alveolar–capillary barriers is a major complication of high-volume mechanical ventilation referred to as “ventilator-induced lung injury.” The stretching force in alveoli is transmitted to endothelial cells, increasing the tension on underlying endothelial plasma membrane. The mechanosensor Piezo1, a plasma membrane cation channel, was inducibly deleted in endothelial cells of mice (Piezo1iEC−/−), which allowed us to study its role in regulating the endothelial barrier response to alveolar stretch. We observed significant increase in lung vascular permeability in Piezo1iEC−/− mice as compared with control Piezo1fl/fl mice in response to high-volume mechanical ventilation. We also observed that human lung endothelial monolayers depleted of Piezo1 and exposed to cyclic stretch had increased permeability. We identified the calcium-dependent cysteine protease calpain as a downstream target of Piezo1. Furthermore, we showed that calpain maintained stability of the endothelial barrier in response to mechanical stretch by cleaving Src kinase, which was responsible for disassembling endothelial adherens junctions. Pharmacological activation of calpain caused Src cleavage and thereby its inactivation, and it restored the disrupted lung endothelial barrier seen in Piezo1iEC−/− mice undergoing high-volume mechanical ventilation. Our data demonstrate that downregulation of Piezo1 signaling in endothelium is a critical factor in the pathogenesis of ventilator-induced lung injury, and thus augmenting Piezo1 expression or pharmacologically activating Piezo1 signaling may be an effective therapeutic strategy.
Keywords: mechanotransduction, calcium signaling, alveolar stretch
Mechanical ventilation with low tidal volumes is the primary and requisite supportive measure in patients with respiratory failure (1). High-volume mechanical ventilation (HVMV), in contrast, injures the lung endothelium and induces pulmonary edema, a condition known as “ventilator-induced lung injury” (VILI) (2). Even low–tidal volume ventilation of injured lungs can overinflate regions of the lung because of uneven distribution of ventilation, and thus can induce VILI (3). Although lung overinflation is known to disrupt the lung endothelial barrier (4), it is not clear why some patients develop VILI and others do not. Thus, a better understanding of underlying signaling mechanisms mediating VILI could lead to therapies targeting pathogenic processes. Pulmonary microvessel endothelial cells (ECs) separated from the alveolar epithelium by a septum of less than 1 μm are subjected to stretch generated by the rise in alveolar pressure (5). The endothelium expresses the mechanosensory channel Piezo1 and responds to plasma membrane stretch (6, 7); therefore, in the present study, we determined the role of alveolar stretch in activation of Piezo1 in ECs and regulation of lung endothelial barrier function.
Piezo channels are essential for a variety of functions in the lungs and the cardiovascular system (8). Piezo2 expressed in airway vagal sensory neurons senses lung inflation and shapes the Hering-Breuer reflex to inhibit lung overinflation (9). In ECs, Piezo1 plays a role in embryonic vasculogenesis (6, 10), shear stress–mediated angiogenesis in the adult (11), hydrostatic pressure–induced pulmonary vascular hyperpermeability and edema (12), and flow-mediated blood pressure regulation (13) through its role as a channel activating calcium influx (14, 15). In this article, we describe an important adaptive function of endothelial Piezo1 in stabilizing pulmonary endothelial adherens junctions (AJs) in response to distention of the lungs. The results of the present study demonstrate a negative feedback adaptive mechanism by which endothelial Piezo1 reinforces EC junctional integrity through stabilizing vascular endothelial (VE)-cadherin adhesion in response to alveolar stretch. We also show that significantly diminished or absent Piezo1 activation results in pulmonary edema during HVMV.
Methods
Mechanical Ventilation in Mice
All procedures were approved by the University of Illinois at Chicago Committee on Use and Care of Animals. The mice were anesthetized with a mixture of ketamine hydrochloride (50 mg/kg) and xylazine (10 mg/kg) intraperitoneally. Orotracheal intubation was conducted using a 20-gauge intravenous catheter (Becton Dickinson Canada). After verification of positioning, the catheter was connected to a ventilator (VentElite; Harvard Apparatus), and ventilation parameters were set according to protocol. To calculate the static compliance of the lung, the ventilator was held at the end of inspiration for 1 second, and airway plateau pressure was recorded. Static compliance was calculated as tidal volume divided by plateau pressure.
Evans Blue Dye Albumin Pulmonary Transvascular Flux Measurements
Evans blue dye albumin (EBA) was injected intravenously at a dose of 8 μl/g body weight. Lungs were perfused with PBS and harvested 30 minutes later. Lung homogenates were incubated with 2 ml of formamide overnight at 60°C, and concentration of EBA was measured at absorbance of 620 nm. EBA concentration was normalized to body weight.
Pulmonary Edema Measurement
Mouse lung tissue was harvested, and wet weight was immediately measured. The lung tissue was then dried in an oven at 60°C for 48 hours to a constant weight, and dry weights were measured.
Myeloperoxidase Assay
Lungs were harvested and weighed after perfusion with PBS to remove all blood and stored at −80°C before myeloperoxidase assay was conducted. Myeloperoxidase activity was assessed as described elsewhere (16).
Cell Culture
Human lung microvascular ECs were cultured in EGM2 medium supplemented with a growth factor BulletKit (Lonza) and 10% heat-inactivated FBS (Thermo Fisher Scientific). Cells were used for cyclic stretch (CS) experiments at passages 5–8.
CS Exposure
CS experiments were conducted with Flexcell FX-5000 Tension System (Flexcell International). Human lung microvascular endothelial cell (HLMVEC) monolayers were exposed to CS of set magnitude (18% elongation) and duration (0–2 h).
Surface Protein Isolation
The Subcellular Protein Fractionation Kit (78840; Thermo Fisher Scientific) was used in accordance with the manufacturer’s protocol for isolation of plasma membrane and cytosolic fractions of VE-cadherin.
Calpain Activity
A fluorometric assay was used to detect cleavage of calpain substrate Ac-LLY-AFC. Output was measured on a microplate reader with 400-nm excitation and 505-nm emission filters. Absorbance values are presented as arbitrary units after subtracting a background value.
Statistics
Data are expressed as mean ± SEM and were analyzed with two-tailed unpaired Student’s t test for comparison of two groups or one-way ANOVA for multiple groups followed by a post hoc Tukey test.
