Abstract
Translational research is essential to the development of reverse-remodeling strategies for the treatment of pulmonary vascular disease, pulmonary hypertension, and heart failure via mechanistic in vivo studies using animal models resembling human pulmonary arterial hypertension (PAH), cardiovascular remodeling, and progressive right heart failure. Since 2007, peroxisome proliferator–activated receptor γ (PPARγ) agonists have emerged as promising novel, antiproliferative, antiinflammatory, insulin-sensitizing, efficient medications for the treatment of PAH. However, early diabetes study results, their subsequent misinterpretations, errors in published review articles, and rumors regarding potential adverse effects in the literature have dampened enthusiasm for considering pharmacological PPARγ activation for the treatment of cardiovascular diseases, including PAH. Most recently, the thiazolidinedione class PPARγ agonist pioglitazone underwent a clinical revival, especially based on the IRIS (Insulin Resistance Intervention After Stroke) study, a randomized controlled trial in 3,876 patients without diabetes status post–transient ischemic attack/ischemic stroke who were clinically followed for 4.8 years. We discuss preclinical basic translational findings and randomized controlled trials related to the beneficial and adverse effects of PPARγ agonists of the thiazolidinedione class, with a particular focus on the last 5 years. The objective is a data-driven approach to set the preclinical and clinical study record straight. The convincing recent clinical trial data on the lack of significant toxicity in high-risk populations justify the timely conduct of clinical studies to achieve “repurposing” or “repositioning” of pioglitazone for the treatment of clinical PAH.
Keywords: peroxisome proliferator–activated receptor, pioglitazone, metabolism, pulmonary hypertension, repurposing drugs
Pulmonary arterial hypertension (PAH) is a progressive, vaso-occlusive disease leading to increased pulmonary vascular resistance and increased pulmonary arterial pressure that can lead to right heart failure and death (1–4). Advanced right ventricular (RV) hypertension, hypertrophy, and dilation lead not only to RV but also to left ventricular (LV) dysfunction through RV–LV interactions. The subsequent heart failure is prognostic in pulmonary hypertension (PH), regardless of etiology. Conversely, PH is a frequent complication of very common left heart diseases that negatively impact symptoms, exercise capacity, and outcome. Moreover, RV dysfunction is common in left heart failure with preserved ejection fraction (in left heart diseases) and is associated with adverse outcome (predicting death), suggesting that RV dysfunction may be a viable therapeutic target in both right and left heart failure. Translational research is thus essential to the development of reverse-remodeling strategies for the treatment of pulmonary vascular disease (PVD), PH, and heart failure through mechanistic in vivo studies using animal models resembling human PAH. Since 2007 (5), peroxisome proliferator–activated receptor γ (PPARγ) agonists have emerged as promising novel, antiproliferative, antiinflammatory, insulin-sensitizing, efficient medications for the treatment of PAH. However, early diabetes study results, their subsequent misinterpretations, errors in published review articles, and rumors regarding potential adverse effects in the literature have dampened enthusiasm for considering pharmacological PPARγ activation for the treatment of cardiovascular diseases, including PAH. The terms PPARγ agonist and antagonist, as well as the distinct thiazolidinedione (TZD) class PPARγ agonistic drugs pioglitazone and rosiglitazone, have frequently been confused with respect to both their pharmacodynamics and/or adverse effects. As stated in a prior review, “Some of the supposed side effects of TZDs were either overblown or idiosyncratic, most notably a putative increase in cardiovascular mortality attributed to the TZD drug rosiglitazone that has been dismissed by the FDA in recent years” (6). Finally, the recent, very large IRIS (Insulin Resistance Intervention After Stroke) trial (7–9) did not find any serious adverse effects of pioglitazone when used in patients with insulin resistance/prediabetes, but so far, pioglitazone has frequently been neglected in review articles on emerging PAH therapies (see below). In the present perspective article, we discuss preclinical basic translational findings (Table 1) and randomized controlled trials (RCTs) (Table 2 and Table E1 in the data supplement) related to the beneficial and adverse effects of PPARγ agonists of the TZD class, with a particular focus on the last 5 years.
Table 1.
Animal and Cell Models
| Reference | Medication | Cell Type/Model | Disease | Effect on Cells | Tissue/Clinical Effect | Toxicity |
|---|---|---|---|---|---|---|
| Calvier et al., 2019 (30) | Pioglitazone 20 mg/kg/d | PASMC/smLRP1−/− LDLR−/− | PAH | — | Inhibits canonical (Smad3) TGFβ1 signaling; and fully reverses PAH, RVH, and pulmonary vascular remodeling induced by LRP1 deficiency in SMC | — |
| Pioglitazone 10 μM | Human PASMC Murine PASMC |
PAH | Pioglitazone inhibits TGFβ1 canonical (Smad3) phosphorylation and downstream target expression (CTGF), all overactivated in LRP1-deficient PASMC | — | — | |
| Legchenko et al., 2018 (39) | Pioglitazone 20 mg/kg/d | PASMC/SuHx rat | PAH | Reduces obliterative pulmonary vascular remodeling | Fully reverses PAH and RVH; prevents RV dilation and failure; reduces RV mass >50%; reduces RVSP 91→34 mm Hg; and decreases NT-proBNP | No cell toxicity with pioglitazone or rosiglitazone (human PAECs, H9c2 rat cardiomyocytes); no changes in blood glucose concentration |
| Pioglitazone 10 μM | Neonatal rat cardiomyocytes | PAH | Induces fatty acid oxidation and ATP production | — | — | |
| SuHx rat | PAH | Decreases RV glucose uptake and decreases cardiomyocyte size | Prevents RV failure | — | ||
| Calvier et al., 2017 (28) | Pioglitazone 10 μM | HPASMC | PAH | Pioglitazone inhibits TGFβ1–induced proliferation, mitochondrial activation, and TGFβ1 canonical (Smad3/4) and noncanonical (Stat3/FoxO1) phosphorylation cascades | — | — |
| Pioglitazone 20 mg/kg/d by mouth for 5 wk | Transgenic TGFβ1 mice | PAH | — | Inhibits canonical (Smad3/4) and noncanonical (Stat3/FoxO1) TGFβ1 signaling; and fully reverses PAH, RVH, and pulmonary vascular remodeling | — | |
| Behringer et al., 2016 (61) | Pioglitazone 40 mg/kg/d by mouth for 2 wk | MCT rats | PAH | — | Reduces PASP, muscularization of small pulmonary arteries, medial wall thickness, Fulton’s index (RV/LV + S), area of fibrotic RV tissue, and inflammatory markers (macrophage number and osteopontin concentration) and improves survival | — |