Results
Endothelium-Specific Deletion of Piezo1 (Piezo1iEC−/−) in Mice Augments Lung Endothelial Hyperpermeability in Response to Lung Distention
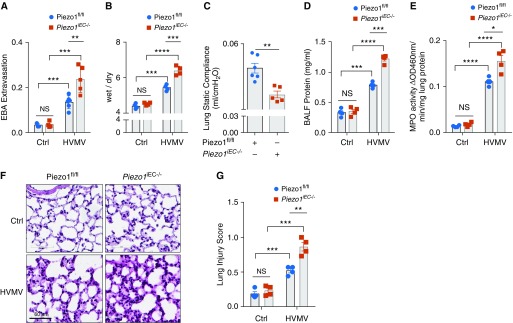
To determine the role of Piezo1 expressed in ECs (17), we generated mice in which Piezo1 was deleted inducibly in ECs (Piezo1iEC−/−) (11, 12). In control Piezo1fl/fl mice, we observed a moderate increase in lung vascular permeability and edema formation in response to HVMV (Figures 1A and 1B). These responses were dependent on the level of tidal volume (Figures E1A–E1C in the data supplement). The responses, however, were significantly elevated in Piezo1iEC−/− but not in Piezo2iEC−/− mice exposed to the same HVMV conditions (Figures 1A, 1B, and E1D), suggesting that endothelial Piezo1 acted in a protective manner to prevent lung vascular leakiness during HVMV.
Figure 1.
Piezo1 deletion in ECs of mice augments high-volume mechanical ventilation (HVMV)-induced lung capillary leakage. (A and B) Endothelium-specific genetic deletion of Piezo1 (Piezo1iEC−/−) augments HVMV-induced pulmonary capillary leakage and edema formation. Piezo1fl/fl and Piezo1iEC−/− mice were mechanically ventilated with tidal volume of 40 ml/kg for 2 hours. Pulmonary transvascular albumin permeability (A) and wet/dry ratio (B) were measured. Plots show mean ± SEM. The data points depict individual mice; n = 3–5. **P < 0.01, ***P < 0.001, and ****P < 0.0001 by ANOVA. (C) Piezo1iEC−/− decreases lung static compliance in mice undergoing mechanical ventilation. Values are shown as mean ± SEM; n = 5–6. **P < 0.01 by two-tailed t test. (D) Piezo1iEC−/− mice show an increase in BAL fluid (BALF) protein concentration in mice undergoing HVMV as in A and B. Mean ± SEM; n = 3–4. ***P < 0.001 and ****P < 0.0001 by ANOVA. (E) Piezo1iEC−/− mice show an increase in HVMV-induced neutrophil sequestration in lungs (assessed by measurement of myeloperoxidase [MPO] activity). Mean ± SEM; n = 4. *P < 0.05 and ****P < 0.0001 by ANOVA. (F and G) Hematoxylin and eosin staining of lung sections (F) and Lung Injury Score (G) showing increased inflammation and lung injury in Piezo1EC−/− mice postventilation as in A and B. Scale bars: 60 μm. **P < 0.01 and ***P < 0.001 by ANOVA. Ctrl = control; EBA = Evans blue-labeled albumin; ECs = endothelial cells; NS = nonsignificant; OD = optical density.
Similar to observations in Piezo1iEC−/− mice, GsmTx-4, a peptide isolated from tarantula venom that inhibits mechanosensitive channels (18), also significantly augmented lung vascular leakage and edema formation as compared with control mice (Figures E1E and E1F). These findings support the role of Piezo1 in protecting the endothelial barrier during lung overinflation.
We also observed significantly reduced lung compliance in Piezo1iEC−/− mice as compared with control littermates (Figure 1C), consistent with observed compliance decreases seen during VILI (19). Protein concentration in BAL fluid and neutrophil infiltration into airways were also significantly elevated in Piezo1iEC−/− mice compared with control mice (Figures 1D and 1E). Histopathological analysis of Piezo1iEC−/− lungs showed severe interstitial and alveolar edema, hyaline membranes, and neutrophil infiltration consistent with augmented lung injury compared with control Piezo1fl/fl mice (Figures 1F and 1G).
Loss of Piezo1 in ECs Increases VE-Cadherin Tyr658 and Tyr685 Phosphorylation and Destabilizes VE-Cadherin Junctions
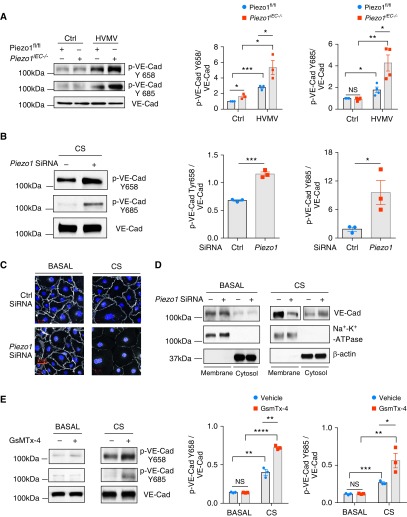
To address the mechanisms of endothelial barrier disruption in Piezo1iEC−/− mouse lungs, we determined alterations in VE-cadherin phosphorylation at Tyr658 and Tyr685, which mediate internalization of VE-cadherin from the plasma membrane and disassembly of AJs (20). We observed significantly increased VE-cadherin phosphorylation at Tyr658 and Tyr685 in response to HVMV in Piezo1iEC−/− mouse lungs as compared with control animals’ lungs (Figure 2A).
Figure 2.
Loss of Piezo1 in ECs augments HVMV-induced vascular endothelial cadherin (VE-Cad) phosphorylation and disruption of adherens junctions (AJs) in lung endothelium. (A) Western blot analysis of VE-Cad phosphorylation in lungs of Piezo1fl/fl and Piezo1iEC−/− mice after mechanical ventilation with tidal volume of 40 ml/kg for 30 minutes. Piezo1iEC−/− increases HVMV-induced phosphorylation of VE-Cad at Y658 and Y685. Mean ± SEM; n = 3–4. *P < 0.05, **P < 0.01, and ***P < 0.001 by ANOVA. (B) Western blot analysis of VE-Cad phosphorylation in HLMVEC monolayers after 18% cyclic stretch (CS) for 30 minutes. Depletion of Piezo1 by siRNA increases stretch-induced phosphorylation of VE-Cad at Y658 and Y685 as compared with scrambled siRNA-treated cells. Mean ± SEM; n = 3. *P < 0.05 and ***P < 0.001 by two-tailed t test. (C) Confocal imaging of VE-Cad (white) and nuclei (DAPI; blue) of HLMVEC monolayers transfected with scrambled or Piezo1 siRNA under basal static conditions and after 30 minutes of 18% CS. Scale bars: 20 μm. (D) Distribution of VE-Cad between plasma membrane and cytosolic pool by Western blot analysis in HLMVEC monolayers transfected with scrambled or Piezo1 siRNA under basal static conditions and after 30 minutes of 18% CS. Depletion of Piezo1 decreased membrane VE-Cad expression and increased cytosolic pool. (E) Western blot analysis of VE-Cad phosphorylation in HLMVEC monolayers treated with 500 nM GsmTx-4 or vehicle control under basal static conditions and after 30 minutes of 18% CS. Pharmacological inhibition of Piezo1 channels increases phosphorylation of VE-Cad at Y658 and Y685. Mean ± SEM; n = 3. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 by two-tailed t test. HLMVEC = human lung microvascular endothelial cell.