| Cardiomyocytes/MCT rats | PAH | Reduces cross-sectional area of cardiomyocytes | Reduces ventricular BNP gene expression | — | ||
| Shiomi et al., 2002 (62) | Pioglitazone 3 mg/kg/d by mouth for 4 wk | Myocardium/CD1 mice with induced myocardial infarction | Myocardial infarction | — | Attenuates progressive LV chamber dilation and dysfunction in a murine model of post-MI heart failure | After 4 wk, plasma glucose concentration was not lowered by pioglitazone; serum AST used to assess potential hepatic toxicity was not increased by pioglitazone |
| Bertero et al., 2014 (33) | Rosiglitazone 20 mg/kg/d by mouth for 3 wk | PASMC overexpression of miR-130a in mice | PAH | — | Reverses miR-130–induced elevated RVSP | — |
| Liu et al., 2012 (63) | Rosiglitazone 10 mg/kg/d | MCT rats | PAH | — | Reduces RVH, PA medial thickening, mitigates 5-HT2BR expression, and inhibits 5-HT2BR–mediated vasoconstriction | — |
| Rosiglitazone 20 mg/kg/d | Chronic hypoxia rats | PH | — | Effects less pronounced than in MCT rats, mitigates 5-HT2BR expression increase, and inhibits 5-HT2BR–mediated vasoconstriction | — | |
| Rosiglitazone pioglitazone 1 μM | PASMC/5-HT–exposed rat | PAH | — | Inhibits upregulation of 5-HT2BR | — | |
| Hansmann et al., 2008 (22) | Rosiglitazone 1 μM | PASMC/SM22α Cre PPARγflox/flox mice | PAH | Reverses SMC proliferation | — | — |
| PASMC human | PAH | Blocks PDGF-BB–induced proliferation of BMPR2 mutant cells | Reverses vascular remodeling | — | ||
| Hansmann et al., 2007 (5) | Rosiglitazone 10 mg/kg/d by mouth for 4 or 10 wk | ApoE−/− mice | PAH | — | Eightfold higher plasma adiponectin concentrations, improves insulin sensitivity, and induces complete regression of PAH, RVH, and abnormal PA muscularization | Mild LV dilation and increased LV mass in apoE-deficient mice, and lower hematocrit in treated mice |
Definition of abbreviations: 5-HT = 5-hydroxytryptamine (or serotonin); 5-HT2BR = serotonin receptor 2B, serotonin; ApoE = apolipoprotein E; AST = aspartate aminotransferase; BMPR2 = bone morphogenetic protein receptor 2; CTGF = connective tissue growth factor; LDLR = low-density lipoprotein receptor; LV = left ventricle; HPASMC = human pulmonary arterial smooth muscle cells; MCT = monocrotaline; MI = myocardial infarction; NT-proBNP = N-terminal prohormone of brain natriuretic peptide; PA = pulmonary artery; PAEC = pulmonary artery endothelial cells; PAH = pulmonary arterial hypertension; PASMC = pulmonary artery smooth muscle cells; PASP = pulmonary arterial systolic pressure; PDGF-BB = platelet-derived growth factor BB; PH = pulmonary hypertension; PPARγ = peroxisome proliferator–activated receptor-γ; RV = right ventricle; RVH = right ventricular hypertrophy; RVSP = right ventricular systolic pressure; S = septum; SMC = smooth muscle cells; smLRP1 = smooth muscle low-density lipoprotein receptor–related protein 1; Stat3 = signal transducer and activator of transcription 3; SuHx = Sugen 5416/hypoxia; TGFβ1 = transforming growth factor β1.
Table 2.
Clinical Trials
| Reference, Study Group, and Treatment | Study Goal, Design, and Years | Disease State and Cohort Size | Inclusion and Major Exclusion Criteria | Primary Outcome and Results | Secondary Outcomes and Results | General and Cardiac Toxicity |
|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Definition of abbreviations: ACS = acute coronary syndrome; BMI = body mass index; BP = blood pressure; BNP = brain natriuretic peptide; CAG = coronary angiography; CI = confidence interval; CRP = C-reactive protein; DM = diabetes mellitus; HbA1c = glycated hemoglobin; HDL = high-density lipoprotein; HF = heart failure; HOMA-IR = homeostatic model assessment of insulin resistance; HR = hazard ratio; IRIS = Insulin Resistance Intervention after Stroke trial; LDL = low-density lipoprotein; LV = left ventricle; LVEDP = left ventricular end-diastolic pressure; LVEF = left ventricular ejection fraction; N/A = not applicable; ns = nonsignificant; NYHA = New York Heart Association; PCI = percutaneous coronary intervention; PIRAMID = Pioglitazone Influence on Triglyceride Accumulation in the Myocardium in Diabetes; PRIDE = Pioglitazone Reduce Inflammation and Restenosis with and without Drug Eluting Stent; T2DM = diabetes mellitus type 2; TIA = transient ischemic attack; TZD = thiazolidinediones; UA = unstable angina; ULN = upper limit of normal.
See data supplement for more detailed table (Table E1).
Pathobiology of PVD and Right Heart Failure
Pulmonary hypertensive vascular disease relates in large part to increased growth factor–mediated cell proliferation, resistance to apoptosis, endothelial dysfunction, and inflammation (with a smaller contribution from vasoconstriction) (2). Moreover, stem cells and progenitor and differentiated blood cells play important roles in progressive, fatal PAH, which has an underestimated prevalence worldwide. Importantly, metabolic dysfunction and epigenetic dysregulation (microRNAs [miRNAs]; histone deacetylases) are emerging major mechanisms involved in both the development and progression of PVD/PH and RV failure—the leading cause of death in PAH. Although until recently it was believed that PAH pathology is restricted to pulmonary arteries, several extrapulmonary organs (heart, skeletal muscle, and adipose tissue) are also affected (10–14). The notion that these tissues share common metabolic abnormalities (i.e., suppression of mitochondrial glucose oxidation and increased glycolysis, disturbed fatty acid oxidation [FAO], and dyslipidemia/insulin resistance) is important for the understanding of PAH, if not a paradigm shift (5, 10, 14–16).
The Vasoprotective Role of PPARγ in PVD
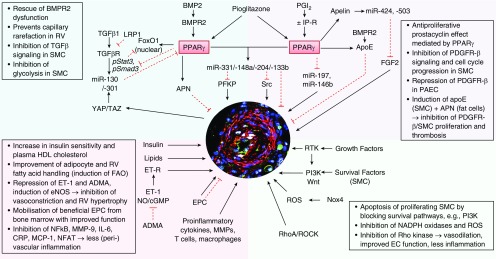
PPARs (PPARα, PPARβ/δ, and PPARγ) are ligand-activated transcription factors belonging to the nuclear receptor superfamily (17). PPARγ is ubiquitously expressed and plays a major role in adipogenesis and glucose metabolism (18), as well as placental and cardiac development (19). Upon ligand activation, PPARγ heterodimerizes with the retinoid X receptor and regulates multiple target genes (i.e., APN [adiponectin], IL6, MCP1 [monocyte chemotactic protein 1; CCL2], ET1 [endothelin 1]), all of which are strongly implicated in the pathobiology of PAH (2, 15). PPARγ agonists have antiproliferative (smooth muscle cells [SMC]), antiinflammatory, proangiogenic, and proapoptotic effects, all of which are beneficial in PAH (Figure 1). Moreover PPARγ has been found to exert protective roles in controlling endothelial metabolic homeostasis and genomic stability (20, 21). Thus, PPARγ agonists have therapeutic potential in PAH and other cardiopulmonary diseases, even in the absence of insulin resistance (15).
Figure 1.