To investigate further whether Piezo1 also mediated endothelial barrier protection in monolayers and matched the response in vivo, we established the CS EC culture model (21) to mimic alveolar stretch and thus study its impact directly on the endothelium. We found that CS of primary HLMVECs treated with control siRNA increased VE-cadherin phosphorylation at Tyr658 and Tyr685 (Figure 2B), consistent with the response seen in mice (Figure 2A). Furthermore, Piezo1 depletion (Figure E2) augmented the increase in CS-induced VE-cadherin phosphorylation at both tyrosine residues (Figure 2B), also consistent with the results in Piezo1iEC−/− mouse lungs (Figure 2A). We observed significantly greater internalization of VE-cadherin and disruption of VE-cadherin junctions in response to CS in Piezo1-depleted ECs (Figures 2C and 2D), in agreement with the effects of VE-cadherin phosphorylation in signaling VE-cadherin internalization through clathrin-mediated endocytosis (20). Treatment of HLMVECs with the Piezo1 inhibitor GsmTx-4 increased VE-cadherin phosphorylation (Figure 2E) in endothelial monolayers subjected to CS, as also seen in Piezo1iEC−/− mouse lungs (Figure 2B).
Loss of Piezo1 in ECs Increases Src Activity to Induce HVMV-mediated Endothelial Hyperpermeability
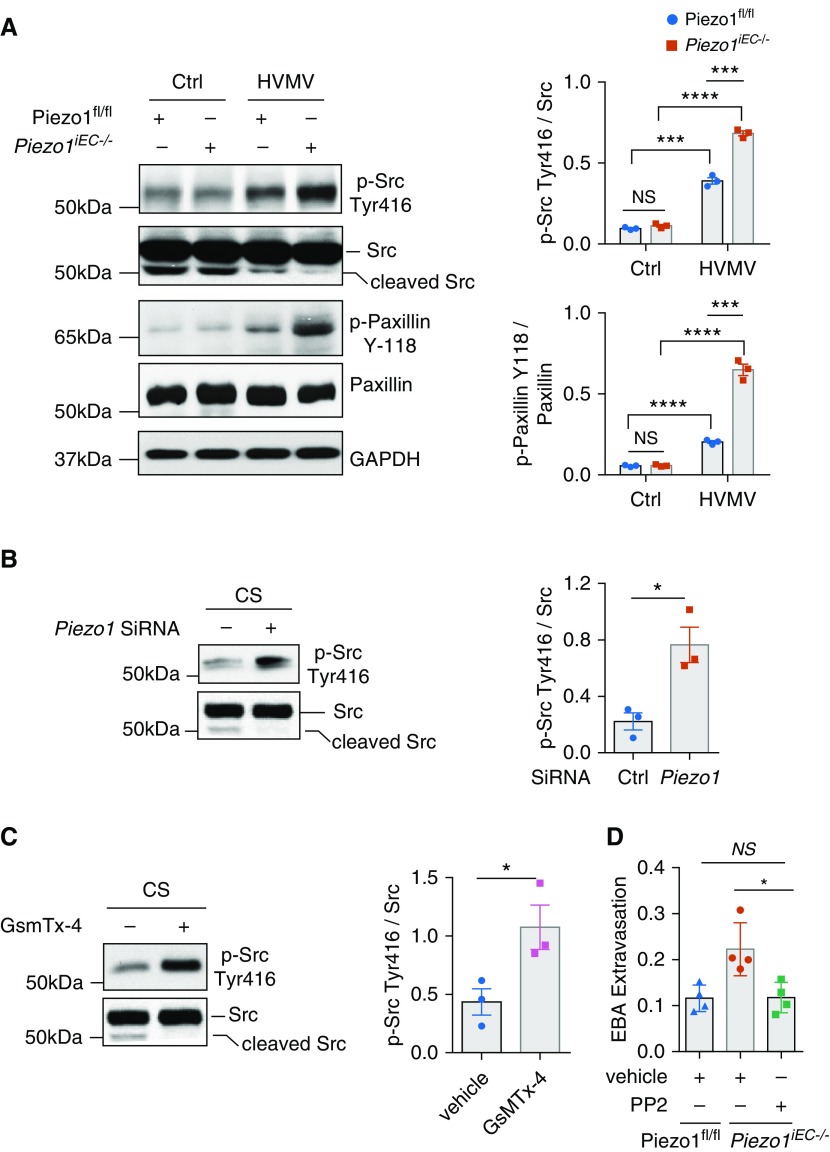
To investigate mechanisms of VE-cadherin phosphorylation and disassembly of AJs seen in Piezo1-depleted ECs of mice in response to HVMV, we next studied the role of Src, which is known to phosphorylate VE-cadherin at Tyr658 and Tyr685 residues and induce its internalization (20). We found that Piezo1iEC−/− mouse lungs showed significantly greater activation of Src, as evidenced by tyrosine autophosphorylation of Src at Y416 as well as phosphorylation of the Src substrate paxillin at Y118 after exposure to HVMV, than in control mice (Figure 3A). Similar results were obtained in HLMVEC monolayers deficient in Piezo1 upon exposure to CS (Figure 3B). Furthermore, treatment of HLMVEC monolayers with the GsmTx-4 inhibitor enhanced CS-induced Src phosphorylation as compared with vehicle-treated cells (Figure 3C). To address the role of Src in mediating increased lung vascular permeability in VILI, we treated Piezo1iEC−/− mice with the Src inhibitor PP2 {4-amino-5-(4-chlorophenyl)-7-(dimethylethyl)pyrazolo[3,4-d]pyrimidine} (22), and we observed that it prevented the augmented lung vascular albumin permeability (EBA) response seen in Piezo1iEC−/− mice subjected to HVMV (Figure 3D).
Figure 3.
Loss of Piezo1 in ECs augments Src-dependent phosphorylation of VE-Cad. (A) Western blot analysis of Src autophosphorylation at Y416 and paxillin phosphorylation at Y118 in lungs of Piezo1fl/fl and Piezo1iEC−/− mice after mechanical ventilation with tidal volume of 40 ml/kg for 30 minutes. Piezo1iEC−/− mice show increased phosphorylation of Src and paxillin, indicative of Src activation. Mean ± SEM; n = 3. ***P < 0.001 and ****P < 0.0001 by ANOVA. (B and C) Western blot analysis of Src phosphorylation in HLMVEC monolayers transfected with control or Piezo1 siRNA (B) or treated with 500 nM GsmTx-4 (C) after exposure to 18% CS for 30 minutes. Genetic depletion of Piezo1 (B) or pharmacological inhibition of Piezo1 (C) augmented Src phosphorylation. Mean ± SEM; n = 3. *P < 0.05 by two-tailed t test. (D) Pulmonary EBA permeability in Piezo1fl/fl and Piezo1iEC−/− mice mechanically ventilated with tidal volume of 40 ml/kg for 2 hours. Piezo1iEC−/− mice received intraperitoneal injection of PP2, an Src inhibitor, at 1 mg/kg body weight before HVMV. Inhibition of Src with PP2 prevents lung microvascular leakage in Piezo1iEC−/− mice. Mean ± SEM. Data points depict individual mice. *P < 0.05 by ANOVA.
Piezo1 Activation of Calpain Suppresses Src Activity to Stabilize VE-Cadherin Junctions
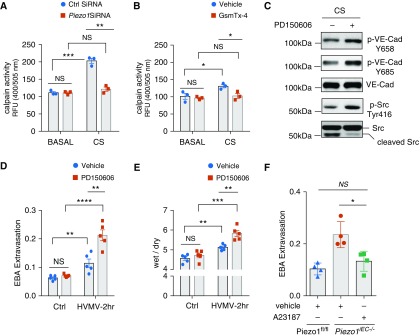
Calpain is a Ca2+-dependent cysteine protease known to be activated by pressure and shear stress stimulation of Piezo1 (6, 12). Calpain also cleaves Src and generates the inactive 52 kD cleavage product of Src, suggesting that calpain activation counters the barrier-disruptive effect of Src. We therefore addressed whether Piezo1-elicited Ca2+ influx was responsible for calpain activation and thus stabilized the endothelial barrier by inactivating Src. We observed that CS induced calpain activation in HLMVEC monolayers and further that the response was significantly reduced in monolayers depleted of Piezo1 or treated with GsmTx-4 (Figures 4A and 4B). Inhibition of calpain with PD160505 (6) prevented the cleavage of Src, increased Src activity, and increased VE-cadherin phosphorylation in HLMVEC monolayers subjected to CS (Figure 4C). In addition, chelation of extracellular Ca2+ with EDTA augmented phosphorylation of Src and VE-cadherin at Tyr658 and Tyr685 in HLMVEC monolayers upon CS (Figure E3). Treatment with PD160505 also significantly increased pulmonary vascular permeability and edema formation in HVMV mice but not in control mice (Figures 4D and 4E). In contrast, treatment with Ca2+ ionophore A23187, a calpain agonist (23), prevented HVMV-induced lung injury in Piezo1iEC−/− mice (Figure 4F).
Figure 4.
Piezo1 prevents Src-dependent phosphorylation of VE-Cad through activation of calpain. (A and B) Calpain activity in HLMVEC monolayers transfected with Piezo1 siRNA (A) or treated with 500 nM GsmTx-4 (B) under basal static conditions and after 30 minutes of 18% CS. Depletion of Piezo1 or inhibition of stretch-activated channels decreases calpain activity. Mean ± SEM; n = 3. *P < 0.05, **P < 0.01, and ***P < 0.001 by two-tailed t test. (C) Western blot analysis of VE-Cad and Src phosphorylation in HLMVEC monolayers challenged with 3 μM PD150606 (calpain inhibitor) or vehicle control and exposed to 18% CS for 30 minutes. Pharmacological inhibition of calpain augments phosphorylation of both VE-Cad and Src in HLMVEC monolayers exposed to 18% CS for 30 minutes. (D and E) Lung transvascular EBA permeability (D) or wet/dry ratio (E) in C57BL/6 mice treated with intravenous injection of PD150606 at 8 μM/kg body weight or vehicle control and exposed to HVMV for 2 hours. Mean ± SEM. Data points depict individual mice. **P < 0.01, ***P < 0.001, and ****P < 0.0001 by two-tailed t test. (F) Lung transvascular EBA lung permeability in Piezo1fl/fl and Piezo1iEC−/− mice treated with intravenous injection of calcium ionophore A23187 at 1 mg/kg body weight or vehicle control as indicated before exposure to mechanical ventilation with tidal volume of 40 ml/kg for 2 hours. Pharmacological activation of calpain prevents lung microvascular leakage in Piezo1iEC−/− mice. Mean ± SEM. Data points depict individual mice; n = 4. *P < 0.05 by ANOVA. RFU = relative fluorescence units.