Regulatory pathways that are controlled by PPARγ and crucial for pulmonary vascular remodeling and reverse remodeling in pulmonary arterial hypertension (PAH). The central immunofluorescence image shows a human pulmonary vascular lesion in PAH. ADMA = asymmetrical dimethylarginine; APN = adiponectin; apoE = apoliprotein E; BMP2 = bone morphogenetic protein 2; BMPR2 = BMP receptor 2; cGMP = cyclic guanosine-3′,5′-monophosphate; CRP = C-reactive protein; EC = endothelial cell(s); eNOS = endothelial nitric oxide synthase; EPC = endothelial progenitor cell(s); ET-1 = endothelin 1; ET-R = ET receptor; FAO = fatty acid oxidation; FGF2 = fibroblast growth factor 2; HDL = high-density lipoprotein; IP-R = prostacyclin receptor; LRP1 = low-density lipoprotein receptor–related protein 1; MCP-1 = monocyte chemoacttractant protein 1; MMP-9 = matrix metallopeptidase 9; NADPH = dihydronicotinamide adenine dinucleotide phosphate; NFAT = nuclear factor of activated T-cells; NO = nitric oxide; NOX4 = nicotinamide adenine dinucleotide phosphate oxidase 4; PAEC = pulmonary arterial endothelial cell(s); PDGFRβ = platelet-derived growth factor receptor-β; PFKP = phosphofructokinase, platelet; PGI2 = prostacyclin; PPARγ = proliferator–activated receptor γ; pStat3 = phosphorylated signal transducer and activator of transcription 3; RhoA = Ras homologous family member A; ROCK = Rho-associated protein kinase; ROS = reactive oxygen species; RTK = receptor tyrosine kinase; RV = right ventricle; SMC = smooth muscle cell(s); STAT3 = signal transducer and activator of transcription 3; TAZ = transcriptional coactivator with a PDZ-binding domain (also known as WWTR1, WW domain containing transcription regulator 1); TGFβ = transforming growth factor β; TGFRβ = TGF β receptor; YAP (also known as YAP1) = yes-associated protein 1.
PPARγ Cross-Talk with Transforming Growth Factor-β Superfamily Members Plays a Major Role in Pulmonary Vascular Homeostasis
TGFβ (transforming growth factor β) receptor superfamily members (BMPR2 [bone morphogenetic protein receptor 2], ACVRL1 [activin A receptor like type 1], endoglin) and their ligands play a critical role in the etiology of PAH. BMP2 (bone morphogenetic protein 2) is a ligand of BMPR2 and a negative regulator of SMC growth. However, in endothelial cells, BMP2 acts as a survival factor and thus may counteract endothelial injury and dysfunction that occur in the early stages of PAH. Loss-of-function mutations in the BMPR2 gene frequently occur in cases of familial/heritable PAH (70%; i.e., germline mutations) and idiopathic pulmonary arterial hypertension (IPAH; 10–20%), and the previous discovery of an antiproliferative BMP2/BMPR2–PPARγ–apolipoprotein E axis (22) in SMC suggests that BMPR2 dysfunction decreases endogenous PPARγ activity (22). Hence, a strategy aimed at activating PPARγ could reverse the PAH phenotype in patients with or without BMPR2 mutations. This concept appears to be even more appealing in light of reports on the decreased pulmonary BMPR2 expression even in the absence of BMPR2 mutations in idiopathic or heritable PAH and in PAH associated with connective tissue or congenital heart disease (23). Of note, patients with PAH have reduced pulmonary mRNA expression of BMP2 (24), PPARγ (25), and apolipoprotein E (24). S100A4-mediated activation of RAGE (receptor for advanced glycation end products) induced STAT3 (signal transducer and activator of transcription 3) phosphorylation and decreased both BMPR2 and PPARγ protein expression in PAH human pulmonary arterial smooth muscle cells (HPASMC) (26). Conversely, RAGE inhibition led to the restoration of the BMPR2–PPARγ axis in HPASMC (26). In addition, PPARγ confers growth-inhibitory signals in hypoxia-exposed HPASMC through suppression of miR-21, and its activation leads to de-repression of PDCD4 (programmed cell death protein 4) that facilitates HPASMC apoptosis (27).
In 2017, PPARγ was identified as a missing link and a key regulator of the functional antagonism between BMP2 and TGFβ1 pathways in human and murine vascular SMC (28, 29). In HPASMC, a novel, noncanonical TGFβ1-pSTAT3–pFoxO1 (phosphorylated FoxO1) pathway is inhibited by PPARγ activation (pioglitazone), as is the canonical TGFβ1–pSmad3/4 axis (28). Moreover, pioglitazone reversed PAH and pulmonary vascular remodeling that develops in TGFβ-overexpressing mice (28). LRP1 (low-density lipoprotein receptor–related protein 1), also named TGFβ receptor 5, interacts with several ligands, such as growth factors, cytokines, lipoproteins, and extracellular matrix glycoproteins. Most recently, it was demonstrated that vascular LRP1 expression is decreased in human PAH and that LRP1 in vascular SMC protects from PAH in vivo (30). Importantly, PPARγ activation by pioglitazone reverses PAH caused by LRP1 deficiency in murine SMC, inhibiting Smad3, Nox4 (nicotinamide adenine dinucleotide phosphate oxidase 4), and CTGF (connective tissue growth factor) (30).
PPARγ and miRNAs in Pulmonary Vascular Homeostasis and Remodeling
Beyond direct studies of PPARγ alone, large numbers of miRNA molecules have been discovered as molecular effectors or modulators of PAH pathogenesis. These miRNAs are expressed in pulmonary vascular and cardiac cells and exert systems-wide regulatory functions in multiple aspects of cardiovascular health and disease (29, 31, 32). In recent years, increasing data have accumulated highlighting the importance of miRNA-based activity in PPARγ-related vascular pathobiology, notably in PAH. Using a combination of in silico and experimental techniques, it was shown that the miR-130/-301 family promotes PH via systems-level regulation of miRNA networks (33–35), with an important centralized role played by PPARγ as a direct target of this miRNA family (Figure 1). Importantly, the major findings were recapitulated in explanted heart and lung tissues from patients with end-stage PAH. For example, increased miR-130a/-301b expression was demonstrated in laser-assisted microdissected pulmonary arteries from patients with IPAH compared with control subjects (28). Moreover, TGFβ1 stimulation decreases PPARγ mRNA via miR-130a/-301b, thus suppressing the BMP2/BMPR2–PPARγ axis in HPASMC. New miRNAs upregulated by the BMP2–PPARγ axis have recently been identified. In HPASMC, miR-331-5p is induced by BMP2 and downregulates the platelet isoform of PFKP (phosphofructokinase) mRNA expression, and PFKP is a rate-limiting enzyme of glycolysis. As such, PFKP has been demonstrated to be a proproliferative factor that is highly expressed in situ in pulmonary arteries of patients with IPAH versus control subjects (28). Activation of the BMP2/BMPR2–PPARγ axis upregulates miR-148a (suspected to repress cell proliferation) and miR-331-5p, thereby inhibiting SMC proliferation and glucose metabolism (28, 29). Therefore, PPARγ activation can restore or sustain TGF-β1–BMP2 homeostasis (balance) by regulating canonical and noncanonical TGF-β1 pathways and by conducting key miRNAs involved in cell proliferation and glucose/lipid metabolism (Figure 1).
BMPR2, FAO, and Lipotoxicity in the Hypertensive Right Ventricle in PAH
In contrast to SMC, the role of BMP2/BMPR2, and particularly that of PPARγ, in cardiomyocytes, cardiac fibroblasts, pericytes, and heart failure with or without RV pressure overload (e.g., PAH or pulmonary arterial banding), is largely unknown. Insulin resistance in PAH is characterized by alterations in lipid and lipoprotein homeostasis axes, manifested by an elevated triglyceride/high-density lipoprotein (HDL) ratio (10, 14). Ceramides have been suggested to accumulate in the right ventricle of deceased patients with PAH and to contribute to lipotoxicity in BMPR2-mutant mice (36, 37). However, a comprehensive analysis in murine sepsis-related cardiac dysfunction did not indicate any association with alteration of ceramide concentrations in the heart (38). Cardiac lipotoxicity (37) may directly arise from the decreased FAO that occurs in the Sugen 5416/hypoxia (SuHx) PAH rat model and end-stage human PAH right ventricles (39), and thus lipotoxicity could be prevented with pioglitazone treatment (through induction of PPARs, adiponectin, FAO-promoting genes, miRNAs, and other mechanisms).