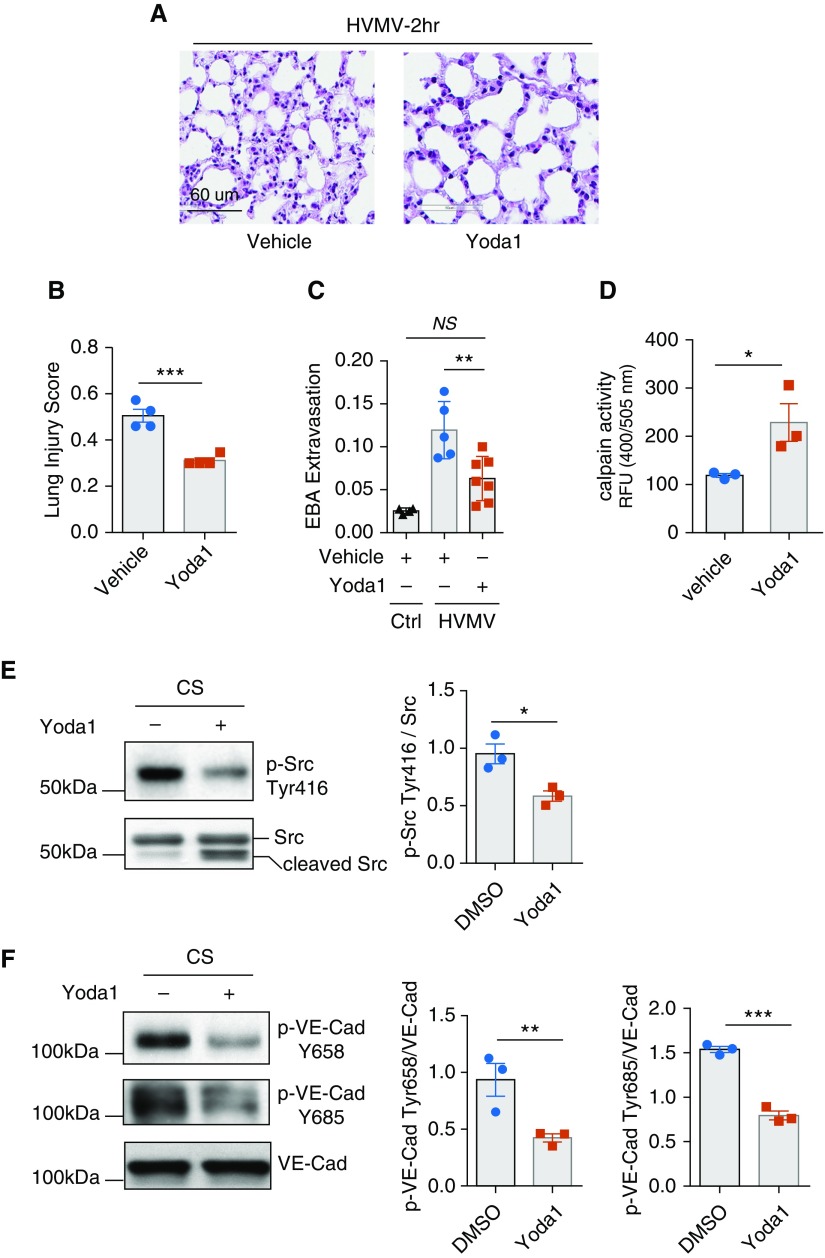
Yoda1 Enhances Calpain Activity and Reduces Src-mediated Phosphorylation of VE-Cadherin
We next focused on identifying potential therapeutic strategies to increase barrier stability via the Piezo1 pathway in the lung endothelium. We determined whether Yoda1, a specific activator of Piezo1 (24), reduces Src-dependent phosphorylation of VE-cadherin and thereby abrogates HVMV-induced increase in lung vascular permeability. We found that intravenous administration of Yoda1 in mice prevented HVMV, as evidenced by the reduced Lung Injury Score (Figures 5A and 5B) and also reduced lung vascular permeability (Figure 5C). Furthermore, treatment of HLMVEC monolayers undergoing CS with Yoda1 increased calpain activity (Figure 5D), suppressed Src activity (Figure 5E), and concomitantly reduced VE-cadherin phosphorylation (Figure 5F).
Figure 5.
Activation of Piezo1 prevents Src-dependent VE-Cad phosphorylation and lung vascular leakage. (A–C) Hematoxylin and eosin staining of lung sections (A), Lung Injury Score (B), and lung transvascular EBA permeability (C) in C57BL/6 mice pretreated with intravenous injection of Piezo1 activator Yoda1 (20 μg/kg body weight) or vehicle control and exposed to HVMV for 2 hours. Yoda1 reduces HVMV-induced lung injury and edema. Mean ± SEM. The data points depict individual mice. n = 4–7. **P < 0.01 by ANOVA and ***P < 0.001 by two-tailed t test. (D) Calpain activity in HLMVEC monolayers pretreated with 100 nM Yoda1 or vehicle control and exposed to 18% CS for 30 minutes. Activation of Piezo1 increases calpain activity. Mean + SEM; n = 3. *P < 0.05 by two-tailed t test. (E and F) Western blot analysis of Src (E) and VE-Cad (F) phosphorylation in HLMVEC monolayers pretreated with 100 nM Yoda1 or vehicle control and exposed to 18% CS for 30 minutes. Activation of Piezo1 decreases phosphorylation of Src and VE-Cad. Mean ± SEM; n = 3. *P < 0.05, **P < 0.01, and ***P < 0.001 by two-tailed t test. Scale bars: 60 μm.
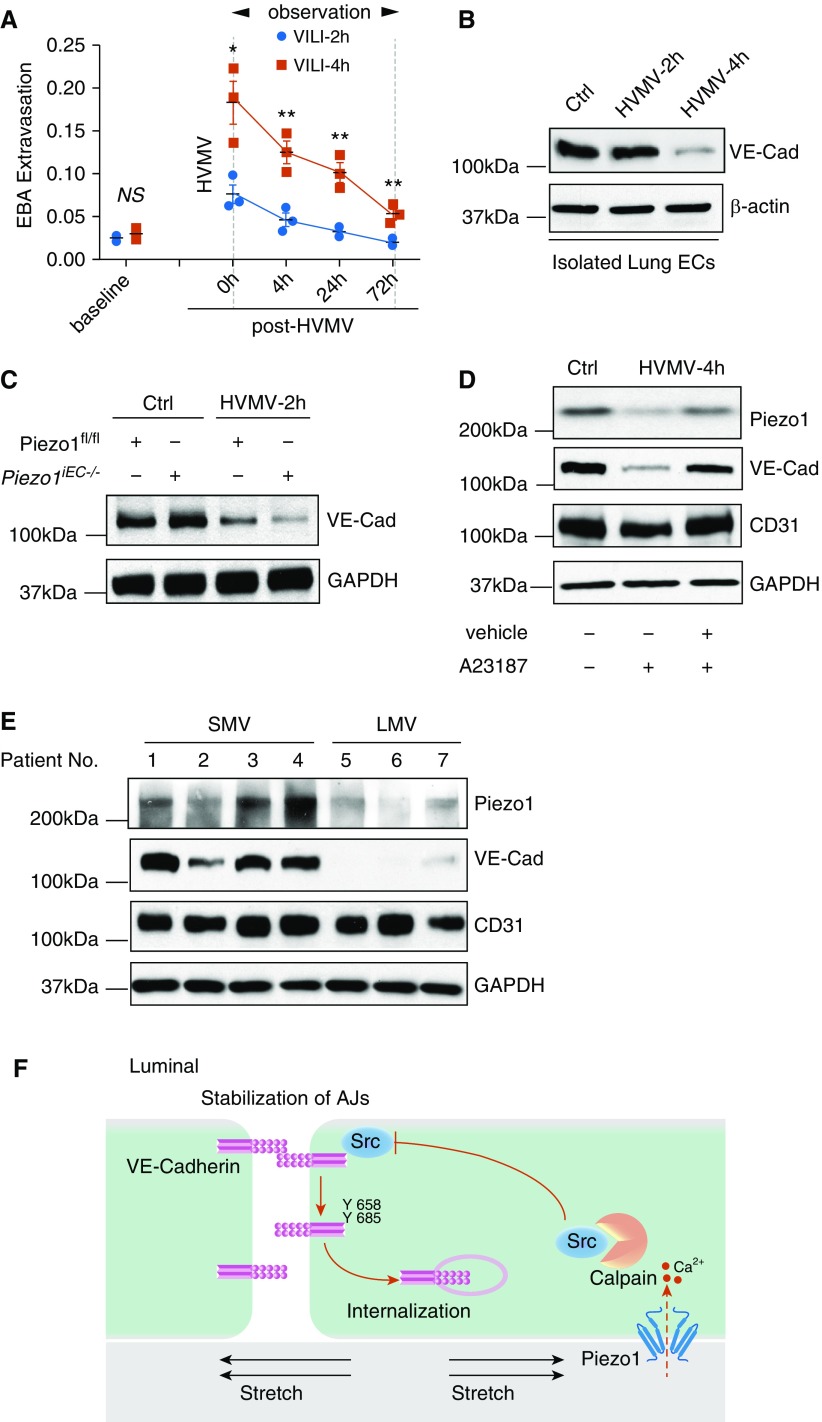
Long-Term Mechanical Ventilation in Mice and Patients Reduces Both Piezo1 and VE-Cadherin Expression in Lungs
We next determined the effects of HVMV in mice (exposed to fourfold greater than normal tidal volume) to study changes in VE-cadherin expression in vivo. We observed that the 4-hour period of HVMV markedly increased lung vascular permeability, which persisted up to 72 hours after restoring normal tidal volume ventilation (Figure 6A). The magnitude of VE-cadherin loss after 4 hours was related to the severity of increase in lung vascular permeability, with the response to 4 hours of HVMV being much greater than that after 2 hours of HVMV (Figure 6B). Furthermore, loss of VE-cadherin was significantly greater in Piezo1iEC−/− mice than in Piezo1fl/fl mice after even 2 hours of HVMV (Figure 6C). On the basis of these findings, we posited that activation of calpain would restore VE-cadherin expression. We found that mice treated with A23187 indeed showed normal expression of both Piezo1 and VE-cadherin after the 4-hour period of HVMV (Figure 6D).
Figure 6.
Mechanical ventilator-induced lung injury (VILI) in mice and humans is characterized by reduced expression of VE-Cad. (A) Long-term mechanical ventilation increases pulmonary transvascular albumin permeability in mice (see Methods). Time course of lung vascular permeability in C57BL/6J mice subjected to 2 or 4 hours of HVMV (see Methods) and followed by monitoring lung vascular permeability up to 72 hours. Mean ± SEM; n = 3. *P < 0.05 and **P < 0.01 by ANOVA. (B) Expression of VE-Cad is significantly reduced in C57BL/6J mice exposed to 4 hours of HVMV. Western blot analysis was performed in freshly isolated lung ECs from these mice. (C) Expression of VE-Cad is reduced in Piezo1iEC−/− mice exposed to 2 hours of HVMV. (D) Pharmacological activation of calpain with A23187 prevents downregulation of both Piezo1 and VE-Cad induced by 4 hours of HVMV. Western blot analysis of VE-Cad, CD31, and GAPDH (as loading control). (E) Human lung tissue samples were collected from patients undergoing short-term mechanical ventilation (SMV) for lobectomy or from organ donors who had undergone long-term mechanical ventilation (LMV). Western blot analysis of Piezo1, VE-Cad, CD31, and GAPDH (loading control) was performed. (F) Model of endothelial Piezo1-mediated stabilization of AJs. Alveolar CS adaptively activates Piezo1 and its downstream target calpain via Ca2+ influx. Calpain, in turn, cleaves Src, reducing Src-dependent VE-Cad phosphorylation and stabilizing AJs.
We next investigated the impact of mechanical ventilation on VE-cadherin expression in human lungs. Control lung tissue was obtained from patients undergoing pulmonary lobectomy who had received short-term periprocedural mechanical ventilation for 30–60 minutes during the surgical procedure (Table E1). Lung tissues from patients who had undergone prolonged (38–252 h) mechanical ventilation were obtained from organ donors in whom lungs were not deemed suitable for transplant (Table E1). We found that patients receiving long-term mechanical ventilation had significantly decreased expression levels of both Piezo1 and VE-cadherin as compared with the short-term mechanical ventilation group (Figure 6D).
Discussion
In the present study, we identified a novel adaptive role of EC-expressed Piezo1 in stabilizing the lung endothelial barrier in response to alveolar stretch. We demonstrated that activation of Piezo1 in ECs elicited calcium signaling and activated calpain to cleave Src, which in turn suppressed Src-mediated VE-cadherin phosphorylation at Y685 and Y658, thereby preventing VE-cadherin internalization from AJs. Our findings uncover an adaptive feedback regulation by which alveolar stretch–induced Piezo1 activation in ECs protects the lung endothelial barrier function. This mechanism is analogous to the recently described adaptive control of lung inflation (Hering-Breuer mechanoreflex) by Piezo2 (9). Although lung distention activated Piezo2 in sensory neurons to prevent lung overinflation, in the present study, we showed that Piezo1 in ECs adaptively suppressed endothelial permeability in response to alveolar stretch. We demonstrated that this adaptive endothelial barrier stabilization response was mediated via a mechanism intrinsic to ECs.
The present findings help to define a fundamental role of Piezo1-mediated calcium signaling in the regulation of endothelial permeability and pathogenesis of VILI. Previous studies focused on the mechanosensitive transient receptor potential superfamily in mediating lung vascular hyperpermeability in response to HVMV (25). Deletion or pharmacological inhibition of TRPV4 (transient receptor potential cation channel subfamily V member 4) prevented HVMV disruption of alveolar–capillary barriers (26), demonstrating a role of TRPV4 in the pathogenesis of VILI. Activation of TRPV4 in ECs did not appear to alter the integrity of AJs; rather, TRPV4 increased hyperpermeability in response to HVMV through activation of metalloproteases and detachment of ECs from the underlying basement membrane (27, 28). Distinct from barrier-disruptive signaling via TRPV4, the present results identify a mechanosensitive, barrier-protective pathway in mice exposed to HVMV that stabilized lung endothelial AJs downstream of Piezo1 activation.