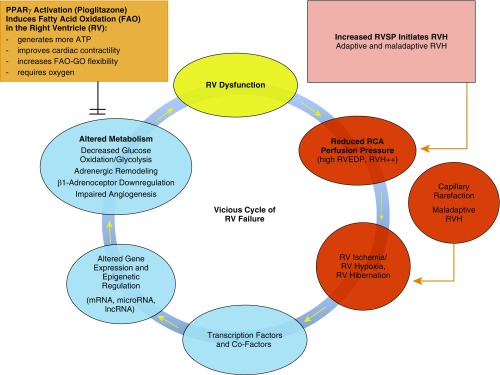
The Epigenetic–Metabolic Transition from a Healthy Heart to RV Hypertrophy, Hibernation, and Failure
The epigenetic–metabolic abnormalities in PVD/PH (Figure 1) and RV failure (Figure 2) include altered transcription factor interplay and epigenetic networks (miRNA; histone deacetylases), less efficient glucose oxidation and increased glycolysis (“glycolytic switch”), downregulation of β1-adrenoceptors, and impaired angiogenesis. Most of these detrimental events are crucial for sustaining the vicious cycle of RV failure, when the right ventricle turns from hibernating to frank failure state (Figure 2), but may be addressed by the interventions we propose, such as the PPARγ agonist pioglitazone (Figure 2). Only few studies so far have explored the dynamic expression and function of miRNAs in the failing right ventricle (e.g., miRs 28, 34a, 93, 126, 146b, 148a, and 197), despite their significant impact on cardiac performance. Targeted deletion of PPARγ in cardiomyocytes leads to biventricular systolic dysfunction in the absence of PAH (39). Intriguingly, 5-week oral treatment with pioglitazone reverses PAH and prevents RV failure in the SuHx rat model by directing distinct mRNA and miRNA networks, restoring mitochondrial function (FAO), and preventing intramyocardial lipid accumulation (39). miR-197 expression and miR-146b were upregulated in the failing SuHx right ventricle and downregulated by pioglitazone (39). The major miRNA findings were recapitulated in human end-stage IPAH because both miR-197 and miR-146b were found to be upregulated in the pressure-overloaded failing human right ventricle (39). In addition, mRNA expression of PPARG (peroxisome proliferator–activated receptor-γ), CPT1B (carnitine palmitoyltransferase 1B), and FABP4 (fatty acid–binding protein 4) was significantly decreased in the failing right ventricles of patients with IPAH (9.7-, 4.3-, and 2-fold, respectively) compared with donor control subjects, whereas all three genes were induced by pioglitazone in cultured cardiomyocytes. Additional mechanistic studies in cardiomyocytes identified a direct link between overexpression of miR-197 and miR-146b and the suppression of genes that drive FAO (39). Adiponectin, an adipocytokine induced by pioglitazone, protects the vasculature (5, 40, 41) and provides a defense mechanism against reactive oxidative stress in the stressed human heart when it is secreted from epicardial fat cells (42). Consistently, pioglitazone modulated myocardial substrate metabolism and improved LV diastolic function in patients with well-controlled type 2 diabetes mellitus (43, 44). Importantly, we did not observe any toxicity with (supra-)physiological doses of pioglitazone in cultured human pulmonary arterial endothelial cells (controls, IPAH) or cardiomyocytes (39). Taken together, PPARγ activation can normalize epigenetic and transcriptional regulation related to disturbed lipid metabolism and mitochondrial morphology/function in the failing right ventricle and the hypertensive pulmonary vasculature, representing a therapeutic approach for PAH and other cardiovascular/pulmonary diseases.
Figure 2.
Key events and mechanisms on tissue, cell, and molecular levels leading to the vicious cycle of RV failure in PAH. GO = glucose oxidation; lncRNA = long noncoding RNA; RCA = right coronary artery; RVEDP = right ventricular end-diastolic pressure; RVH = right ventricular hypertrophy; RVSP = right ventricular systolic pressure.
Revival of Pioglitazone, a PPARγ Agonist of the TZD Class
Pioglitazone and rosiglitazone are PPARγ agonists of the TZD class that have the potential to reverse advanced PVD (15); however, their effects in the heart are less clear (45, 46). The TZDs received “bad press” in 2007–2011 for potential adverse effects (mainly reversible heart failure and bladder cancer). Most notably, a putative increase in cardiovascular mortality was attributed to the TZD drug rosiglitazone but has been dismissed by the U.S. Food and Drug Administration in recent years after readjudication of the RECORD (Rosiglitazone Evaluated for Cardiac Outcomes and Regulation of Glycaemia in Diabetes) trial (6).
Potential Adverse Events with the Use of Pioglitazone in High-Risk Populations
TZDs are not believed to cause cardiac dysfunction directly, but rather to exacerbate preexisting heart failure via fluid retention in susceptible patients with multiple cardiovascular risk factors such as diabetes (45). Two mechanisms likely contribute to fluid retention and edema with TZDs such as pioglitazone: salt and water retention due to effects on the renal tubular epithelial sodium channel and other effects in the collecting duct (47) and perhaps increased vascular permeability (48). Several actions to address the problem of fluid retention and edema are possible. First, a lower final dose of pioglitazone (7.5–30 mg) after dose titration could be effective. Even 7.5 mg of pioglitazone per day appears to confer much of the benefit of the drug with less weight gain and fluid retention (49). Second, potassium-sparing, weak diuretic medications such as the mineralocorticoid receptor antagonists spironolactone and eplerenone or, alternatively, amiloride, a specific antagonist of the epithelial sodium channel, may be effective in counteracting the weight gain and fluid retention that may occur with pioglitazone. Mineralocorticoid receptor antagonists are first-line medications in left heart failure and are frequently used in patients with PAH with or without ventricular dysfunction. Importantly, an increase in incident heart failure was not observed with pioglitazone in the large IRIS trial (7–9), but concerns have nonetheless been raised about heart failure as an adverse effect of pioglitazone.
Recent data from the IRIS trial, which included 3,876 patients (64 ± 11 yr old) with insulin resistance/prediabetes status post–recent transient ischemic attack (TIA)/ischemic stroke (7), offer the most contemporary findings regarding the side effect profile of pioglitazone. Pioglitazone was associated with an increased occurrence of bone fractures in this high-risk population (number needed to harm [NNH], 125 in the on-treatment analysis) (9). More specifically, the number of serious fractures in the on-treatment analysis (≥80% drug compliance) was 3.6% (23 of 644) in those receiving pioglitazone versus 2.8% in those receiving pioglitazone placebo (23 of 810), leading to an event difference of 0.8% (9). Interestingly, there were more fractures in the patients enrolled in the IRIS trial who did not take pioglitazone faithfully (4.9%) than in the ones who did (3.6%) (D. Spence, M.D., personal communication, July 24, 2019). Furthermore, edema was defined as self-reported new or worse swelling of the feet or lower legs (NNH, 8), and weight gain was considered any change in body weight of 10% or more from baseline at any time in the trial (NNH, 7) (9). Importantly, there was no significant between-group difference in the number of patients with heart failure (74 vs. 71; P = 0.80) or in the number of patients hospitalized for heart failure (51 vs. 42; P = 0.35) in the IRIS trial, regardless of pioglitazone therapy (7). Incident bladder cancer also occurred in 12 patients in the pioglitazone group and in 8 in the placebo group (P = 0.37). The total incidence of cancer did not differ significantly between the two groups (133 patients and 150 patients, respectively; P = 0.29) (7). According to a meta-analysis of controlled studies published by 2013, over 20,000 patients would need to be treated with pioglitazone to cause one additional case of bladder cancer (50). Finally, there was no significant between-group difference in the incidence of other monitored adverse events in the IRIS trial (7), with the exception of a change in the alanine aminotransferase concentration, which was more favorable with pioglitazone. Overall, the IRIS investigators suggested that the clinical benefits of pioglitazone outweigh the risks of bone fracture and fluid retention, according to a post hoc analysis (9). The aforementioned postanalyses of the IRIS trial did not find any evidence that pioglitazone is associated with an elevated serious event rate for heart failure or bladder cancer (51).