VE-cadherin, the key component of AJs controlling the integrity of endothelial junctions (29), regulates endothelial barrier permeability through the assembly of homotypic VE-cadherin trans-interaction in ECs (30). We demonstrated that deletion of Piezo1 increased the phosphorylation of VE-cadherin at Y685 and Y658, the “phosphoswitch” responsible for internalizing VE-cadherin and thus destabilizing AJs (20). In contrast, we observed that pharmacological activation of Piezo1 reduced phosphorylation at these residues, both in mouse lungs and in confluent human endothelial monolayers, in response to alveolar CS. Phosphorylation of VE-cadherin at Y685 and Y658 is known to be mediated by Src and signal VE-cadherin internalization via clathrin-mediated endocytosis, resulting in disassembly of AJs (20). We observed that increased activation of Src augmented VE-cadherin phosphorylation in human lung ECs depleted of Piezo1 as well as in Piezo1iEC−/− mice. Pharmacological inhibition of Src in Piezo1iEC−/− mice reduced VE-cadherin phosphorylation and restored endothelial permeability to levels seen in control mice subjected to similar HVMV. Thus, these results demonstrate that activation of Piezo1 in lung ECs prevents lung endothelial barrier breakdown in response to alveolar stretch through suppression of Src-induced VE-cadherin phosphorylation.
Piezo1 is known to activate calcium-dependent calpain proteases (6, 31), a family of highly conserved proteolytic enzymes (32). The mechanism of calpain activation in the context of lung alveolar stretch is unclear. Our results based on both inhibiting and activating calpain suggest a role of Piezo1 in activating calpain secondary to Piezo1-induced calcium signaling in ECs (6). Calpain cleaves Src at N-terminal domain and results in Src accumulation in cytosol (33). In Piezo1iEC−/− mouse endothelium exposed to alveolar stretch, we observed that activation of calpain with the calcium ionophore A23187 reduced Src activity as well as phosphorylation of VE-cadherin to levels observed in control mice, which thereby normalized lung barrier function. This is in contrast to Piezo1-mediated activation of calpain-dependent degradation of both VE-cadherin and catenin proteins (12, 34) observed in the conditions of elevated hydrostatic pressure in the lung (12). These differences in Piezo1 function can be explained by the direction and magnitude of mechanical forces experienced by ECs. Although HVMV transmits forces through the basement membrane, a hydrostatic pressure activates Piezo1 localized at the luminal surface of ECs.
Furthermore, we established the clinical relevance of our findings by comparing lung tissue samples from patients exposed to short-term mechanical ventilation and organ donor patients exposed to longer periods of mechanical ventilation. We found that only long-term mechanical ventilation was associated with loss of both Piezo1 and VE-cadherin in human lungs that was characteristic of VILI. We also observed a similar decreased VE-cadherin expression after long-term mechanical ventilation in mice, indicating that mechanical ventilation itself can disrupt the integrity of VE-cadherin junctions, leading to pulmonary edema. Although the mechanism of VE-cadherin downregulation in response to prolonged mechanical ventilation is unknown, our findings in human and mouse lung ECs showing that Piezo1 signaling preserves VE-cadherin junctions suggest a key role of Piezo1 in maintaining endothelial integrity during VILI. These results raise the possibility that VILI likely develops when expression of Piezo1 is downregulated in ECs exposed to protracted exposure to mechanical stresses.
In conclusion, we describe a novel lung endothelial Piezo1-regulated mechanism involved in preventing the disassembly of lung endothelial VE-cadherin junctions, breakdown of the endothelial barrier, and fulminant pulmonary edema resulting from alveolar stretch. These findings suggest a strategy of preventing VILI through activation of Piezo1 signaling in the lung endothelium that thereby may shift the balance toward Piezo1-mediated barrier protective signaling.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Dr. Weiguo Chen (University of Illinois at Chicago) for help with the cyclic stretch method and Drs. Hong Fan, Songtao Xu, Kang Song, and Shenghao Ye (Zhongshan Hospital Fudan University) for providing human tissue samples. Piezo1fl/fl mice were a generous gift from Dr. David J. Beech (University of Leeds, Leeds, UK).
Footnotes
Supported by National Institutes of Health grant R01HL045638 (Y.A.K. and A.B.M.).
Author Contributions: Y.A.K. and A.B.M. conceived of the study. M.Z., J.R., Y.A.K., and A.B.M. designed the experiments. M.Z., W.W., H.K., Z.H., S.X., and X.G. performed the experiments and analyzed the data. M.Z., J.R., Y.A.K., and A.B.M. wrote the paper. All authors reviewed and edited the manuscript.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2019-0024OC on August 13, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med. 2017;377:562–572. doi: 10.1056/NEJMra1608077. [DOI] [PubMed] [Google Scholar]
- 2.Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med. 2013;369:2126–2136. doi: 10.1056/NEJMra1208707. [DOI] [PubMed] [Google Scholar]
- 3.Gattinoni L, Marini JJ, Pesenti A, Quintel M, Mancebo J, Brochard L. The “baby lung” became an adult. Intensive Care Med. 2016;42:663–673. doi: 10.1007/s00134-015-4200-8. [DOI] [PubMed] [Google Scholar]
- 4.Schwingshackl A. The role of stretch-activated ion channels in acute respiratory distress syndrome: finally a new target? Am J Physiol Lung Cell Mol Physiol. 2016;311:L639–L652. doi: 10.1152/ajplung.00458.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.West JB. Invited review: pulmonary capillary stress failure. J Appl Physiol (1985) 2000;89:2483–2489. doi: 10.1152/jappl.2000.89.6.2483. [Discussion, p. 2497.] [DOI] [PubMed] [Google Scholar]