Efficacy of Pioglitazone in Reducing Cardiovascular Events
On the basis of evolving evidence and reanalysis of previous clinical studies in the last two decades, pioglitazone was proposed by leading scientists for therapeutic revival because it is quite a safe drug with strong therapeutic potential beyond insulin resistance, with a net benefit of 40 fewer deaths per 100,000 population (45). Clinical studies on the impact of pioglitazone on RV function do not exist; however, pioglitazone did improve LV diastolic function in patients with diabetes (44) and without diabetes (52). Pioglitazone has less off-target effects and a better side effect profile than rosiglitazone, and it does improve systolic and diastolic LV function in mice, rats, and patients (44, 52). Importantly, a recent landmark publication demonstrated that genetic variation determines PPARγ function and antidiabetic drug response in vivo (53): Single-nucleotide polymorphism (SNP) alters the genome-wide binding of the transcription factor PPARγ, impacting the response of mice to antidiabetic drugs and affecting individual risk for metabolic disease in humans (53). Thus, natural genetic variation in PPARγ genomic occupancy determines individual disease risk and drug response. One PPARγ motif-altering SNP is associated with HDL concentrations and other metabolic syndrome parameters (53), making it highly relevant for PAH that is strongly associated with insulin resistance and low HDL cholesterol (10). It is entirely possible that SNPs alter the binding pattern of PPARγ not only in human fat but also in cardiovascular cells (SMC, endothelial cells, pericytes, and cardiomyocytes), suggesting SNP testing as a precision medicine approach before starting pioglitazone.
Pioglitazone prevented the development of LV fibrosis and left heart failure with preserved ejection fraction (severe diastolic LV dysfunction) in a rat model, at least partly due to attenuated Wnt–β-catenin signaling (54). The clinical effects of pioglitazone on the heart in the presence or absence of insulin resistance are not quite clear (45), but they were partly addressed in the IRIS trial (n = 3,876 patients without diabetes status post–TIA/ischemic stroke, clinically followed for 4.8 yr) (7). The risk of stroke or myocardial infarction was significantly lower among patients who received pioglitazone (n = 1,939) than among those who received placebo (n = 1,937; follow-up, 4.8 yr; hazard ratio, 0.76; 95% confidence interval, 0.62–0.93; P = 0.007) (7). Additional RCT data regarding the efficacy of pioglitazone in preventing cardiovascular events, in addition to potential adverse effects/toxicity, are listed in Tables 2 and E1, and overall argue in favor of pioglitazone.
Pioglitazone for PAH: Potential Trial Design
The average delay between onset of symptoms and PAH diagnosis is approximately 2 years, so that hardly any symptomatic patient with PAH is likely to be in a very early disease stage. Upfront combination PAH-targeted therapy is superior to and recommended versus monotherapy right after diagnosis in World Health Organization functional class 2 or 3 in most instances (1). Advanced/end-stage PAH requires (additional) intravenous pharmacotherapy with prostacyclin analogs and/or bilateral lung transplant. Thus, a promising trial design (among several possibilities) could include pioglitazone versus placebo as add-on medication to dual oral PAH-targeted pharmacotherapy, consisting of a PDE5 inhibitor (sildenafil, tadalafil) or soluble guanylate cyclase stimulator (riociguat) plus an endothelin receptor antagonist (macitentan, bosentan, ambrisentan) and no medication changes within the preceding 3 months. It will be key to use add-on pioglitazone in the early and midterm PAH disease stages. Enrollment of patients with stable PAH in such a double-blind RCT should occur within the first 5 years after diagnosis and in World Health Organization functional class 2 or 3. Eligible patients should be in the low- or intermediate-risk class according to the guidelines of the European Society of Cardiology/European Respiratory Society and World Symposium on Pulmonary Hypertension 2018 (1) to ensure that the patient is not experiencing disease progression and is not in very advanced or end-stage PAH at the time of enrollment. Treatment-naive, usually newly diagnosed patients can also be enrolled to receive upfront pioglitazone or placebo plus simultaneous dual oral combination PAH-targeted pharmacotherapy (see above), as long as their condition is stable at the time of diagnosis. The World Symposium on Pulmonary Hypertension 2018 recommends triple sequential combination therapy if patients with PAH cannot be brought to low risk within 6 months of combination therapy. We suggest that patients with a predefined insufficient response to any treatment (any study arm) can be treated with add-on prostaglandin I2 receptor (IP receptor) receptor agonist (selexipag) or parenteral prostacyclin analog (PCA) (iloprost/treprostinil by inhalation, treprostinil s.c., treprostinil/epoprostenol i.v.). The duration of this trial of pioglitazone in PAH should be 6 months, followed by an open-label phase of at least 12 months (with or without crossover). All patients should receive a mineralocorticoid receptor antagonist (spironolactone, eplerenone) for at least 3 months before randomization. Patients should be allowed to receive (additional) moderately dosed diuretics when predefined weight gain/fluid retention occurs.
In terms of prognostic outcome variables, a recent post hoc analysis of the GRIPHON (Selexipag [ACT-293987] in Pulmonary Arterial Hypertension) RCT established the prognostic relevance of NT-proBNP (N-terminal prohormone of brain natriuretic peptide) concentrations in PAH and provided the first evidence for the association of NT-proBNP concentration and treatment response (55). Using two similar sets of thresholds, these analyses support the relevance of the low, medium, and high NT-proBNP categories as part of the multiparametric risk assessment approach outlined in the European Society of Cardiology/European Respiratory Society guidelines for the management of patients with PAH (55). Because enrollment in phase 2 studies is usually less than 100 subjects, a combined morbidity/mortality event as a primary outcome (as in the GRIPHON RCT) is likely not feasible. However, the primary outcome of a phase 2 RCT of pioglitazone in PAH could be a combination of change in the prognostic NT-proBNP serum concentration (two-tailed) and change in 6-minute-walk distance.
In conclusion, we believe that the existing, extensive data on the efficacy of pioglitazone in preclinical cell and animal models (Figures 1 and 2 and Table 1), as well as the convincing recent clinical trial data on the lack of significant toxicity in high-risk populations (Tables 2 and E1), justify the timely conduct of clinical studies to achieve “repurposing” or “repositioning” of pioglitazone for the treatment of clinical PAH. This concept has been supported by recent publications, including a contemporary and extensive review on 22 drugs with the potential to be repurposed for the treatment of PAH (56). Of the 22 medications that were very critically reviewed by Prins and colleagues (57), pioglitazone currently reaches the second highest “numerical score of preclinical rigor.” The rigor score includes the number of animal models with drug efficiency and whether human tissues have been studied, among other criteria. The TGFβ-overexpressing mouse (28) is the fifth PAH animal model in which PPARγ agonists improve or reverse PAH, bringing the preclinical rigor score to 9 (dichloroacetate score, 11; metformin score, 8). Moreover, new non-TZD class PPARγ agonistic drugs (including mitochondrial target of TZD (mTOT) mitochondrial modulators) are being tested in preclinical and clinical studies (45), but not in PAH or heart failure yet. Interestingly, prostacyclin analogs (58), the most effective PAH drug class used clinically so far, and sildenafil (59) exert their antiproliferative, beneficial effects in PAH via PPARγ activation. The convincing recent clinical trial data on the lack of significant toxicity in high-risk populations justify the timely conduct of clinical studies to on the use pioglitazone (or other PPARγ-activating agents) for the treatment of PAH in humans.
Supplementary Material
Footnotes
Supported by the German Research Foundation (DFG; HA4348/2-2 and HA4348/6 KFO311), Kinderherzen (W-H-001-2014), and the European Pediatric Pulmonary Vascular Disease Network (www.pvdnetwork.org) (G.H.). L.C. is the recipient of a DFG Postdoctoral Fellowship (CA 1303/1-1). S.Y.C. was supported by National Institutes of Health grants R01 HL124021, HL 122596, HL 138437, and UH2 TR002073 as well as the American Heart Association Established Investigator Award (18EIA33900027).
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2019-0226PS on October 2, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Galiè N, Channick RN, Frantz RP, Grünig E, Jing ZC, Moiseeva O, et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J. 2019;53:1801889. doi: 10.1183/13993003.01889-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Humbert M, Guignabert C, Bonnet S, Dorfmüller P, Klinger JR, Nicolls MR, et al. Pathology and pathobiology of pulmonary hypertension: state of the art and research perspectives. Eur Respir J. 2019;53:1801887. doi: 10.1183/13993003.01887-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hansmann G. Pulmonary hypertension in infants, children, and young adults. J Am Coll Cardiol. 2017;69:2551–2569. doi: 10.1016/j.jacc.2017.03.575. [DOI] [PubMed] [Google Scholar]
- 4.Hansmann G, Koestenberger M, Alastalo TP, Apitz C, Austin ED, Bonnet D, et al. 2019 updated consensus statement on the diagnosis and treatment of pediatric pulmonary hypertension: the European Pediatric Pulmonary Vascular Disease Network (EPPVDN), endorsed by AEPC, ESPR and ISHLT. J Heart Lung Transplant. 2019;38:879–901. doi: 10.1016/j.healun.2019.06.022. [DOI] [PubMed] [Google Scholar]
- 5.Hansmann G, Wagner RA, Schellong S, Perez VA, Urashima T, Wang L, et al. Pulmonary arterial hypertension is linked to insulin resistance and reversed by peroxisome proliferator-activated receptor-γ activation. Circulation. 2007;115:1275–1284. doi: 10.1161/CIRCULATIONAHA.106.663120. [DOI] [PubMed] [Google Scholar]
- 6.Lazar MA. Reversing the curse on PPARγ. J Clin Invest. 2018;128:2202–2204. doi: 10.1172/JCI121392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kernan WN, Viscoli CM, Furie KL, Young LH, Inzucchi SE, Gorman M, et al. IRIS Trial Investigators. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med. 2016;374:1321–1331. doi: 10.1056/NEJMoa1506930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young LH, Viscoli CM, Curtis JP, Inzucchi SE, Schwartz GG, Lovejoy AM, et al. IRIS Investigators. Cardiac outcomes after ischemic stroke or transient ischemic attack: effects of pioglitazone in patients with insulin resistance without diabetes mellitus. Circulation. 2017;135:1882–1893. doi: 10.1161/CIRCULATIONAHA.116.024863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spence JD, Viscoli CM, Inzucchi SE, Dearborn-Tomazos J, Ford GA, Gorman M, et al. Pioglitazone therapy in patients with stroke and prediabetes: a post hoc analysis of the iris randomized clinical trial. JAMA Neurol. 2019;76:526–535. doi: 10.1001/jamaneurol.2019.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zamanian RT, Hansmann G, Snook S, Lilienfeld D, Rappaport KM, Reaven GM, et al. Insulin resistance in pulmonary arterial hypertension. Eur Respir J. 2009;33:318–324. doi: 10.1183/09031936.00000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malenfant S, Potus F, Fournier F, Breuils-Bonnet S, Pflieger A, Bourassa S, et al. Skeletal muscle proteomic signature and metabolic impairment in pulmonary hypertension. J Mol Med (Berl) 2015;93:573–584. doi: 10.1007/s00109-014-1244-0. [DOI] [PubMed] [Google Scholar]
- 12.Jafri S, Ormiston ML. Immune regulation of systemic hypertension, pulmonary arterial hypertension, and preeclampsia: shared disease mechanisms and translational opportunities. Am J Physiol Regul Integr Comp Physiol. 2017;313:R693–R705. doi: 10.1152/ajpregu.00259.2017. [DOI] [PubMed] [Google Scholar]
- 13.Culley MK, Chan SY. Mitochondrial metabolism in pulmonary hypertension: beyond mountains there are mountains. J Clin Invest. 2018;128:3704–3715. doi: 10.1172/JCI120847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hemnes AR, Luther JM, Rhodes CJ, Burgess JP, Carlson J, Fan R, et al. Human PAH is characterized by a pattern of lipid-related insulin resistance. JCI Insight. 2019;4:123611. doi: 10.1172/jci.insight.123611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansmann G, Zamanian RT. PPARγ activation: a potential treatment for pulmonary hypertension. Sci Transl Med. 2009;1:12ps14. doi: 10.1126/scitranslmed.3000267. [DOI] [PubMed] [Google Scholar]
- 16.Bertero T, Oldham WM, Cottrill KA, Pisano S, Vanderpool RR, Yu Q, et al. Vascular stiffness mechanoactivates YAP/TAZ-dependent glutaminolysis to drive pulmonary hypertension. J Clin Invest. 2016;126:3313–3335. doi: 10.1172/JCI86387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Derosa G, Sahebkar A, Maffioli P. The role of various peroxisome proliferator-activated receptors and their ligands in clinical practice. J Cell Physiol. 2018;233:153–161. doi: 10.1002/jcp.25804. [DOI] [PubMed] [Google Scholar]
- 18.Dubois V, Eeckhoute J, Lefebvre P, Staels B. Distinct but complementary contributions of PPAR isotypes to energy homeostasis. J Clin Invest. 2017;127:1202–1214. doi: 10.1172/JCI88894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR, et al. PPARγ is required for placental, cardiac, and adipose tissue development. Mol Cell. 1999;4:585–595. doi: 10.1016/s1097-2765(00)80209-9. [DOI] [PubMed] [Google Scholar]