- 6.Li J, Hou B, Tumova S, Muraki K, Bruns A, Ludlow MJ, et al. Piezo1 integration of vascular architecture with physiological force. Nature. 2014;515:279–282. doi: 10.1038/nature13701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang H, Liu C, Zhou RM, Yao J, Li XM, Shen Y, et al. Piezo2 protein: a novel regulator of tumor angiogenesis and hyperpermeability. Oncotarget. 2016;7:44630–44643. doi: 10.18632/oncotarget.10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhong M, Komarova Y, Rehman J, Malik AB. Mechanosensing Piezo channels in tissue homeostasis including their role in lungs. Pulm Circ. 2018;8:2045894018767393. doi: 10.1177/2045894018767393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nonomura K, Woo SH, Chang RB, Gillich A, Qiu Z, Francisco AG, et al. Piezo2 senses airway stretch and mediates lung inflation-induced apnoea. Nature. 2017;541:176–181. doi: 10.1038/nature20793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ranade SS, Qiu Z, Woo SH, Hur SS, Murthy SE, Cahalan SM, et al. Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proc Natl Acad Sci USA. 2014;111:10347–10352. doi: 10.1073/pnas.1409233111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang H, Hong Z, Zhong M, Klomp J, Bayless KJ, Mehta D, et al. Piezo1 mediates angiogenesis through activation of MT1-MMP signaling. Am J Physiol Cell Physiol. 2019;316:C92–C103. doi: 10.1152/ajpcell.00346.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedrich EE, Hong Z, Xiong S, Zhong M, Di A, Rehman J, et al. Endothelial cell Piezo1 mediates pressure-induced lung vascular hyperpermeability via disruption of adherens junctions. Proc Natl Acad Sci USA. 2019;116:12980–12985. doi: 10.1073/pnas.1902165116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang S, Chennupati R, Kaur H, Iring A, Wettschureck N, Offermanns S. Endothelial cation channel PIEZO1 controls blood pressure by mediating flow-induced ATP release. J Clin Invest. 2016;126:4527–4536. doi: 10.1172/JCI87343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bagriantsev SN, Gracheva EO, Gallagher PG. Piezo proteins: regulators of mechanosensation and other cellular processes. J Biol Chem. 2014;289:31673–31681. doi: 10.1074/jbc.R114.612697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewis AH, Grandl J. Mechanical sensitivity of Piezo1 ion channels can be tuned by cellular membrane tension. Elife. 2015;4:e12088. doi: 10.7554/eLife.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gong H, Rehman J, Tang H, Wary K, Mittal M, Chaturvedi P, et al. HIF2α signaling inhibits adherens junctional disruption in acute lung injury. J Clin Invest. 2015;125:652–664. doi: 10.1172/JCI77701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyamoto T, Mochizuki T, Nakagomi H, Kira S, Watanabe M, Takayama Y, et al. Functional role for Piezo1 in stretch-evoked Ca2+ influx and ATP release in urothelial cell cultures. J Biol Chem. 2014;289:16565–16575. doi: 10.1074/jbc.M113.528638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gnanasambandam R, Ghatak C, Yasmann A, Nishizawa K, Sachs F, Ladokhin AS, et al. GsMTx4: mechanism of inhibiting mechanosensitive ion channels. Biophys J. 2017;112:31–45. doi: 10.1016/j.bpj.2016.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hughes R, May AJ, Widdicombe JG. The effect of pulmonary congestion and oedema on lung compliance. J Physiol. 1958;142:306–313. doi: 10.1113/jphysiol.1958.sp006017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orsenigo F, Giampietro C, Ferrari A, Corada M, Galaup A, Sigismund S, et al. Phosphorylation of VE-cadherin is modulated by haemodynamic forces and contributes to the regulation of vascular permeability in vivo. Nat Commun. 2012;3:1208. doi: 10.1038/ncomms2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Birukov KG, Jacobson JR, Flores AA, Ye SQ, Birukova AA, Verin AD, et al. Magnitude-dependent regulation of pulmonary endothelial cell barrier function by cyclic stretch. Am J Physiol Lung Cell Mol Physiol. 2003;285:L785–L797. doi: 10.1152/ajplung.00336.2002. [DOI] [PubMed] [Google Scholar]
- 22.Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ, et al. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor: study of Lck- and FynT-dependent T cell activation. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 23.Oda A, Druker BJ, Ariyoshi H, Smith M, Salzman EW. pp60src is an endogenous substrate for calpain in human blood platelets. J Biol Chem. 1993;268:12603–12608. [PubMed] [Google Scholar]
- 24.Syeda R, Xu J, Dubin AE, Coste B, Mathur J, Huynh T, et al. Chemical activation of the mechanotransduction channel Piezo1. Elife. 2015;4:e07369. doi: 10.7554/eLife.07369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamanaka K, Jian MY, Weber DS, Alvarez DF, Townsley MI, Al-Mehdi AB, et al. TRPV4 initiates the acute calcium-dependent permeability increase during ventilator-induced lung injury in isolated mouse lungs. Am J Physiol Lung Cell Mol Physiol. 2007;293:L923–L932. doi: 10.1152/ajplung.00221.2007. [DOI] [PubMed] [Google Scholar]
- 26.Michalick L, Erfinanda L, Weichelt U, van der Giet M, Liedtke W, Kuebler WM. Transient receptor potential vanilloid 4 and serum glucocorticoid-regulated kinase 1 are critical mediators of lung injury in overventilated mice in vivo. Anesthesiology. 2017;126:300–311. doi: 10.1097/ALN.0000000000001443. [DOI] [PubMed] [Google Scholar]
- 27.Alvarez DF, King JA, Weber D, Addison E, Liedtke W, Townsley MI. Transient receptor potential vanilloid 4-mediated disruption of the alveolar septal barrier: a novel mechanism of acute lung injury. Circ Res. 2006;99:988–995. doi: 10.1161/01.RES.0000247065.11756.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Villalta PC, Townsley MI. Transient receptor potential channels and regulation of lung endothelial permeability. Pulm Circ. 2013;3:802–815. doi: 10.1086/674765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giannotta M, Trani M, Dejana E. VE-cadherin and endothelial adherens junctions: active guardians of vascular integrity. Dev Cell. 2013;26:441–454. doi: 10.1016/j.devcel.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 30.Komarova YA, Kruse K, Mehta D, Malik AB. Protein interactions at endothelial junctions and signaling mechanisms regulating endothelial permeability. Circ Res. 2017;120:179–206. doi: 10.1161/CIRCRESAHA.116.306534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McHugh BJ, Buttery R, Lad Y, Banks S, Haslett C, Sethi T. Integrin activation by Fam38A uses a novel mechanism of R-Ras targeting to the endoplasmic reticulum. J Cell Sci. 2010;123:51–61. doi: 10.1242/jcs.056424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ono Y, Sorimachi H. Calpains: an elaborate proteolytic system. Biochim Biophys Acta. 2012;1824:224–236. doi: 10.1016/j.bbapap.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 33.Hossain MI, Roulston CL, Kamaruddin MA, Chu PW, Ng DC, Dusting GJ, et al. A truncated fragment of Src protein kinase generated by calpain-mediated cleavage is a mediator of neuronal death in excitotoxicity. J Biol Chem. 2013;288:9696–9709. doi: 10.1074/jbc.M112.419713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miyazaki T, Taketomi Y, Takimoto M, Lei XF, Arita S, Kim-Kaneyama JR, et al. m-Calpain induction in vascular endothelial cells on human and mouse atheromas and its roles in VE-cadherin disorganization and atherosclerosis. Circulation. 2011;124:2522–2532. doi: 10.1161/CIRCULATIONAHA.111.021675. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.