- 20.Li CG, Mahon C, Sweeney NM, Verschueren E, Kantamani V, Li D, et al. PPARγ interaction with UBR5/ATMIN promotes DNA repair to maintain endothelial homeostasis. Cell Rep. 2019;26:1333–1343, e7. doi: 10.1016/j.celrep.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diebold I, Hennigs JK, Miyagawa K, Li CG, Nickel NP, Kaschwich M, et al. BMPR2 preserves mitochondrial function and DNA during reoxygenation to promote endothelial cell survival and reverse pulmonary hypertension. Cell Metab. 2015;21:596–608. doi: 10.1016/j.cmet.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansmann G, de Jesus Perez VA, Alastalo TP, Alvira CM, Guignabert C, Bekker JM, et al. An antiproliferative BMP-2/PPARγ/apoE axis in human and murine SMCs and its role in pulmonary hypertension. J Clin Invest. 2008;118:1846–1857. doi: 10.1172/JCI32503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Atkinson C, Stewart S, Upton PD, Machado R, Thomson JR, Trembath RC, et al. Primary pulmonary hypertension is associated with reduced pulmonary vascular expression of type II bone morphogenetic protein receptor. Circulation. 2002;105:1672–1678. doi: 10.1161/01.cir.0000012754.72951.3d. [DOI] [PubMed] [Google Scholar]
- 24.Geraci MW, Moore M, Gesell T, Yeager ME, Alger L, Golpon H, et al. Gene expression patterns in the lungs of patients with primary pulmonary hypertension: a gene microarray analysis. Circ Res. 2001;88:555–562. doi: 10.1161/01.res.88.6.555. [DOI] [PubMed] [Google Scholar]
- 25.Ameshima S, Golpon H, Cool CD, Chan D, Vandivier RW, Gardai SJ, et al. Peroxisome proliferator-activated receptor γ (PPARγ) expression is decreased in pulmonary hypertension and affects endothelial cell growth. Circ Res. 2003;92:1162–1169. doi: 10.1161/01.RES.0000073585.50092.14. [DOI] [PubMed] [Google Scholar]
- 26.Meloche J, Courchesne A, Barrier M, Carter S, Bisserier M, Paulin R, et al. Critical role for the advanced glycation end-products receptor in pulmonary arterial hypertension etiology. J Am Heart Assoc. 2013;2:e005157. doi: 10.1161/JAHA.112.005157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Green DE, Murphy TC, Kang BY, Bedi B, Yuan Z, Sadikot RT, et al. Peroxisome proliferator-activated receptor-γ enhances human pulmonary artery smooth muscle cell apoptosis through microRNA-21 and programmed cell death 4. Am J Physiol Lung Cell Mol Physiol. 2017;313:L371–L383. doi: 10.1152/ajplung.00532.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calvier L, Chouvarine P, Legchenko E, Hoffmann N, Geldner J, Borchert P, et al. PPARγ links BMP2 and TGFβ1 pathways in vascular smooth muscle cells, regulating cell proliferation and glucose metabolism. Cell Metab. 2017;25:1118–1134, e7. doi: 10.1016/j.cmet.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 29.Calvier L, Chouvarine P, Legchenko E, Hansmann G. Transforming growth factor β1– and bone morphogenetic protein 2/PPARγ-regulated microRNAs in pulmonary arterial hypertension [letter] Am J Respir Crit Care Med. 2017;196:1227–1228. doi: 10.1164/rccm.201705-0923LE. [DOI] [PubMed] [Google Scholar]
- 30.Calvier L, Boucher P, Herz J, Hansmann G. LRP1 deficiency in vascular smc leads to pulmonary arterial hypertension that is reversed by PPARγ activation. Circ Res. 2019;124:1778–1785. doi: 10.1161/CIRCRESAHA.119.315088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chun HJ, Bonnet S, Chan SY. Translating microRNA biology in pulmonary hypertension: it will take more than “miR” words. Am J Respir Crit Care Med. 2017;195:167–178. doi: 10.1164/rccm.201604-0886PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang YF, Xu HM, Yu F, Wang M, Li MY, Xu T, et al. Crosstalk between microRNAs and peroxisome proliferator-activated receptors and their emerging regulatory roles in cardiovascular pathophysiology. PPAR Res. 2018;2018:8530371. doi: 10.1155/2018/8530371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bertero T, Lu Y, Annis S, Hale A, Bhat B, Saggar R, et al. Systems-level regulation of microRNA networks by miR-130/301 promotes pulmonary hypertension. J Clin Invest. 2014;124:3514–3528. doi: 10.1172/JCI74773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bertero T, Cottrill K, Krauszman A, Lu Y, Annis S, Hale A, et al. The microRNA-130/301 family controls vasoconstriction in pulmonary hypertension. J Biol Chem. 2015;290:2069–2085. doi: 10.1074/jbc.M114.617845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bertero T, Cottrill KA, Lu Y, Haeger CM, Dieffenbach P, Annis S, et al. Matrix remodeling promotes pulmonary hypertension through feedback mechanoactivation of the YAP/TAZ-miR-130/301 circuit. Cell Rep. 2015;13:1016–1032. doi: 10.1016/j.celrep.2015.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hemnes AR, Brittain EL, Trammell AW, Fessel JP, Austin ED, Penner N, et al. Evidence for right ventricular lipotoxicity in heritable pulmonary arterial hypertension. Am J Respir Crit Care Med. 2014;189:325–334. doi: 10.1164/rccm.201306-1086OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brittain EL, Talati M, Fessel JP, Zhu H, Penner N, Calcutt MW, et al. Fatty acid metabolic defects and right ventricular lipotoxicity in human pulmonary arterial hypertension. Circulation. 2016;133:1936–1944. doi: 10.1161/CIRCULATIONAHA.115.019351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drosatos K, Khan RS, Trent CM, Jiang H, Son NH, Blaner WS, et al. Peroxisome proliferator-activated receptor-γ activation prevents sepsis-related cardiac dysfunction and mortality in mice. Circ Heart Fail. 2013;6:550–562. doi: 10.1161/CIRCHEARTFAILURE.112.000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Legchenko E, Chouvarine P, Borchert P, Fernandez-Gonzalez A, Snay E, Meier M, et al. PPARγ agonist pioglitazone reverses pulmonary hypertension and prevents right heart failure via fatty acid oxidation. Sci Transl Med. 2018;10:eaao0303. doi: 10.1126/scitranslmed.aao0303. [DOI] [PubMed] [Google Scholar]
- 40.Hansmann G, Rabinovitch M. The protective role of adiponectin in pulmonary vascular disease [editorial] Am J Physiol Lung Cell Mol Physiol. 2010;298:L1–L2. doi: 10.1152/ajplung.00367.2009. [DOI] [PubMed] [Google Scholar]
- 41.Margaritis M, Antonopoulos AS, Digby J, Lee R, Reilly S, Coutinho P, et al. Interactions between vascular wall and perivascular adipose tissue reveal novel roles for adiponectin in the regulation of endothelial nitric oxide synthase function in human vessels. Circulation. 2013;127:2209–2221. doi: 10.1161/CIRCULATIONAHA.112.001133. [DOI] [PubMed] [Google Scholar]
- 42.Antonopoulos AS, Margaritis M, Verheule S, Recalde A, Sanna F, Herdman L, et al. Mutual regulation of epicardial adipose tissue and myocardial redox state by PPAR-γ/adiponectin signalling. Circ Res. 2016;118:842–855. doi: 10.1161/CIRCRESAHA.115.307856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van der Meer RW, Rijzewijk LJ, de Jong HW, Lamb HJ, Lubberink M, Romijn JA, et al. Pioglitazone improves cardiac function and alters myocardial substrate metabolism without affecting cardiac triglyceride accumulation and high-energy phosphate metabolism in patients with well-controlled type 2 diabetes mellitus. Circulation. 2009;119:2069–2077. doi: 10.1161/CIRCULATIONAHA.108.803916. [DOI] [PubMed] [Google Scholar]
- 44.Hughes AD, Park C, March K, Coady E, Khir A, Chaturvedi N, et al. A randomized placebo controlled double blind crossover study of pioglitazone on left ventricular diastolic function in type 2 diabetes. Int J Cardiol. 2013;167:1329–1332. doi: 10.1016/j.ijcard.2012.03.179. [DOI] [PubMed] [Google Scholar]
- 45.Soccio RE, Chen ER, Lazar MA. Thiazolidinediones and the promise of insulin sensitization in type 2 diabetes. Cell Metab. 2014;20:573–591. doi: 10.1016/j.cmet.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vega RB, Kelly DP. Cardiac nuclear receptors: architects of mitochondrial structure and function. J Clin Invest. 2017;127:1155–1164. doi: 10.1172/JCI88888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bełtowski J, Rachańczyk J, Włodarczyk M. Thiazolidinedione-induced fluid retention: recent insights into the molecular mechanisms. PPAR Res. 2013;2013:628628. doi: 10.1155/2013/628628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakamura A, Osonoi T, Terauchi Y. Relationship between urinary sodium excretion and pioglitazone-induced edema. J Diabetes Investig. 2010;1:208–211. doi: 10.1111/j.2040-1124.2010.00046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adachi H, Katsuyama H, Yanai H. The low dose (7.5 mg/day) pioglitazone is beneficial to the improvement in metabolic parameters without weight gain and an increase of risk for heart failure. Int J Cardiol. 2017;227:247–248. doi: 10.1016/j.ijcard.2016.11.126. [DOI] [PubMed] [Google Scholar]
- 50.Ferwana M, Firwana B, Hasan R, Al-Mallah MH, Kim S, Montori VM, et al. Pioglitazone and risk of bladder cancer: a meta-analysis of controlled studies. Diabet Med. 2013;30:1026–1032. doi: 10.1111/dme.12144. [DOI] [PubMed] [Google Scholar]
- 51.Young LH, Viscoli CM, Schwartz GG, Inzucchi SE, Curtis JP, Gorman MJ, et al. IRIS Investigators. Heart failure after ischemic stroke or transient ischemic attack in insulin-resistant patients without diabetes mellitus treated with pioglitazone. Circulation. 2018;138:1210–1220. doi: 10.1161/CIRCULATIONAHA.118.034763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Horio T, Suzuki M, Suzuki K, Takamisawa I, Hiuge A, Kamide K, et al. Pioglitazone improves left ventricular diastolic function in patients with essential hypertension. Am J Hypertens. 2005;18:949–957. doi: 10.1016/j.amjhyper.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 53.Soccio RE, Chen ER, Rajapurkar SR, Safabakhsh P, Marinis JM, Dispirito JR, et al. Genetic variation determines PPARγ function and anti-diabetic drug response in vivo. Cell. 2015;162:33–44. doi: 10.1016/j.cell.2015.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kamimura D, Uchino K, Ishigami T, Hall ME, Umemura S. Activation of peroxisome proliferator-activated receptor γ prevents development of heart failure with preserved ejection fraction; inhibition of Wnt-β-catenin signaling as a possible mechanism. J Cardiovasc Pharmacol. 2016;68:155–161. doi: 10.1097/FJC.0000000000000397. [DOI] [PubMed] [Google Scholar]
- 55.Chin KM, Rubin LJ, Channick R, Di Scala L, Gaine S, Galiè N, et al. Association of N-terminal pro brain natriuretic peptide and long-term outcome in patients with pulmonary arterial hypertension. Circulation. 2019;139:2440–2450. doi: 10.1161/CIRCULATIONAHA.118.039360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Prins KW, Thenappan T, Weir EK, Kalra R, Pritzker M, Archer SL. Repurposing medications for treatment of pulmonary arterial hypertension: what’s old is new again. J Am Heart Assoc. 2019;8:e011343. doi: 10.1161/JAHA.118.011343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prins KW, Archer SL, Pritzker M, Rose L, Weir EK, Sharma A, et al. Interleukin-6 is independently associated with right ventricular function in pulmonary arterial hypertension. J Heart Lung Transplant. 2018;37:376–384. doi: 10.1016/j.healun.2017.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Falcetti E, Hall SM, Phillips PG, Patel J, Morrell NW, Haworth SG, et al. Smooth muscle proliferation and role of the prostacyclin (IP) receptor in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2010;182:1161–1170. doi: 10.1164/rccm.201001-0011OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang J, Yang K, Xu L, Zhang Y, Lai N, Jiang H, et al. Sildenafil inhibits hypoxia-induced transient receptor potential canonical protein expression in pulmonary arterial smooth muscle via cGMP-PKG-PPARγ axis. Am J Respir Cell Mol Biol. 2013;49:231–240. doi: 10.1165/rcmb.2012-0185OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ryan JJ, Archer SL. The right ventricle in pulmonary arterial hypertension: disorders of metabolism, angiogenesis and adrenergic signaling in right ventricular failure. Circ Res. 2014;115:176–188. doi: 10.1161/CIRCRESAHA.113.301129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Behringer A, Trappiel M, Berghausen EM, Ten Freyhaus H, Wellnhofer E, Odenthal M, et al. Pioglitazone alleviates cardiac and vascular remodelling and improves survival in monocrotaline induced pulmonary arterial hypertension. Naunyn Schmiedebergs Arch Pharmacol. 2016;389:369–379. doi: 10.1007/s00210-015-1205-3. [DOI] [PubMed] [Google Scholar]
- 62.Shiomi T, Tsutsui H, Hayashidani S, Suematsu N, Ikeuchi M, Wen J, et al. Pioglitazone, a peroxisome proliferator-activated receptor-γ agonist, attenuates left ventricular remodeling and failure after experimental myocardial infarction. Circulation. 2002;106:3126–3132. doi: 10.1161/01.cir.0000039346.31538.2c. [DOI] [PubMed] [Google Scholar]
- 63.Liu Y, Tian XY, Mao G, Fang X, Fung ML, Shyy JY, et al. Peroxisome proliferator-activated receptor-γ ameliorates pulmonary arterial hypertension by inhibiting 5-hydroxytryptamine 2B receptor. Hypertension. 2012;60:1471–1478. doi: 10.1161/HYPERTENSIONAHA.112.198887. [DOI] [PubMed] [Google Scholar]
- 64.Viscoli CM, Inzucchi SE, Young LH, Insogna KL, Conwit R, Furie KL, et al. IRIS Trial Investigators. Pioglitazone and risk for bone fracture: safety data from a randomized clinical trial. J Clin Endocrinol Metab. 2017;102:914–922. doi: 10.1210/jc.2016-3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tanaka A, Komukai S, Shibata Y, Yokoi H, Iwasaki Y, Kawasaki T, et al. Pioglitazone Reduce Inflammation and Restenosis with and without Drug Eluting Stent (PRIDE) Study Investigators. Effect of pioglitazone on cardiometabolic profiles and safety in patients with type 2 diabetes undergoing percutaneous coronary artery intervention: a prospective, multicenter, randomized trial. Heart Vessels. 2018;33:965–977. doi: 10.1007/s00380-018-1143-3. [DOI] [PubMed] [Google Scholar]
- 66.Breunig IM, Shaya FT, McPherson ML, Snitker S. Development of heart failure in Medicaid patients with type 2 diabetes treated with pioglitazone, rosiglitazone, or metformin. J Manag Care Spec Pharm. 2014;20:895–903. doi: 10.18553/jmcp.2014.20.9.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.